Abstract
Background:
Patients undergoing amputation of the lower extremity for the complications of peripheral artery disease and/or diabetes are at risk of treatment failure and the need for reamputation at a higher level. The aim of this study was to develop a patient-specific reamputation risk prediction model.
Methods:
Patients with incident unilateral transmetatarsal, transtibial or transfemoral amputation between 2004 and 2014 secondary to diabetes and/or peripheral artery disease, and who survived 12 months after amputation, were identified using Veterans Health Administration databases. Procedure codes and natural language processing were used to define subsequent ipsilateral reamputation at the same or higher level. Stepdown logistic regression was used to develop the prediction model. It was then evaluated for calibration and discrimination by evaluating the goodness of fit, area under the receiver operating characteristic curve (AUC) and discrimination slope.
Results:
Some 5260 patients were identified, of whom 1283 (24·4 per cent) underwent ipsilateral reamputation in the 12 months after initial amputation. Crude reamputation risks were 40·3, 25·9 and 9·7 per cent in the transmetatarsal, transtibial and transfemoral groups respectively. The final prediction model included 11 predictors (amputation level, sex, smoking, alcohol, rest pain, use of outpatient anticoagulants, diabetes, chronic obstructive pulmonary disease, white blood cell count, kidney failure and previous revascularization), along with four interaction terms. Evaluation of the prediction characteristics indicated good model calibration with goodness-of-fit testing, good discrimination (AUC 0·72) and a discrimination slope of 11·2 per cent.
Conclusion:
A prediction model was developed to calculate individual risk of primary healing failure and the need for reamputation surgery at each amputation level. This model may assist clinical decision-making regarding amputation-level selection.
Introduction
Amputation-level decision-making in the context of diabetes and peripheral artery disease (PAD) is extremely challenging1. The preservation of mobility and its impact on quality of life2 are key considerations3,4. The goal of preserving mobility has led to a focus on attempting the most distal amputation procedure possible: preserving the knee joint by performing a transtibial (TT) rather than a transfemoral (TF) amputation4, or by attempting a limb salvage transmetatarsal (TM) amputation rather than a major amputation3. The importance of preserving extremity length by performing more distal amputations, especially at the TM level, has been questioned because of the associated risk of healing failure5 and the uncertainty about potential mobility advantages4-6. Failure of primary healing results in the need for additional reamputation surgery and/or prolonged wound care with restricted ambulation1,7-9 which may adversely affect future mobility7 and increase the risk of death10.
There is a lack of evidence to assist surgeons and their patients in determining the magnitude of the probable risk of healing failure and need for additional surgery at each amputation level7,9,11. Clinical judgement continues to be a key factor in estimating reamputation risk8,12. Current clinical algorithms used to predict healing failure risk and future mobility have limitations7, and there is a perceived need for a more individualized approach to decision-making13,14 and improved predictive data12,14,15. Providing patients with information about their individual outcome probabilities permits a more informed decision-making process that can help ensure that outcomes match their values, but also help set realistic outcome expectations3,12.
The primary aim of this study, therefore, was to develop a predictive model for 12-month risk of reamputation in patients undergoing an incident amputation at the TM, TT or TF level secondary to complications of diabetes and PAD, who survived for 12months after amputation. The goal was to predict the need for reamputation so that patients and surgeons can be informed better about risks during amputation-level shared decision-making discussions.
Methods
This study used administrative, quality improvement and clinical data from two primary sources: the Veterans Affairs (VA) Surgical Quality Improvement Program (VASQIP) and the VA Corporate Data Warehouse (CDW). The study was conducted in accordance with procedures approved by the participating institution’s human subjects review board.
Veterans Affairs Surgical Quality Improvement Program
The VASQIP database was used to define the inception cohort as well as several preamputation risk predictors. It includes information on 30-day surgical outcomes, and preoperative, perioperative and postoperative co-variables from 110 VA Medical Center inpatient surgical programmes. VASQIP is a surgical quality improvement data set developed to monitor the quality of surgical care in the VA Health System. Data are collected on approximately 70 per cent of all major operations and about 25 per cent of all operations in the VA Health System16,17. The process for assignment to the data collection group is random. Eligible non-cardiac procedures include those performed by a physician that require general, spinal or epidural anaesthesia.
Corporate Data Warehouse
The VA CDW includes inpatient and outpatient data, as well as demographic information. Data from the CDW were used to determine whether the VASQIP surgical procedure was an incident amputation (explained below). The CDW was also used to acquire additional predictor variables (such as weight and laboratory values) that were not available through VASQIP. The CDW Vital Status File was used to identify those who died within the first year after incident amputation18.
Study sample
The target population comprised patients who survived for 1 year after undergoing their first unilateral TM, TT or TF amputation between 1 October 2004 and 31 December 2014 secondary to diabetes and/or PAD (based on the co-existence of ICD-9-CM codes; PAD: 440.22, 440.23, 440.24, 440.4, 442.3, 444.22; diabetes: 249.7, 250.7, 443.81, 785.4, 249.8, 250.8, 707.1, 707.11, 707.12, 707.13, 707.14, 707.15, 707.19), and were aged over 40 years19. Amputation level was determined using the following codes: TM (Current Procedural Terminology (CPT) 28800, 28805; ICD-9 84.12), TT (CPT 27880, 27881, 27882, 27888, 27889; ICD-9 84.14, 84.15) or TF amputation (CPT 27590, 27591, 27598; ICD-9 84.16, 84.17, 84.18). Subjects were excluded if they had a preoperative diagnosis of coma, paraplegia, quadriplegia, disseminated cancer, tumour of the central nervous system, were ventilator-dependent, their amputation laterality could not be ascertained, or they died within 1 year of amputation. Patients were also excluded if their height, weight or BMI was considered implausible (less than 1·2 or more than 2·1 m, below 34 or above 318 kg20, and less than 15 or over 52 kg/m2 respectively) because these were likely to be implausible values owing to data entry errors.
