Abstract
Exposure to ionizing radiation can induce harmful biological effects on the human body, particularly in cases of high-dose γ-irradiation affecting the gastrointestinal tract, bone marrow, skin and lung. Such exposures lead to lethal outcomes as individuals experience a breakdown in their immune system’s ability to defend against pathogens, predisposing them to sepsis-induced multiple organ failures. Mesenchymal stromal/stem cells (MSCs) possess diverse biological characteristics, including immunomodulation, anti-inflammation and tissue regeneration. Off-the-shelf culture-expanded human bone marrow- or adipose tissue-derived MSCs are clinically available to treat graft-versus-host disease following hematopoietic cell transplantation and perianal fistulas in Crohn’s disease in Japan. While preclinical studies showcase encouraging outcomes in radiation-induced injuries, the effectiveness of MSC transplantation in addressing acute radiation syndrome affecting organs in irradiated individuals is limited. Recent studies have highlighted MSC-releasing extracellular vesicles as nanoparticle substances responsible for outlining the mechanism of action and have identified various components, including proteins and microRNA, that serve as functional molecules. MSC-releasing extracellular vesicle-based therapy emerges as a promising avenue, offering a potential solution to the challenges posed by radiation-induced injuries. However, further investigation is required, especially regarding whether MSC-releasing extracellular vesicles have regenerative effects on tissue-resident stem cells. These unresolved issues represent key aspects that need to be addressed to optimize the therapeutic potential of cell-based and extracellular vesicle-based MSC therapies for interventions in the context of radiation-induced injuries.
Keywords: acute radiation syndrome, radiation disaster, gastrointestinal tract, skin, bone marrow, tissue-resident stem cell, mesenchymal stromal/stem cells, extracellular vesicles
INTRODUCTION
Exposure to ionizing radiation can induce harmful biological effects on the human body. The classification of radiation effects encompasses deterministic and stochastic effects, immediate and delayed responses, as well as genetic, tumorigenic and teratogenic outcomes (Table 1). Most deterministic effects manifest as immediate injuries, with symptoms appearing within several weeks after exposure. These result from a cascade of biological responses culminating in cell death in affected organs/tissues [1]. Actively dividing cells, particularly those less differentiated, exhibit higher radiosensitivity and vice versa. Deterministic effects become evident only upon exposure to radiation surpassing a certain level, dependent on radiosensitivity (Table 2). In the event of an accidental radiation disaster, high-dose irradiation, primarily from γ rays, induces severe injuries that extend beyond the responses in the exposed tissues and organs. Notably, injuries to the gastrointestinal (GI) tract, bone marrow (BM), skin and lung can be lethal because the breakdown of these tissue and organ systems predisposes the affected individuals to infection, leading to sepsis-mediated multiple organ failure [2]. This article reviews the current knowledge in cell therapy using culture-expanded mesenchymal stromal/stem cells (MSCs) and nanoparticle therapy employing MSC-producing extracellular vesicles (MSC-EVs) for the restoration of immediate injuries in acute radiation syndrome (ARS) affecting organ systems (Fig. 1).
Table 1.
Classification of radiation effects on human body
| Symbolic symptoms | Affected organs/tissues | Latency | Mechanisms | |
|---|---|---|---|---|
| ARS | Cytopenia | BM | Immediate (−weeks) | Death of cells: Deterministic effects |
| Bleeding | GI tract | |||
| Erosion | Skin | |||
| Dyspnea | Lung | |||
| Vomit | Central nervous system | |||
| Sterility | Reproductive organs | |||
| Cataract | Eyes | Late (months–years) | ||
| Cancers | Various | Mutations in cells: Stochastic effects | ||
| Hereditary effects | Reproductive organs | |||
The phases of ARS are prodromal: 0–2 days from exposure, latent: 2–20 days and manifest illness: 21–60 days from exposure.
Table 2.
The threshold values of ionizing irradiation in various organs/tissues. Threshold doses for symptoms with clear clinical abnormalities (doses causing effects on 1% of people).
| Affected organs/tissues and symptoms | Threshold/Severe doses (Gy, approx.) (acute) | Incubation period |
|---|---|---|
| Testis: sterility (temporal) | 0.2/− | <1 month |
| Eyes: cataract | 0.5/5 | >1 year |
| Ovary: sterility (temporal) | 0.5/− | <1 month |
| BM: cytopenia | 0.5/2 | 2 weeks |
| GI tract: diarrhea, bleeding | 2/5 | 1 week |
| Skin: erosion | 2/10 | 3 weeks |
| Lung: hypo-oxygenation | 2/10 | 4 weeks |
| Ovary: sterility (permanent) | 2.5/− | <1 month |
| Testis: sterility (permanent) | 3.5/− | <1 month |
| Central nervous system: Encephalitis | 15/30 | Variable |
| Soft tissues | >25/− | Variable |
Fig. 1.
