Abstract
Background:
Productive cough with sputum is a prominent sign generally associated with respiratory diseases, including chronic obstructive pulmonary disease (COPD). Airway clearance devices are an option for COPD management, but physicians’ preferences for and clinical practice with them are not known.
Objective:
This study aims to explore preferences for and clinical practice with airway clearance devices among physicians in Saudi Arabia.
Design:
An observational, cross-sectional survey.
Methods:
A self-administered questionnaire was conducted between October 2022 and September 2023, which included a review of respiratory medication prescriptions by physicians for patients with COPD. The analysis was performed using the Statistical Package for the Social Sciences.
Results:
The participants were 445 physicians. The majority were female, accounting for 64.3% of the sample. Flutter and Acapella were the most commonly preferred airway clearance devices (45.8% and 20.7%, respectively). Among the participants, 12.6% reported unfamiliarity with any of the mentioned devices. Of the participants, 43.6% “usually” suggested the devices for patients with daily, difficult-to-clear, thick sputum, while 27% “sometimes” recommended them to COPD patients who had experienced four exacerbations or more. In routine clinical practice, physicians prescribe pharmacological therapies as the main treatment. The prescribing data showed that in the last year, there was no record of prescribed airway clearance devices for COPD by physicians.
Conclusion:
Family and pulmonary physicians prefer Flutter and Acapella devices, but a significant number of physicians are unaware of such devices. Prescribing data showed no record of prescribed airway clearance devices for COPD management. Further initiatives are needed to increase awareness in clinical practice.
Keywords: airway clearance, COPD, cough, physician, respiratory care
Background
Coughing is an important protective mechanism that helps to clear the airway and inhibit pulmonary complications.1,2 Productive cough with sputum is a prominent sign generally associated with respiratory diseases, including chronic obstructive pulmonary disease (COPD).2,3 During a pathological state of the respiratory tract, tracheobronchial mucus formation exceeds its clearance due to the inhibition of mucociliary function. 2 This leads to the excessive accumulation of secretions in the airway, which promotes breathing difficulty and increases the risk of exacerbation in COPD patients. 2 Therefore, airway clearance therapy is one of the most crucial aspects of treatment for individuals with COPD. 4
One important treatment shown to enhance airway clearance in COPD patients is the use of mucolytic agents. 5 These agents help to decrease sputum viscosity and facilitate its removal during coughing. Moreover, it has been shown that treatment with mucolytic drugs is associated with a slight reduction in the probability of developing exacerbation in COPD and chronic bronchitis patients.5 –7 Airway clearance techniques, particularly airway clearance devices, are non-pharmacological therapies that can be used with or without mucolytic medications to improve airway function and decrease the risk of respiratory exacerbation. Airway clearance devices work on the concept of applying high-frequency vibrations during exhalation, resulting in a reduction in secretion viscoelasticity and the augmentation of mucus mobility. 8 Accordingly, this might prevent the risks associated with secretion accumulation, which positively impacts hospital admission rates and quality of life for COPD patients.9,10 Notably, several clinical studies have suggested that using Oscillatory Positive Expiratory Pressure (OPEP) devices with COPD patients enhances clinical outcomes and prevents subsequent severe diseases after discharge.11 –13 Thus, these findings offer some important insights regarding COPD management that healthcare providers should consider when promoting secretion removal.
The current COPD management guidelines in Saudi Arabia include non-pharmacological strategies alongside other treatment regimens.14,15 However, there are questions about the knowledge and attitudes of family and pulmonary physicians regarding adherence and the implementation of these guidelines. 16 It has been suggested that there is a need for more awareness of international COPD guidelines, 17 understanding disease epidemiology, or applying available beneficial treatments among family and pulmonary physicians in primary care settings. 16 Despite the importance of airway clearance devices in managing patients, data on the understanding of these devices in clinical practice are still limited. Hence, this study aims to explore preferences and the clinical practice of airway clearance devices among family and pulmonary physicians in Saudi Arabia.
Methods
Study design
This cross-sectional observational study was conducted between October 2022 and September 2023 using a self-administered questionnaire. The questionnaire was previously used in COPD clinical studies and was only available in English (Supplemental digital content 1).18,19 The questionnaire was employed to evaluate the preferences and clinical practices of airway clearance devices among family and pulmonary physicians in Saudi Arabia. The prescription data for respiratory medications with COPD from October 2022 to September 2023 was reviewed. This study adheres to the guidelines outlined in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 20
Questionnaire
The questionnaire was initially designed and validated by a team of respiratory medicine experts who also assessed its face and content validity. 19 It had been previously employed in clinical studies on COPD and was only available in English; demographics, preferences, and clinical practices of using airway clearance devices were the themes of this survey. The term airway clearance device refers to any physical device that helps clear mucus.18,21 COPD exacerbation was defined as any deterioration in the symptoms requiring medical assistance.17,22 A 5-point Likert scale (i.e., “always,” “usually,” “sometimes,” “rarely,” and “never”) was used to answer the multiple-choice questions. The purpose and summary of the study, as well as information about the principal investigator, were presented to the participants before they began filling out the questionnaire. While demographic information was collected, no personal identifiable information was gathered, and participation was voluntary. The following additional statement was included in the survey: ‘By responding “Yes” or “No” to the survey questions, you give your consent to your anonymous data being used for research purposes.’ When a participant answered “Yes,” the survey page opened, and if they answered “No,” the survey closed.