These exclusions were used to create a cohort that was not at a high risk of death related to causes not typically associated with diabetes, PAD and their complications, and also to exclude those whose severe co-morbidity profile was consistent with a patient who would typically undergo a TF amputation. The goal was to create a cohort of individuals undergoing an incident lower extremity amputation for whom the most appropriate amputation level was uncertain, making the prediction model a useful tool to augment decision-making.
Definition of incident amputation
After defining the initial subject population using the VASQIP database, the CDW was then used to provide a look-back window between 2 days and 5 years before the amputation procedure. The presence of any diagnostic or procedure code related to amputation or its treatment resulted in the exclusion of these subjects. For guillotine procedures at the TT (CPT 27881; ICD-9 84·13) and TF (CPT 27592) levels, the presumption was that a closure procedure would be performed within 3 weeks of the guillotine procedure; therefore, research staff searched forward 3 weeks for the next procedure code to classify the incident level. If the next subsequent procedure was more than 3 weeks after the initial guillotine procedure, an error in the initial coding was presumed and the guillotine code was recorded as a definitive amputation. Some 328 patients were coded as having guillotine procedures, but did not have a subsequent closure procedure from VASQIP or CDW. For these, professional medical record annotators (professionals who review and interpret medical records for retrospective study projects) were used to identify the definitive closure level. This became the level of incident amputation.
Classification of laterality for incident and subsequent amputations
Using electronic health records from the CDW, a natural language processing (NLP) system was developed to classify the laterality of the incident amputation, subsequent amputation, revascularization procedures and ankle : brachial pressure index (ABPI) values21. Where laterality of the amputations could not be classified using the NLP process, chart annotation was used to classify laterality. The accuracy of validation (comparison of the NLP classifications with a random sample of 100 reviewed charts) for incident amputation, subsequent amputation and revascularization was 95·0, 92·0 and 91·0 per cent respectively.
Outcome
The primary outcome of this study was ipsilateral surgical reamputation occurring within 1 year of index amputation. Reamputation was identified by an amputation procedure code in the CDW that occurred 3 weeks or more after the incident amputation and was ipsilateral to the incident procedure. Three separate categories of reamputation were identified and were all included in the study definition of reamputation: soft tissue revisions after TT (CPT 27884) and TF (CPT 27594) procedures, reamputations at the same level after TT (CPT 27886) and TF (CPT 27596) procedures, and definitive amputation at a higher level after TT (CPT 27880, 27881) and TF (CPT 27590, 27591) procedures. A search was also undertaken for ICD-9 code 84.3 representing a revision. Because TM amputations do not have codes for soft tissue revision or reamputation at the same level, all ICD-9 84.3 codes after an incident TM amputation were coded as a subsequent TM amputation, and were classified as a reamputation at the same level.
Candidate predictor variables
The databases described above were used to retrieve 37 potential candidate predictor variables, identified a priori through a review of the literature and expert clinical opinion; 28 variables were ultimately considered as candidates in developing a predictive model (Table 1) after dropping or combining nine because of a large number of missing values, difficulty in measuring that would limit future clinical utility, or because they were combined with other variables (Table S1, supporting information). When VASQIP laboratory values were missing, the most proximate CDW value within 3 months before the date of surgery was used; a similar process was used for missing values for other VASQIP predictors that were also recorded in the CDW. For calculation of BMI, the median CDW value for height and the VASQIP-recorded value for weight were used, as these are documented just before surgery in the hospital setting. Patients with diabetes (requiring oral agents or insulin) were identified from VASQIP. Patients not identified as having diabetes in VASQIP were classified as having diabetes if one of the ICD-9 codes listed above existed in the CDW. A well accepted, validated or standardized measure of symptomatic PAD does not exist or is very difficult to obtain from retrospective databases. Therefore, three predictors that are thought to be associated clinically with symptomatic or more severe PAD were chosen: abnormal ABPI (less than 0·9 measured in the past year), history of revascularization in the past year (open, endovascular or both), and a diagnosis of rest pain or gangrene within 30 days before surgery.
Table 1.
