Abstract
Antifreeze activity is induced by cold temperatures in winter rye (Secale cereale) leaves. The activity arises from six antifreeze proteins that accumulate in the apoplast of winter rye leaves during cold acclimation. The individual antifreeze proteins are similar to pathogenesis-related proteins, including glucanases, chitinases, and thaumatin-like proteins. The objective of this study was to study the regulation of antifreeze activity in response to ethylene and salicyclic acid, which are known regulators of pathogenesis-related proteins induced by pathogens. Nonacclimated plants treated with salicylic acid accumulated apoplastic proteins with no antifreeze activity. In contrast, when nonacclimated plants were exposed to ethylene, both antifreeze activity and the concentration of apoplastic protein increased in rye leaves. Immunoblotting revealed that six of the seven accumulated apoplastic proteins consisted of two glucanases, two chitinases, and two thaumatin-like proteins. The ethylene-releasing agent ethephon and the ethylene precursor 1-aminocyclopropane-1-carboxylate also induced high levels of antifreeze activity at 20°C, and this effect could be blocked by the ethylene inhibitor AgNO3. When intact rye plants were exposed to 5°C, endogenous ethylene production and antifreeze activity were detected within 12 and 48 h of exposure to cold, respectively. Rye plants exposed to drought produced both ethylene and antifreeze activity within 24 h. We conclude that ethylene is involved in regulating antifreeze activity in winter rye in response to cold and drought.
Overwintering plants become freezing tolerant only after they are exposed to cold temperatures. During this process of cold acclimation, plants accumulate novel proteins, including antifreeze proteins (AFPs) that presumably promote plant survival during freezing (Guy, 1990; Duman and Olsen 1993; Griffith and Antikainen, 1996). AFPs are located in the apoplast, where they bind to the surface of ice and modify its growth within intercellular spaces of plant tissues (Griffith et al., 1992; Antikainen et al., 1996; Pihakaski-Maunsbach et al., 1996). When the six AFPs from winter rye (Secale cereale) were characterized, they were found to be similar to three types of pathogenesis-related (PR) proteins: two are β-1,3-endoglucanases, two are endochitinases, and two are thaumatin-like proteins (TLPs; Hon et al., 1995). Cold-induced accumulation of chitinases has also been reported in barley (Tronsmo et al., 1993), wheat (Ergon et al., 1998), and bermudagrass (Gatschet et al., 1996). At the molecular level, transcripts of β-1,3-endoglucanases and endochitinases have been shown to be weakly expressed at warm temperature and strongly up-regulated by cold in winter wheat and rye both in controlled environments and in the field (Ergon et al., 1998; Gaudet et al., 2000; Yeh et al., 2000). Because the AFPs retain their enzymatic activities, they may also have antifungal properties that are important in disease resistance, particularly against low-temperature pathogens such as snow molds (Ergon et al., 1998; Hiilovaara-Teijo et al., 1999).
At this time, little is known about the regulation of AFPs in any plant. Although many genes regulated by cold are also expressed in response to drought and abscisic acid (ABA; reviewed by Hughes and Dunn, 1996; Xin and Browse, 2000), we have recently shown that the rye AFPs accumulate in response to drought but not ABA (Yu and Griffith, 2001). Chitinases and β-1,3-glucanases are known to be induced by stress-related ethylene in defense responses against pathogens (Boller et al., 1983), and by both ethylene-dependent and -independent pathways during pea seed germination (Petruzzelli et al., 1999). Furthermore, exogenously applied ethylene induces accumulation of β-1,3-endoglucanase and endochitinase and increases their enzymatic activities in bean leaves (Mauch et al., 1992). In addition, ethylene-responsive elements have been identified in the regulatory regions of a bean chitinase gene (Roby et al., 1991) and a β-1,3-endoglucanase gene (Ohme-Takagi and Shinshi, 1995).
Many studies have demonstrated that salicylic acid (SA) is an endogenous activator of the accumulation of PR proteins and systemic acquired disease resistance in plants (Malamy et al., 1990; Kessmann et al., 1994). In tobacco mosaic virus-infected tobacco (Nicotiana tabacum) plants (Malamy et al., 1990) and tobacco necrosis virus-infected cucumbers (Métraux et al., 1990), the accumulation of endogenously synthesized SA is correlated with the induction of PR proteins (Yalpani et al., 1991). The exogenous application of SA induces both resistance to tobacco mosaic virus and the accumulation of PR proteins in tobacco plants (White, 1979). Moreover, transgenic tobacco plants that express the gene encoding salicylate hydroxylase, which degrades SA into catechol, do not accumulate either SA or systemic acquired disease resistance-related PR proteins and show no resistance to pathogen infection (Gaffney et al., 1993).
We hypothesized that ethylene and/or SA could regulate the accumulation of the rye AFPs because of their similarity to PR proteins. Our approach was to treat plants grown at warm temperature with ethylene or SA and assay antifreeze activities of apoplastic extracts from the treated leaves. Where antifreeze activity was present, we examined the apoplastic proteins by SDS-PAGE and immunoblotting. We also assayed endogenous ethylene production in winter rye plants exposed to cold and drought. Our results show, for the first time, that ethylene induces the accumulation of AFPs that are normally induced by low temperature in leaves of winter rye plants.