Data collection and sampling strategy
Methods for convenience cross-sectional sampling were employed to recruit the study participants. The sample size determination was based on a report published by the General Authority of Statistics, which enumerated approximately 65,316 physicians in Saudi Arabia. 23 Employing a 95% confidence level, an anticipated response rate of 50%, and a 5% margin of error, the minimum sample size was calculated to be 382 physicians. The questionnaire was distributed online. Physicians, particularly family and pulmonary specialists, who were more inclined to perform standard assessments for COPD patients and monitor their health status, were included. These physicians must hold an active license, practice in Saudi Arabia, and agree to participate in the study. Two professional bodies managing respiratory diseases were invited to assist in data collection. These were the Saudi Society of Family and Community Medicine and the Saudi Thoracic Society. Prescriptions of medications by family and pulmonary physicians were collected retrospectively from Al-Noor Specialist Hospital and the Security Forces Hospital in Makkah using a standard spreadsheet.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, V.26, IBM Corporation, Armonk, NY, USA) was used for the analysis. Categorical variables were reported as percentages and frequencies. The statistical significance of the categorical variables was determined using a chi-square test. We used a 95% confidence interval to determine the ideal sample size. However, the significance level of p < 0.01 was used to determine the significance threshold.
Results
The study included 445 physicians. The majority of the participants were female, accounting for 64.3% of the sample, with most falling in the 20–30 age group (50.8%), followed by the 31–40 age range (31.5%). Most of the physicians who participated in the study were Saudi nationals (84.3%) and worked in specialist hospitals (44%). Geographically, the southern region (29.4%) and the eastern region (24.3%) had the highest representation among the participants. The majority held a medical bachelor’s degree (66.5%) and identified as family physicians (67.1%). The largest groups had 3–4 years (31.2%) of experience with COPD patients, followed by 5–6 years (28.1%) and 1–2 years (23.4%), as depicted in Table 1.
Table 1.
Characteristics of the survey participants (n = 445).
| Characteristics | Frequency (n) | Percentage |
|---|---|---|
| Gender | ||
| Male | 159 | 35.7 |
| Female | 286 | 64.3 |
| Age | ||
| 20–30 | 226 | 50.8 |
| 31–40 | 140 | 31.5 |
| 41–50 | 54 | 12.1 |
| 51–60 | 23 | 5.2 |
| >60 | 2 | 0.4 |
| Nationality | ||
| Saudi | 375 | 84.3 |
| Non-Saudi | 70 | 15.7 |
| Medical centers | ||
| Clusters | 25 | 5.6 |
| General practice | 100 | 22.5 |
| Specialized hospitals | 196 | 44 |
| Medical cities | 67 | 15.1 |
| Private hospitals | 57 | 12.8 |
| Geographical location | ||
| Central region | 83 | 18.7 |
| Eastern region | 108 | 24.3 |
| Northern region | 70 | 15.7 |
| Southern region | 131 | 29.4 |
| Western region | 53 | 11.9 |
| Academic and clinical qualifications | ||
| Medical bachelor’s degree (MD) | 296 | 66.5 |
| MD and master’s degree | 68 | 15.3 |
| MD and medical board residency/fellowship | 54 | 12.1 |
| MD and PhD degree | 27 | 6.1 |
| Profession | ||
| Family physicians | 299 | 67.1 |
| Pulmonary physicians | 146 | 33.0 |
| Years of experience with COPD patients | ||
| 1–2 years | 104 | 23.4 |
| 3–4 years | 139 | 31.2% |
| 5–6 years | 125 | 28.1 |
| 7–8 years | 36 | 8.1 |
| >8 years | 41 | 9.2 |
COPD, chronic obstructive pulmonary disease; MD, medical doctor.
Physicians’ preferences for prescribing airway clearance devices
The second theme in the survey focused on the physicians’ preferences for prescribing airway clearance devices (the survey included pictures of the devices). Approximately two-thirds of the participants selected Flutter (45.8%) and Acapella (20.7%). Conversely, Aerosure (2.5%), Bubble PEP (4.7%), Aerobika (5.8%), and PEP masks (7.9%) were the least selected devices. Notably, 12.6% of respondents reported not being familiar with any of the airway clearance devices mentioned (Table 2).
Table 2.