Candidate predictors for the AMPREDICT 1-year reamputation risk prediction model
| % of patients* | ||
|---|---|---|
| No reamputation (n = 3977) |
Reamputation (n = 1283) |
|
| Amputation | ||
| Level | ||
| Transmetatarsal | 18·5 | 38·7 |
| Transtibial | 45·7 | 49·4 |
| Transfemoral | 35·8 | 11·9 |
| Guillotine (any level) | 12·2 | 14·4 |
| Demographics | ||
| Age (years)† | 66·6(10·2) | 64·9(9·5) |
| Men | 99·0 | 99·4 |
| Married | 41·4 | 36·8 |
| Race | ||
| Caucasian | 58·6 | 61·1 |
| Black | 32·2 | 31·6 |
| Hispanic | 8·3 | 6·2 |
| Other | 1·0 | 1·1 |
| Co-morbidities | ||
| Diabetes | 74·2 | 74·2 |
| Dialysis in 2 weeks before operation | 8·5 | 10·8 |
| History of COPD | 12·2 | 14·7 |
| Health factors | ||
| Smoker within 1 year before operation | 41·6 | 46·9 |
| >2 alcoholic drinks/day in past 2 weeks | 8·1 | 11·2 |
| Illicit drugs within 1 year before operation | 11·9 | 14·4 |
| Nutritional status | ||
| >10% weight loss in 6 months before operation | 8·4 | 9·4 |
| BMI (kg/m2)† | 26·7(6·1) | 26·9(6·2) |
| Mental health | ||
| Any mental health diagnosis‡ | 43·0 | 41·6 |
| Mental health outpatient visits (≥1 past year) | 15·4 | 17·9 |
| Physical function | ||
| Independent | 56·0 | 61·0 |
| Partially dependent | 35·8 | 33·0 |
| Totally dependent | 8·3 | 6·1 |
| Medications | ||
| Outpatient anticoagulation using warfarin | 12·1 | 14·0 |
| Outpatient antiplatelet medication | 17·0 | 19·8 |
| Preoperative laboratory values | ||
| Blood urea nitrogen (mg/dl)† | 21·5(14·6) | 22·4(16·4) |
| WBC count <11 000/μl | 62·8 | 51·3 |
| EGFR <15 ml per min per 1·73 m2§ | 5·9 | 9·0 |
| Platelet count (× 106/ml)† | 341·2(140·3) | 347·8(145·6) |
| Potassium (mEq/l)† | 4·22(0·52) | 4·22(0·53) |
| Haematocrit (%)† | 32·2(5·2) | 32·0(5·2) |
| Vascular/limb status | ||
| Rest pain/gangrene within 30 days before operation¶ | 70·8 | 77·5 |
| Any revascularization in past year | 19·4 | 26·7 |
| Abnormal ABPI (< 0·9) | 49·5 | 53·5 |
Unless indicated otherwise
values are mean(s.d.).
Any diagnosis of depression, anxiety, post-traumatic stress disorder, bipolar disorder or schizophrenia.
Estimated glomerular filtration rate (eGFR) that is considered kidney failure.
Rest pain in the 30 days preceding amputation defined as a more severe form of ischaemic pain due to occlusive disease which occurs at rest and manifests as a severe, unrelenting pain aggravated by elevation and often preventing sleep. This definition includes gangrene (marked skin discoloration and disruption indicative of death and decay of tissues in the extremities due to severe and prolonged ischaemia), and ischaemic ulceration and/or tissue loss related to peripheral vascular disease. COPD, chronic obstructive pulmonary disease; WBC, white blood cell; ABPI, ankle : brachial pressure index.
Model development and validation
The initial strategy was to develop a reamputation risk prediction model using patient data from the East, South and Midwest VA regions, and then to validate it externally in the West and Mountain West/Texas regions. However, geographical variability in reamputation frequency and predictor distributions led the authors to pursue the strategy of developing the model in all regions and then undertaking internal validation through bootstrapping.
There were few missing data for the 28 candidate predictor variables and so a complete-case analysis was used, as opposed to other approaches, such as multiple imputation of missing values. Candidate predictors were evaluated rigorously by univariable exploratory data analysis followed by bivariable analysis, evaluating the association between each predictor and 1-year reamputation. Twelve potential interactions based on clinical experience were considered. Seven with TM amputation (married, smoking, alcohol abuse, illicit drug use, diabetes, kidney failure (based on estimated glomerular filtration rate (eGFR) values), and rest pain/gangrene), one with TT amputation (guillotine amputation), one with TF amputation (smoking) and three with diabetes (abnormal ABPI, rest pain/gangrene and history of revascularization). Associations with continuous predictors were assumed to be linear (on the logit scale).
A logistic regression model with all 28 candidate predictors and 12 interaction terms was fitted to provide a reference for comparison with more parsimonious models. Variable selection for more parsimonious prediction models using both backward stepwise and stepdown logistic regression methods22 was considered. For the backwards stepwise variable selection approach, a P value cut-off of 0·157 was chosen, which approximates the best subset of predictors using the Akaike information criterion23. For the stepdown variable selection approach, models that explained 99 and 95 per cent of the variability in the risk predictions from the full model were considered. Calibration was evaluated by means of the Hosmer–Lemeshow (H–L) goodness-of-fit test21. A plot of observed fraction of 1-year reamputation versus the mean of model-estimated risks of 1-year reamputation for each decile of predicted risk was also assessed visually. Discrimination was assessed quantitatively by calculating the area under the receiver operating characteristic curve (AUC), the discrimination slope (difference in mean predicted 1-year reamputation probabilities for those who did and those who did not undergo reamputation within the first year), and the difference in mean estimated risk in the highest and lowest deciles of predicted risk.
The final model was validated internally with bootstrap sampling to obtain estimates of the optimism (discrepancy due to overfitting) of the estimated AUC and the difference in predicted probabilities for those who did or did not undergo reamputation (discrimination slope). Bootstrap samples were drawn with replacement and with the same size as the original sample. Model selection was carried out for each bootstrap sample and model performance assessment compared with that of the original sample. This was repeated 500 times to obtain stable estimates of the mean optimism of the AUC and discrimination slope for each model.
Results
The complete-case cohort included 5260 individuals who underwent lower extremity amputation owing to the complications of PAD and/or diabetes. There were 1231 TM (23·4 per cent), 2452 TT (46·6 per cent) and 1577 TF (30·0 per cent) amputees (Fig. 1). This represented 95·6 per cent of those eligible. The distributions of the 28 candidate predictors evaluated in the prediction model are summarized by reamputation status in Table 1.
