Abstract
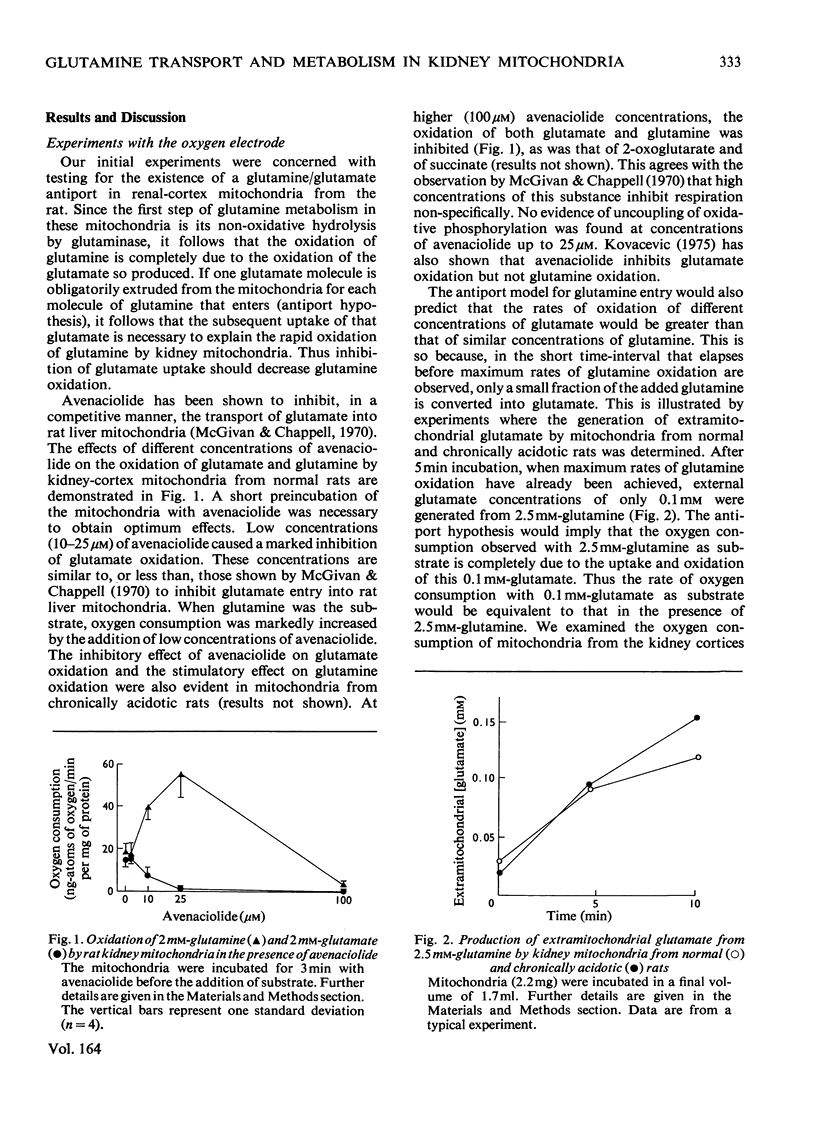
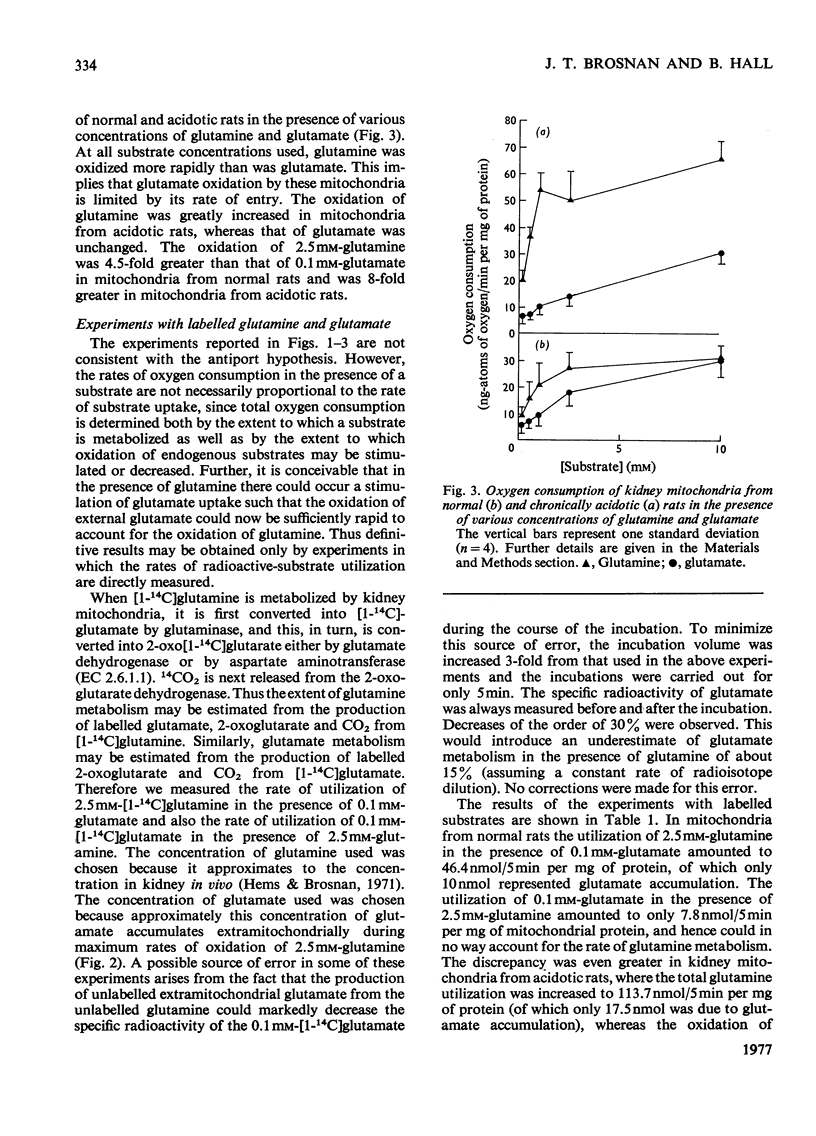
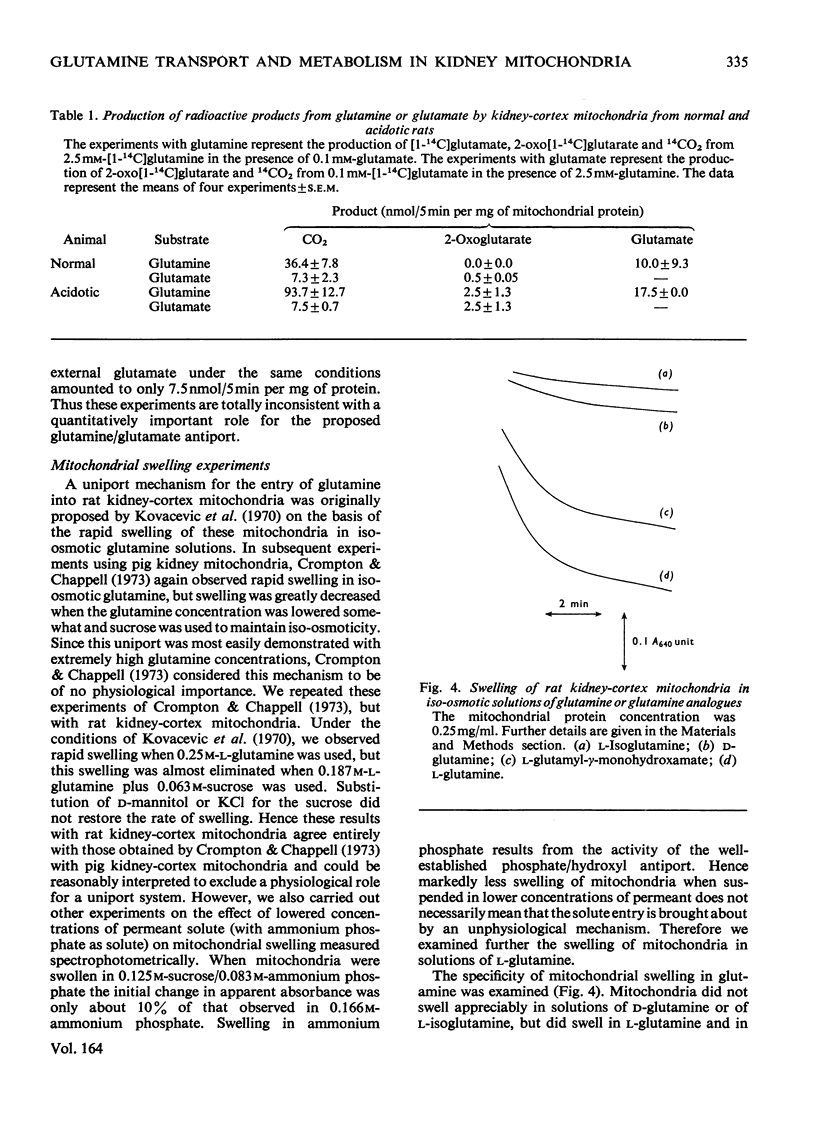
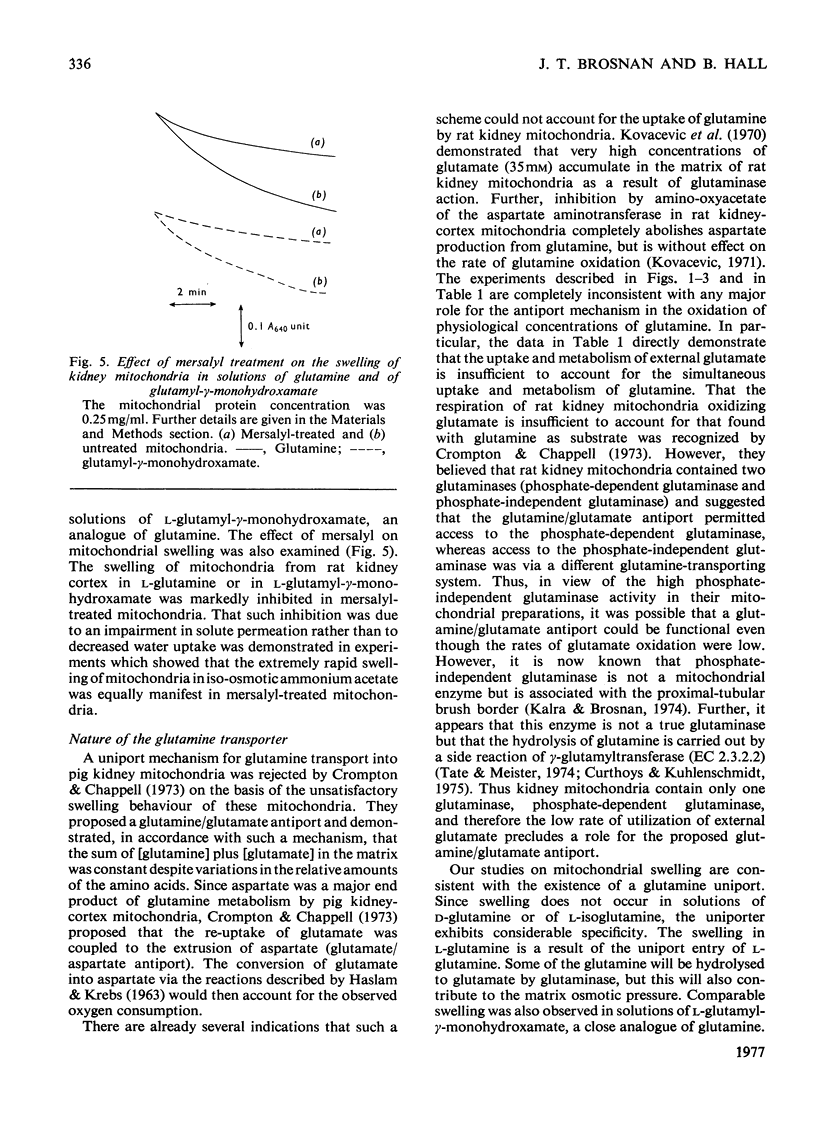
1. The oxidation of glutamine by kidney-cortex mitochondria from normal and acidotic rats was not inhibited by avenaciolide, which did inhibit glutamate uptake and oxidation. The oxidation of glutamine by these mitochondria was always greater than that of glutamate. Direct measurements of the metabolism of [1-14C]glutamine in the presence of glutamate, and of [1-14C]glutamate in the presence of glutamine, demonstrated that the uptake and metabolism of external glutamate is insufficient to account for the observed rate of glutamine uptake and metabolism. Thus the postulated glutamine/glutamate antiport does not play a quantitatively important role in the metabolism of glutamine by renal mitochondria. 2. Rapid swelling of these mitochondria was observed in iso-osmotic solutions of L-glutamine and L-glutamyl-gamma-monohydroxamate but not in D-glutamine or L-isoglutamine (1-amido-2-aminoglutaric acid). Thus a relatively specific glutamine uniport exists in these mitochondria. 