RESULTS
Antifreeze Activity of Ethylene- and SA- Treated Samples
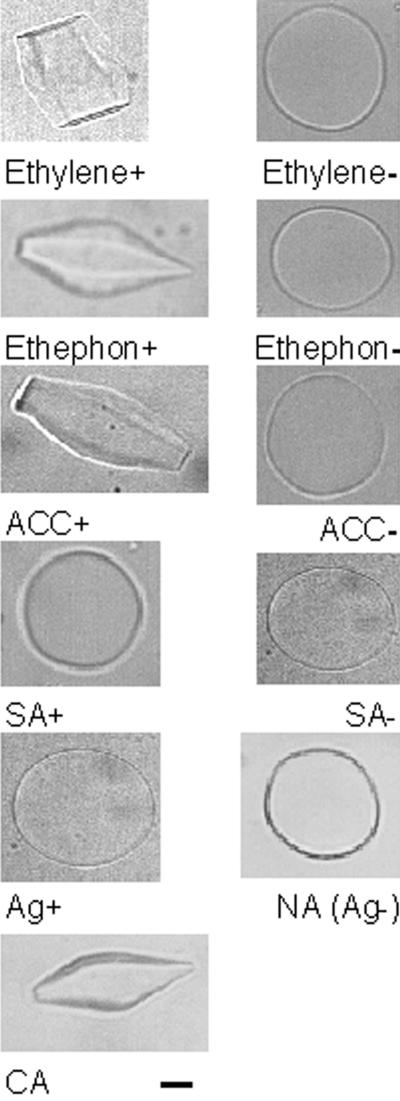
Apoplastic extracts from leaves of nonacclimated (NA) plants treated with ethylene, the ethylene-releasing agent 2-chloroethylphosphonic acid (ethephon), the ethylene precursor 1-aminocyclopropane-1-carboxylate (ACC), the ethylene inhibitor AgNO3, and SA were assayed for antifreeze activity. A low level of antifreeze activity was detected in NA leaves after a 72-h exposure to either ethylene or ethephon and after a 96-h exposure to ACC (data not shown). After the plants were treated with ethylene for 120 h, ice crystals grown in the apoplastic extracts were shaped like hexagonal columns, which indicated a high level of antifreeze activity (Fig. 1, Ethylene+). A similar high level of antifreeze activity was detected in the ethephon and ACC treatments at 168 and 144 h, respectively (Fig. 1, Ethephon+ and ACC+), when apoplastic protein concentrations reached their maxima (Fig. 2). One way to compare the relative amounts of antifreeze activity is to perform a dilution series and determine the dilution where the ice crystals lose their hexagonal shapes and become completely round and flat. The levels of ethephon- and ACC-induced antifreeze activities were equivalent to that found in cold-acclimated (CA) leaf extracts (Fig. 1, CA) because the ability of the extracts to modify the growth of ice disappeared at the same dilution (20-fold) of the apoplastic extracts from all three treatments. In contrast, no antifreeze activity was detected in extracts obtained from SA-treated plants (Fig. 1, SA+), even though PR proteins accumulated in the apoplast of these plants (data not shown). There was also no antifreeze activity in any of the extracts from control plants (Fig. 1, Ethylene−, Ethephon−, ACC−, and SA−), as indicated by the growth of disc-shaped ice crystals.
Figure 1.
Antifreeze activity of rye leaves treated to manipulate ethylene levels. Antifreeze activity was determined by observing the morphology of ice crystals grown in crude leaf apoplastic extracts of plants treated with 1 μL L−1 ethylene for 120 h (Ethylene+) and its control (Ethylene−); 10 mm ethephon for 168 h (Ethephon+) with 2 mm HCl and 2 mm H2PO3 used as its control (Ethephon−); 10 mm ACC for 168 h (ACC+) and its control (ACC−); 200 μm salicylic acid (SA+) and its control (SA−) at 192 h; and 200 μm AgNO3 (Ag+) and its control (Ag−) at 168 h. Ice crystals grown in extracts from plants that were grown under cold-acclimating conditions (CA) for 7 weeks or nonacclimating conditions (NA) for 3 weeks are shown for comparison. A representative crystal obtained from one of three independent experiments is shown. For all circular ice crystals, the basal plane is parallel to the plane of the page. For all hexagonal ice crystals, the basal plane is perpendicular to the plane of the page. Bar = 10 μm.
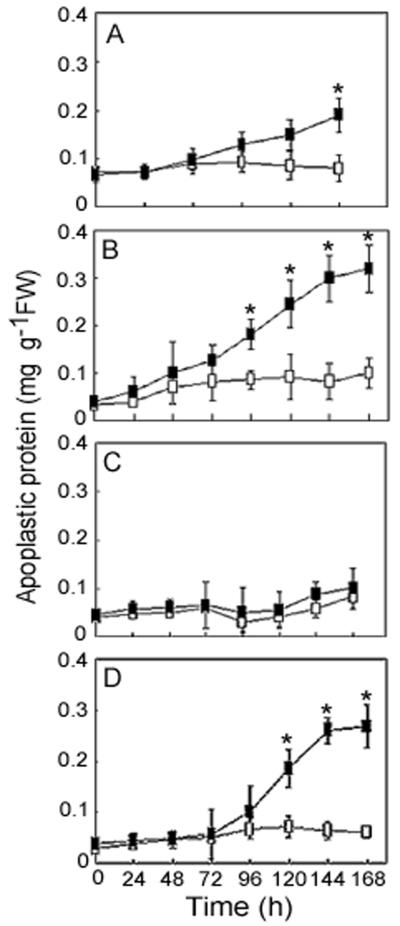
Figure 2.
Time course of accumulation of apoplastic proteins in rye leaves treated to manipulate ethylene levels. Apoplastic proteins were extracted at 24-h intervals from the leaves of NA rye plants treated with 1 μL L−1 ethylene (▪) or air (□) as a control inside a closed chamber (A), sprayed with 10 mm ethephon and 0.005% (v/v) Tween 20 (▪) or with 2 mm HCl, 2 mm H2PO3, and 0.005% (v/v) Tween 20 (□) as a control (B), sprayed with 10 mm ethephon and 0.005% (v/v) Tween 20 then watered with Hoagland solution containing 200 μm AgNO3 (▪) or sprayed with 2 mm HCl, 2 mm H2PO3, and 0.005% (v/v) Tween 20 (□) then watered with Hoagland solution as a control (C), or sprayed with 10 mm ACC and 0.005% (v/v) Tween 20 (▪) or with 0.005% (v/v) Tween 20 as a control (□) (D). Total apoplastic proteins were measured using the Bio-Rad method with bovine serum albumin as the standard protein and are presented as the means ± se (n = 3). An asterisk indicates that the total apoplastic protein content of treated winter rye leaves was significantly different (P = 0.05) from the control leaves at that time point.
