Abstract
Background:
Despite the revolutionary impact of immune checkpoint inhibitors (ICIs) on the treatment of metastatic urothelial carcinoma (mUC), the clinical utility of reliable prognostic biomarkers to foresee survival outcomes remains underexplored.
Objectives:
The purpose of this study was to ascertain the prognostic significance of serum inflammatory markers in mUC patients undergoing ICI therapy.
Design:
This is a retrospective, multicenter study.
Methods:
Data were collected from two independent medical centers in Taiwan, encompassing a validation and a training cohort (TC). Patients with histopathologically confirmed urothelial carcinoma who received at least one cycle of ICI monotherapy were included. Serum inflammatory markers such as neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and systemic immune-inflammation index (SII) were calculated prior to ICI therapy. Statistical analyses involved the use of receiver operating characteristic (ROC) curves to determine optimal biomarker cutoffs and Cox proportional hazards models to evaluate the independent predictive capability of these markers.
Results:
A total of 192 patients were enrolled. In the univariate analysis, serum markers such as NLR, PLR, SII, and Hb were significantly associated with overall survival (OS) in both the training and validation cohorts (VC). White blood cells, NLR, and SII demonstrated a robust correlation with progression-free survival across both cohorts. Multivariate analysis revealed that Eastern Cooperative Oncology Group performance status ⩾2 (p < 0.001), visceral metastasis (p < 0.001), leukocytosis (p < 0.001), Hb levels ⩾10 mg/dL (p = 0.008), and NLR ⩾5 (p = 0.032) as independent predictors of OS. A prognostic nomogram integrating these independent factors yielded a C-index for a 3-year OS of 0.769 in the TC and 0.657 in the VC.
Conclusion:
Serum inflammatory markers, combined with clinicopathologic factors, provide a practical prognostic tool in mUC treatment with ICIs.
Keywords: immune checkpoint inhibitor, leukocytosis, metastatic urothelial carcinoma, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, systemic immune-inflammation index
Plain language summary
Using blood tests and health information to predict survival in bladder cancer patients receiving immune therapy
Study overview
Despite the success of new immune therapies in treating advanced bladder cancer, we still need better ways to predict how well patients will respond to treatment. Our study looked at certain blood tests that measure inflammation to see if they can help predict patient outcomes.
What we did
We reviewed the medical records of 192 patients with advanced bladder cancer who were treated at two medical centers in Taiwan. These patients had received immune therapy, and we analyzed their blood for signs of inflammation before they started treatment. We specifically looked at the ratios of different types of blood cells involved in inflammation and immune response.
What we found
Certain blood markers were linked to how long patients lived after treatment. For example, patients with higher levels of certain inflammatory markers tended to have shorter survival times. We used this information along with other medical data to create a tool that helps predict how patients might do with treatment.
What it means
Our findings suggest that checking for inflammation in the blood could help doctors better understand who will benefit most from immune therapy for advanced bladder cancer. This could help tailor treatments more effectively for each patient.
Introduction
Metastatic urothelial cancer (mUC) poses a significant clinical challenge, with a 5-year survival rate of only 5.5%. 1 For decades, cisplatin-based chemotherapy has stood as the standard first-line treatment for metastatic urothelial carcinoma (mUC),2 –4 yielding an objective response rate (ORR) of approximately 40%–50%. 5 However, most of the patients experience disease progression soon after treatment initiation, with a median survival of about 14–15 months. 5 Moreover, approximately 50% of patients with mUC are deemed ineligible for cisplatin-based chemotherapy due to poor Eastern Cooperative Oncology Group (ECOG) performance status or impaired renal function, which restricts the application of treatment and adversely affects survival outcomes.6,7
The therapeutic paradigm for mUC has evolved toward the use of immune checkpoint inhibitors (ICIs) that target programmed death-ligand 1 (PD-L1) and programmed death 1 (PD-1). The encouraging results from the Keynote-045 study demonstrated that pembrolizumab, when used as a second-line treatment, significantly prolonged overall survival (OS) than conventional chemotherapy in patients with mUC.8,9 In the IMVigor 210 study, atezolizumab exhibited efficacy in cisplatin-ineligible patients, with a complete response rate of 9%, an ORR of 23%, and an excellent median OS of 15.9 months. 10 Based on these promising findings, pembrolizumab and atezolizumab have received FDA approval as second-line treatments for patients with mUC who have experienced progression following prior platinum-based therapy. Furthermore, avelumab has demonstrated improved OS in the maintenance setting for patients with mUC who responded to first-line platinum-based chemotherapy. This maintenance strategy has significantly reshaped the treatment landscape of mUC, increasing the median OS from 14.3 to 21.4 months. 11
Despite these advancements, a substantial proportion of patients—approximately 60%–70%—remain unresponsive to ICIs. 12 Therefore, it is crucial to identify effective prognostic factors and who might benefit from ICIs. While severe studies have demonstrated a positive correlation between PD-L1 expression and ICI response, patients with negative PD-L1 status can still derive clinical benefits from ICI treatment or combination therapies.13 –15 This discrepancy may be attributed to factors such as tumor sampling variability, assay inconsistencies, and limited uniformity in PD-L1 immunohistochemistry. 16
Systemic inflammation plays an important role in tumor promotion and progression. 17 Some hematologic parameters, such as the systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR), are good indicators that reflect the systemic inflammatory response.18 –20 Previous studies have demonstrated the relationship between these systemic inflammatory biomarkers and tumorigenesis, disease progression, and clinical prognosis, particularly in melanoma and non-small-cell lung cancer (NSCLC). 21 Elevated pre-treatment levels of NLR and PLR have been associated with reduced OS and progression-free survival (PFS), as well as lower response rates in patients with metastatic NSCLC undergoing nivolumab therapy.21,22 Similar trends have been observed in melanoma patients treated with PD-1 inhibitor monotherapy. 23
However, the relationship between peripheral blood biomarkers and survival outcomes in mUC patients undergoing ICI therapy has not been thoroughly studied or confirmed. Although several studies have explored this issue, their findings remain inconclusive, often focusing on a narrow set of markers, such as NLR.24,25 Therefore, we conducted the present study to explore the prognostic significance of serum inflammatory biomarkers in patients with mUC treated with ICIs.
