Abstract
Background:
Differences between virtual bronchoscopic navigation (VBN) systems and their impacts on the diagnostic yield of transbronchial biopsy (TBB) of peripheral pulmonary nodules (PPNs) remain unclear.
Objectives:
To compare the Synapse 3D system (Version 4.4, Fujifilm, Japan) and DirectPath system (Version 2.0, Olympus, Japan) in the VBN application of PPNs.
Design:
Retrospective study with self-paired design and exploratory study with retrospective cohort design.
Methods:
The study analyzed patients with PPNs using the Synapse 3D system (Group S) and DirectPath system (Group D) and compared differences between the two groups in bronchial tree reconstruction, navigation pathway planning, and VBN-assisted TBB of PPNs.
Results:
In all, 289 patients were analyzed ultimately. Bronchial tree reconstruction quality was better in Group S (p < 0.001). Navigation pathway planning duration in Group S was longer than that in Group D (median 1.35 vs 1.04 s, p < 0.001). Automated navigation pathway planning success rate in Group S was higher than that in Group D (36.7% vs 19.7%, p < 0.001), and CT image reconstruction parameter and nodule diameter, bronchus sign, and distance from the hilum had significant effects on it in both groups. Fifty-six patients in Group S and forty-two patients in Group D were analyzed ultimately. The localization success rate and diagnostic yield of PPNs between the two groups were not significantly different (85.3% vs 91.2% and 67.6% vs 61.8%, respectively, p > 0.05).
Conclusion:
Synapse 3D system (Version 4.4) and DirectPath system (Version 2.0) had their own merits. Localization success rate and diagnostic yield of VBN-assisted TBB were of no statistical difference for these two VBN systems. Improvements in segmentation algorithms of VBN systems and using the most suitable chest CT scan data for them may be the breakthrough to improve the efficiency of VBN, especially for poor experienced interventional physicians.
Keywords: diagnostic yield, DirectPath, localization success rate, navigation pathway planning success rate, peripheral pulmonary nodules, Synapse 3D, transbronchial biopsy, virtual bronchoscopic navigation
Introduction
Global Cancer Statistics 2020 data indicated that the incidence and mortality rate of lung cancer worldwide ranked second and first, respectively. 1 Early-stage lung cancer patients have a better prognosis. 2 Therefore, early diagnosis of lung cancer is critical. Surgery, transthoracic needle biopsy (TTNB), and transbronchial biopsy (TBB) are recommended for the diagnosis of PPNs. 3 Surgery is traumatic, costly, and risky. Although the overall sensitivity of CT-guided TTNB in the diagnosis of peripheral pulmonary lesions (PPLs) can reach 94%, 4 its clinical implementation is suboptimal since this excellent diagnostic yield comes at the cost of considerable complications including pneumothorax and hemorrhage.4 –6 Flexible bronchoscopy with a safer profile provides another available modality for the diagnosis of PPLs. However, the yield of conventional TBB is suboptimal. With the advancement of novel bronchoscopic techniques such as radial endobronchial ultrasound (R-EBUS), electromagnetic navigation bronchoscope (ENB), and virtual bronchoscopic navigation (VBN), the diagnostic yield of PPLs has significantly improved.7 –9 Consequently, performing TBB for patients with PPLs has gained popularity in recent years.
VBN is a technique that uses chest CT scan data to simulate the pathway to guide the bronchoscope to the lesion and biopsy. Compared with ENB, bronchoscopic transparenchymal nodule access, and robotic bronchoscopy, VBN is more affordable, easier to master, and has less radiation exposure to both the operator and patient. 10 Several commercial VBN systems have been applied to the TBB of PPNs. However, the diagnostic yield of VBN-assisted TBB still varies widely. Differences between VBN systems and their impacts on diagnostic yield remain unclear. DirectPath system (Olympus, Japan) is one of the earliest and most commonly used VBN systems, and DirectPath (Version 2.0, Olympus, Japan) was launched in China in 2022. In recent years, the Synapse 3D system (Fujifilm, Japan) has also been increasingly applied.11,12 This was the first study aimed to explore differences between VBN systems and to lay the foundation for VBN to play a more active role in TBB in diagnosing PPNs.