Accumulation of Apoplastic Proteins in Ethylene-Treated Leaves
When NA plants were treated with 1 μL L−1 ethylene, the total apoplastic protein extracted from the leaves increased slowly, beginning 24 h after the initiation of ethylene treatment (Fig. 2A). After 120 h, the ethylene-treated leaves had accumulated 0.19 ± 0.02 mg apoplastic protein g−1 leaf fresh weight (mean ± se, n = 3), whereas only 0.08 ± 0.04 mg apoplastic protein g−1 leaf fresh weight was found in control leaves. During ethylene treatment, the NA plants were kept in a closed chamber and became wilted and chlorotic after 72 h, which could have been caused by ethylene itself and/or other factors, such as low O2 and high CO2. To eliminate the effects of a closed system, the ethylene-releasing agent ethephon was used to spray the NA plants. As shown in Figure 2B, NA plants that had been treated with ethephon for more than 72 h accumulated significantly more (P = 0.05) apoplastic proteins in their leaves than the control plants. After 144 h of ethephon treatment, the leaves accumulated 0.30 ± 0.02 mg apoplastic protein g−1 leaf fresh weight. By comparison, previous studies showed that apoplastic AFPs accumulate to levels of 0.3 and 0.1 mg protein g−1 fresh weight in winter rye and wheat leaves, respectively, after 7 weeks of cold acclimation (Marentes et al., 1993; Chun et al., 1998).
When taken up by plants, ethephon decomposes into ethylene, hydrochloric acid, and phosphoric acid. Therefore, the induction of apoplastic proteins in NA leaves by ethephon could be the effect of any one or a combination of the three compounds. To assess these possibilities, the control plants were sprayed with 2 mm hydrochloric acid and 2 mm phosphoric acid. The concentration of 2 mm of acids was chosen because treatment with 10 mm of either acid caused severe necrosis of the leaves, which was not seen in leaves treated with 10 mm ethephon, and because the decomposition of ethephon is a gradual process (Abeles, 1973) which does not normally result in a high concentration of acids released at one time on the leaf surface. Treatment of the leaves with 2 mm acids caused pinpoint necroses and mimicked the symptoms observed on leaves treated with 10 mm ethephon. The fact that apoplastic proteins accumulated specifically in ethephon-treated leaves, but not in the control leaves treated with acids (Fig. 2B), indicates that it was the ethylene that induced production of apoplastic proteins in NA rye leaves. The role of ethylene in the production of AFPs was also supported by the fact that ethephon-induced antifreeze activity (Fig. 1) and accumulation of apoplastic proteins (Fig. 2B) were both blocked by concurrently applying the ethylene inhibitor AgNO3 (Figs. 1 and 2C).
To further test the hypothesis that ethylene is involved in the induction of AFPs in NA winter rye, ACC, an immediate precursor to ethylene in the ethylene biosynthetic pathway (Yang and Hoffman, 1984), was used to elevate endogenous levels of ethylene. As shown in Figure 2D, the total apoplastic protein gradually increased in leaves of NA plants after a 72-h application of 10 mm ACC and reached a maximum level of 0.27 ± 0.03 mg protein g−1 leaf fresh weight at 144 h, which was significantly higher (P = 0.05) than that in the control. Compared with the time course of induction of apoplastic proteins by ethephon (Fig. 2B), there was a 24-h delay in the accumulation of apoplastic protein in leaves treated with ACC (Fig. 2D), which suggests that additional time was required to convert ACC to ethylene.
Analysis of Ethylene-Induced Apoplastic Proteins by SDS-PAGE and Immunoblotting
The apoplastic proteins extracted from ethephon-treated and control leaves were denatured and examined by SDS-PAGE and immunoblotting. Compared with the controls, seven polypeptides with molecular masses of 35, 32, 28, 25, 16, 14, and 11 kD, were rapidly induced by ethephon (Fig. 3A). The accumulation of these polypeptides closely followed the time course of total apoplastic proteins accumulated in NA leaves treated with ethephon as shown in Figure 2B. At the end of the induction experiment, the pattern of ethephon-induced polypeptides (Fig. 3A, lane 168 h, +) separated by SDS-PAGE was very similar to that of cold-induced polypeptides (Fig. 3A, lane CA) in terms of total numbers of polypeptides and the molecular mass of each polypeptide.
Figure 3.
Examination of polypeptides present in apoplastic extracts of ethephon-treated winter rye leaves by SDS-PAGE and immunoblotting. A, For SDS-PAGE, apoplastic proteins were extracted at 24-h intervals from NA rye leaves sprayed with 10 mm ethephon (+), from NA leaves sprayed with 2 mm HCl/H2PO3 (−) as a negative control, and from CA leaves (CA) as positive control and were denatured, separated on 15% (w/v) polyacrylamide gels, and stained with Coomassie Brilliant Blue R-250 (Bio-Rad, Mississauga, Canada). An equal volume of each apoplastic extract per gram leaf fresh weight (30 μL) was loaded on each lane. For immunoblotting, SDS-polyacrylamide gels loaded with 10 μL per lane of each apoplastic extract were blotted and probed with antisera produced against the cold-induced winter rye 32-kD glucanase (B), the cold-induced winter rye 35-kD chitinase (C), and the cold-induced winter rye 25-kD TLP (D). Low-range prestained SDS-PAGE standards (M) were used in both SDS-PAGE and immunoblotting analysis to determine the molecular mass (kD). The molecular mass of each immunodetected polypeptide is indicated on the right.
Immunoblots revealed that six of the seven abundant polypeptides induced by ethephon were positively detected by AFP antisera (Antikainen et al., 1996). As shown in Figure 3, a 32-kD glucanase, a 35-kD chitinase, and a 25-kD TLP were found in both ethephon-treated and control apoplastic extracts, but they accumulated to greater levels in the ethephon treatment. On the other hand, the 35-kD glucanase, 28-kD chitinase, and 16-kD TLP were detected only in extracts from ethephon-treated leaves, thus indicating that they were specifically induced by ethylene. Interestingly, these three proteins appeared at different timepoints: The 35-kD glucanase was detected 24 h after ethephon treatment began (Fig. 3B), the 28-kD chitinase was detected after 48 h (Fig. 3C), and the 16-kD TLP was not present until 96 h. Apoplastic extracts from ethylene- and ACC-treated plants also contained seven polypeptides similar to those induced by cold and by ethephon, including two glucanases, two chitinases, and two TLPs (data not shown).