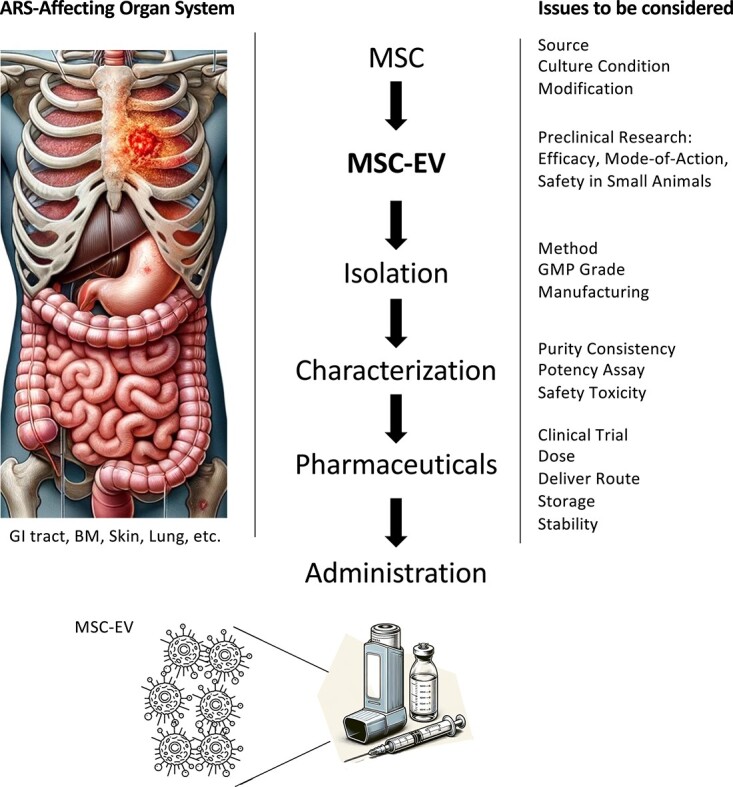
Clinical application trajectories of MSC-EVs. This figure illustrates the comprehensive pathway from laboratory research to clinical implementation, focusing on the use of MSC-EVs in therapeutic interventions. It highlights the critical steps and considerations in the transition from bench work to bedside, emphasizing the application of MSC-EVs in the treatment of organ systems affected by ARS [61], as well as their potential use in managing various other disease conditions. ARS= acute radiation syndrome, BM = bone marrow, MSC = mesenchymal stromal/stem cells, EV = extracellular vesicle, GMP = good manufacturing practice, GI = gastrointestinal.
THE GENERAL MODE OF ACTION OF MSC-BASED CELL AND EV THERAPY
Culture-expanded MSCs have been employed in the treatment of various intractable diseases, anticipating anti-inflammatory and immunomodulatory properties, as well as facilitating the regeneration of damaged tissues [3]. While the primary sources of human MSCs are BM and adipose tissues, exploration into alternative sources such as umbilical cord and umbilical cord blood that can be obtained in a non-invasive manner has been conducted (Table 3) [4]. When human culture-expanded MSCs are administered to the human body, they are expected to migrate to damaged tissues and secrete soluble factors such as cytokines and chemokines [5]. In Japan, allogeneic off-the-shelf BM-MSCs or adipose tissue-derived MSCs (ADSCs) have been used in clinics for the treatment of acute graft-versus-host disease following hematopoietic cell transplantation since 2015 [6] and perianal fistulas in Crohn’s disease since 2021 [7], respectively. Internationally, the use of allogeneic MSCs has been approved for the treatment of critical limb ischemia (India, 2017) and knee cartilage defects (South Korea, 2012).
Table 3.
A list of MSCs and MSC-EVs. EVs are produced by almost all cell types, including various sources of MSCs. Based on biogenesis and size, EVs are classified as exosomes, microvesicles and apoptotic bodies. Essentially, EVs mirror the characteristics and state of their originating cells. The recent position statement by ISEV (MISEV2018) suggests naming medium/large EVs (m/l EVs) and small EVs (sEVs) based on size, where sEVs are defined as being ≤200 nm and m/l EVs as >200 nm. Exosomes are categorized as sEVs [71].