3. The utilization of glutamine was increased about 3-fold in mitochondria from chronically acidotic rats. Thus mitochondrial adaptations play an important part in the renal response to metabolic acidosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam W., Simpson D. P. Glutamine transport in rat kidney mitochondria in metabolic acidosis. J Clin Invest. 1974 Jul;54(1):165–174. doi: 10.1172/JCI107738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., Chappell J. B. Transport of glutamine and glutamate in kidney mitochondria in relation to glutamine deamidation. Biochem J. 1973 Jan;132(1):35–46. doi: 10.1042/bj1320035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M., McGivan J. D., Chappell J. B. The intramitochondrial location of the glutaminase isoenzymes of pig kidney. Biochem J. 1973 Jan;132(1):27–34. doi: 10.1042/bj1320027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys N. P., Kuhlenschmidt T. Phosphate-independent glutaminase from rat kidney. Partial purification and identity with gamma-glutamyltranspeptidase. J Biol Chem. 1975 Mar 25;250(6):2099–2105. [PubMed] [Google Scholar]
- Goldstein L. Glutamine transport by mitochondria isolated from normal and acidotic rats. Am J Physiol. 1975 Oct;229(4):1027–1033. doi: 10.1152/ajplegacy.1975.229.4.1027. [DOI] [PubMed] [Google Scholar]