Materials and methods
Patient and treatment
This retrospective cohort study analyzed patient data from two independent medical centers in Taiwan: Kaohsiung Chang Gung Memorial Hospital, serving as the validation cohort (VC), and Linkou Chang Gung Memorial Hospital, serving as the training cohort (TC). All patients had a histopathologically confirmed diagnosis of urothelial carcinoma and were clinically diagnosed with metastatic disease. Each patient received at least one cycle of ICI monotherapy, specifically pembrolizumab, nivolumab, avelumab, durvalumab, or atezolizumab. Patients with only localized disease or those whose ICI treatment lasted less than 1 month were excluded from the analysis. This study received approval from the Institutional Review Board of the Chang Gung Medical Foundation (201901248B0). The reporting of this study conforms to the STROBE statement. 26
Clinical data and response evaluation
We extracted data from medical records, including key parameters such as age, sex, ECOG performance status, laboratory results, Bajorin risk score, primary or metastatic sites, and PD-L1 expression levels. Serological markers, such as white blood cell (WBC) count, hemoglobin, platelet count, neutrophil count, and lymphocyte count, were obtained prior to the first dose of ICI. Tumor response assessments were conducted using computed tomography or magnetic resonance imaging, following the Response Evaluation Criteria in Solid Tumors (RECIST version 1.1). PD-L1 immunohistochemistry staining was performed using the Dako 22C3 anti-human PD-L1 antibody and was interpreted by a certified pathologist (C.-C.C.).
Calculation of serum inflammatory markers
The formulas used to calculate pre-treatment serum inflammation markers are as follows: NLR = neutrophil count/lymphocyte counts; PLR = platelet counts/lymphocyte counts; SII = platelet counts × neutrophil counts/lymphocyte counts.
Statistics
Our statistical analysis utilized the IBM SPSS version 25.0 (SPSS Inc., Chicago, IL, USA) and R software 4.3.3. We assessed categorical variables using Pearson’s Chi-square test or Fisher’s exact test, depending on the data. Optimal cutoff values for PLR and SII were determined using data from the TC and analyzed with X-tile software (Version 3.6.1; Yale University School of Medicine, USA). We also plotted receiver operating characteristic (ROC) curves to evaluate the sensitivity, specificity, and area under the curve (AUC) differences for the prognostic factors identified. For survival analysis, we estimated PFS and OS using the Kaplan–Meier method and assessed differences using the log-rank test. We compared treatment subgroups by analyzing hazard ratios (HRs) and their 95% confidence intervals (CIs) using an unstratified Cox proportional hazards regression model that included both univariate and multivariate analyses.
Results
Patient characteristics
We enrolled a total of 192 patients diagnosed with mUC, with 123 patients in the TC and 69 in the VC, respectively. A detailed description of the clinical characteristics of both cohorts is provided in Table 1. The median age of the patients was 70 years, ranging from 63 to 78 years. Among the total cohort, 113 patients (58.9%) were male, and 104 patients (54.2%) had upper tract urothelial carcinoma (UTUC). The analysis demonstrated no significant differences in age, sex, and ECOG performance status between the TC and VC. However, significant differences in renal function and primary site were noted. The VC exhibited a higher proportion of patients with impaired renal function (76.8% compared to 58.3%). This disparity may be due to the higher prevalence of upper tract primary sites in the VC (65.2% compared to 48.0%). In addition, a significant difference in liver metastasis was observed, with a higher prevalence in the TC (27.0% compared to 11.6%). The majority of patients had an ECOG score between 0 and 1 (80.6%), and about half of the patients presented with visceral metastasis (52.4%). Notably, there were no significant differences in terms of visceral metastasis, Bajorin prognostic factor, and PD-L1 expression between the two cohorts.
Table 1.
Clinical characteristics of the training and validation cohorts.