Comparing physicians’ preferences in prescribing airway clearance devices.
| Airway clearance devices | Total | Family | Pulmonary | Chi-square (p value) |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| Flutter (Allergan, Dublin, Ireland) | 204 (45.8) | 138 (46.5) | 66 (44.6) | <0.001* |
| Acapella (Smiths-Medical, Dublin, OH, USA) | 92 (20.7) | 58 (19.5) | 34 (23) | 0.005* |
| PEP mask | 35 (7.9) | 25 (8.3) | 12 (8.1) | <0.001* |
| Aerobika (Monaghan Medical, Plattsburgh, NY, USA) |
26 (5.8) | 9 (3) | 17(11.5) | <0.001* |
| Bubble PEP | 21 (4.7) | 7 (2.4) | 12 (8.1) | 0.05 |
| Aerosure (Actegy, Bracknell, UK) | 11 (2.5) | 7 (2.4) | 4 (2.7) | 0.05 |
| I don’t know any of these devices | 56 (12.6) | 55 (18.5) | 1 (0.7) | <0.001* |
Percentages compared with the chi-square test.
Denotes that the difference between groups was statistically significant.
PEP, positive expiratory pressure.
Difference between physicians’ preferences for prescribing airway clearance devices
Factors related to physicians’ preferences for prescribing airway clearance devices were explored based on demographics. In general, the analysis revealed that age, place of work, region, and academic qualifications could be potential factors that influence the prescription of airway clearance devices among family and pulmonary physicians (Table 3). An additional analysis comparing family and pulmonary physicians who exclusively prescribed airway devices identified an additional influencing factor: nationality (Supplemental digital content 2).
Table 3.
Comparing demographic factors related to prescribing airway clearance devices.
| Variables | Comparing factors related to airway clearance device prescription | p Value | |||
|---|---|---|---|---|---|
| Family | Pulmonary | ||||
| n | % | n | % | ||
| Gender | |||||
| Female | 112 | 37.5 | 47 | 32.2 | 0.071 |
| Male | 187 | 62.5 | 99 | 67.8 | |
| Age | |||||
| 20–30 | 158 | 52.8 | 68 | 46.6 | 0.008 |
| 31–40 | 95 | 31.8 | 45 | 30.8 | |
| 41–50 | 27 | 9.0 | 27 | 18.5 | |
| 51–60 | 19 | 6.4 | 4 | 2.7 | |
| Nationality | |||||
| Saudi | 259 | 86.6 | 116 | 79.5 | 0.680 |
| Non-Saudi | 40 | 13.4 | 30 | 20.5 | |
| Medical center | |||||
| Clusters | 19 | 6.4 | 6 | 4.1 | 0.004 |
| General practice | 88 | 29.4 | 12 | 8.2 | |
| Specialized hospitals | 130 | 43.5 | 66 | 45.2 | |
| Medical cities | 30 | 10.0 | 37 | 25.3 | |
| Private hospitals | 32 | 10.7 | 25 | 17.1 | |
| Regions | |||||
| Central region | 58 | 19.4 | 25 | 17.1 | <0.001 |
| Eastern region | 80 | 26.8 | 28 | 19.2 | |
| Northern region | 50 | 16.7 | 20 | 13.7 | |
| Southern region | 80 | 26.8 | 51 | 34.9 | |
| Western region | 31 | 10.4 | 22 | 15.1 | |
| Academic and clinical qualifications | |||||
| Medical bachelor’s degree (MD) | 149 | 49.8 | 92 | 63.0 | <0.001 |
| MD and master’s degree | 34 | 11.4 | 34 | 23.3 | |
| MD and medical board residency/fellowship | 52 | 17.4 | 2 | 1.4 | |
| MD and PhD degree | 23 | 7.7 | 4 | 2.7 | |
| Years of experience with COPD patients | |||||
| 1–2 years | 81 | 27.1 | 23 | 15.8 | 0.080 |
| 3–4 years | 89 | 29.8 | 50 | 34.2 | |
| 5–6 years | 69 | 23.1 | 56 | 38.4 | |
| 7–8 years | 25 | 8.4 | 11 | 7.5 | |
| >8 years | 35 | 11.7 | 6 | 4.1 | |
MD, medical doctor; PhD, Doctor of Philosophy.
Physicians’ Recommendations for Airway Clearance Devices
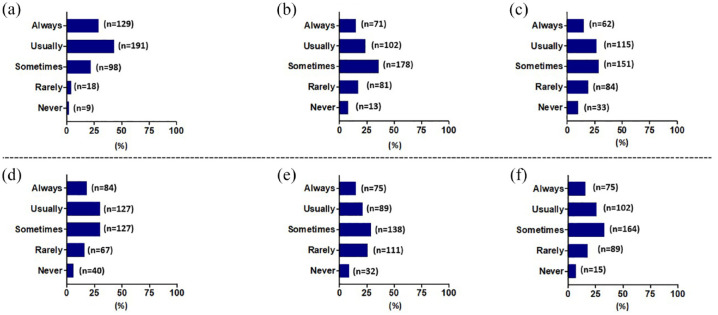
When the physicians were asked about their recommendations for using airway clearance devices for different COPD severity categories, 43.6% of them “usually” suggested them for patients with daily, difficult-to-clear, thick sputum. Physicians also “sometimes” (40%) or “usually” (23%) recommended these devices to COPD patients who produced sputum throughout the day but could clear it. In addition, 34% of physicians “sometimes” recommended cough devices for patients with COPD who had morning sputum only. Regarding recommending airway clearance devices for COPD exacerbations, 37% of the physicians “sometimes” recommended airway clearance devices, with an increased percentage when exacerbations occurred four or more times a year (Figure 1).