Methods
Patients
First, the retrospective study with the self-paired design was performed to compare differences between Synapse 3D system (Version 4.4, Fujifilm, Japan; Group S) and DirectPath system (Version 2.0, Olympus, Japan) (Group D) in bronchial tree reconstruction and navigation pathway planning. Patients who presented to the Department of Respiratory and Critical Care Medicine, the First Affiliated Hospital of Navy Medical University from July 2021 to December 2021 and were diagnosed with PPNs by chest CT in the hospital were screened. Inclusion criteria were as follows: (I) The nodules were 6–30 mm in diameter on axial CT images, surrounded by pulmonary parenchyma, which could not be found by routine bronchoscopy, (II) inspiratory CT scans, ⩾64 slices, slice thickness ⩽ 1.0 mm, and (III) chest CT scans must cover entire lung. Exclusion criteria were as follows: (I) age < 18 years, (II) patients with a history of lung surgery, (III) patients with obvious airway stenosis, severe airway deformity, severe emphysema, severe pulmonary bullae, severe diffuse interstitial lung disease, obvious atelectasis, or postoperative status of airway stent implantation, which obviously affected bronchial tree and airway reconstruction, (IV) patients with multiple pulmonary nodules and unable to determine main lesion, and (V) chest CT scans showed insufficient inspiration, poor breath holding, or obvious image artifacts.
Second, the exploratory study with a retrospective cohort design was performed to compare localization success rate and diagnostic yield using two VBN systems for VBN-assisted TBB of PPNs. Synapse 3D system (Version 4.4, Fujifilm, Japan) (Group S) and DirectPath system (Version 2.0, Olympus, Japan) (Group D) were used for navigation pathway planning for all patients who underwent VBN-assisted TBB of PPNs in this Respiratory Endoscopy Center from December 2021 to June 2022 and from July 2022 to February 2023, respectively. Inclusion criteria were as described above, and chest CT scans must be done within 1 week before VBN-assisted TBB. Exclusion criteria were as described above, but patients with a history of lung surgery or multiple pulmonary nodules could be reserved.
The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 13
Procedures, clinical and technical variables, and data collection
Two researchers were trained by a respiratory interventional physician with more than 10 years of experience in VBN utilization and TBB of PPNs, and system application engineers from Fujifilm Corporation and Olympus Corporation. Then two researchers operated the Synapse 3D system (Version 4.4) and DirectPath system (Version 2.0), respectively.
The study began after two researchers and the respiratory interventional physician mutually agreed on the data acquisition method and details. Two researchers jointly determined the optimal location and diameter of target lesions on chest CT images for all nodules (in case of doubt or disagreement, the respiratory interventional physician made final judgment), and independently mutually conducted subsequent data acquisition.
Bronchial tree reconstruction
Bronchial tree reconstruction was automatically performed using the Synapse 3D system and DirectPath system. If the bronchial tree was not reconstructed, at most three additional automatic bronchial tree reconstructions were allowed.
Bronchial tree reconstruction quality
Following bronchial tree reconstruction success, RB1a, RB6a, and RB10a were selected as evaluation bronchi and bronchial tree reconstruction quality was classified as good, medium, and poor, according to the study from Huang et al. 14
Navigation pathway planning duration
The duration from completion of target lesion placement and the beginning of automatic pathway planning to the end of that was defined as “navigation pathway planning duration.”
Navigation pathway classification and navigation pathway planning success
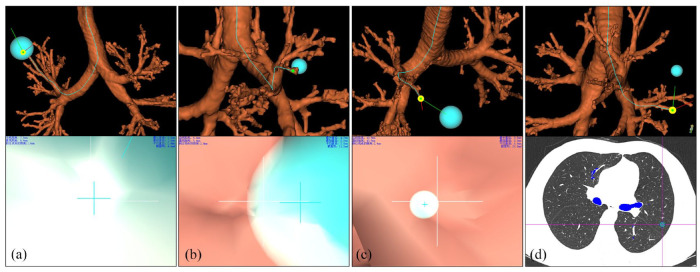
The navigation pathway was classified into four categories based on the optimal segmentation pathway by Diez-Ferrer et al. 15 (I) Category I pathway: the navigation endpoint was within or about to reach, or pass through the nodule (Figure 1(a)), (II) navigation endpoint was outside the nodule, and the distance from the edge of the nodule was ⩽5 mm (Category II pathway; Figure 1(b)) or >5 mm (Category III pathway; Figure 1(c)), and (III) Category IV pathway: bronchial tree reconstruction failure, across interlobar pleura navigation pathway, or segmental bronchus or above where the nodule was located could not be reconstructed and adjacent segmental bronchus or below could not reach the nodule (Figure 1(d)). The best classification was selected as the navigation pathway classification for each patient. Category I or Category II pathway was defined as “navigation pathway planning success,” and Category III or Category IV pathway was defined as “navigation pathway planning failure.”