Fig. 1. STROBE diagram showing total numbers acquired by the Veterans Affairs Surgical Quality Improvement Program and total number excluded to achieve the final inception cohort.
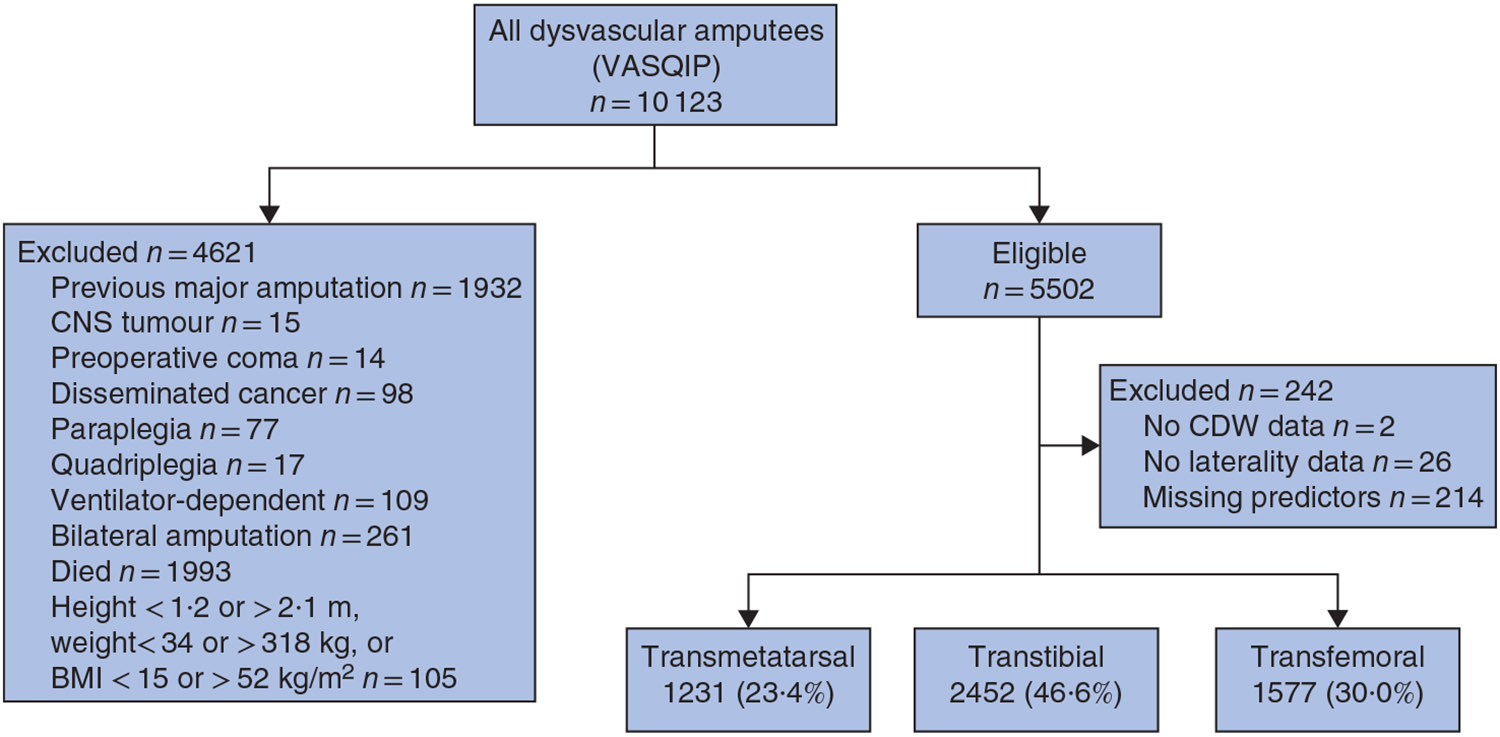
VASQIP, Veterans Affairs Surgical Quality Improvement Program; CNS, central nervous system; CDW, Veterans Affairs Corporate Data Warehouse.
Outcomes
A total of 1283 ipsilateral reamputations (24·4 per cent) occurred within the first year after incident amputation. The risk of ipsilateral reamputation was 40·3 per cent among TM amputees (496 of 1231), 25·9 per cent in TT amputees (634 of 2452) and 9·7 per cent in TF amputees (153 of 1577).
Risk prediction model development
The backwards stepwise variable selection resulted in a model with 16 variables. The 99 per cent stepdown variable selection resulted in a model with 19 variables and the 95 per cent stepdown variable selection resulted in a model with 11 variables. These 11 variables were a subset of the 16 variables from the backwards stepwise model. The three predictor models were compared with the full model as the criterion standard. All models performed similarly to the full model with respect to qualitative (graphical) and quantitative (AUC and discrimination slope) assessments (Table S2 and Fig. S1, supporting information). Given this, the 11-variable model was chosen (Table 2), as it was more parsimonious, resulting in a lower clinical burden for the provider to obtain the information. This 11-predictor model contained nine main effects and four interactions. Table 2 shows the components of the modelled reamputation risk score. Both TM and TT amputees were at higher risk of 1-year reamputation than TF amputees. The modelled reamputation risk in TT and TM amputees was similar, except for TM amputees who had diabetes or were in kidney failure (based on eGFR values), who had a higher risk. Other predictive factors associated with increased risk of reamputation were current smoking in TT and TF amputees, alcohol abuse, presence of rest pain/gangrene, use of outpatient anticoagulants, previous diagnosis of chronic obstructive pulmonary disease, and white blood cell counts of 11 000/μl or higher. Having both diabetes and a history of an ipsilateral revascularization in the previous year increased the risk for TM amputees.