| MSCs | Sources | Effects | |
|---|---|---|---|
| BM | Anti-inflammation Immunomodulation Tissue/organ regeneration |
||
| Adipose tissue | |||
| Umbilical cord and blood | |||
| Placenta | |||
| Amniotic fluid | |||
| Menstrual blood | |||
| Dental tissue | |||
| Pluripotent stem cell | |||
| EVs | Classification | Diameter | Origin |
| Exosomes | 30–100 nm | Endosomes | |
| Microvesicles | 50–1000 nm | Plasma membranes | |
| Apoptotic bodies | 100–5000 nm | Dead cell membranes | |
Since recent research has highlighted that the therapeutic effects of MSCs can be replicated by EVs released from MSCs, exploration into the application of MSC-EVs for diseases targeted by MSC-based cell therapy has been prompted [8]. As well as the MSC population itself, MSC-EVs show anti-inflammatory and immunomodulatory effects; they repress antigen uptake and maturation of dendritic cells, promote M2 macrophage polarization, preserve regulatory T cells and inhibit peripheral differentiation of T cells [9]. Key proteins such as transforming growth factor (TGF)-β1 [10], galectin-1 [11] and PD-L1 [12], along with various microRNAs, including miR-223 [13–15], contribute to mediating these therapeutic effects. In addition, MSC-EVs demonstrate direct regenerative effects on damaged cells. In a skin wound model, MSC-EVs enhance the proliferation of human dermal fibroblasts and suppress their differentiation by inhibiting TGF-β receptor signaling [16]. In an acute kidney injury model, MSC-EVs mitigate apoptosis and heightened autophagy of tubular epithelial cells by inhibiting caspase 1 and Ras-related GTP-binding D [17]. They also reduce the apoptosis of lung epithelial cells during lung injury [18]. In a model of autoimmune hepatitis, MSC-EVs protect hepatocytes by downregulating nuceotide-binding and oligomerization domain (NOD)-like receptor family pyrin domain-containing 3 and caspase-1 expression [19]. Cardiomyocytes benefit from protection through the downregulation of semaphorin 3A and STAT3 [20] along with the reduction of reactive oxygen species [21]. Additionally, MSC-EVs contribute to the recovery of neural cells by upregulating nerve growth factor signaling [22]. MSC-EVs influence different types of programmed cell death by transporting various microRNAs and proteins that modulate apoptosis-, pyroptosis-, ferroptosis- and necroptosis-related proteins [23]. In the context of radiation injuries to tissues/organs, both MSCs and MSC-EVs are anticipated to exhibit therapeutic effects by directly supporting cell regeneration in injured tissues, particularly in tissue-resident stem and progenitor cells. Furthermore, their indirect effects are expected to extend mitigating adverse inflammation and immune reactions.
GI TRACT
The histological findings associated with immediate effects of ionizing irradiation in the GI tract commonly include epithelial injury, submucosal changes with inflammatory cell infiltration, endothelial cell swelling and muscle atrophy. In the esophagus, immediate effects primarily impact the basal cells of the stratified squamous epithelium, which are characterized by the presence of apoptotic bodies and mitotic figures [24]. Subsequently, swelling of vascular endothelial cells occurs in the submucosal layer [25]. Limited studies have directly explored the effects of MSCs or MSC-SVs on the regeneration of esophageal layers in preclinical models. In a murine model of C57/B6 exposed to X-rays with twice-weekly doses totaling 10 Gy, human ADSCs were locally injected the day after the final irradiation. The results showed that the MSC-treated mice exhibited improved basal cell division and reduced thickness of the esophageal submucosa, although details about effector molecules, including EVs, were not provided [26].
In the stomach, the immediate response often occurs in the antrum [27]. Histological observations demonstrate atrophy of epithelial cells, degeneration of parietal cells and chief cells, inflammation in the lamina propria and submucosal edema [28]. Gastric epithelial progenitor or stem cells are essential for the regeneration of simple columnar epithelium in the stomach mucosa by differentiating into normal gastric lineage cells [29]. Such cells include B lymphoma Mo-MLV insertion region 1 homolog (Bmi)-1- or leucine-rich repeats and immunoglobulin-like domain protein 1 (Lrig1)-expressing cells located in the isthmal region of gastric glands in the corpus and antrum [30] and leucine-rich repeat-containing G protein-coupled receptor 5 (Lgr5)-expressing cells located in the base region of gastric glands in the antrum [31, 32]. As of now, there are no PubMed-indexed studies examining the direct effects of MSCs or MSC-EVs on the regeneration of such gastric epithelial progenitor or stem cells in preclinical models.
Radiation-induced damage to the small intestine is of particular concern due to its extensive length along the GI tract and its crucial role in water and nutrient absorption, as well as electrolyte balance. The intestinal epithelial cells undergo differentiation from Bmi-1-expressing or Lgr5-expressing intestinal stem cells located in the crypts [33, 34]. Thereafter, they migrate and undergo apoptosis at the apex of the villi approximately 3–4 days after the initiation of differentiation. Radiation-induced stem cell death results in immediate damage to the simple columnar epithelium with an incubation period of ~1 week. Severe injury occurs with >5 Gy irradiation, often resulting in death within 3–5 days without medical intervention. Multiple studies have demonstrated that MSCs improve intestinal epithelial repair in mouse models of radiation injury [35, 36]. Additionally, a significant increase in Lgr5-expressing intestinal stem cells is demonstrated, involving the activation of the Wnt/β-catenin signaling pathway in C57BL/6 mice that received abdominal irradiation >12 Gy [37]. Regarding MSC-EVs, the direct effect of MSC-EVs on intestinal stem cells after radiation has not been studied yet. In nude mice that received whole-body irradiation at 10 Gy, intravenous administration of EVs produced from human embryonic stem cell-derived MSCs improved the renewal of the small intestinal epithelium by stimulating proliferation and inhibiting apoptosis of the epithelial crypt cells [38].
Lgr5-expressing cells are not only found in the stem cell population of the small intestine but also in the large intestine (colon), where they divide approximately once per day [33]. Given that the epithelial cell turnover rate is 3–5 days for the small intestine and 5–7 days for the colon [39], the colon is believed to have lower radiosensitivity compared with the small intestine. There is a report involving SD rats that received local irradiation with a single dose of 27 Gy and were subsequently treated with systemic administration of BM-MSCs. This resulted in the proliferation of crypt epithelial cells with increased Wnt4 expression and a limited reduction in the number of Sox9-high cells [36]. However, as of now, there appears to be no report that directly examines the efficacy of MSC-EVs on the tissue regeneration of colon epithelium after irradiation.