Figure 1.
Navigation pathway classification. (a): Category I pathway: Nodule semi-diameter was 12.9 mm and DEC was 6.6 mm, (b): Category II pathway: Nodule semi-diameter was 5.0 mm and DEC was 6.4 mm, (c): Category III pathway: Nodule semi-diameter was 10.9 mm and DEC was 43.9 mm, (d): Category IV pathway: The nodule was located in left upper lobe with the navigation pathway leading to left lower lobe.
DEC, the distance from the navigation endpoint to the center of the lesion.
Most distantly visible bronchial branch generation and optimal bronchial branch generation
For patients with a non-Category IV pathway, along the navigation pathway to the target lesion, the carina was regarded as generation 0 and each bronchial bifurcation increased by one generation (Supplemental Figure 1). When the bronchial wall was not continuous or/and complete or bronchial bifurcation was not distinguished smoothly, the previous branch was regarded as optimal bronchial branch generation.
The distance from the navigation endpoint to the center of the lesion (DEC)
For patients with non-Category IV pathway, in Group S, DEC was the sum of the distance from the navigation endpoint to the edge of the target lesion and the semidiameter of that. When the navigation endpoint passed through the nodule along the navigation pathway, DEC was the negative of the sum above (Supplemental Figure 2).
VBN-assisted TBB
One designated researcher (same as a previous study) was responsible for navigation pathway planning using the Synapse 3D system from December 2021 to June 2022; another designated researcher (same as the previous study) was responsible for navigation pathway planning using the DirectPath system from July 2022 to February 2023.
Following navigation pathway planning, the respiratory interventional physician who intended to perform VBN-assisted TBB confirmed the navigation pathway and performed the procedure.
All procedures were performed under intravenous sedation and analgesia. The interventional physician advanced the bronchoscope toward the nodule along with the VBN pathway and reached insertable most distal bronchi, and then performed TBB under R-EBUS (with or without GS), fluoroscopy or R-EBUS-GS combined with fluoroscopy as described previously.16 –18 Localization success rate was defined as the ratio of the number of successfully localized nodules to that of all nodules. Generally, sampling procedures were performed in the order of forceps biopsy, bronchial brushing, and bronchoalveolar lavage (if fine-needle aspiration biopsy was performed, it was usually performed first. Sampling methods and sequence were determined by the interventional physician). At least six specimens were collected with biopsy forceps, bronchial brushing was performed 1–2 times, and fine-needle aspiration biopsy was performed 1–2 times. Bronchoalveolar lavage was usually performed last.
Diagnosis
For patients with high suspicion of benign or malignant tumors, if histological or cytological results suggested benign or malignant tumors, patients were considered to be diagnosed. For patients with high suspicion of infectious diseases, if histological or cytological results suggested they were consistent with inflammatory diseases, such as epithelioid granuloma or caseous necrosis, or pathogenic examination was positive, and patients whose nodules disappeared or significantly shrank after standardized antibiotic or anti-tuberculosis treatment were also considered to be diagnosed. When histological, cytological, and pathogenic results were temporarily unable to diagnose, a clinical follow-up of at least 6 months was recommended as a subsequent strategy. Diagnostic yield was defined as the ratio of the number of successfully diagnosed nodules to that of all nodules.
Data analyses
Continuous variables with normal distribution were described as mean ± standard deviation (SD), and those with non-normal distribution were described as median (lower quartile, upper quartile) (Q1, Q3). Continuous variables between the two groups were compared using the Wilcoxon signed-rank test and Mann–Whitney U test. Categorical variables were described as numbers and percentages. Categorical variables were compared using McNemar’s chi-square test, Pearson’s chi-square test, and Fisher’s exact test. Binary logistic regression analyses were performed to analyze factors affecting automatic navigation pathway planning success rate in Group S or Group D. Rank variables were compared using the Wilcoxon signed-rank test, Mann–Whitney U test, and Kruskal–Wallis H test. Above data analysis was performed using IBM SPSS Statistics 26. Using R software (Version 4.0.4, The R Foundation), confounding factors were controlled for two groups using the propensity score matching (PSM) method, with the caliper value set to 0.1 propensity score SD and the matching ratio of 1:1. p < 0.05 (two-sided) was considered statistically significant.