| All (n, %) | Training cohort (n, %) | Validation cohort (n, %) | p Value | |
|---|---|---|---|---|
| N | 192 | 123 | 69 | |
| Age (mean, SD), years | 68.9 ± 12.1 | 69.0 ± 11.5 | 68.7 ± 13.4 | 0.54 |
| Median (range) | 70 (63–78) | 70 (63–78) | 70 (64–79) | |
| Age | 0.54 | |||
| <60 | 35 (18.2) | 24 (19.5) | 11 (15.9) | |
| ⩾60 | 157 (81.8) | 99 (80.5) | 58 (84.1) | |
| Gender | 0.47 | |||
| Female | 79 (41.1) | 53 (43.1) | 26 (37.7) | |
| Male | 113 (58.9) | 70 (56.9) | 43 (62.3) | |
| ECOG | 0.14 | |||
| 0–1 | 154 (80.6) | 103 (83.7) | 51 (75.0) | |
| ⩾2 | 37 (19.4) | 20 (16.3) | 17 (25.0) | |
| Renal function (mL/min) | 0.01 | |||
| CCr ⩾ 60 | 66 (34.9) | 50 (41.7) | 16 (23.2) | |
| CCr < 60 | 123 (65.1) | 70 (58.3) | 53 (76.8) | |
| Primary site | 0.007 | |||
| Bladder | 86 (44.8) | 64 (52.0) | 22 (31.9) | |
| Upper tract | 104 (54.2) | 59 (48.0) | 45 (65.2) | |
| Both | 2(1.0) | 0(0) | 2(2.9) | |
| Visceral metastasis | 0.35 | |||
| No | 91 (47.6) | 55 (45.1) | 36 (52.2) | |
| Yes | 100 (52.4) | 67 (54.9) | 33 (47.8) | |
| Lymph node metastasis | 0.96 | |||
| No | 53 (27.7) | 34 (27.9) | 19 (27.5) | |
| Yes | 138 (72.3) | 88 (72.1) | 50 (72.5) | |
| Liver metastasis | 0.012 | |||
| No | 150 (78.5) | 89 (73.0) | 61 (88.4) | |
| Yes | 41 (21.5) | 33 (27.0) | 8 (11.6) | |
| Lung metastasis | 0.27 | |||
| No | 126 (66.0) | 77 (63.1) | 49 (71.0) | |
| Yes | 65 (34.0) | 45 (36.9) | 20 (29.0) | |
| Bone metastasis | 0.68 | |||
| No | 152 (79.6) | 96 (78.7) | 56 (81.2) | |
| Yes | 39 (20.4) | 26 (21.3) | 13 (18.8) | |
| WBC (×103/μL) | 0.75 | |||
| <10 | 139 (72.4) | 90 (73.2) | 49 (71.0) | |
| ⩾10 | 53 (27.6) | 33 (26.8) | 20 (29.0) | |
| NLR | 0.81 | |||
| <5 | 108 (56.3) | 70 (58.9) | 38 (55.1) | |
| ⩾5 | 84 (43.8) | 53 (43.1) | 31 (44.9) | |
| Hemoglobin (g/dL) | 0.16 | |||
| ⩾10 | 121 (63.0) | 82 (66.7) | 39 (56.5) | |
| <10 | 71 (37.0) | 41 (33.3) | 30 (43.5) | |
| Bajorin prognostic factor | 0.90 | |||
| 0 | 73 (38.2) | 47 (38.2) | 26 (38.2) | |
| 1 | 98 (51.3) | 64 (52.0) | 34 (50.0) | |
| 2 | 20 (10.5) | 12 (9.8) | 8 (11.8) | |
| PD-L1 (22C3) | 0.08 | |||
| <10 | 72 (37.5) | 35 (28.5) | 37 (53.6) | |
| ⩾10 | 56 (29.2) | 36 (29.3) | 20 (29.0) | |
| Missing | 64 (33.3) | 52 (42.3) | 12 (17.4) | |
CCr, clearance of creatinine; ECOG, Eastern Cooperative Oncology Group; NLR, neutrophil-to-lymphocyte ratio; PD-L1, programmed cell death ligand-1; WBC, white blood cell count.
Determine the optimal level of serum optimal cutoff level of immune biomarkers
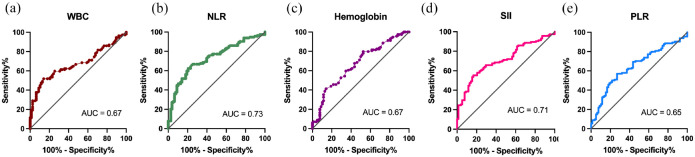
ROC curves were generated to assess the predictive efficacy of various biomarkers on patient prognosis. The AUC values obtained were as follows: WBC (0.67), NLR (0.73), Hb (0.67), PLR (0.65), and SII (0.71) (see Figure 1). These results demonstrate the significant predictive validity of WBC, NLR, Hb, PLR, and SII in prognostication. To determine the optimal cutoff levels for immune biomarkers, the following values were established: WBC (10,000/μL), NLR (5), and Hb (10.0 g/L). Furthermore, X-tile plots identified 2205 and 194.84 as the cutoff points for SII and PLR, respectively. Based on these biomarkers, patients were subsequently stratified into two groups for further analysis.
Figure 1.
The survival prediction ROC curves are determined by the following biomarkers. (a) WBC count, (b) NLR, (c) hemoglobin, (d) SII, and (e) PLR.
NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ROC, receiver operating characteristic; SII, systemic immune-inflammation index; WBC, white blood cell count.
Survival outcomes and inflammation markers
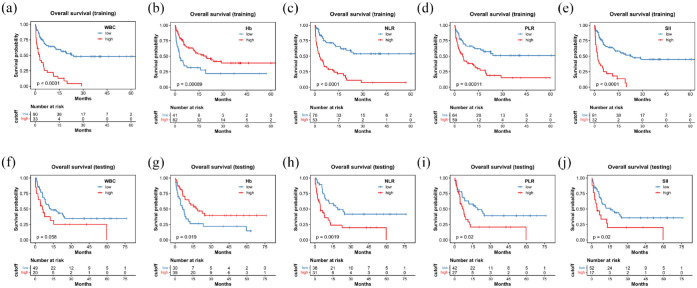
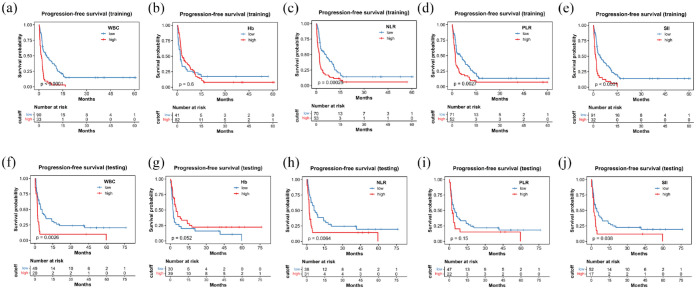
During the median follow-up period of 28.8 months, a total of 114 deaths occurred. In the TC, the OS significantly decreased when inflammatory marker levels exceeded predefined thresholds. The median OS was 22.6 months compared to 2.6 months (p < 0.0001) for WBC; it was not reached versus 2.9 months (p < 0.0001) for NLR; 18.0 versus 2.9 months (p = 0.006) for hemoglobin; it was not reached versus 4.9 months (p = 0.0001) for PLR; and 22.6 versus 1.9 months (p < 0.0001) for SII (Figure 2). Similarly, PFS also showed significant differences using these cutoff values for WBC, NLR, PLR, and SII, as depicted in Figure 3. In the VC, patients with higher NLR (18.9 vs 4.5 months; p = 0.002), PLR (18.9 vs 7.0 months; p = 0.02), SII (15.4 vs 4.4 months; p = 0.02), and lower levels of hemoglobin (17.5 vs 7.5 months; p = 0.02) demonstrated shorter OS (Figure 2). A comparable pattern was observed in PFS for WBC, NLR, and SII (Figure 3).