Collectively, the administration of MSCs demonstrates therapeutic effects on radiation-induced injuries throughout the GI tract, likely modulating the adverse inflammation and immune response as well as supporting the regeneration of epithelial cells by acting on intestinal stem cells. While the efficacy of MSC-EV therapy appears promising in non-irradiation disease models, there is still limited literature related to MSC-EV therapy specifically for radiation-induced damage in the GI tract.
BONE MARROW
Some populations of hematopoietic stem cells (HSCs) in the G0 phase have been identified as radioresistant, even when exposed to 6 Gy irradiation [40]. When radiation exposure exceeds 10 Gy, the autonomous recovery of hematopoiesis becomes impractical. In such cases, allogeneic HSC transplantation is the sole modality to reconstitute hematopoiesis in irradiated individuals, and indeed, the engraftment of donor-derived HSCs has been achieved in multiple cases [41]. Nevertheless, individuals exposed to radiation over 4 Gy often succumb to non-BM toxicity despite allogeneic HSC rescue.
In preclinical study, lipopolysaccharide-primed MSC-derived EVs can educate monocytes, and such monocytes promote hematopoietic recovery and survival in small animal models [42]. The simultaneous administration of culture-expanded MSCs with HSCs has been investigated as clinical trials since the 1990s, anticipating the promotion of hematopoiesis and the avoidance of rejection post-medical myelosuppressive conditioning, involving irradiation and/or chemotherapy [43]. Despite historical exploration, a recent systematic review and meta-analysis, acknowledging limitations in study design and small sample sizes, reported no statistically significant effects of MSCs in co-transplantation with HSCs [44]. In a specific case of accidental exposure to 14.5 Gy γ-radiation in 2008, a combined treatment approach involving intravenous administration of HLA-mismatched haploidentical peripheral blood stem cells from a sibling and intra-BM injection of allogeneic BM-MSCs led to engraftment on Day 8 post-transplantation, implying the contribution of MSCs to the rapid engraftment of donor HSCs [45]. Although the hematopoiesis was sustained and the skin ulceration was improved with additional local injection of BM-MSCs, the patient faced a fatal outcome with multiple organ failure on Day 55 post-transplantation, i.e. Day 62 after irradiation exposure. Autopsy findings unveiled a severe epithelial injury in the GI tract.
Multiple studies have demonstrated that the administration of MSC-derived EV promotes multiple lineages of hematopoietic recovery in irradiated mice [46], possibly attributed to the reversal of radiation-induced DNA damage and of subsequent apoptosis in hematopoietic cells [47]. It is reported that human BM-MSC-derived EVs are incorporated into human CD34-expressing cells, resulting in decreased caspase-3 activity, increased colony-forming unit-granulocyte-macrophage (CFU-GM) formation and enhanced lodging in 3.5 Gy-irradiated NOD/SCID mice [48]. Thus, MSC-EVs are likely to have direct regenerative effects on HSCs and support the generation of hematopoietic cells from HSCs into consideration, the potential impact of ionizing irradiation on the hematopoiesis-supportive characteristics of MSCs becomes a significant issue. In in vitro co-culture experiments, exposure of human BM-MSCs to ionizing radiation at 0.1 Gy showed an increase in the generation of CD34+CD38+ double positive cells from CD34-expressing cells, along with elevated IL-6 levels [49]. Additionally, the generation of CD19-expressing cells from CD34-expressing cells decreased with reduced IL-7 levels when human BM-MSCs were exposed to ionizing radiation at over 2 Gy [50]. These effects are likely attributed to radiation-induced DNA damage in BM-MSCs, although it remains unknown whether this change in irradiated BM-MSCs is transient or permanent [49, 50]. In a 16 Gy irradiation-induced bone loss SD rat model, EVs derived from normal BM-MSCs restored the function of irradiated BM-MSCs by accelerating DNA repair, activating Wnt/β-catenin pathway and promoting restored differentiation potential [51]. However, it remains unknown whether normal BM-MSC-EVs can restore the hematopoiesis-supportive capability of irradiated BM-MSCs.
To summarize, in the context of established HSC administration for radiation-induced BM injuries, the simultaneous use of MSCs is expected to target non-BM tissue/organ injuries rather than primarily supporting the recovery of BM injuries. Ongoing research suggests that MSC-EVs have the potential to serve a dual purpose, embodying a ‘hit two and more birds with one stone’ strategy. This approach holds promise not only for hematopoiesis recovery but also for the restoration of injured non-hematopoietic organs and tissues. Further exploration of MSC-EVs is poised to offer comprehensive therapeutic benefits in the context of radiation-induced injuries.