Results
Part I comparison of two VBN systems in bronchial tree reconstruction and navigation pathway planning
Patients, chest CT scan data, and nodules
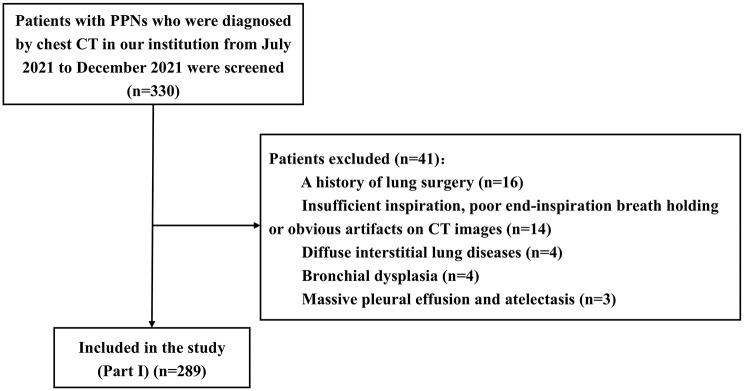
A total of 330 patients were enrolled, and 289 patients with PPNs (median 9.5 mm in diameter) were finally analyzed (Figure 2). The patients, chest CT scan data, and nodule characteristics are shown in Supplemental Tables 1 and 2.
Figure 2.
Flow of patients enrolled in Part I of the study.
Bronchial tree reconstruction success rate and quality
Bronchial tree reconstruction success rate in Group S and Group D was 100.0% and 98.3%, respectively. In Group D, the bronchial tree reconstruction success rate using chest CT plain scan sequence was 100.0%, which was higher than that using chest CT contrast-enhanced scan sequence (93.9%; p = 0.002; Supplemental Table 3).
Bronchial tree reconstruction quality in Group S was better than that in Group D (p < 0.001). However, bronchial tree reconstruction quality using B-type CT data was not significantly different between the two groups (p = 0.088; Supplemental Table 3). In Group D, bronchial tree reconstruction quality using B-type CT data was the best (Supplemental Table 3).
Navigation pathway planning duration
Navigation pathway planning duration in Group S was longer than that in Group D (1.35 (1.01, 1.99) s vs 1.04 (0.95, 1.17) s, p < 0.001).
Automated navigation pathway planning success rate
The automated navigation pathway planning success rate in Group S was superior to that in Group D (36.7% vs 19.7%, p < 0.001; Table 1). In all subgroups, it was higher in Group S than in Group D (all p < 0.05) (Table 1).
Table 1.
Automated navigation pathway planning success rate of 289 patients for two VBN systems.
| Variables | Group S | Group D | p Value |
|---|---|---|---|
| Total | 106 (36.7%) | 57 (19.7%) | <0.001 a |
| Chest CT scan sequence | |||
| Plain scan sequence | 67 (32.4%) | 36 (17.4%) | <0.001 a |
| Contrast-enhanced scan sequence | 39 (47.6%) | 21 (25.6%) | <0.001 a |
| Image reconstruction parameter | |||
| 1 mm × 1 mm, L (L-type CT data) | 66 (39.1%) | 35 (20.7%) | <0.001 a |
| 1 mm × 0.8 mm, LARGE-FC 56/55 (FC-type CT data) | 15 (23.4%) | 4 (6.3%) | 0.001 a |
| 0.67 mm × 0.67 mm, B (B-type CT data) | 25 (44.6%) | 18 (32.1%) | 0.039 a |
| Lesion size (diameter on CT) | |||
| ⩽10 mm | 27 (17.2%) | 13 (8.3%) | 0.001 a |
| 10–⩽20 mm | 52 (50.5%) | 27 (26.2%) | <0.001 a |
| 20–⩽30 mm | 27 (93.1%) | 17 (58.6%) | 0.002 a |
| Texture on CT | |||
| Solid | 64 (44.1%) | 39 (26.9%) | <0.001 a |
| Part-solid | 23 (43.4%) | 12 (22.6%) | 0.003 a |
| Pure ground glass | 19 (20.9%) | 6 (6.6%) | <0.001 a |
| Lobe location | |||
| Upper lobe | 49 (38.3%) | 24 (18.8%) | <0.001 a |
| Middle lobe (Lingula) | 27 (41.5%) | 14 (21.5%) | 0.001 a |
| Lower lobe | 30 (31.3%) | 19 (19.8%) | 0.007 a |
| Bronchus sign | |||
| Absent | 18 (12.0%) | 9 (6.0%) | 0.012 a |
| Present | 88 (63.3%) | 48 (34.5%) | <0.001 a |
| Nodule location from the hilum | |||
| Peripheral 1/3 | 54 (25.4%) | 22 (10.3%) | <0.001 a |
| Inner 2/3 | 52 (68.4%) | 35 (46.1%) | <0.001 a |
Binomial distribution used.