Figure 1.
Physicians’ recommendations for airway clearance devices based on severity categories: (a) A COPD patient with daily difficulties clearing thick sputum, (b) A COPD patient producing sputum throughout the day but able to clear it, (c) A COPD patient with morning sputum only, (d) A COPD patient who only has sputum with exacerbations or has 0-1 exacerbation/year, (e) A COPD patient who only has sputum with exacerbations and has 2-3 exacerbations /year and (f) A COPD Patient who only has sputum with exacerbations and has >4 exacerbations/year.
Clinical practices of prescribing airway clearance devices
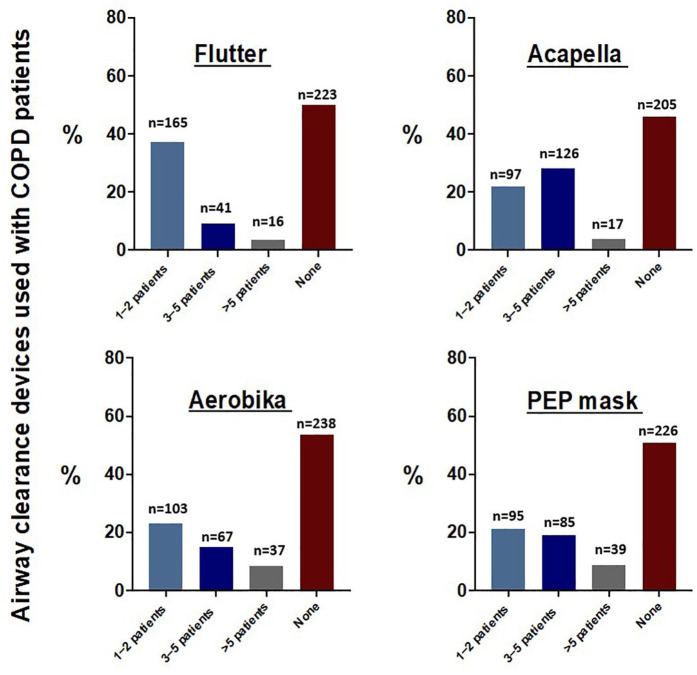
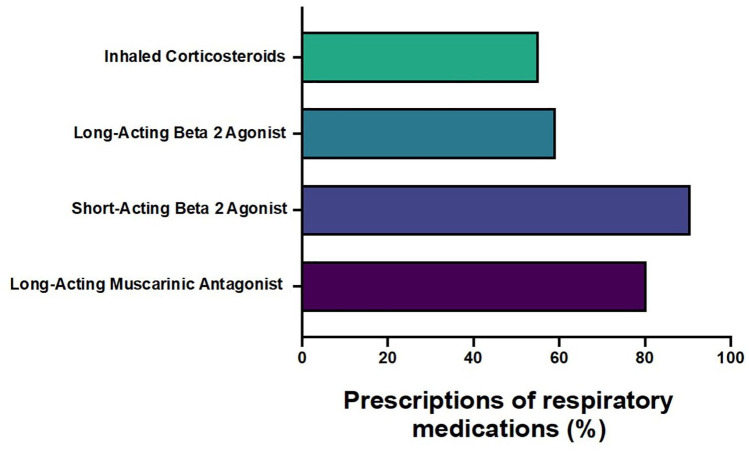
In clinical practice, physicians have reported the prescription of different airway clearance devices for patients with COPD. Questionnaire data indicated that 38% of physicians prescribed the Flutter device, 22% recommended the Acapella, 23% opted for the Aerobika, and 21% selected the PEP mask for one or two COPD patients (Figure 2). When assessing the real prescription data by family and pulmonary physicians, the retrospective data showed that there was no record of prescribed airway clearance devices over the reported year (the report covers the period from October 2022 to September 2023). On the other hand, drugs for obstructive airway diseases were prescribed to manage cough and sputum in COPD: 90.7% were prescribed short-acting beta-2 agonists (SABA), followed by long-acting muscarinic antagonists (LAMA) (80.3%), long-acting beta-2 agonists (LABA; 59.2%), and inhaled corticosteroids (55.2%) (Figure 3).
Figure 2.
Clinical practices of prescribing airway clearance devices used with COPD patients. Family and pulmonary physicians’ survey.