Endogenous Ethylene Levels in Cold- and Drought-Treated Plants
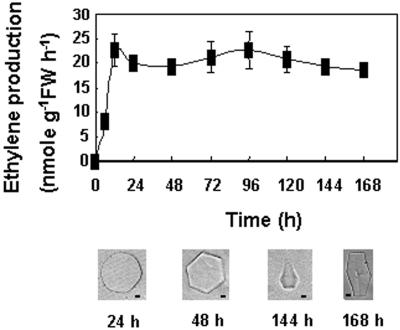
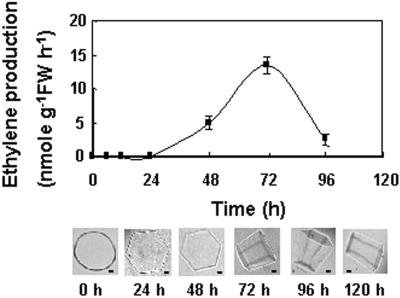
Three-week-old NA rye plants transferred to cold temperature began to produce ethylene quickly and peaked at a rate of 22.6 ± 3.2 nmol ethylene g−1 leaf fresh weight h−1 (mean ± se, n = 3) within 12 h (Fig. 4). The production of ethylene was sustained at this level throughout the 1-week course of the experiment. No endogenous ethylene production was detected in the NA control plants. A low level of antifreeze activity was detected in apoplastic extracts of the ethylene-treated NA plants 48 h after the transfer to cold (Fig. 4). By the end of a week at cold temperature, the ice crystals grown in the apoplastic extracts formed hexagonal columns and partial bipyramids (Fig. 4). Rye plants exposed to water stress responded differently than plants exposed to cold because ethylene was produced transiently. An increase in ethylene concentration was detected 24 h after watering stopped and peaked at 72 h (Fig. 5), when the maximum rate of ethylene production was 13.5 ± 1.3 nmol ethylene g−1 leaf fresh weight h−1 (mean ± se, n = 3). Ethylene levels then decreased during the phase when the plants were minimally rewatered. No ethylene production was detected in the corresponding well-watered NA control plants. A very low level of antifreeze activity was first detected after 24 h of water stress, which showed that the response to ethylene at 20°C was much quicker than observed at 5°C. After watering was stopped for 72 h, the ice crystals grown in leaf apoplastic extracts formed hexagonal columns.
Figure 4.
Time course of ethylene produced endogenously by plants transferred to low temperature. Ethylene content was measured by gas chromatography with analyzed ethylene standards and is presented as the means ± se (n = 3). Antifreeze activity was also determined in leaf apoplastic extracts at various time points after the plants were transferred to cold temperature. Representative ice crystals are shown for each time point. The two crystals on the left are shown with the basal plane parallel to the plane of the page. The two crystals on the right are shown with the basal plane normal to the plane of the page. Bars = 5 μm.
Figure 5.
Time course of ethylene produced endogenously by plants exposed to drought. Ethylene content was measured by gas chromatography with analyzed ethylene standards and is presented as the means ± se (n = 3). Antifreeze activity was also measured in leaf apoplastic extracts at various time points after the plants were subjected to water stress. Representative ice crystals are shown for each time point. The three crystals on the left are shown with the basal plane parallel to the plane of the page. The three crystals on the right are shown with the basal plane normal to the plane of the page. Bars = 5 μm.
DISCUSSION
Although SA and ethylene both act as signaling molecules to induce the accumulation of PR proteins in defense responses against pathogens, we have now shown that only ethylene is effective in signaling the development of antifreeze activity (Fig. 1). In response to ethylene treatment at warm temperature, winter rye quickly developed high levels of antifreeze activity (Fig. 1, Ethylene+). Moreover, the ethylene-induced apoplastic proteins were very similar to the cold-induced apoplastic proteins in concentration (Fig. 2; Marentes et al., 1993), molecular mass (Fig. 3A), and immunodetection by antisera to glucanases, chitinases, and TLPs (Fig. 3, B–D; Hon et al., 1995; Antikainen et al., 1996). The induction of antifreeze activity in winter rye by ethylene was further confirmed by application of the ethylene-releasing agent ethephon or the precursor of ethylene biosynthetic precursor ACC at warm temperature (Fig. 1, Ethephon+ and ACC+). Both ethephon and ACC induced levels of antifreeze activity and apoplastic glucanases, chitinases, and TLPs (data not shown) similar to those induced by low temperature and ethylene (Figs. 1–3). Additional evidence implicating ethylene in induction of rye AFPs is provided by the observation that the ethephon-induced appearance of both antifreeze activity and AFPs was inhibited by AgNO3 (Figs. 1 and 2), a known competitor for the binding site of ethylene (Davies et al., 1990).
Is ethylene involved in the accumulation of AFPs at low temperature in planta? Our experiments suggest that it may be. Winter rye plants produced ethylene endogenously within hours after being transferred to cold temperature, and this was followed by an increase in antifreeze activity and apoplastic protein content (Fig. 4). These results confirm earlier observations showing that antifreeze activity was detected within a week of transferring winter rye plants to cold temperature (Hon et al., 1995). Moreover, the sustained production of ethylene at cold temperature (Fig. 4) could be the signal that leads to the high localized levels of AFP accumulation required to effectively control the growth of ice. However, our data showing that individual AFPs accumulated differentially in response to ethylene (Fig. 3) do indicate that ethylene is probably not the sole regulator of antifreeze activity and that there must be other interacting factors that influence the accumulation of all AFPs. Although antifreeze activity was also induced by limiting water availability to the plants (Yu and Griffith, 2001), ethylene was produced only transiently during water stress (Fig. 5), so the high levels of antifreeze activity produced in response to the ethylene pulse are unlikely to be sustained. We found only one study that reports the induction of a gene by both cold and ethylene. The gene encodes a dehydrin and is expressed in spruce seedlings in response to cold, drought, and ethylene, as well as treatment with ABA or jasmonic acid or wounding (Richard et al., 2000). The AFPs in winter rye are not induced by ABA (Yu and Griffith, 2001) or wounding (X.-M. Yu and M. Griffith, unpublished data), so the signaling pathway(s) for the dehydrin may be different from those for the AFPs.