Table 2.
Reamputation risk score coefficients for individual predictors and interaction terms in the 11-variable reamputation prediction model
| Coefficient | |
|---|---|
| Baseline for TM | −2·293 |
| Baseline for TT | −2·460 |
| Baseline for TF | −3·806 |
| Male sex | +0·891 |
| Current smoker | +0·367 |
| Current smoker and TM | −0·456 |
| Alcohol abuse | +0·318 |
| Rest pain/gangrene | +0·373 |
| Outpatient anticoagulant use | +0·280 |
| Diabetes | −0·446 |
| Diabetes and TM | +0·878 |
| Diabetes and revascularization | +0·329 |
| Previous diagnosis of COPD | +0·321 |
| WBC ≥11 000/μl | +0·583 |
| Kidney failure and TM | +0·818 |
The risk score, S, for an individual is the sum of the coefficients for all the components that apply to that individual. Predicted 1-year reamputation risk = eS/(1 + eS). For example, the risk score for a male transfemoral amputee who is a current smoker with diabetes and revascularization is calculated as: S = −3·806 + 0·891 + 0·367 – 0·446 + 0·329 = −2·665. Predicted 1-year reamputation risk = e−2·665/(1 + e−2·665) = 0·065; this represents a 6·5 per cent predicted risk of reamputation in 1 year. TM, transmetatarsal; TT, transtibial; COPD, chronic obstructive pulmonary disease; WBC, white blood cell.
The mean 1-year reamputation risk in the cohort of 5260 patients predicted using the 11-variable risk prediction model was 24·4 (range 2·0 to 75·6) per cent. The estimated AUC was 0·72 and the H-L goodness-of-fit test for this model indicated good calibration (P = 0·918) (Table 3). The discrimination slope was 11·2 per cent. The difference in mean predicted risk of reamputation in the highest versus lowest deciles of predicted risk was 47·7 per cent (Fig. 2).
Table 3.
Goodness-of-fit assessment from the final 11-variable prediction model (5260 patients)
| Decile of predicted risk |
Predicted probabilities (%) |
n | Observed reamputations |
Expected reamputations |
|---|---|---|---|---|
| 1 | 0·2–6·8 | 542 | 32 | 28 |
| 2 | 6·9–11·1 | 513 | 44 | 45 |
| 3 | 11·2–15·5 | 523 | 68 | 66 |
| 4 | 15·6–16·9 | 536 | 88 | 87 |
| 5 | 17·0–21·9 | 518 | 102 | 107 |
| 6 | 22·0–25·7 | 556 | 125 | 139 |
| 7 | 25·8–31·4 | 519 | 150 | 148 |
| 8 | 31·5–35·5 | 501 | 174 | 170 |
| 9 | 35·6–43·9 | 527 | 215 | 215 |
| 10 | 44·0–75·6 | 525 | 285 | 278 |
Fig. 2.
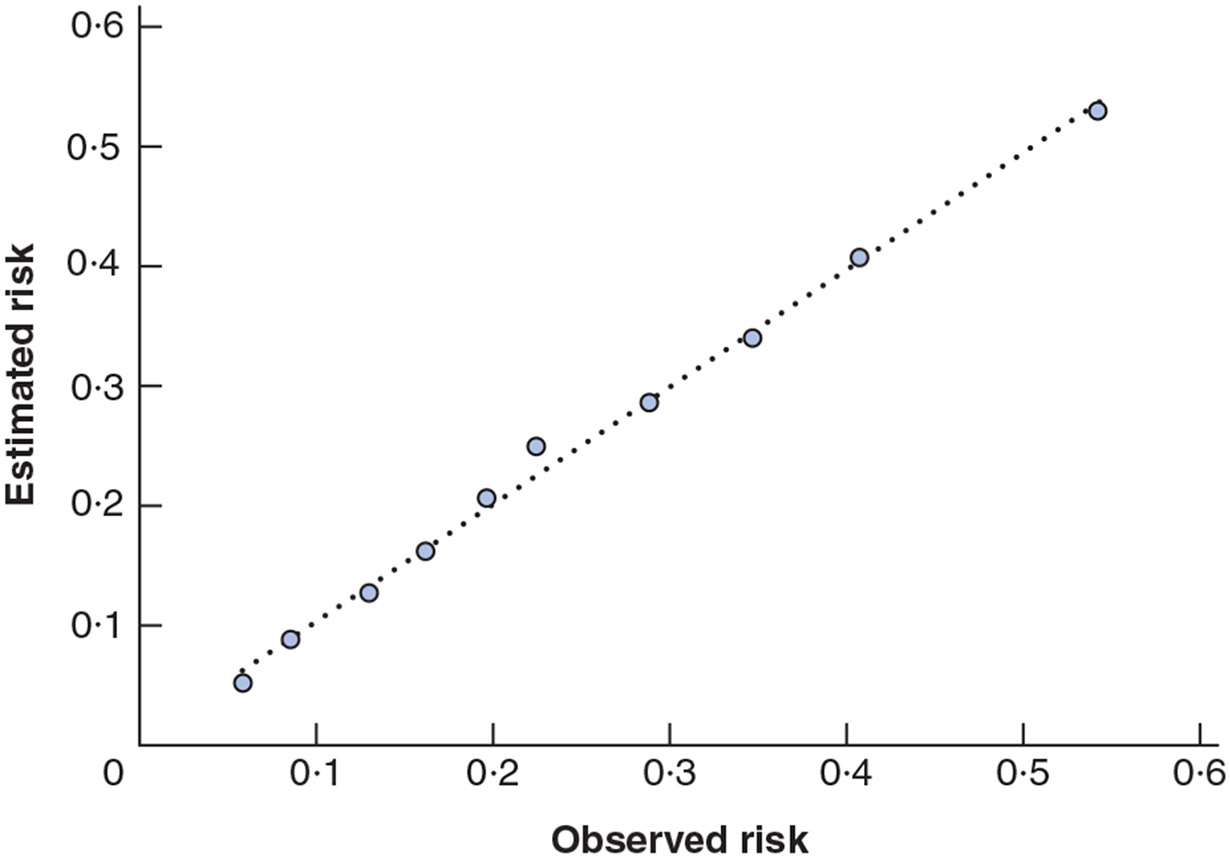
Observed versus predicted risk of 1-year reamputation by decile of risk in the internal validation sample
Internal validation of risk prediction model
Bootstrap estimates of the optimism for both the AUC and discrimination slope were 0·01, indicating that there was negligible optimism in these estimated accuracy characteristics when using the same data to develop and validate the model.