COPD, chronic obstructive pulmonary disease.
Figure 3.
Prescribing data of respiratory medications by family and pulmonary physicians to manage cough and sputum per total number of patients. The data represent the prescriptions for 700 patients with a COPD diagnosis. The percentages in the figure represent the percentage of patients who received respiratory medications prescribed by family and pulmonary physicians. Prescribing data showed that there was no record of airway clearance devices being prescribed.
COPD, chronic obstructive pulmonary disease.
Discussion
Generally, family and pulmonary physicians are aware of airway clearance devices, but preferences and reasons for prescribing them for individuals with COPD vary. The findings show a disparity between physicians’ preferences and the prescribing data for airway clearance devices. There was no record of prescribed airway clearance devices for COPD in the reported year. This study makes an important contribution toward a better understanding of the current clinical practices related to airway clearance devices among family and pulmonary physicians in Saudi Arabia. It has been well documented that airway clearance devices can lead to a reduction in exacerbation frequency, improvements in sputum clearance, and a reduction in symptoms in individuals with COPD.13,18,24 Therefore, the current understanding and utilization of airway clearance devices among physicians must be updated continuously to facilitate the use of low-cost, high-value, non-pharmacological treatments in COPD care rather than respiratory medications. In parallel, clinical practice guidelines for COPD care in Saudi Arabia are still missing clinical data that present the benefits of using airway clearance devices. 18
Our study revealed that, among the participants, Flutter and Acapella were the two most popular devices in clinical practice. However, there was a significant difference in preferences between family and pulmonary physicians. Family physicians favored Flutter, PEP masks, and Aerobika devices the most. This observation may stem from familiarity with the devices depicted in the survey, which included images. Furthermore, the existing evidence substantiates the use of these devices in aiding sputum clearance for COPD patients. Another contributing factor could be that the majority of participants held a medical bachelor’s degree and were relatively young, with fewer than 4 years of independent clinical practice, which may have influenced their familiarity with the devices. Additionally, the popularity of certain devices over others can be attributed to another study that found comparable performance characteristics between Acapella and Flutter. Notably, Acapella’s performance is not gravity-dependent; this means it does not rely on the device’s orientation. This feature might make it easier for some patients to use, particularly at a lower expiratory flow. 25
It is important to emphasize the need for future studies to explore the reasons behind the low preference for other devices, including Aerosure, Bubble PEP, Aerobika, and PEP masks. It is pivotal to determine whether the limited use of these devices stems from availability, clinical rationale, or simply a lack of awareness.
From this study, we can observe that a significant number of physicians recommend these devices for clearing thick mucus in the airways, mainly for individuals with COPD, who have a large amount of secretions. They also stated that these devices are often recommended during COPD exacerbations. Furthermore, our findings corroborate other studies, providing collective evidence that airway clearance devices can improve sputum clearance and reduce exacerbation frequency and symptoms in stable COPD.26 –28 These findings encourage future studies to investigate the short- and long-term impacts of using these devices on individuals with COPD.
Our analysis of the clinical data revealed that the majority of individuals with COPD were prescribed SABA, LAMA, and LABA along with inhaled corticosteroids. In contrast, there were no records of airway clearance devices being prescribed. This suggests a disparity in the management of COPD symptoms. This discrepancy can also be explained by the underutilization of airway clearance devices, as well as the lack of awareness of the existence of these devices. In addition, Saudi guidelines for the diagnosis and management of COPD did not refer to using airway clearance devices or chest physiotherapy when describing “non-pharmacological therapies” in COPD with sputum production.14,15 Thus, informing and reinforcing local guidelines with recent evidence about the benefits of non-pharmacological therapies must be established.5,13,16,18,24 However, our prescribing data do reveal a concern about the underutilization of airway clearance devices in treating COPD patients.
Clinical implementation
Based on the findings of our study, it is evident that there is a need for continuous medical education within the physician community. This requirement is especially pertinent among younger healthcare professionals, as a substantial section of this demographic is unaware of the existence and efficacy of various airway clearance devices for COPD. To enhance the quality of care and streamline the prescription process, it is essential to establish precise guidelines for device utilization and documentation. This is particularly critical for patients dealing with persistent and challenging sputum. By aligning prescription practices with evidence-based guidelines and subjecting them to clinical reviews, we can enhance patient outcomes. Furthermore, there is an exigent need to intensify patient education efforts regarding the potential advantages of non-pharmacological devices. Such initiatives could potentially lead to an increased frequency of device prescriptions following the latest evidence-based recommendations. A strength of this study was its reporting of preferences for airway clearance devices, which are rarely documented in clinical practice. Additionally, it provides a comparison between pharmacological treatments and airway clearance devices for COPD in routine care.