What evidence is available to implicate ethylene in the processes of cold acclimation or development of freezing tolerance in plants? Although ethylene has been long implicated in the development of chilling tolerance in annual crop plants, this ethylene is produced transiently only after chilled plants are returned to warmer temperatures (Field, 1984; Ciardi et al., 1997). The production of ethylene in response to low temperatures by freezing-tolerant plants is less well known. In one case, rhododendron plants treated with ethephon early in cold acclimation were shown to increase their freezing tolerance by several degrees, and rhododendron plants exposed to a sublethal freeze produced stress ethylene endogenously (Harber and Fuchigami, 1989). In another case, winter rape plants exposed to a sublethal freezing event also produced ethylene and increased their freezing tolerance (Kacperska and Kubacka-Zebalska, 1989).
Molecular studies have implicated ethylene in the transcriptional regulation of genes encoding PR proteins. Studies using promoter deletion analysis showed that an ethylene-responsive element or GCC box regulates transcription of a tobacco β-1,3-glucanase gene (Ohme-Takagi and Shinshi, 1995), a tomato basic chitinase gene (Zhou et al., 1997), and the PR-5d gene encoding an isoform of a tobacco TLP (Sato et al., 1996). However, Ohta and coworkers (2000) demonstrated that ethylene-responsive elements do not confer a simple response to ethylene because each ethylene-responsive factor (ERF) that binds specifically to the GCC box produces a different effect. In tobacco, ERF2 and ERF4 both function as transactivators of gene expression, with ERF4 inducing much greater levels of transcription than ERF2, whereas ERF3 represses gene expression. One subfamily of ERFs includes the transcriptional activators C-repeat binding factor (CBF1) and drought-responsive element binding proteins (DREBs), which enhance transcription of cold- and drought-responsive genes in Arabidopsis (Yamaguchi-Shinozaki and Shinozaki, 1994; Stockinger et al., 1997; Shinwari et al., 1998). CBF1 and the DREBs bind to a conserved 9-bp cis-acting element known as the C-repeat or drought-responsive element and contain a single copy of the same DNA-binding domain found in ERFs (Ohta et al., 2000). The core sequence CCGAC was shown to be required for cold-responsive expression of the Brassica napus gene BN115 as well (Jiang et al., 1996). At this time, it is not known whether CBF1 or the DREBs are responsive to ethylene. However, because winter rye AFPs are also PR proteins composed of β-1,3-glucanase, chitinase, and TLPs (Hon et al., 1995), it is reasonable to hypothesize that low temperature activates endogenous ethylene production in winter rye, followed by transactivation of AFP gene expression, leading to accumulation of glucanases, chitinases, and TLPs at low temperature.
We have not proven that ethylene acts alone in promoting the accumulation of antifreeze proteins. If ethylene does not act directly on gene expression, then it may serve to make the plants more sensitive to other hormones or it may enhance antifreeze activity by turning on a second, additive pathway governing a similar response. There is evidence that additive pathways are at work in the accumulation of PR proteins at low temperature. Recently Hiilovaara-Teijo et al. (1999) found that winter rye plants accumulated three classes of apoplastic PR proteins (glucanases, chitinases, and TLPs) in response to the infection by pink snow mold (Microdochium nivale), a low-temperature parasitic fungus, at warm temperature. Apoplastic extracts from snow mold-infected NA leaves exhibited both glucanase and chitinase activities but lacked antifreeze activity, which suggests that an ethylene-independent pathway was triggered by the pathogen. Rye plants transferred to cold temperature and immediately infected with snow mold showed the highest level of antifreeze activity, which may indicate the pathways are additive. There is also evidence that the production of ethylene at low temperature may sensitize the tissues of winter cereals. Ergon et al. (1998) showed that glucanase and chitinase gene expression occurred more quickly and reached higher levels when CA plants were infected by snow mold than could be accounted for by the additive effect of induction individually by pathogens and by cold.
We conclude that ethylene is a regulator of antifreeze activity and the accumulation of AFPs in response to cold and drought stress. Winter rye plants subjected to either cold temperature or drought may produce endogenous ethylene as a signal to initiate synthesis of AFPs directly, or it may interact with other mediators of gene expression.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of winter rye (Secale cereale L. cv Musketeer) were obtained from Dr. Grant McLeod (Agriculture Canada, Swift Current, SK, Canada). Seeds surface-sterilized with a 0.28% sodium hypochlorite solution for 5 min were planted in 15-cm pots of coarse vermiculite and germinated at 20°C/16°C (day/night) with a 16-h day length and a photosynthetic photon flux density (PPFD) of 300 μmol m−2 s−1 for a week. NA plants were grown under these conditions for an additional 2 weeks. CA plants were transferred to 5°C/2°C (day/night) with an 8-h day length and a PPFD of 300 μmol m−2 s−1 for an additional 7 weeks. NA plants grown at 20°C/16°C for 3 weeks have similar physiological age to the CA plants grown at 5°C/2°C for 7 weeks (Krol et al., 1984; Griffith and McIntyre, 1993). Plants were watered as needed with Hoagland solution (Hoagland and Arnon, 1950).
Hormonal Treatments
For ethylene treatment, one pot of NA plants was transferred into a 143-L sealed plexiglass chamber. Ethylene was administered by injecting 143 mL of 1,000 μL L−1 ethylene (Alltech, Deerfield, IL) to make a concentration of 1 μL L−1 in the chamber. Control plants were kept in a similar chamber with no ethylene. For ethephon treatment, leaves of NA plants were sprayed daily with 10 mm ethephon (Sigma, St. Louis; Brederode et al., 1991; Cabello et al., 1994) and 0.005% (v/v) Tween 20 at the beginning of dark period. Control plants were sprayed daily with 2 mm hydrochloric acid, 2 mm phosphoric acid, and 0.005% (v/v) Tween 20. For ACC treatments, NA plants were sprayed daily with 10 mm ACC (CalBiochem, San Diego) and 0.005% (v/v) Tween 20. Control plants were sprayed with only 0.005% (v/v) Tween 20. For ethylene inhibitor treatments, NA plants were watered daily with 200 μm AgNO3 (Smalle et al., 1997) in Hoagland nutrient solution and sprayed with 10 mm ethephon and 0.005% (v/v) Tween 20. Control plants were sprayed daily with 2 mm hydrochloric acid and 2 mm phosphoric acid as well as 0.005% (v/v) Tween 20 and watered daily with Hoagland solution. For salicylic acid treatment, NA plants were sprayed daily with 200 μm salicylic acid (Sigma) (Janda et al., 1999) and 0.005% (v/v) Tween 20. Control plants were sprayed with 0.005% (v/v) Tween 20. Three independent experiments were carried out for each of the above treatments and each treatment lasted for 120 to 168 h. Leaves of treated and control plants were harvested for apoplastic extraction at 24-h intervals.