Discussion
A novel reamputation risk prediction model (AMPRE-DICT Reamputation) was developed and validated internally in this study. It can be used to quantify the individual risk of reamputation within 1 year of the incident amputation procedure in patients who require TM, TT or TF amputation because of complications of diabetes and/or PAD, and who survive the first year after incident amputation. The model was developed using a large data set of 5260 individuals undergoing amputation, of whom 1283 had an ipsilateral reamputation in the subsequent year. The model demonstrated good prediction characteristics which confirmed its potential in communicating individual patient risk. The predicted reamputation risks vary substantially based on an individual’s risk factors. Therefore, the amputation-level choice surgeons and their patients make may differ considerably depending on how the magnitude of risk and the downstream consequences of this risk are viewed.
Other studies have indicated that the risk of reamputation is dependent on amputation level, increasing the more distal the amputation. TM amputation has been associated with a 29–35 per cent reamputation risk15, whereas TT and TF amputations have been associated with risk of 12–25 per cent and 8 per cent respectively1,24. In addition, multiple health factors have been shown to affect reamputation risk, including male sex25, smoking25, alcohol use26, rest pain/gangrene9,27-29, anticoagulant and aspirin use9, increased international normalized ratio30, increased white blood cell count/sepsis31 and history of revascularization32,33. These summaries inform population risk, but do not inform individual patient risk, which is essential for incorporation into treatment planning decisions.
Informing patients and surgeons about individual reamputation risk is critical because of its effect on key health and quality-of-life outcomes. Reamputation surgery after TM amputation imposes an additional mortality risk, which has been estimated at 5 per cent10. In a mixed population of TM, TT and TF amputees, who required hospital readmission primarily for wound-related complications, there was a twofold increase in risk of death28. In addition to increased mortality risk, failure of primary healing is complicated by the need for additional wound care, hospital admissions, and often multiple operations which result in a substantial burden to patients and families30. There is also concern that reamputation may lead to a reduction in mobility and quality of life that exceeds that of an initial higher-level amputation5. The extremely poor 1- and 5-year survival rates after dysvascular amputation also create an imperative to better inform patients about the outcome risks associated with amputation at each level, so that their personal priorities regarding how they wish to spend their remaining life are incorporated into the surgical decision34.
The AMPREDICT Reamputation prediction model is a novel tool that could be used in conjunction with a previously published mobility prediction model6 and mortality prediction model35 to provide an individualized multidimensional view of key outcomes associated with each amputation level. These tools can be used to balance the risks and benefits of other key outcomes, including body image, social stigma and self-esteem, in making the amputation-level choice2,34,36,37. Patients want to participate in the amputation-level decision24 and want to be better informed. The proposed reamputation risk prediction model, in conjunction with the mobility and mortality risk prediction models, enables an assessment of individual-patient and amputation-level risk12,14 that can be used to better inform patient and surgeon, so they can develop an individualized plan of care13,14 that incorporates patient priorities in arriving at an amputation-level decision3,12,14. When such assessments are used in a shared decision-making environment, patients will be better informed, which should result in more value-concordant healthcare decisions. Patient involvement and empowerment may result in more positive health and psychological outcomes24.