Figure 2.
Kaplan–Meier curve for OS stratified by WBC, Hb, NLR, PLR, and SII in the training cohort (a–e) and the testing cohort (f–j).
Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; WBC, white blood cell count.
Figure 3.
Kaplan–Meier curve for PFS stratified by WBC, Hb, NLR, PLR, and SII in the training cohort (a–e) and the testing cohort (f–j).
Hb, hemoglobin; NLR, neutrophil-to-lymphocyte ratio; PFS, progression-free survival; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; WBC, white blood cell count.
Univariate and multivariate analyses of OS
We performed the univariate and multivariate Cox regression analyses to analyze variables that influence OS. The detailed results are shown in Table 2. In the univariate analysis, significant prognostic factors for OS included ECOG performance status ⩾ 2 (p < 0.001), Bajorin risk score 2 (p < 0.001), visceral metastasis (p < 0.001), WBC ⩾ 10 × 103/μL (p < 0.001), hemoglobin levels ⩾ 10 mg/dL (p < 0.001), NLR ⩾ 5 (p < 0.001), SII ⩾ 2205 (p < 0.001), and PLR ⩾ 194.84 (p < 0.001). The multivariate Cox regression analysis, adjusting for ECOG performance status, visceral metastasis, WBC, hemoglobin level, NLR, SII, and PLR, identified independent prognostic indicators. These included ECOG performance status ⩾ 2 (HR 2.35; 95% CI: 1.52–3.64; p < 0.001), visceral metastasis (HR 2.08; 95% CI: 1.41–3.08; p < 0.001), WBC ⩾ 10 × 103/μL (HR 1.88; 95% CI: 1.15–3.08; p = 0.012), hemoglobin levels ⩾ 10 mg/dL (HR 0.59; 95% CI: 0.39–0.87; p = 0.008), and NLR ⩾ 5 (HR 1.90; 95% CI: 1.06–3.40; p = 0.032).
Table 2.
Univariate and multivariate analyses of OS.
| Characteristics | Median OS | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| (month) | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age (year) | 0.28 | ||||
| <60 | 15.3 | 1 | |||
| ⩾60 | 6.2 | 1.31 (0.81–2.12) | |||
| Gender | 0.77 | ||||
| Female | 6.1 | 1 | |||
| Male | 7.5 | 1.06 (0.73–1.54) | |||
| ECOG | <0.001 | <0.001 | |||
| 0–1 | 9.3 | 1 | 1 | ||
| ⩾2 | 3.0 | 2.76 (1.81–4.20) | 2.35 (1.52–3.64) | ||
| Bajorin risk score | <0.001 | ||||
| 0–1 | 8.4 | 1 | |||
| 2 | 2.2 | 3.33 (1.99–5.58) | |||
| Visceral metastasis | <0.001 | <0.001 | |||
| No | 12.5 | 1 | 1 | ||
| Yes | 3.7 | 2.05 (1.40–3.00) | 2.08 (1.41–3.08) | ||
| WBC (×103/μL) | <0.001 | 0.012 | |||
| <10 | 10.5 | 1 | 1 | ||
| ⩾10 | 2.9 | 2.94 (2.02–4.30) | 1.88 (1.15–3.08) | ||
| Hb (g/dL) | <0.001 | 0.008 | |||
| <10 | 3.7 | 1 | 1 | ||
| ⩾10 | 11.6 | 0.48 (0.33–0.69) | 0.59 (0.39–0.87) | ||
| NLR | <0.001 | 0.03 | |||
| <5 | 14.2 | 1 | 1 | ||
| ⩾5 | 3.7 | 3.30 (2.26–4.82) | 1.90 (1.06–3.40) | ||
| SII | <0.001 | 0.62 | |||
| Low (<2205) | 10.2 | 1 | 1 | ||
| High (⩾2205) | 2.8 | 2.93 (1.99–4.29) | 0.85 (0.46–1.58) | ||
| PLR | <0.001 | 0.50 | |||
| Low (<194.84) | 11.7 | 1 | 1 | ||
| High (⩾194.84) | 4.8 | 2.29 (1.57–3.33) | 1.21 (0.69–2.12) | ||
| PD-L1 | 0.25 | ||||
| <10 | 7.5 | 1 | |||
| ⩾10 | 12.3 | 0.76 (0.48–1.21) | |||
CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; HR, hazard ratio; NLR, neutrophil-to-lymphocyte ratio; OS, overall survival; PD-L1, programmed cell death ligand-1; PLR, platelet-to-lymphocyte ratio; SII, systemic immune-inflammation index; WBC, white blood cell count.