Apoplastic Protein Extraction
Apoplastic proteins were extracted as described in Hon et al. (1994). Briefly, the leaves were cut into 3-cm sections, rinsed several times with deionized water, then vacuum-infiltrated with a solution of 20 mm ascorbic acid and 20 mm CaCl2, followed by centrifugation at 900g to recover the apoplastic contents. Total apoplastic protein content was measured using the Bradford (1976) method, as modified by Bio-Rad, with bovine serum albumin as the standard protein. The two-sample t test (two-tailed; SAS software, version 8.0, SAS Institute, Cary, NC) was used at the 95% level of confidence to detect differences in apoplastic protein accumulation between treated and control plants at each time point (n = 3).
Protein Electrophoresis and Immunoblotting
Apoplastic proteins extracted from treated and control rye leaves were denatured, and the polypeptides were separated in a 15% (w/v) SDS-polyacrylamide gel and stained with Coomassie Brilliant Blue R-250 (Bio-Rad) according to Laemmli (1970). An equal volume of each apoplastic extract per gram leaf fresh weight (30 μL) was loaded on each lane. In immunoblotting experiments, an equal volume of each apoplastic extract per gram leaf fresh weight (10 μL) was loaded on each lane. Low-range prestained SDS-PAGE molecular standards (Bio-Rad) were used to determined the apparent molecular masses of polypeptides. For immunoblotting, polypeptides separated by SDS-PAGE were transferred onto 0.45-μm nitrocellulose membranes (Bio-Rad) using a buffer composed of 25 mm Tris (pH 8.3), 192 mm Gly, and 20% (v/v) methanol, and the Mini Trans-Blot cell (Bio-Rad) according to the manufacturer's instructions. The blots were probed with rabbit anti-glucanase serum (dilution 1:2,000), or chitinase antiserum (dilution 1:2,000), or TLP antiserum (dilution 1:10,000) produced against isolated winter rye AFPs similar to 35-kD glucanase, 35-kD chitinase, and 25-kD TLP, respectively (Hon et al., 1994; Antikainen et al., 1996). The immunoreactions were detected by alkaline phosphatase conjugated to goat anti-rabbit IgG (Sigma) with 5-bromo-4-chloro-3-indolylphosphate-toluidine salt (BioShop, Burlington, ON, Canada) and nitroblue tetrazolium (Sigma) as substrates.
Antifreeze Activity
Antifreeze activity was assayed in 10-nL aliquots of apoplastic extracts obtained at 24-h intervals after the initiation of a treatment. The antifreeze assay is a qualitative one that involves examining the concentration-dependent morphology of ice crystals grown in solution (De Vries, 1986; Hon et al., 1994; Chun et al., 1998). The growth of a single ice crystal in each sample was controlled using the freezing stage of a nanoliter osmometer (Clifton Technical Physics, Hartford, NY), and the morphology of the ice crystal was examined using a phase-contrast photomicroscope (BHT, Olympus, Tokyo). In this assay, ice grown in a solution with no AFPs forms a round, flat crystal whose face is the basal plane and whose circumference is composed of prism faces. As the temperature is lowered, the crystal becomes larger in diameter as water molecules bind to its circumference. In the presence of a low (nanomolar) concentration of AFPs, the ice crystal forms a flat hexagon, due to adsorption of AFPs on the prism faces and subsequent inhibition of the binding of additional water molecules. In a higher (micromolar) concentration of AFPs, it becomes energetically favorable for water to bind to the basal plane of the ice crystal, which leads to crystal growth perpendicular to the basal plane and the formation of a hexagonally shaped column or bipyramid.
Endogenous Ethylene Measurements
For each measurement of endogenous ethylene production in response to cold, a single 3-week-old NA rye plant was placed in a test tube (2.5 cm in diameter, 25 cm in height) with its roots immersed in Hoagland nutrient solution and transferred to 5°C/2°C (day/night) with an 8-h day length and a PPFD of 300 μmol m−2 s−1 (time 0). Corresponding control plants were maintained under nonacclimating conditions at 20°C/16°C (day/night). When assaying ethylene production, the tube was sealed with a rubber cap. After 30 min, a 1-mL sample of gas was withdrawn using a gas-tight syringe and was analyzed using a gas chromatograph (model GC-17A, Shimadzu Scientific Instruments, Inc., Columbia, MD) equipped with an aluminum column (1.5 m long, 0.55 mm i.d.) and a flame ionization detector. The column temperature was isothermal at 100°C, and the injector and detector oven temperatures were 150°C. Analyzed ethylene standards (Alltech) were used to quantify the samples.
For drought treatments, pots of 3-week-old NA plants were not watered until they were visibly wilted. The first wilt symptoms usually appeared within 48 h from the last watering. After 72 h, the plants were rewatered daily with 100 mL of Hoagland nutrient solution, whereas the control plants were watered to excess with 500 mL. This watering regime lowered the relative water content of the plants from 85% to 76% (Yu and Griffith, 2001). For sampling gases and measuring ethylene, a pot of either drought-treated plants or well-watered control plants was placed in a plastic bag sealed with a rubber septum for 30 min. Cold- and drought-treated plants were sampled for ethylene production at 24-h intervals, and three independent experiments were conducted per treatment.