SKIN
Given the principle that rapidly dividing and undifferentiated cells exhibit greater radiosensitivity than slowly dividing and differentiated cells, the epidermis is more radiosensitive compared with the dermis, with the basal layer identified as the utmost radiosensitive component [52]. Within the epidermis, epidermal stem cells reside in the basal layer of the interfollicular epithelium, orchestrating the routine regeneration of the various layers of the epidermis [53]. A preceding study unveiled that the human keratinocyte population endowed with stem cell characteristics demonstrated a relative resistance to 2 Gy γ-irradiation compared with the human keratinocyte population with progenitor characteristics [52]. The study revealed that the former cells occur at a frequency of 1%, while the latter constitutes 10% of the population. While the direct impact of MSCs or MSC-EVs on epidermal stem cells remains unsolved, numerous reports from both preclinical and clinical trials underscore that MSCs are promising agents for managing radiation-induced skin injuries [45, 54]. Particularly, the injection of MSCs into the perilesional sites of local radiation-induced skin injuries appears to clinically result in complete epithelium regeneration [45, 55]. MSC-EVs, through their modulation of immunoregulation, angiogenesis and fibroblast proliferation, contribute to wound healing and skin regeneration [56]. Furthermore, in vitro assessments demonstrated the re-epithelialization effects of MSC-EVs, where they facilitated proliferation and migration while inhibiting apoptosis in human keratinocyte line HaCaT cells damaged by H2O2 through miR-93-3p-targeted apoptotic peptidase activating factor 1 [57]. Based on the current literature, the efficacy of MSC-EVs in the context of radiation-induced skin injury remains undetermined.
In summary, MSC therapy exhibits clinical efficacy in the management of radiation-induced skin injuries. For the comprehensive treatment of extensive or widespread radiation skin injuries necessitating systemic intervention, the consideration of MSC-EVs over MSCs may be prudent, leveraging their advantageous distribution throughout the human body.
LUNG
Ionizing radiation can lead to radiation-induced lung injury, characterized by diffuse alveolar damage, in its acute phase [58]. Both Type I (AT1) and Type II (AT2) alveolar epithelial cells are susceptible to this damage; however, AT2 cells that survive have the capability to proliferate and differentiate into AT1 cells, akin to stem cells in alveolar repair and regeneration [59]. Studies indicate that MSCs directly assist AT2 cells in differentiating into AT1 cells by suppressing the Yes-associated protein in models of lipopolysaccharide-induced lung injury [60]. A potential advantage of MSC therapy over MSC-EV therapy in treating radiation-induced lung injury is that intravenously administered MSCs tend to accumulate in the lungs [61]. Once there, they secrete anti-inflammatory substances, including interleukin-10 [62], and help reduce pro-inflammatory cytokines such as tumor necrosis factor-α and interleukin-6 [63], contributing to the healing of the damaged lung tissue. Despite promising results in preclinical studies for MSC therapy in radiation-induced lung injury [64], no clinical trials have been registered on ClinicalTrials.gov regarding treatments with MSCs or MSC-EVs for this specific injury [65]. However, the recent COVID-19 epidemic highlighted the potential of MSC-EVs in treating COVID-19 pneumonia [66], especially since SARS-CoV-2 infects AT2 cells via the ACE2 receptor, causing alveolar damage and potentially leading to severe acute respiratory distress syndrome (ARDS) [67]. A study involving 24 patients with moderate-to-severe ARDS from SARS-CoV-2 infection indicated improved oxygenation after MSC-EV intravenous infusion, although no significant pathological or radiological amelioration of alveolar damage was mentioned [68]. Another pilot study for COVID-19 pneumonia explored the feasibility and efficacy of nebulization of MSC-EV and reported radiographic improvements without noticeable side effects [69]. MSC-EVs are internalized by AT2 cells [70]. In vitro studies on Mycobacterium tuberculosis infection reveal that this internalization of MSC-EVs leads to the transfer of miR-20b, which helps inhibit the production of inflammatory factors in AT2 cells by targeting the Toll-like receptor and nuclear factor of activated T-cell 5 signaling pathways [70]. Together, although the significant clinical effectiveness of MSCs and MSC-EVs on radiation-induced lung injury has not yet been established, employing MSC-EVs via inhalation could be a reasonable approach to enhance clinical outcomes in the lungs, given the exosome’s size (30–100 nm) and its ability to efficiently reach the alveolar space (300 μm in diameter) for targeted therapeutic effects.
CONCLUSION
As ionizing radiation induces a complex landscape of injuries across various organ systems, the diverse characteristics of MSCs, including regeneration, anti-inflammatory properties and immunomodulation, position them as agents for mitigating radiation-induced injuries. While preclinical studies showcase encouraging outcomes, the effectiveness of MSC transplantation on ARS affecting organs in irradiated individuals, especially those exposed to high-dose γ-irradiation, appears primarily limited to skin lesions. Severe GI tract injuries induced by radiation lead to fatalities, often due to sepsis-mediated multiple organ failure. Moreover, the capability of endogenous MSCs in high-dose γ-irradiated individuals to sustain long-term hematopoiesis remains uncertain. Anticipated to overcome limitations in MSC-cell therapy, MSC-EV-based therapy emerges as a promising avenue, offering a potential solution to the challenges posed by radiation-induced injuries. However, further investigation is especially required to determine whether MSC-EVs have regenerative effects on tissue-resident stem cells—key issues that need resolution.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
Program of the Network-type Joint Usage/Research Centre for Radiation Disaster Medical Science (Hiroshima University) (Y.M. and T.I.).