Construction and validation of a nomogram for OS
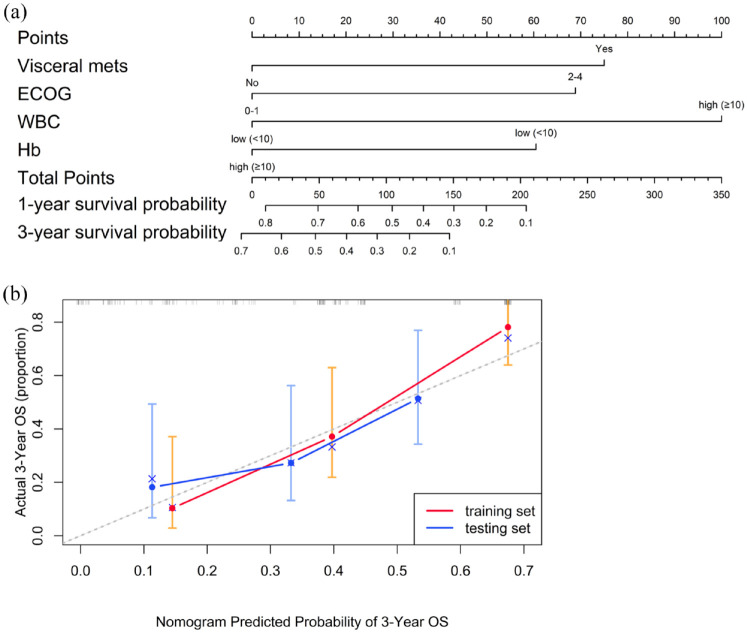
We developed a prognostic nomogram based on independent factors identified in the multivariate Cox regression analysis, including visceral metastasis, ECOG performance status, WBC, and Hb levels (Figure 4). Each factor was assigned a specific point value in the nomogram, allowing for the prediction of 1- and 3-year OS for patients with mUC undergoing ICI treatment by summing these points. To assess the precision of the nomogram in predicting OS, we used the C-index and generated calibration curves. The C-index values for the nomogram were 0.769 (95% CI, 0.72–0.82) in the TC and 0.657 (95% CI, 0.57–0.74) in the VC. In addition, the calibration plots for 3-year OS, shown in Figure 4, revealed a strong correlation between the predicted and actual outcomes in both cohorts, underscoring the nomogram’s robustness and reliability.
Figure 4.
(a) A nomogram designed to predict OS in mUC patients receiving ICIs. (b) A calibration curve of the nomogram for predicting 3-year OS in the training and validation cohorts.
ICI, immune checkpoint inhibitors; mUC, metastatic urothelial carcinoma; OS, overall survival.
Discussion
In this study, we have highlighted the significance of inflammatory biomarkers such as WBC, Hb, SII, NLR, and PLR in predicting OS for patients with mUC undergoing ICI therapy. Our analysis identified WBC, Hb, and NLR are independent prognostic factors, similar to well-established indicators such as visceral metastasis and ECOG performance status. To the best of our knowledge, this finding represents the first research of systemic inflammatory biomarkers as prognostic indicators in mUC treated with ICIs, specifically within an Asian population, while concurrently evaluating multiple biomarkers, including WBC, Hb, SII, NLR, and PLR. These hematological tests and biomarkers are basic and widely accessible, potentially serving as reliable indicators to help clinicians decide on the use of ICI for treating mUC.
Although recent advancements have significantly improved the treatment of mUC and extended OS through the combination of ICIs with gemcitabine/cisplatin or antibody–drug conjugates, ICIs remain the cornerstone of therapy for mUC patients.27,28 Understanding the key predictive and prognostic factors for ICI treatment is crucial in managing mUC. Several clinicopathological features have been widely discussed, including liver metastasis, tertiary lymphoid structures (TLS), primary tumor location, and the number of metastatic sites. Among recent advances in oncoimmunotherapy, the identification of TLS has emerged as a promising predictive and prognostic marker for ICI treatment across various cancers.29,30 TLS is an ectopic secondary lymphoid structure composed of dendritic cells, B cells, and high endothelial venules. Studies have shown that the presence of TLS can predict and is associated with better outcomes in mUC patients treated with ICIs.31,32 Besides, the prognostic impact of primary tumor location in mUC treated with chemotherapy has been reported variably, yet its influence on OS in mUC patients receiving ICI treatment remains uncertain. 33 A Japanese study comparing oncologic outcomes between UTUC and lower tract urothelial carcinoma (LTUC) in patients receiving second-line pembrolizumab demonstrated similar OS. 34 However, a recent analysis using the SEER database revealed that LTUC patients had better OS compared to UTUC patients after multivariable Cox regression adjustment (8 vs 7 months, p = 0.034). 35 By contrast, our data did not demonstrate a significant effect of primary tumor location in either univariate or multivariate analyses, suggesting that tumor location may not serve as a prognostic factor in mUC.
Inflammation has been increasingly recognized as a key contributor to cancer pathogenesis, progression, and response to therapeutic interventions.36,37 Persistent inflammation promotes the recruitment of regulatory immune cells such as T-regulatory cells and myeloid-derived suppressor cells that inhibit the effective functioning of cytotoxic T-cells and natural killer cells. 38 Early indicators of pre-treatment acute inflammation have been linked to unfavorable responses to ICI and shortened response durations. Hematological markers like NLR, PLR, and SII are key indicators of inflammation and immune response. Elevated pre-treatment levels of these markers are consistently linked to poor outcomes in various cancers treated with ICIs, including NSCLC, melanoma, gastric cancer, and pancreatic cancer.17,18,20,23,39,40 Our findings further substantiate the prognostic relevance of pretreatment NLR, SII, and PLR in mUC, where higher values of these biomarkers are associated with poor outcomes following ICI therapy.
The prognostic significance of the NLR in various solid tumors is well documented. 41 While the optimal NLR value is not fixed, many studies have identified 5.0 as the most effective threshold for predicting outcomes in patients with NSCLC and melanoma undergoing treatment with ICIs. 42 Our study showed that NLR ⩾ 5 remained as independent prognostic factors in mUC patients, guiding clinicians to determine treatment decisions for future patients. Although the precise mechanisms connecting NLR with the effectiveness of ICIs and OS are not fully understood, it likely indicates the intricate balance between the innate immune response (mediated by neutrophils) and the adaptive immune response (mediated by lymphocytes). 43 Neutrophilia, commonly observed in cancer-associated chronic inflammation, promotes tumor proliferation, while lymphopenia compromises the cell-mediated adaptive immune response essential for effective antitumor immunity induced by ICIs.44 –46 Therefore, elevated NLR serves as a surrogate marker for poor prognosis in patients receiving ICIs. In addition to the baseline NLR value, recent studies have highlighted the prognostic significance of dynamic changes in NLR before and after ICI treatment. Yamamoto et al. 47 demonstrated that a decrease in NLR following pembrolizumab treatment was associated with significantly improved OS (p = 0.0002) and ORR (p = 0.0023) in mUC patients. Similarly, another multicenter retrospective study identified that changes in NLR at 6 weeks of pembrolizumab treatment had a prognostic value for OS in mUC. 48 Our data did not include dynamic NLR changes, as our focus was on identifying prognostic factors present before the initiation of ICI treatment, rather than those observed after treatment to capture dynamic shifts.