ACKNOWLEDGMENTS
We thank Dr. Grant McLeod (Agriculture Canada) for the Musketeer rye seeds and Vicky Jackson (University of Waterloo) for growing the plants. We would also like to thank Drs. Bernard R. Glick, Barbara A. Moffatt, and Robert W. Johnson (University of Waterloo) for both technical assistance and helpful discussions.
Footnotes
This work was supported by the Natural Science and Engineering Research Council of Canada (research grant to M.G.).
LITERATURE CITED
- Abeles FB. Ethylene in Plant Biology. New York: Academic Press; 1973. [Google Scholar]
- Antikainen M, Griffith M, Zhang J, Hon WC, Yang DSC, Pihakaski-Maunsbach K. Immunolocalization of antifreeze proteins in winter rye leaves, crowns and roots by tissue printing. Plant Physiol. 1996;110:845–857. doi: 10.1104/pp.110.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Gehri A, Mauch F, Voegeli U. Chitinase in bean leaves: induction by ethylene, purification, properties, and possible function. Planta. 1983;157:22–31. doi: 10.1007/BF00394536. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:341–347. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brederode FT, Linthorst HJM, Bol JF. Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Mol Biol. 1991;17:1117–1125. doi: 10.1007/BF00028729. [DOI] [PubMed] [Google Scholar]
- Cabello F, Jorrin JV, Tena M. Chitinase and p-1,3-glucanase activities in chickpea (Cicer arietinum): induction of different isoenzymes in response to wounding and ethephon. Physiol Plant. 1994;92:654–660. [Google Scholar]
- Chun JU, Yu XM, Griffith M. Heritability of antifreeze proteins and their correlation with winter survival in wheat. Euphytica. 1998;102:219–226. [Google Scholar]
- Ciardi JA, Deikman J, Orzolek MD. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol Plant. 1997;101:333–340. [Google Scholar]
- Davies KM, Hobson GE, Grierson D. Differential effect of silver ions on the accumulation of ripening related mRNAs in tomato fruit. J Plant Physiol. 1990;135:708–713. [Google Scholar]
- De Vries AL. Antifreeze glycopeptides and peptides: interaction with ice and water. Methods Enzymol. 1986;127:293–303. doi: 10.1016/0076-6879(86)27024-x. [DOI] [PubMed] [Google Scholar]
- Duman JG, Olsen TM. Thermal hysteresis protein activity in bacteria, fungi and phylogenetically diverse plants. Cryobiology. 1993;30:322–328. [Google Scholar]
- Ergon A, Klemsdal SS, Tronsmo AM. Interactions between cold hardening and Microdochium nivale infection on expression of pathogenesis-related genes in winter wheat. Physiol Mol Plant Pathol. 1998;53:301–310. [Google Scholar]
- Field RJ. The role of 1-aminocyclopropane-1-carboxylic acid in the control of low temperature induced ethylene production in leaf tissue of Phaseolus vulgaris L. Ann Bot. 1984;54:61–67. [Google Scholar]
- Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J. Requirement of salicylic acid for the induction of systemic acquired resistance. Science. 1993;261:754–756. doi: 10.1126/science.261.5122.754. [DOI] [PubMed] [Google Scholar]
- Gatschet MJ, Taliaferro CM, Porter DR, Anderson MP, Anderson JA, Jackson KW. A cold-regulated protein from Bermudagrass crowns is a chitinase. Crop Sci. 1996;36:712–718. [Google Scholar]
- Gaudet DA, Laroche A, Frick M, Davoren J, Puchalski B, Ergon A. Expression of plant defense-related (PR-protein) transcripts during hardening and dehardening of winter wheat. Physiol Mol Plant Pathol. 2000;57:15–24. [Google Scholar]
- Griffith M, Ala P, Yang DSC, Hon WC, Moffatt BA. Antifreeze protein produced endogenously in winter rye leaves. Plant Physiol. 1992;100:593–596. doi: 10.1104/pp.100.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith M, Antikainen M. Extracellular ice formation in freezing-tolerant plants. Adv Low-Temp Biol. 1996;3:107–139. [Google Scholar]
- Griffith M, McIntyre HCH. The interrelationship of growth and frost tolerance in winter rye. Physiol Plant. 1993;87:335–344. [Google Scholar]
- Guy CL. Cold acclimation and freezing stress tolerance: role of protein metabolism. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:187–223. [Google Scholar]
- Harber RM, Fuchigami LH. Ethylene-induced stress resistance. In: Li PH, editor. Low Temperature Stress Physiology in Crops. Boca Raton, FL: CRC Press; 1989. pp. 81–89. [Google Scholar]
- Hiilovaara-Teijo M, Hannukkala A, Griffith M, Yu XM, Pihakaski-Maunsbach K. Snow-mold-induced apoplastic proteins in winter rye leaves lack antifreeze activity. Plant Physiol. 1999;121:665–674. doi: 10.1104/pp.121.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DR, Arnon DI. The water-culture method for growing plants without soil. Calif Agric Exp Stn Circ. 1950;347:1–32. [Google Scholar]
- Hon WC, Griffith M, Chong P, Yang DCS. Extraction and isolation of antifreeze proteins from winter rye (Secale cereale L.) leaves. Plant Physiol. 1994;104:971–980. doi: 10.1104/pp.104.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hon WC, Griffith M, Mlynarz A, Kwok YA, Yang DCS. Antifreeze proteins in winter rye are similar to pathogenesis-related proteins. Plant Physiol. 1995;109:879–889. doi: 10.1104/pp.109.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes M, Dunn MA. The molecular biology of plant acclimation to low temperature. J Exp Bot. 1996;47:291–305. [Google Scholar]
- Janda T, Szalai G, Tari I, Paldi E. Hydroponic treatment with salicylic acid decreases the effects of chilling injury in maize (Zea mays L.) plants. Planta. 1999;208:17–180. [Google Scholar]
- Jiang C, Iu B, Singh J. Requirement of a CCGAC cis-acting element for cold induction of the BN115 gene from winter Brassica napus. Plant Mol Biol. 1996;30:679–684. doi: 10.1007/BF00049344. [DOI] [PubMed] [Google Scholar]