This model has a number of strengths as well as potential limitations. The validity of the model is supported by the agreement between the predictors identified in this model and factors that have been associated with reamputation risk in previous studies. From a methodological perspective, the study has strengths in its approach to defining reamputation; previous studies used ICD-9 codes that do not define laterality or CPT laterality modifier codes, which raises uncertainty regarding whether the second procedure was ipsilateral or contralateral to the initial surgery. To overcome this limitation, NLP and chart annotation were employed to ensure that subsequent amputations were ipsilateral to the initial amputation. Furthermore, guillotine amputations are also commonly employed as an emergency procedure to control infection, with a planned subsequent definitive amputation. The amputation after the guillotine procedure was defined as the definitive amputation level, and only the next subsequent ipsilateral amputation was counted as a reamputation. One primary limitation is the generalizability of the findings. Some caution is advisable when applying the model to women, Hispanics and patients who fall into the ‘other’ race category, such as Asians, Pacific Islanders and Native Americans, owing to the limited number of subjects in these categories. Patients who experienced a previous major amputation or had severe co-morbidities that typically lead to TF amputation were excluded, as this study was interested in a cohort of patients in whom the amputation decision level would be less certain. Only patients who survived the first year after amputation were included; as mortality is a competing risk with reamputation, this study sought to predict the risk of reamputation among those who survive. The model was also developed using a specific population comprising US veterans exclusively, and external validation in other populations is needed. However, prediction models developed for other purposes in VA populations have shown strong external validation characteristics with non-veteran populations38,39. Potential predictors with a large number of missing values such as albumin levels and haemoglobin (Hb) A1c levels were dropped from consideration. Interestingly, neither albumin nor HbA1c levels were associated with reamputation in bivariable assessments among subjects for whom these variables were recorded (data not shown). Despite reasonable discrimination and good calibration, the discrimination slope of 11·2 per cent is modest, probably because 90 per cent of the predicted reamputation probabilities for the cohort were below 44 per cent (Table 3). Some of this may be explained by unmeasured characteristics in these patients that the study was unable to account for and inclusion of which might have further improved the performance of the model. Some examples include patient frailty and social support structures; however, marital status was included as a surrogate for social support, and independence in self-care as a potential surrogate for frailty. Neither of these surrogate measures affected reamputation risk. The challenge in evaluating any potential effect of these variables on model enhancement is that there would be a need for widespread agreement on which specific frailty and social support measures to use, and to have them implemented routinely as part of patient care practices. Because this prediction model is designed to inform probable risk of reamputation at the time of surgical decision-making, it does not incorporate operative or postoperative factors which may modify the risk of healing. Finally, as with all prediction models, this model requires periodic recalibration to ensure adequate future performance.
With completion of the development of validated prediction models for mobility, mortality and reamputation, future work is needed to determine how best to communicate these risks to patients using patient decision aids, and to surgeons using clinical decision support tools. Implementation studies will be critical to help ensure that these models can be incorporated into clinical care pathways and are clinically useful.
Supplementary Material
Acknowledgements
This material is based on work supported by the US Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development (Merit Review RX001474-01A1; principal investigator J.M.C). The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or the US Government.
Footnotes
Disclosure: The authors declare no conflict of interest.
Supporting information
Additional supporting information can be found online in the Supporting Information section at the end of the article.
References
- 1.O’Brien PJ, Cox MW, Shortell CK, Scarborough JE. Risk factors for early failure of surgical amputations: an analysis of 8878 isolated lower extremity amputation procedures. J Am Coll Surg 2013; 216: 836–842. [DOI] [PubMed] [Google Scholar]
- 2.Suckow BD, Goodney PP, Nolan BW, Veeraswamy RK, Gallagher P, Cronenwett JL et al. Domains that determine quality of life in vascular amputees. Ann Vasc Surg 2015; 29: 722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landry GJ, Silverman DA, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011; 146: 1005–1009. [DOI] [PubMed] [Google Scholar]
- 4.Columbo JA, Nolan BW, Stucke RS, Rzucidlo EM, Walker KL, Powell RJ et al. Below-knee amputation failure and poor functional outcomes are higher than predicted in contemporary practice. Vasc Endovascular Surg 2016; 50: 554–558. [DOI] [PubMed] [Google Scholar]
- 5.Dillon MP, Fatone S. Deliberations about the functional benefits and complications of partial foot amputation: do we pay heed to the purported benefits at the expense of minimizing complications? Arch Phys Med Rehabil 2013; 94: 1429–1435. [DOI] [PubMed] [Google Scholar]
- 6.Czerniecki JM, Turner AP, Williams RM, Thompson ML, Landry G, Hakimi K et al. The development and validation of the AMPREDICT model for predicting mobility outcome after dysvascular lower extremity amputation. J Vasc Surg 2017; 65: 162–171.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony T, Roberts J, Modrall JG, Huerta S, Asolati M, Neufeld J et al. Transmetatarsal amputation: assessment of current selection criteria. Am J Surg 2006; 192: e8–e11. [DOI] [PubMed] [Google Scholar]
- 8.Stone PA, Flaherty SK, Aburahma AF, Hass SM, Jackson JM, Hayes JD et al. Factors affecting perioperative mortality and wound-related complications following major lower extremity amputations. Ann Vasc Surg 2006; 20: 209–216. [DOI] [PubMed] [Google Scholar]