Univariate and multivariate logistic regression analysis showed that CT image reconstruction parameters and nodule diameter, bronchus sign, and distance from the hilum had significant effects on automated navigation pathway planning success rate in Group S and Group D (all p < 0.05; Table 2).
Table 2.
Factors affecting automated navigation pathway planning success rate in two VBN systems.
| Variables | Synapse 3D | DirectPath | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| p Value | p Value | OR a | 95% CI b for OR | p Value | p Value | OR a | 95% CI b for OR | |||
| Lower | Upper | Lower | Upper | |||||||
| Chest CT scan sequence | 0.016 | 0.422 | 0.116 | 0.727 | ||||||
| Plain scan sequence | Reference | Reference | ||||||||
| Contrast-enhanced scan sequence | — | — | — | — | — | — | ||||
| Image reconstruction parameter | 0.037 | 0.048 | 0.004 | 0.001 | ||||||
| 1 mm × 1 mm, L (L-type CT data) | 0.303 | 0.117 | 0.786 | 0.168 | 0.063 | 0.450 | ||||
| 1 mm × 0.8 mm, LARGE-FC 56/55 (FC-type CT data) | 0.489 | 0.167 | 1.431 | 0.104 | 0.024 | 0.455 | ||||
| 0.67 mm × 0.67 mm, B (B-type CT data) | Reference | Reference | ||||||||
| Lesion size (diameter on CT) | <0.001 | <0.001 | <0.001 | 0.030 | ||||||
| ⩽10 mm | 0.029 | 0.005 | 0.163 | 0.211 | 0.062 | 0.717 | ||||
| 10–⩽20 mm | 0.056 | 0.010 | 0.310 | 0.284 | 0.097 | 0.828 | ||||
| 20–⩽30 mm | Reference | Reference | ||||||||
| Texture on CT | 0.001 | 0.749 | 0.002 | 0.058 | ||||||
| Pure ground glass | Reference | Reference | ||||||||
| Part-solid | — | — | — | — | — | — | ||||
| Solid | — | — | — | — | — | — | ||||
| Lobe location | 0.899 | — | 0.899 | — | ||||||
| Upper lobe | Reference | Reference | ||||||||
| Middle lobe (Lingula) | — | — | — | — | — | — | ||||
| Lower lobe | — | — | — | — | — | — | ||||
| Bronchus sign | <0.001 | <0.001 | <0.001 | 0.002 | ||||||
| Absent | Reference | Reference | ||||||||
| Present | 9.505 | 4.334 | 20.848 | 4.889 | 1.821 | 13.123 | ||||
| Nodule location from the hilum | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| Peripheral 1/3 | Reference | Reference | ||||||||
| Inner 2/3 | 9.504 | 4.383 | 20.606 | 10.869 | 4.764 | 24.795 | ||||
Odds ratio.
95% confidence interval.
Most distantly visible and optimal bronchial branch generation, and the distance from the navigation endpoint to the center of the lesion
For the same patients with non-Category IV pathways in both groups, we chose top-ranked non-Category IV pathways for matching. The most distantly visible bronchial branch generation (MVG) in Group S and Group D were both 6.0 (5.0, 7.5) generation (p = 0.971). Optimal bronchial branch generation (OG) was 6.0 (5.0, 7.0) generation in Group S and 5.0 (4.0, 6.0) generation in Group D (p < 0.001). DEC was 14.6 (9.3, 24.0) mm in Group S and 24.3 (12.3, 33.5) mm in Group D (p < 0.001). The most distantly and optimal matched bronchial branch generation was 5.0 (4.0, 6.0) generation and 4.0 (3.0, 6.0) generation, respectively.