Similarly, elevated SII and PLR have been associated with unfavorable outcomes in various cancers, although the evidence is less conclusive compared to NLR. 49 The components of SII and PLR, including thrombocythemia, neutrophilia, and lymphopenia, contribute to their prognostic value. Platelets play multifaceted roles in cancer progression, directly stimulating tumor cells and protecting them from immune cell cytotoxicity, potentially impacting the efficacy of immunotherapy.50,51 Despite the promising implications of these biomarkers, the lack of consensus on optimal cutoff values poses a challenge to their clinical utility. Previous research has reported a wide range of cutoff values for SII and PLR in cancer patients undergoing immunotherapy. For instance, the cutoff value for the SII has been reported to range from 268.8 to 1375, while that for the PLR has varied from 111 to 241, according to meta-analysis.49,52 In our study, we identified cutoff values of SII = 2205 and PLR = 195. The cutoff level of PLR is closely aligned with values reported in previous studies. However, optimal SII levels in previous studies conflicted with our findings. This discrepancy may be attributed to differences in analysis methods, as not all studies used ROC curves to determine the suitable SII cutoff value. In addition, variations in cancer types and immunotherapy regimens included in meta-analyses may have contributed to the observed differences.
In our Cox multivariate analysis, we found that lower levels of Hb and higher WBC count were significantly associated with decreased OS in mUC patients treated with ICIs. Paraneoplastic leukocytosis, a phenomenon characterized by abnormal elevation of WBC counts in the absence of an apparent cause, has been previously linked to a poor prognosis in patients with urothelial cell carcinoma.53,54 Consistent with these findings, our study demonstrated a similarly unfavorable OS response to ICIs in patients with paraneoplastic leukocytosis. In addition, anemia, which is often indicative of aggressive tumor biology, has been associated with poor outcomes in UC patients and may serve as a marker for identifying patients who are likely to benefit from ICIs.55,56 Our study further corroborated these associations.
Our study faces several limitations that should be taken into account. First, there is variation in the use of different ICIs, such as pembrolizumab, nivolumab, avelumab, durvalumab, and atezolizumab, which could affect our findings. In addition, being a retrospective study with a small cohort from only two medical centers may limit the wider applicability of our results. Also, the lack of PD-L1 expression data in up to 40% of patients might have limited our analysis related to treatment outcomes. Moreover, we did not include immune-related adverse events (irAEs) in our analysis, which are significant in assessing the safety of ICI therapy. This omission prevents us from linking hematological inflammatory markers with irAEs, which could have offered further insights. Besides, due to limitations in our database and patient information, we could not include the Charlson Comorbidity Index, which assesses comorbidities and may impact treatment decisions and ICI efficacy. Lastly, unlike specific pathological markers, serum inflammatory biomarkers can be influenced by other inflammatory conditions or physical states not related to the cancer itself, adding complexity to interpreting our results. This inherent variability introduces a level of complexity that may impact the interpretation of our results.
Conclusion
Combining serum inflammatory markers with clinicopathologic factors creates an effective and easily accessible prognostic tool. This tool can predict treatment outcomes and assist in making therapeutic decisions for mUC patients treated with ICIs.
Acknowledgments
We thank the multidisciplinary team of genitourinary cancer at our hospital for their generous assistance and cooperation.
Footnotes
ORCID iD: Harvey Yu-Li Su  https://orcid.org/0000-0002-1289-473X
https://orcid.org/0000-0002-1289-473X
Contributor Information
Liang-Yun Cheng, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Po-Jung Su, Division of Hematology–Oncology, Chang Gung Memorial Hospital at Linkou and Chang Gung University College of Medicine, Taoyuan, Taiwan.
Ming-Chun Kuo, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Chang-Ting Lin, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Hao-Lun Luo, Department of Urology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Chih-Chi Chou, Department of Pathology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Shih-Yu Huang, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Chia-Che Wu, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Chien-Hsu Chen, Department of Urology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Chun-Chieh Huang, Department of Radiation Oncology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Kai-Lung Tsai, Department of Colorectal Surgery, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan.
Harvey Yu-Li Su, Division of Hematology–Oncology, Department of Internal Medicine, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, No. 123, Dapi Road, Niaosong District, Kaohsiung City 833, Taiwan; Genomic and Proteomic Core Laboratory, Department of Medical Research, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; Cancer Center, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan; School of Medicine, College of Medicine, National Sun Yat-sen University, Kaohsiung, Taiwan.
Declarations
Ethics approval and consent to participate: Approval for the retrospective study was granted by the Institutional Review Board of Chang Gung Medical Foundation (Approval No: 201901248B0). The research was performed in accordance with the Declaration of Helsinki and all methods were carried out in accordance with relative guidelines and regulations. Individual consent for this retrospective analysis was waived.
Consent for publication: Not applicable.
Author contributions: Liang-Yun Cheng: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Po-Jung Su: Data curation; Methodology.
Ming-Chun Kuo: Data curation; Methodology.
Chang-Ting Lin: Data curation; Formal analysis.
Hao-Lun Luo: Data curation; Investigation.
Chih-Chi Chou: Data curation; Software.
Shih-Yu Huang: Data curation; Investigation.
Chia-Che Wu: Data curation; Methodology.
Chien-Hsu Chen: Data curation; Validation.
Chun-Chieh Huang: Data curation; Validation.