Limitations
However, this study is not free of certain limitations that should be taken into consideration. First, the findings may not be readily generalizable to other regions of Saudi Arabia, as the prescribing data collection process exclusively focused on hospitals within a specific geographical area; they did not include data from hospitals in all the regions of the country. Additionally, information from two facilities that provide airway clearance devices through medical prescriptions indicates that their situation may differ from other healthcare facilities. Consequently, these data should be interpreted carefully. Moreover, it is pivotal to note that this study relied on self-reported responses from physicians, thereby introducing the possibility of response bias, whereby they may not have provided completely accurate or unbiased information. Finally, as a cross-sectional study, our research design did not permit us to create the temporality between our variables of interest. Future studies with different designs are warranted to investigate causal relationships in greater depth.
Conclusion
Notwithstanding the wide range of airway clearance devices available for COPD patients, the Flutter and Acapella devices are preferred by physicians. Intriguingly, a significant number of physicians are unaware of any such devices. When prescribing airway clearance devices, the frequency of exacerbations and the patients’ pattern of sputum production significantly impact the recommendations for them. Thus, pharmaceutical therapies are the main therapeutic strategy compared to non-pharmacological therapies in COPD patients with frequent sputum production. This suggests that, while physicians can identify the significance of airway clearance devices in treating COPD, their actual usage might not align with their current practice, thus highlighting the need for further initiatives to increase awareness about using airway clearance devices in clinical settings.
Supplemental Material
Supplemental material, sj-docx-1-tar-10.1177_17534666241307066 for Observational study on the prescription practices of family and pulmonary physicians for airway clearance devices in chronic obstructive pulmonary disease management by Saeed Mardy Alghamdi, Abdulaziz A. Alzahrani, Mohammad S. Dairi, Hassan Alwafi, Abdulelah M. Aldhahir, Jaber S. Alqahtani, Mohammed M. Alqahtani, Abdullah M. Alanazi, Abdullah A. Alqarni, Rayan A. Siraj, Noha Saeed Alghamdi, Hassan A. Alzahrani and Abdulghani A. Alhindi in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666241307066 for Observational study on the prescription practices of family and pulmonary physicians for airway clearance devices in chronic obstructive pulmonary disease management by Saeed Mardy Alghamdi, Abdulaziz A. Alzahrani, Mohammad S. Dairi, Hassan Alwafi, Abdulelah M. Aldhahir, Jaber S. Alqahtani, Mohammed M. Alqahtani, Abdullah M. Alanazi, Abdullah A. Alqarni, Rayan A. Siraj, Noha Saeed Alghamdi, Hassan A. Alzahrani and Abdulghani A. Alhindi in Therapeutic Advances in Respiratory Disease
Acknowledgments
We would like to express our deepest gratitude to our co-author, Abdullah M. Alanazi, who sadly passed away during the revision of this manuscript. We are profoundly grateful for Abdullah M. Alanazi’s commitment and passion, which will continue to inspire us. We also acknowledge that the abstract was previously presented as a poster at the International Conference for the American Thoracic Society on May 19, 2024, San Diego Convention Center, USA.
Footnotes
ORCID iDs: Saeed Mardy Alghamdi  https://orcid.org/0000-0002-6677-1110
https://orcid.org/0000-0002-6677-1110
Abdulaziz A Alzahrani  https://orcid.org/0000-0001-5973-2802
https://orcid.org/0000-0001-5973-2802
Hassan Alwafi  https://orcid.org/0000-0001-5627-1633
https://orcid.org/0000-0001-5627-1633
Jaber S. Alqahtani  https://orcid.org/0000-0003-1795-5092
https://orcid.org/0000-0003-1795-5092
Abdullah A. Alqarni  https://orcid.org/0000-0002-2460-3901
https://orcid.org/0000-0002-2460-3901
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Saeed Mardy Alghamdi, Clinical Technology Department, Respiratory Care Program, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah 21961, Saudi Arabia.
Abdulaziz A. Alzahrani, Clinical Technology Department, Respiratory Care Program, Faculty of Applied Medical Sciences, Umm Al-Qura University, Makkah, Saudi Arabia
Mohammad S. Dairi, Department of Medicine, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia
Hassan Alwafi, Department of Pharmacology and Toxicology, College of Medicine, Umm Al-Qura University, Makkah, Saudi Arabia.
Abdulelah M. Aldhahir, Respiratory Therapy Program, Nursing Department, College of Nursing and Health Sciences, Jazan University, Jazan, Saudi Arabia
Jaber S. Alqahtani, Department of Respiratory Care, Prince Sultan Military College of Health Sciences, Dammam, Saudi Arabia
Mohammed M. Alqahtani, Department of Respiratory Therapy, College of Applied Medical Sciences, King Saud Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
Abdullah M. Alanazi, Department of Respiratory Therapy, College of Applied Medical Sciences, King Saud Abdulaziz University for Health Sciences, King Abdullah International Medical Research Center, Riyadh, Saudi Arabia
Abdullah A. Alqarni, Department of Respiratory Therapy, Faculty of Medical Rehabilitation Sciences, King Abdulaziz University, Jeddah, Saudi Arabia Respiratory Therapy Unit, King Abdulaziz University Hospital, Jeddah, Saudi Arabia.