AUTHOR CONTRIBUTIONS
Y.M., S.F. and T.I. designed and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Contributor Information
Yasuo Miura, Department of Hematology and Oncology, Research Institute for Radiation Biology and Medicine, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan; Department of Transfusion Medicine and Cell Therapy, Fujita Health University School of Medicine, 1-93 Dengakugakubo, Kutsukakecho, Toyoake, Aichi 470-1192, Japan.
Sumie Fujii, Department of Transfusion Medicine and Cell Therapy, Fujita Health University School of Medicine, 1-93 Dengakugakubo, Kutsukakecho, Toyoake, Aichi 470-1192, Japan.
Tatsuo Ichinohe, Department of Hematology and Oncology, Research Institute for Radiation Biology and Medicine, Hiroshima University, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8553, Japan.
REFERENCES
- 1. Reisz JA, Bansal N, Qian J, et al. Effects of ionizing radiation on biological molecules—mechanisms of damage and emerging methods of detection. Antioxid Redox Signal 2014;21:260–92. 10.1089/ars.2013.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. BOOKLET to Provide Basic Information Regarding Health Effects of Radiation . Radiosensitivity of Organs and Tissues. https://www.env.go.jp/en/chemi/rhm/basic-info/1st/03-02-06.html (2 December 2023, date last accessed).
- 3. Miura Y. Human bone marrow mesenchymal stromal/stem cells: current clinical applications and potential for hematology. Int J Hematol 2016;103:122–8. [DOI] [PubMed] [Google Scholar]
- 4. Yoshioka S, Miura Y, Iwasa M, et al. Isolation of mesenchymal stromal/stem cells from small-volume umbilical cord blood units that do not qualify for the banking system. Int J Hematol 2015;102:218–29. [DOI] [PubMed] [Google Scholar]
- 5. Chapel A, Bertho JM, Bensidhoum M, et al. Mesenchymal stem cells home to injured tissues when co-infused with hematopoietic cells to treat a radiation-induced multi-organ failure syndrome. J Gene Med 2003;5:1028–38. [DOI] [PubMed] [Google Scholar]
- 6. Muroi K, Miyamura K, Okada M, et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol 2016;103:243–50. [DOI] [PubMed] [Google Scholar]
- 7. Panés J, García-Olmo D, Van Assche G, et al. Long-term efficacy and safety of stem cell therapy (Cx601) for complex perianal fistulas in patients with Crohn's disease. Gastroenterology 2018;154:1334–1342.e4. 10.1053/j.gastro.2017.12.020. [DOI] [PubMed] [Google Scholar]
- 8. Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells 2017;35:851–8. [DOI] [PubMed] [Google Scholar]
- 9. Fujii S, Miura Y. Immunomodulatory and regenerative effects of MSC-derived extracellular vesicles to treat acute GVHD. Stem Cells 2022;40:977–90. [DOI] [PubMed] [Google Scholar]
- 10. Kordelas L, Rebmann V, Ludwig AK, et al. MSC-derived exosomes: a novel tool to treat therapy-refractory graft-versus-host disease. Leukemia 2014;28:970–3. [DOI] [PubMed] [Google Scholar]
- 11. Fajka-Boja R, Urbán VS, Szebeni GJ, et al. Galectin-1 is a local but not systemic immunomodulatory factor in mesenchymal stromal cells. Cytotherapy 2016;18:360–70. [DOI] [PubMed] [Google Scholar]
- 12. Yang N, Liu X, Chen X, et al. Stem cells from exfoliated deciduous teeth transplantation ameliorates Sjögren's syndrome by secreting soluble PD-L1. J Leukoc Biol 2022;111:1043–55. [DOI] [PubMed] [Google Scholar]
- 13. He X, Dong Z, Cao Y, et al. MSC-derived exosome promotes M2 polarization and enhances cutaneous wound healing. Stem Cells Int 2019;2019:7132708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gholampour MA, Abroun S, Nieuwland R, et al. Mesenchymal stem cell-derived extracellular vesicles conditionally ameliorate bone marrow failure symptoms in an immune-mediated aplastic anemia mouse model. J Cell Physiol 2021;236:6055–67. [DOI] [PubMed] [Google Scholar]
- 15. Lu FB, Chen DZ, Chen L, et al. Attenuation of experimental autoimmune hepatitis in mice with bone mesenchymal stem cell-derived exosomes carrying microRNA-223-3p. Mol Cell 2019;42:906–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhang Y, Pan Y, Liu Y, et al. Exosomes derived from human umbilical cord blood mesenchymal stem cells stimulate regenerative wound healing via transforming growth factor-β receptor inhibition. Stem Cell Res Ther 2021;12:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang C, Shang Y, Chen X, et al. Supramolecular nanofibers containing arginine-glycine-aspartate (RGD) peptides boost therapeutic efficacy of extracellular vesicles in kidney repair. ACS Nano 2020;14:12133–47. [DOI] [PubMed] [Google Scholar]
- 18. Li JW, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol 2019;852:68–76. [DOI] [PubMed] [Google Scholar]