- Kacperska A, Kubacka-Zebalska M. Formation of stress ethylene depends both on ACC synthesis and the activity of free-radical generating system. Physiol Plant. 1989;77:231–237. [Google Scholar]
- Kessmann H, Staub T, Hofmaan C, Maetzke T, Herzog J. Induction of systemic acquired disease resistance in plants by chemicals. Annu Rev Phytopathol. 1994;32:439–459. doi: 10.1146/annurev.py.32.090194.002255. [DOI] [PubMed] [Google Scholar]
- Krol M, Griffith M, Huner NPA. An appropriate physiological control for environmental temperature studies: comparative growth kinetics of winter rye. Can J Bot. 1984;62:1062–1068. [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malamy J, Carr JP, Klessig DP, Raskin I. Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science. 1990;250:1002–1004. doi: 10.1126/science.250.4983.1002. [DOI] [PubMed] [Google Scholar]
- Marentes EM, Griffith M, Mlynarz A, Brush RA. Proteins accumulate in the apoplast of winter rye leaves during cold acclimation. Physiol Plant. 1993;87:499–507. [Google Scholar]
- Mauch F, Meehl JB, Staehelin LA. Ethylene-induced chitinase and β-1,3-glucanase accumulate specifically in the lower epidermis and along vascular stands of bean leaves. Planta. 1992;186:367–375. doi: 10.1007/BF00195317. [DOI] [PubMed] [Google Scholar]
- Métraux JP, Signer H, Ryals J, Ward E, Wyss-Benz M, Gaudin J, Raschdorf K, Schmid E, Blum W, Inverardi B. Increase in salicylic acid at the onset of systemic acquired resistance in cucumber. Science. 1990;250:1004–1006. doi: 10.1126/science.250.4983.1004. [DOI] [PubMed] [Google Scholar]
- Ohme-Takagi M, Shinshi H. Ethylene-inducible DNA binding proteins that interact with an ethylene-responsive element. Plant Cell. 1995;7:173–182. doi: 10.1105/tpc.7.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta M, Ohme-Takagi M, Shinshi H. Three ethylene-responsive transcription factors in tobacco with distinct transactivation functions. Plant J. 2000;22:29–38. doi: 10.1046/j.1365-313x.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Kunz C, Waldvogel R, Meins F, Jr, Leubner-Metzger G. Distinct ethylene- and tissue-specific regulation of β-1,3-glucanases and chitinases during pea seed germination. Planta. 1999;209:195–201. doi: 10.1007/s004250050622. [DOI] [PubMed] [Google Scholar]
- Pinakaski–Maunsbach K, Griffith M, Antikainen M, Maunsbach AB. Immunogold localization of glucanase–like antifreeze protein in cold–acclimated winter rye. Protoplasma. 1996;191:115–125. [Google Scholar]
- Richard S, Morency M-J, Drevet C, Jouanin L, Séguin A. Isolation and characterization of a dehydrin gene from white spruce induced upon wounding, drought and cold stresses. Plant Mol Biol. 2000;43:1–10. doi: 10.1023/a:1006453811911. [DOI] [PubMed] [Google Scholar]
- Roby D, Broglie K, Gaynor J, Broglie R. Regulation of chitinase gene promoter by ethylene and elicitors in bean protoplasts. Plant Physiol. 1991;97:433–439. doi: 10.1104/pp.97.1.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Kilajima S, Koyama T, Yamada Y. Ethylene-induced gene expression of osmotin-like protein, a neutral isoform of tobacco PR-5, is mediated by AGCCGCC cis-sequence. Plant Cell Physiol. 1996;37:249–255. doi: 10.1093/oxfordjournals.pcp.a028939. [DOI] [PubMed] [Google Scholar]
- Shinwari ZH, Nakashima K, Miura S, Kasuga M, Seki M, Yamaguchi-Shinozaki K, Shinozaki K. An Arabidopsis gene family encoding DRE/CRT binding proteins involved in low-temperature-responsive gene expression. Biochem Biophys Res Commun. 1998;250:161–170. doi: 10.1006/bbrc.1998.9267. [DOI] [PubMed] [Google Scholar]
- Smalle J, Haegman M, Kurepa J, Van Montagu M, Van Der Straeten D. Ethylene can stimulate Arabidopsis hypocotyl elongation in the light. Proc Natl Acad Sci USA. 1997;94:2756–2761. doi: 10.1073/pnas.94.6.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional factor that binds to the C-repeat/DRE, a cis-acting element that stimulates transcription in response to low temperature and water deficit. Proc Natl Acad Sci USA. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronsmo AM, Gregersen P, Hjeljord L, Sandal T, Bryngelsson T, Collinge DB. Cold-induced disease resistance. In: Fritig B, Legrand M, editors. Mechanisms of Plant Defense. Dordrecht: Kluwer Academic Publishers; 1993. p. 369. [Google Scholar]
- White RF. Acetylsalicylic (aspirin) induces resistance to tobacco mosaic virus in tobacco. Virology. 1979;99:410–412. doi: 10.1016/0042-6822(79)90019-9. [DOI] [PubMed] [Google Scholar]
- Xin Z, Browse J. Cold comfort farm: the acclimation of plants to freezing temperatures. Plant Cell Environ. 2000;23:893–902. [Google Scholar]
- Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in viral-infected tobacco. Plant Cell. 1991;3:809–810. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]
- Yeh S, Moffatt B, Griffith M, Xiong F, Yang DSC, Wiseman SB, Sarhan F, Danyluk J, Xue YQ, Hew CL. Chitinase genes responsive to cold encode antifreeze proteins in winter cereals. Plant Physiol. 2000;124:1251–1265. doi: 10.1104/pp.124.3.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu XM, Griffith M. Winter rye antifreeze activity increases in response to cold and drought, but not abscisic acid. Physiol Plant. 2001;112:78–86. doi: 10.1034/j.1399-3054.2001.1120111.x. [DOI] [PubMed] [Google Scholar]
- Zhou J, Tang X, Martin GB. The Pto kinase conferring resistance to tomato bacterial speck disease interacts with proteins that bind to a cis-element of pathogenesis-related genes. EMBO J. 1997;16:3207–3218. doi: 10.1093/emboj/16.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]