Rayan A. Siraj, Department of Respiratory Care, College of Applied Medical Sciences, King Faisal University, Al-Ahasa, Saudi Arabia
Noha Saeed Alghamdi, University College London, Global Business School for Health, London, UK.
Hassan A. Alzahrani, Department of Respiratory Care, Medical Cities at the Minister of Interior, MCMOl, Riyadh, Saudi Arabia
Abdulghani A. Alhindi, Respiratory Care Unit, Security Forces Hospital, Makkah, Saudi Arabia
Declarations
Ethics approval and consent to participate: Ethical approval for the study was obtained from the Institutional Review Boards (IRB) at Al-Noor Specialist Hospital at Makkah, (IRB number: H02K0760523951) and Security Force Hospital at Makkah (IRB number: 0560070223). Written consent was obtained prior to participation in the study.
Consent for publication: Not applicable.
Author contributions: Saeed Mardy Alghamdi: Conceptualization; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Abdulaziz A. Alzahrani: Conceptualization; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Mohammad S. Dairi: Data curation; Formal analysis; Investigation; Writing – review & editing.
Hassan Alwafi: Data curation; Formal analysis; Investigation; Writing – review & editing.
Abdulelah M. Aldhahir: Data curation; Formal analysis; Investigation; Writing – review & editing.
Jaber Alqahtani: Data curation; Formal analysis; Investigation; Writing – review & editing.
Mohammed M. Alqahtani: Formal analysis; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Abdullah M. Alanazi: Formal analysis; Investigation; Visualization; Writing – original draft; Writing – review & editing.
Abdullah A. Alqarni: Formal analysis; Investigation; Validation; Visualization; Writing – review & editing.
Rayan A. Siraj: Formal analysis; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing.
Noha Saeed Alghamdi: Formal analysis; Validation; Writing – review & editing.
Hassan A. Alzahrani: Conceptualization; Investigation; Resources; Writing – review & editing.
Abdulghani A. Alhindi: Conceptualization; Investigation; Resources; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: All pertinent data are contained within the paper.
References
- 1. Chung KF, McGarvey L, Song W-J, et al. Cough hypersensitivity and chronic cough. Nat Rev Dis Primers 2022; 8: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hughes R, Rapsomaniki E, Janson C, et al. Frequent productive cough: symptom burden and future exacerbation risk among patients with asthma and/or COPD in the NOVELTY study. Respir Med 2022; 200: 106921. [DOI] [PubMed] [Google Scholar]
- 3. Miravitlles M. Cough and sputum production as risk factors for poor outcomes in patients with COPD. Respir Med 2011; 105: 1118–1128. [DOI] [PubMed] [Google Scholar]
- 4. Tse J, Wada K, Wang Y, et al. Impact of oscillating positive expiratory pressure device use on post-discharge hospitalizations: a retrospective cohort study comparing patients with COPD or chronic bronchitis using the Aerobika® and Acapella® devices. Int J Chron Obstruct Pulmon Dis 2020; 15: 2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bourbeau J, McIvor RA, Devlin HM, et al. Oscillating positive expiratory pressure (OPEP) device therapy in Canadian respiratory disease management: review, care gaps and suggestion for use. Can J Respir Crit Care Sleep Med 2019; 3: 233–240. [Google Scholar]
- 6. Poole P, Sathananthan K, Fortescue R. Mucolytic agents versus placebo for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database of Syst Rev 2019; 5(5): CD001287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadopoulou E, Hansel J, Lazar Z, et al. Mucolytics for acute exacerbations of chronic obstructive pulmonary disease: a meta-analysis. Eur Respir Rev 2023; 32: 32(167): 220141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brooks D, Newbold E, Kozar LF, et al. The flutter device and expiratory pressures. J Cardiopulm Rehabil Prev 2002; 22: 53–57. [DOI] [PubMed] [Google Scholar]
- 9. Lindberg A, Sawalha S, Hedman L, et al. Subjects with COPD and productive cough have an increased risk for exacerbations and death. Respir Med 2015; 109: 88–95. [DOI] [PubMed] [Google Scholar]
- 10. Langenderfer B. Alternatives to percussion and postural drainage: a review of mucus clearance therapies: percussion and postural drainage, autogenic drainage, positive expiratory pressure, flutter valve, intrapulmonary percussive ventilation, and high-frequency chest compression with the ThAIRapy vest. J Cardiopulm Rehabil Prevent 1998; 18: 283–289. [DOI] [PubMed] [Google Scholar]
- 11. Lewis A, Osadnik CR. Changing practice by changing pressures: a role for oscillating positive expiratory pressure in chronic obstructive pulmonary disease. BMJ Publishing Group Ltd, UK, 2023, p. 113–115. [DOI] [PubMed] [Google Scholar]