- 19. Chen L, Lu FB, Chen DZ, et al. BMSCs-derived miR-223-containing exosomes contribute to liver protection in experimental autoimmune hepatitis. Mol Immunol 2018;93:38–46. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Gu H, Qin D, et al. Exosomal miR-223 contributes to mesenchymal stem cell-elicited cardioprotection in polymicrobial sepsis. Sci Rep 2015;5:13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sánchez-Sánchez R, Gómez-Ferrer M, Reinal I, et al. miR-4732-3p in extracellular vesicles from mesenchymal stromal cells is cardioprotective during myocardial ischemia. Front Cell Dev Biol 2021;9:734143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Y, Lai X, Wu D, et al. Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther 2021;12:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Wu Y, Yao R, et al. The role of mesenchymal stem cell-derived extracellular vesicles in inflammation-associated programmed cell death. Nano Today 2023;50:101865. [Google Scholar]
- 24. Coia LR, Myerson RJ, Tepper JE. Late effects of radiation therapy on the gastrointestinal tract. Int J Radiat Oncol Biol Phys 1995;31:1213–36. [DOI] [PubMed] [Google Scholar]
- 25. Trowers E, Thomas C Jr, Silverstein FE. Chemical- and radiation-induced esophageal injury. Gastrointest Endosc Clin N Am 1994;4:657–75. [PubMed] [Google Scholar]
- 26. Kim IG, Cho H, Shin J, et al. Regeneration of irradiation-damaged esophagus by local delivery of mesenchymal stem-cell spheroids encapsulated in a hyaluronic-acid-based hydrogel. Biomater Sci 2021;9:2197–208. [DOI] [PubMed] [Google Scholar]
- 27. Chao CJ, Shin JS, Hsu WC, Wang PM. Endoscopic features of radiation gastritis after irradiation of hepatocellular carcinoma. Endoscopy 2013;45:E280–1. [DOI] [PubMed] [Google Scholar]
- 28. Chen G, Yu Z, Zhang Y, et al. Radiation-induced gastric injury during radiotherapy: molecular mechanisms and clinical treatment. J Radiat Res 2023;64:870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao S, Zhou L. Gastric stem cells: physiological and pathological perspectives. Front Cell Dev Biol 2020;8:571536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Choi E, Lantz TL, Vlacich G, et al. Lrig1+ gastric isthmal progenitor cells restore normal gastric lineage cells during damage recovery in adult mouse stomach. Gut 2018;67:1595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yoshioka T, Fukuda A, Araki O, et al. Bmi1 marks gastric stem cells located in the isthmus in mice. J Pathol 2019;248:179–90. [DOI] [PubMed] [Google Scholar]
- 32. Barker N, Huch M, Kujala P, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 2010;6:25–36. [DOI] [PubMed] [Google Scholar]
- 33. Barker N, van Es JH, Kuipers J, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–7. [DOI] [PubMed] [Google Scholar]
- 34. Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet 2008;40:915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saha S, Bhanja P, Kabarriti R, et al. Bone marrow stromal cell transplantation mitigates radiation-induced gastrointestinal syndrome in mice. PLoS One 2011;6:e24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sémont A, Demarquay C, Bessout R, et al. Mesenchymal stem cell therapy stimulates endogenous host progenitor cells to improve colonic epithelial regeneration. PLoS One 2013;8:e70170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong W, Guo M, Han Z, et al. Mesenchymal stem cells stimulate intestinal stem cells to repair radiation-induced intestinal injury. Cell Death Dis 2016;7:e2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Accarie A, l’Homme B, Benadjaoud MA, et al. Extracellular vesicles derived from mesenchymal stromal cells mitigate intestinal toxicity in a mouse model of acute radiation syndrome. Stem Cell Res Ther 2020;11:371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barker N. Adult intestinal stem cells: critical drivers of epithelial homeostasis and regeneration. Nat Rev Mol Cell Biol 2014;15:19–33. [DOI] [PubMed] [Google Scholar]
- 40. Inoue T, Hirabayashi Y, Mitsui H, et al. Survival of spleen colony-forming units (CFU-S) of irradiated bone marrow cells in mice: evidence for the existence of a radioresistant subfraction. Exp Hematol 1995;23:1296–300. [PubMed] [Google Scholar]
- 41. Chiba S, Saito A, Ogawa S, et al. Transplantation for accidental acute high-dose total body neutron- and gamma-radiation exposure. Bone Marrow Transplant 2002;29:935–9. [DOI] [PubMed] [Google Scholar]
- 42. Forsberg MH, Kink JA, Thickens AS, et al. Exosomes from primed MSCs can educate monocytes as a cellular therapy for hematopoietic acute radiation syndrome. Stem Cell Res Ther 2021;12:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant 1995;16:557–64. [PubMed] [Google Scholar]
- 44. Kallekleiv M, Larun L, Bruserud Ø, Hatfield KJ. Co-transplantation of multipotent mesenchymal stromal cells in allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. Cytotherapy 2016;18:172–85. [DOI] [PubMed] [Google Scholar]