- 9.Phair J, DeCarlo C, Scher L, Koleilat I, Shariff S, Lipsitz EC et al. Risk factors for unplanned readmission and stump complications after major lower extremity amputation. J Vasc Surg 2018; 67: 848–856. [DOI] [PubMed] [Google Scholar]
- 10.Stone PA, Back MR, Armstrong PA, Flaherty SK, Keeling WB, Johnson BL et al. Midfoot amputations expand limb salvage rates for diabetic foot infections. Ann Vasc Surg 2005; 19: 805–811. [DOI] [PubMed] [Google Scholar]
- 11.Swaminathan A, Vemulapalli S, Patel MR, Jones WS. Lower extremity amputation in peripheral artery disease: improving patient outcomes. Vasc Health Risk Manag 2014; 10: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attinger CE, Brown BJ. Amputation and ambulation in diabetic patients: function is the goal. Diabetes Metab Res Rev 2012; 28(Suppl 1): 93–96. [DOI] [PubMed] [Google Scholar]
- 13.Game F. Choosing life or limb. Improving survival in the multi-complex diabetic foot patient. Diabetes Metab Res Rev 2012; 28(Suppl 1): 97–100. [DOI] [PubMed] [Google Scholar]
- 14.Klaphake S, de Leur K, Mulder PG, Ho GH, de Groot HG, Veen EJ et al. Mortality after major amputation in elderly patients with critical limb ischemia. Clin Interv Aging 2017; 12: 1985–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg 2016; 55: 591–599. [DOI] [PubMed] [Google Scholar]
- 16.Khuri SF, Daley J, Henderson W, Hur K, Demakis J, Aust JB et al. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 1998; 228: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson WG, Daley J. Design and statistical methodology of the National Surgical Quality Improvement Program: why is it what it is? Am J Surg 2009; 198(Suppl): S19–S27. [DOI] [PubMed] [Google Scholar]
- 18.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr 2006; 4: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feinglass J, Pearce WH, Martin GJ, Gibbs J, Cowper D, Sorensen M et al. Postoperative and late survival outcomes after major amputation: findings from the Department of Veterans Affairs National Surgical Quality Improvement Program. Surgery 2001; 130: 21–29. [DOI] [PubMed] [Google Scholar]
- 20.Das SR, Kinsinger LS, Yancy WS Jr, Wang A, Ciesco E, Burdick M et al. Obesity prevalence among veterans at Veterans Affairs medical facilities. Am J Prev Med 2005; 28: 291–294. [DOI] [PubMed] [Google Scholar]
- 21.Singh J, DuVall S. Novel natural language processing algorithm can ascertain laterality in veterans undergoing total joint replacement. Value Health 2013; 16: A23. [Google Scholar]
- 22.Harrell FE Jr, Lee KL, Califf RM, Pryor DB, Rosati RA. Regression modelling strategies for improved prognostic prediction. Stat Med 1984; 3: 143–152. [DOI] [PubMed] [Google Scholar]
- 23.Sauerbrei W. The use of resampling methods to simplify regression models in medical statistics. J R Stat Soc Ser C Appl Stat 1999; 48: 313–329. [Google Scholar]
- 24.Columbo JA, Davies L, Kang R, Barnes JA, Leinweber KA, Suckow BD et al. Patient experience of recovery after major leg amputation for arterial disease. Vasc Endovascular Surg 2018; 52: 262–268. [DOI] [PubMed] [Google Scholar]
- 25.Acar E, Kacira BK. Predictors of lower extremity amputation and reamputation associated with the diabetic foot. J Foot Ankle Surg 2017; 56: 1218–1222. [DOI] [PubMed] [Google Scholar]
- 26.Belmont PJ Jr, Davey S, Orr JD, Ochoa LM, Bader JO, Schoenfeld AJ. Risk factors for 30-day postoperative complications and mortality after below-knee amputation: a study of 2911 patients from the National Surgical Quality Improvement Program. J Am Coll Surg 2011; 213: 370–378. [DOI] [PubMed] [Google Scholar]
- 27.Kono Y, Muder RR. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg 2012; 26: 1120–1126. [DOI] [PubMed] [Google Scholar]
- 28.Curran T, Zhang JQ, Lo RC, Fokkema M, McCallum JC, Buck DB et al. Risk factors and indications for readmission after lower extremity amputation in the American College of Surgeons National Surgical Quality Improvement Program. J Vasc Surg 2014; 60: 1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip VS, Teo NB, Johnstone R, Robertson AG, Robertson JH, Welch GH et al. An analysis of risk factors associated with failure of below knee amputations. World J Surg 2006; 30: 1081–1087. [DOI] [PubMed] [Google Scholar]
- 30.Hasanadka R, McLafferty RB, Moore CJ, Hood DB, Ramsey DE, Hodgson KJ. Predictors of wound complications following major amputation for critical limb ischemia. J Vasc Surg 2011; 54: 1374–1382. [DOI] [PubMed] [Google Scholar]
- 31.Blume P, Salonga C, Garbalosa J, Pierre-Paul D, Key J, Gahtan V et al. Predictors for the healing of transmetatarsal amputations: retrospective study of 91 amputations. Vascular 2007; 15: 126–133. [DOI] [PubMed] [Google Scholar]
- 32.Beaulieu RJ, Grimm JC, Lyu H, Abularrage CJ, Perler BA. Rates and predictors of readmission after minor lower extremity amputations. J Vasc Surg 2015; 62: 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Font-Jimenez I, Llauradó-Serra M, Pallarés-Martí À, García-Hedrera F. Psycho-social factors involved in amputation. Systematic review of the literature. Aten Primaria 2016; 48: 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dunning T, Martin P. Palliative and end of life care of people with diabetes: issues, challenges and strategies. Diabetes Res Clin Pract 2018; 143: 454–463. [DOI] [PubMed] [Google Scholar]
- 35.Norvell DC, Thompson ML, Boyko EJ, Landry G, Littman AJ, Henderson WG et al. Mortality prediction following non-traumatic amputation of the lower extremity. Br J Surg 2019; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Quigley FG, Faris IB, Xiouruppa H. Transmetatarsal amputation for advanced forefoot tissue loss in elderly patients. Aust N Z J Surg 1995; 65: 339–341. [DOI] [PubMed] [Google Scholar]
- 37.Rybarczyk B, Nyenhuis D, Nicholas JJ, Cash SM, Kaiser J. Body image, perceived social stigma, and the prediction of psychosocial adjustment to leg amputation. Rehabil Psychol 1995; 40: 95–110. [Google Scholar]
- 38.Brown JR, MacKenzie TA, Maddox TM, Fly J, Tsai TT, Plomondon ME et al. Acute kidney injury risk prediction in patients undergoing coronary angiography in a National Veterans Health Administration cohort with external validation. J Am Heart Assoc 2015; 4: e002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deppen SA, Blume JD, Aldrich MC, Fletcher SA, Massion PP, Walker RC et al. Predicting lung cancer prior to surgical resection in patients with lung nodules. J Thorac Oncol 2014; 9: 1477–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