- 12. Svenningsen S, Paulin GA, Sheikh K, et al. Oscillatory positive expiratory pressure in chronic obstructive pulmonary disease. COPD 2016; 13: 66–74. [DOI] [PubMed] [Google Scholar]
- 13. Alghamdi SM, Alsulayyim AS, Alasmari AM, et al. Oscillatory positive expiratory pressure therapy in COPD (O-COPD): a randomised controlled trial. Thorax 2023; 78: 136–143. [DOI] [PubMed] [Google Scholar]
- 14. Khan JH, Lababidi HM, Al-Moamary MS, et al. The Saudi guidelines for the diagnosis and management of COPD. Ann Thorac Med 2014; 9: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Al-Jahdali H, Al-Lehebi R, Lababidi H, et al. The Saudi Thoracic Society Evidence-based guidelines for the diagnosis and management of chronic obstructive pulmonary disease. Ann Thorac Med 2024; 9(2): 55–76. [Google Scholar]
- 16. Daynes E, Greening N, Singh SJ. Randomised controlled trial to investigate the use of high-frequency airway oscillations as training to improve dyspnoea (TIDe) in COPD. Thorax 2022; 77: 690–696. [DOI] [PubMed] [Google Scholar]
- 17. Agustí A, Celli BR, Criner GJ, et al. Global initiative for chronic obstructive lung disease 2023 report: GOLD executive summary. Am J Respir Crit Care Med 2023; 207: 819–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alghamdi SM, Alzahrani A, Alshahrani YM, et al. Perception and clinical practice regarding mucus clearance devices with chronic obstructive pulmonary disease: a cross-sectional study of healthcare providers in Saudi Arabia. BMJ Open 2023; 13: e074849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barker R, Laverty AA, Hopkinson NS. Adjuncts for sputum clearance in COPD: clinical consensus versus actual use. BMJ Open Respir Res 2017; 4: e000226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 21. Osadnik CR, McDonald CF, Holland AE. Airway clearance techniques in acute exacerbations of COPD: a survey of Australian physiotherapy practice. Physiotherapy 2013; 99: 101–106. [DOI] [PubMed] [Google Scholar]
- 22. Tamondong-Lachica DR, Skolnik N, Hurst JR, et al. GOLD 2023 Update: implications for clinical practice. Int J Chron Obstruct Pulmon Dis 2023; 18: 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Physicians in health care centers of MOH by specialization and nationality, https://www.stats.gov.sa/ar/6174 (2024, accessed 7 November 2024).
- 24. Alghamdi SM, Barker RE, Alsulayyim AS, et al. Use of oscillatory positive expiratory pressure (OPEP) devices to augment sputum clearance in COPD: a systematic review and meta-analysis. Thorax 2020; 75: 855–863. [DOI] [PubMed] [Google Scholar]
- 25. Volsko TA, DiFiore J, Chatburn RL. Performance comparison of two oscillating positive expiratory pressure devices: acapella versus flutter. Respir Care 2003; 48: 124–130. [PubMed] [Google Scholar]
- 26. Daynes E, Jones AW, Greening NJ, et al. The use of airway clearance devices in the management of chronic obstructive pulmonary disease. A systematic review and meta-analysis of randomized controlled trials. Ann Am Thorac Society 2021; 18: 308–320. [DOI] [PubMed] [Google Scholar]
- 27. Osadnik CR, McDonald CF, Jones AP, et al. Airway clearance techniques for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012: Cd008328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lisy K, White H, Pearson A. Overview of reviews: mechanical interventions for the treatment and management of chronic obstructive pulmonary disease. Int J Nurs Pract 2014; 20: 701–708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tar-10.1177_17534666241307066 for Observational study on the prescription practices of family and pulmonary physicians for airway clearance devices in chronic obstructive pulmonary disease management by Saeed Mardy Alghamdi, Abdulaziz A. Alzahrani, Mohammad S. Dairi, Hassan Alwafi, Abdulelah M. Aldhahir, Jaber S. Alqahtani, Mohammed M. Alqahtani, Abdullah M. Alanazi, Abdullah A. Alqarni, Rayan A. Siraj, Noha Saeed Alghamdi, Hassan A. Alzahrani and Abdulghani A. Alhindi in Therapeutic Advances in Respiratory Disease
Supplemental material, sj-docx-2-tar-10.1177_17534666241307066 for Observational study on the prescription practices of family and pulmonary physicians for airway clearance devices in chronic obstructive pulmonary disease management by Saeed Mardy Alghamdi, Abdulaziz A. Alzahrani, Mohammad S. Dairi, Hassan Alwafi, Abdulelah M. Aldhahir, Jaber S. Alqahtani, Mohammed M. Alqahtani, Abdullah M. Alanazi, Abdullah A. Alqarni, Rayan A. Siraj, Noha Saeed Alghamdi, Hassan A. Alzahrani and Abdulghani A. Alhindi in Therapeutic Advances in Respiratory Disease