- 45. Guo M, Dong Z, Qiao J, et al. Severe acute radiation syndrome: treatment of a lethally 60Co-source irradiated accident victim in China with HLA-mismatched peripheral blood stem cell transplantation and mesenchymal stem cells. J Radiat Res 2014;55:205–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Preciado S, Muntión S, Sánchez-Guijo F. Improving hematopoietic engraftment: potential role of mesenchymal stromal cell-derived extracellular vesicles. Stem Cells 2021;39:26–32. [DOI] [PubMed] [Google Scholar]
- 47. Wen S, Dooner M, Cheng Y, et al. Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia 2016;30:2221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Preciado S, Muntión S, Corchete LA, et al. The incorporation of extracellular vesicles from mesenchymal stromal cells into CD34+ cells increases their clonogenic capacity and bone marrow lodging ability. Stem Cells 2019;37:1357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fujishiro A, Miura Y, Iwasa M, et al. Effects of acute exposure to low-dose radiation on the characteristics of human bone marrow mesenchymal stromal/stem cells. Inflamm Regen 2017;37:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iwasa M, Fujii S, Fujishiro A, et al. Impact of 2 Gy γ-irradiation on the hallmark characteristics of human bone marrow-derived MSCs. Int J Hematol 2021;113:703–11. [DOI] [PubMed] [Google Scholar]
- 51. Zuo R, Liu M, Wang Y, et al. BM-MSC-derived exosomes alleviate radiation-induced bone loss by restoring the function of recipient BM-MSCs and activating Wnt/β-catenin signaling. Stem Cell Res Ther 2019;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rachidi W, Harfourche G, Lemaitre G, et al. Sensing radiosensitivity of human epidermal stem cells. Radiother Oncol 2007;83:267–76. [DOI] [PubMed] [Google Scholar]
- 53. Li A, Simmons PJ, Kaur P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc Natl Acad Sci USA 1998;95:3902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fang Z, Chen P, Tang S, et al. Will mesenchymal stem cells be future directions for treating radiation-induced skin injury? Stem Cell Res Ther 2021;12:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kotenko K, Moroz B, Nadezhina N, et al. Successful treatment of localised radiation lesions in rats and humans by mesenchymal stem cell transplantation. Radiat Prot Dosim 2012;151:661–5. [DOI] [PubMed] [Google Scholar]
- 56. Zhang W, Ling Y, Sun Y, et al. Extracellular vesicles derived from mesenchymal stem cells promote wound healing and skin regeneration by modulating multiple cellular changes: a brief review. Genes (Basel) 2023;14:1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sun C, Shi C, Duan X, et al. Exosomal microRNA-618 derived from mesenchymal stem cells attenuate the progression of hepatic fibrosis by targeting Smad4. Bioengineered 2022;13:5915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xu T, Zhang Y, Chang P, et al. Mesenchymal stem cell-based therapy for radiation-induced lung injury. Stem Cell Res Ther 2018;9:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013;123:3025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen XY, Chen KY, Feng PH, et al. YAP-regulated type II alveolar epithelial cell differentiation mediated by human umbilical cord-derived mesenchymal stem cells in acute respiratory distress syndrome. Biomed Pharmacother 2023;159:114302. [DOI] [PubMed] [Google Scholar]
- 61. Nanduri LSY, Duddempudi PK, Yang WL, et al. Extracellular vesicles for the treatment of radiation injuries. Front Pharmacol 2021;12:662437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang H, Yang YF, Zhao L, et al. Hepatocyte growth factor gene-modified mesenchymal stem cells reduce radiation-induced lung injury. Hum Gene Ther 2013;24:343–53. [DOI] [PubMed] [Google Scholar]
- 63. Jiang X, Jiang X, Qu C, et al. Intravenous delivery of adipose-derived mesenchymal stromal cells attenuates acute radiation-induced lung injury in rats. Cytotherapy 2015;17:560–70. [DOI] [PubMed] [Google Scholar]
- 64. Rezvani M. Therapeutic potential of mesenchymal stromal cells and extracellular vesicles in the treatment of radiation lesions-a review. Cell 2021;10:427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Available online: http://www.clinicaltrials.gov (27 January 2024, date last accessed).
- 66. Serretiello E, Ballini A, Smimmo A, et al. Extracellular vesicles as a translational approach for the treatment of COVID-19 disease: an updated overview. Viruses 2023;15:1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev 2020;29:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chu M, Wang H, Bian L, et al. Nebulization therapy with umbilical cord mesenchymal stem cell-derived exosomes for COVID-19 pneumonia. Stem Cell Rev Rep 2022;18:2152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Yan K, Xu G, Li Z. MicroRNA-20b carried by mesenchymal stem cell-derived extracellular vesicles protects alveolar epithelial type II cells from mycobacterium tuberculosis infection in vitro. Infect Genet Evol 2022;101:105292. [DOI] [PubMed] [Google Scholar]
- 71. Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]