Kai-Lung Tsai: Data curation; Validation.
Harvey Yu-Li Su: Conceptualization; Formal analysis; Funding acquisition; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by a grant from Chang Gung Memorial Hospital, Kaohsiung, Taiwan (CPRPG8J0012, CORPG8L0621).
The authors declare that there is no conflict of interest.
Availability of data and materials: The data underpinning this study’s conclusions are accessible upon request from the corresponding author. These data are not publicly accessible due to concerns over privacy and ethical considerations.
References
- 1. Beigi A, Vafaei-Nodeh S, Huang L, et al. Survival outcomes associated with first and second-line palliative systemic therapies in patients with metastatic bladder cancer. Curr Oncol 2021; 28: 3812–3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Necchi A, Pond GR, Raggi D, et al. Efficacy and safety of gemcitabine plus either taxane or carboplatin in the first-line setting of metastatic urothelial carcinoma: a systematic review and meta-analysis. Clin Genitourin Cancer 2017; 15(1): 23–30.e2. [DOI] [PubMed] [Google Scholar]
- 3. Witjes JA, Bruins HM, Carrión A, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2023 guidelines. Eur Urol 2024; 85: 17–31. [DOI] [PubMed] [Google Scholar]
- 4. Powles T, Bellmunt J, Comperat E, et al. ESMO clinical practice guideline interim update on first-line therapy in advanced urothelial carcinoma. Ann Oncol 2024; 35: 485–490. [DOI] [PubMed] [Google Scholar]
- 5. Maase H von der, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 2000; 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- 6. Lin C-T, Su P-J, Huang S-Y, et al. First-line immune checkpoint inhibitor versus immune checkpoint inhibitor with chemotherapy for cisplatin-ineligible metastatic urothelial carcinoma: evidence from a real-world, multicenter analysis. J Immunother 2022; 45: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cathomas R, Santis MD, Galsky MD. First-line treatment of metastatic disease cisplatin-ineligible patients. Hematol Oncol Clin North Am 2015; 29: 329–340. [DOI] [PubMed] [Google Scholar]
- 8. Bellmunt J, Wit R de, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376: 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balar AV, Castellano DE, Grivas P, et al. Efficacy and safety of pembrolizumab in metastatic urothelial carcinoma: results from KEYNOTE-045 and KEYNOTE-052 after up to 5 years of follow-up. Ann Oncol 2023; 34: 289–299. [DOI] [PubMed] [Google Scholar]
- 10. Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 2017; 389: 67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020; 383: 1218–1230. [DOI] [PubMed] [Google Scholar]
- 12. Thana M, Wood L. Immune checkpoint inhibitors in genitourinary malignancies. Curr Oncol 2020; 27: 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rouquette I, Taranchon-Clermont E, Gilhodes J, et al. Immune biomarkers in thymic epithelial tumors: expression patterns, prognostic value and comparison of diagnostic tests for PD-L1. Biomark Res 2019; 7: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rao A, Patel MR. A review of avelumab in locally advanced and metastatic bladder cancer. Ther Adv Urol 2019; 11: 1756287218823485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17: e542–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eckstein M, Cimadamore A, Hartmann A, et al. PD-L1 assessment in urothelial carcinoma: a practical approach. Ann Transl Med 2019; 7: 690–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shang J, Han X, Zha H, et al. Systemic immune-inflammation index and changes of neutrophil–lymphocyte ratio as prognostic biomarkers for patients with pancreatic cancer treated with immune checkpoint blockade. Front Oncol 2021; 11: 585271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Li S, Zhang S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab. J Clin Lab Anal 2019; 33: e22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Biswas T, Kang KH, Gawdi R, et al. Using the systemic immune-inflammation index (SII) as a mid-treatment marker for survival among patients with stage-III locally advanced non-small cell lung cancer (NSCLC). Int J Environ Res Public Health 2020; 17: 7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mesti T, Kuhar CG, Ocvirk J. Biomarkers for outcome in metastatic melanoma in first line treatment with immune checkpoint inhibitors. Biomedicines 2023; 11: 749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diem S, Schmid S, Krapf M, et al. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017; 111: 176–181. [DOI] [PubMed] [Google Scholar]
- 22. Li C, Wu J, Jiang L, et al. The predictive value of inflammatory biomarkers for major pathological response in non-small cell lung cancer patients receiving neoadjuvant chemoimmunotherapy and its association with the immune-related tumor microenvironment: a multi-center study. Cancer Immunol Immunother 2023; 72: 783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bartlett EK, Flynn JR, Panageas KS, et al. High neutrophil-to-lymphocyte ratio (NLR) is associated with treatment failure and death in patients who have melanoma treated with PD-1 inhibitor monotherapy. Cancer 2020; 126: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chawla NS, Sayegh N, Tripathi N, et al. Genomic and clinical prognostic factors in patients with advanced urothelial carcinoma receiving immune checkpoint inhibitors. Clin Genitourin Cancer 2023; 21: 69–75. [DOI] [PubMed] [Google Scholar]
- 25. Abuhelwa AY, Bellmunt J, Kichenadasse G, et al. Enhanced Bellmunt risk score for survival prediction in urothelial carcinoma treated with immunotherapy. Clin Genitourin Canc 2022; 20(2): 132–138. [DOI] [PubMed] [Google Scholar]
- 26. Elm E von, Altman DG, Egger M, et al.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573. [DOI] [PubMed] [Google Scholar]
- 27. Powles T, Valderrama BP, Gupta S, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 2024; 390: 875–888. [DOI] [PubMed] [Google Scholar]