Part II: Utilization of two VBN systems in assisting TBB of peripheral pulmonary nodules
Patients, chest CT scan data, and nodules
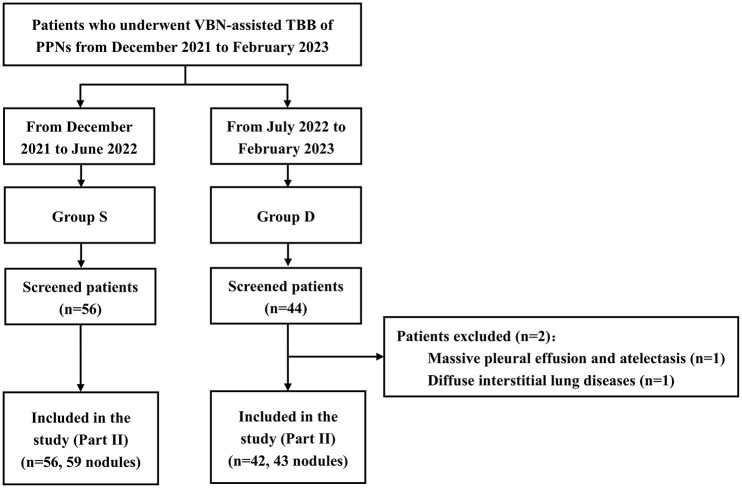
A total of 56 patients with 59 PPNs (Group S) and 44 patients (Group D) were enrolled, and 2 patients in Group D were excluded (Figure 3). The patients, chest CT scan data, and nodule characteristics are shown in Supplemental Tables 4 and 5. The baseline demographic, chest CT scan data, and pulmonary nodule characteristics were balanced in both groups.
Figure 3.
Flow of patients enrolled in Part II of the study.
PPNs, peripheral pulmonary nodules; TBB, transbronchial biopsy; VBN, virtual bronchoscopic navigation.
Bronchoscopic procedures
The flexible bronchoscope type, lesion confirmation technique, and the number of sampling methods used in VBN-assisted TBB are shown in Supplemental Table 5. Baseline characteristics were not statistically different in both groups.
Localization success rate and diagnostic yield
There was no significant difference in localization success rate and diagnostic yield between Group S and Group D (84.7% vs 86.0%, p = 0.855 and 64.4% vs 60.5%, p = 0.684, respectively), and the sampling rate was 100% in both groups.
There was no significant difference in baseline variables between the two groups after PSM (all p > 0.05; Table 3), indicating that baseline characteristics were well balanced between the two groups after PSM. The distribution of propensity scores before and after PSM is shown in Supplemental Figure 3. The differences in localization success rate and diagnostic yield were not significant in both groups after PSM (Table 3).
Table 3.
After PSM, a baseline characteristics, localization success rate, and diagnostic yield of VBN-assisted transbronchial biopsy.