- HASLAM R. J., KREBS H. A. THE METABOLISM OF GLUTAMATE IN HOMOGENATES AND SLICES OF BRAIN CORTEX. Biochem J. 1963 Sep;88:566–578. doi: 10.1042/bj0880566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A., Brosnan J. T. Effects of metabolic acidosis and starvation on the content of intermediary metabolites in rat kidney. Biochem J. 1971 Jul;123(3):391–397. doi: 10.1042/bj1230391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra J., Brosnan J. T. The subcellular localization of glutaminase isoenzymes in rat kidney cortex. J Biol Chem. 1974 May 25;249(10):3255–3260. [PubMed] [Google Scholar]
- Kalra J., Brosnan J. T. The subcellular localization of glutaminase isoenzymes in rat kidney cortex. J Biol Chem. 1974 May 25;249(10):3255–3260. [PubMed] [Google Scholar]
- Kovacević Z., McGivan J. D., Chappell J. B. Conditions for activity of glutaminase in kidney mitochondria. Biochem J. 1970 Jun;118(2):265–274. doi: 10.1042/bj1180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacević Z. Possible mechanisms of the efflux of glutamate from kidney mitochondria generated by the activity of mitochondrial glutaminase. Biochim Biophys Acta. 1975 Sep 8;396(3):325–334. doi: 10.1016/0005-2728(75)90139-5. [DOI] [PubMed] [Google Scholar]
- Kovacević Z. The pathway of glutamine and glutamate oxidation in isolated mitochondria from mammalian cells. Biochem J. 1971 Dec;125(3):757–763. doi: 10.1042/bj1250757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PITTS