- 28. Heijden MS van der, Sonpavde G, Powles T, et al. Nivolumab plus gemcitabine–cisplatin in advanced urothelial carcinoma. N Engl J Med 2023; 389: 1778–1789. [DOI] [PubMed] [Google Scholar]
- 29. Sautès-Fridman C, Petitprez F, Calderaro J, et al. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019; 19: 307–325. [DOI] [PubMed] [Google Scholar]
- 30. Dijk N van, Gil-Jimenez A, Silina K, et al. The tumor immune landscape and architecture of tertiary lymphoid structures in urothelial cancer. Front Immunol 2021; 12: 793964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang L, Zhang R, Jin D, et al. Synergistic induction of tertiary lymphoid structures by chemoimmunotherapy in bladder cancer. Br J Cancer 2024; 130: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou L, Xu B, Liu Y, et al. Tertiary lymphoid structure signatures are associated with survival and immunotherapy response in muscle-invasive bladder cancer. Oncoimmunology 2021; 10: 1915574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsieh M-C, Chiang P-H, Rau K-M, et al. The comparison of oncologic outcomes between metastatic upper tract urothelial carcinoma and urothelial carcinoma of the bladder after cisplatin-based chemotherapy. Urol Oncol 2015; 33: 495.e9–495.e14. [DOI] [PubMed] [Google Scholar]
- 34. Nishiyama N, Kita Y, Ito K, et al. Second-line pembrolizumab for metastatic urothelial carcinoma: differences in treatment outcomes according to the primary site. Anticancer Res 2023; 43: 5041–5050. [DOI] [PubMed] [Google Scholar]
- 35. Bello FD, Siech C, Jannello LMI, et al. Contemporary survival in metastatic bladder cancer patients: a population-based study. Int J Cancer 2024; 155: 1762–1768. [DOI] [PubMed] [Google Scholar]
- 36. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674. [DOI] [PubMed] [Google Scholar]
- 37. Zhao H, Wu L, Yan G, et al. Inflammation and tumor progression: signaling pathways and targeted intervention. Signal Transduct Target Ther 2021; 6: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang M, Chen S, He X, et al. Targeting inflammation as cancer therapy. J Hematol Oncol 2024; 17: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Qu Z, Wang Q, Wang H, et al. The effect of inflammatory markers on the survival of advanced gastric cancer patients who underwent anti-programmed death 1 therapy. Front Oncol 2022; 12: 783197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kauffmann-Guerrero D, Kahnert K, Kiefl R, et al. Systemic inflammation and pro-inflammatory cytokine profile predict response to checkpoint inhibitor treatment in NSCLC: a prospective study. Sci Rep 2021; 11: 10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heshmat-Ghahdarijani K, Sarmadi V, Heidari A, et al. The neutrophil-to-lymphocyte ratio as a new prognostic factor in cancers: a narrative review. Front Oncol 2023; 13: 1228076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang N, Jiang J, Tang S, et al. Predictive value of neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in non-small cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Int Immunopharmacol 2020; 85: 106677. [DOI] [PubMed] [Google Scholar]
- 43. Li M, Spakowicz D, Burkart J, et al. Change in neutrophil to lymphocyte ratio during immunotherapy treatment is a non-linear predictor of patient outcomes in advanced cancers. J Cancer Res Clin Oncol 2019; 145: 2541–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Howard R, Kanetsky PA, Egan KM. Exploring the prognostic value of the neutrophil-to-lymphocyte ratio in cancer. Sci Rep 2019; 9: 19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ménétrier-Caux C, Ray-Coquard I, Blay J-Y, et al. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer 2019; 7: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Treffers LW, Hiemstra IH, Kuijpers TW, et al. Neutrophils in cancer. Immunol Rev 2016; 273: 312–328. [DOI] [PubMed] [Google Scholar]
- 47. Yamamoto Y, Yatsuda J, Shimokawa M, et al. Prognostic value of pre-treatment risk stratification and post-treatment neutrophil/lymphocyte ratio change for pembrolizumab in patients with advanced urothelial carcinoma. Int J Clin Oncol 2021; 26: 169–177. [DOI] [PubMed] [Google Scholar]
- 48. Nishio K, Higashio T, Komura K, et al. Predicting objective response of pembrolizumab in platinum-refractory urothelial carcinoma based on neutrophil–lymphocyte ratio fluctuation and liver metastases. Oncology 2024; 102: 457–464. [DOI] [PubMed] [Google Scholar]
- 49. Tian B-W, Yang Y-F, Yang C-C, et al. Systemic immune-inflammation index predicts prognosis of cancer immunotherapy: systemic review and meta-analysis. Immunotherapy 2022; 14: 1481–1496. [DOI] [PubMed] [Google Scholar]
- 50. Schmied L, Höglund P, Meinke S. Platelet-mediated protection of cancer cells from immune surveillance—possible implications for cancer immunotherapy. Front Immunol 2021; 12: 640578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis. Nat Rev Cancer 2011; 11: 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bao Y, Wang Y, Li X, et al. Prognostic significance of platelet-to-lymphocyte ratio in urothelial carcinoma patients: a meta-analysis. Cancer Cell Int 2019; 19: 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Izard JP, Gore JL, Mostaghel EA, et al. Persistent, unexplained leukocytosis is a paraneoplastic syndrome associated with a poor prognosis in patients with urothelial carcinoma. Clin Genitourin Cancer 2015; 13: e253–e258. [DOI] [PubMed] [Google Scholar]
- 54. Warli SM, Andy A, Prapiska FF, et al. Poor prognosis of urothelial carcinoma in patients presented with persistent paraneoplastic leukocytosis with anemia. Urol Ann 2022; 14: 328–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kurashina R, Ando K, Inoue M, et al. Pretreatment hemoglobin levels and platelet-to-lymphocyte ratio predict survival benefit from pembrolizumab in advanced urothelial carcinoma. Cancer Diagn Progn 2023; 3: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rink M, Sharifi N, Fritsche H-M, et al. Impact of preoperative anemia on oncologic outcomes of upper tract urothelial carcinoma treated with radical nephroureterectomy. J Urol 2014; 191: 316–322. [DOI] [PubMed] [Google Scholar]