R. F., PILKINGTON L. A., DEHAAS J. C. N15 TRACER STUDIES ON THE ORIGIN OF URINARY AMMONIA IN THE ACIDOTIC DOG, WITH NOTES ON THE ENZYMATIC SYNTHESIS OF LABELED CLUTAMIC ACID AND GLUTAMINES. J Clin Invest. 1965 May;44:731–745. doi: 10.1172/JCI105186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts R. F. Symposium on acid-base homeostasis. Control of renal production of ammonia. Kidney Int. 1972 May;1(5):297–305. doi: 10.1038/ki.1972.42. [DOI] [PubMed] [Google Scholar]
- Roobol A., Alleyne G. A. Control of renal cortex ammoniagenesis and its relationship to renal cortex gluconeogenesis. Biochim Biophys Acta. 1974 Aug 7;362(1):83–91. doi: 10.1016/0304-4165(74)90029-4. [DOI] [PubMed] [Google Scholar]
- Simpson D. P., Adam W. Glutamine transport and metabolism by mitochondria from dog renal cortex. General properties and response to acidosis and alkalosis. J Biol Chem. 1975 Oct 25;250(20):8148–8158. [PubMed] [Google Scholar]
- Tate S. S., Meister A. Stimulation of the hydrolytic activity and decrease of the transpeptidase activity of gamma-glutamyl transpeptidase by maleate; identity of a rat kidney maleate-stimulated glutaminase and gamma-glutamyl transpeptidase. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3329–3333. doi: 10.1073/pnas.71.9.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]