| Variables | Group S (n = 34) |
Group D (n = 34) |
p Value |
|---|---|---|---|
| Age, mean ± SD, b years | 64 ± 13 | 64 ± 12 | 0.881 |
| Sex | 1.000 | ||
| Female | 26.5% (9) | 26.5% (9) | |
| Male | 73.5% (25) | 73.5% (25) | |
| Lesion size | 0.790 | ||
| ⩽20 mm | 32.4% (11) | 26.5% (9) | |
| 20–⩽30 mm | 67.6% (23) | 73.5% (25) | |
| Texture on CT | 1.000 | ||
| Solid | 91.2% (31) | 91.2% (31) | |
| Ground glass | 8.8% (3) | 8.8% (3) | |
| Lobe location | 0.946 | ||
| Upper lobe | 41.2% (14) | 44.1% (15) | |
| Middle lobe (Lingula) | 20.6% (7) | 17.6% (6) | |
| Lower lobe | 38.2% (13) | 38.2% (13) | |
| Bronchus sign | 1.000 | ||
| Absent | 14.7% (5) | 11.8% (4) | |
| Present | 85.3% (29) | 88.2% (30) | |
| Nodule location from the hilum | 1.000 | ||
| Inner 2/3 | 61.8% (21) | 61.8% (21) | |
| Peripheral 1/3 | 38.2% (13) | 38.2% (13) | |
| Bronchoscope type | 0.861 | ||
| BF-1T260/1TQ290 | 17.6% (6) | 17.6% (6) | |
| BF-260/Q290 | 52.9% (18) | 47.1% (16) | |
| BF-P260F/P290 | 29.4% (10) | 35.3% (12) | |
| Lesion confirmation technique | 1.000 | ||
| X-ray fluoroscopy | 2.9% (1) | 2.9% (1) | |
| R-EBUS | 94.1% (32) | 94.1% (32) | |
| R-EBUS + X-ray fluoroscopy | 2.9% (1) | 2.9% (1) | |
| Localization success rate | 85.3% (29) | 91.2% (31) | 0.709 |
| Diagnostic yield | 67.6% (23) | 61.8% (21) | 0.800 |
PSM, Propensity score matching. Matched factors included gender and age of the subjects, diameter, texture, lobe location, bronchus sign and distance from the hilum of pulmonary nodules, bronchoscope type, and lesion confirmation technique.
SD, Standard deviation.
Discussion
Diagnostic yield of VBN-assisted TBB for PPNs varied from 46.0% to 80.4%,12,19,20 and it is still controversial that combined VBN can improve diagnostic yield. VBN systems used in different studies are not identical. Therefore, exploring differences between VBN systems may bring new light to VBN-assisted TBB. To the best of our knowledge, this was the first study directly comparing both different VBN systems by themselves and localization success rates and diagnostic yield in VBN-assisted TBB of PPNs.
Bronchial tree reconstruction success rate was not significantly different between the two groups. However, bronchial trees of five patients with chest CT contrast-enhanced scan sequence were not reconstructed successfully in Group D. Less attention has been paid to the effect of intravenous contrast medium on VBN before chest CT scans. Airway segmentation by the VBN system is based on a threshold algorithm. Due to the partial volume effect, chest CT scans after intravenous injection of contrast media would increase CT values in airway walls and lumens adjacent to or concomitant with vessels, resulting in failed or wrong segmentation by VBN systems. Therefore, chest CT contrast-enhanced scan sequence on VBN should be supposed to be paid attention.
The overall bronchial tree reconstruction quality was better in Group S than in Group D. However, the difference was not significant using chest CT scan data with thinner reconstruction slice thickness, interval, and algorithm B (B-type CT data). CT image reconstruction slice thickness and algorithm have been considered to be significant parameters affecting airway segmentation.21,22 In Group D, the CT image reconstruction parameter was a significant factor affecting bronchial tree reconstruction quality. Therefore, CT scans data with thinner reconstruction slice thickness and interval, and specific algorithms should be explored and utilized.
Based on the classification from Diez-Ferrer et al., 15 we defined the automated navigation pathway planning success rate. The overall and subgroups’ automated navigation pathway planning success rate in Group S was significantly superior to that in Group D, which indicates that the spatial segmentation algorithm of the Synapse 3D system may be superior. As with diagnostic yield of TBB, nodule diameter,23 –25 bronchus sign,24 –26 and distance from hilum15,25,27 were crucial factors influencing automated navigation pathway planning success rate in both groups. The diagnostic yield of TBB for PPNs located in the inner 2/3 and peripheral 1/3 lung field was 71% and 63%, 25 respectively. In Group S, the automated navigation pathway planning success rate of PPNs located in the inner 2/3 lung field was 68.4%, which was consistent with the diagnostic yield, while that of PPNs located in the peripheral 1/3 lung field was only 25.4%. However, the automated navigation pathway planning success rate of PPNs located in the peripheral 1/3 lung field was more unsatisfactory in Group D. These suggested that the two VBN systems did not adequately make bronchi segmented in the peripheral 1/3 lung field. Multivariate analysis demonstrated that chest CT scan sequence and nodule texture on CT image did not significantly influence automated navigation pathway planning success rate, which may be due to that patients with larger size nodules tend to be performed chest CT contrast-enhanced scans and the diameter of solid nodules is commonly longer than that of ground-glass nodules.
OG was less than MVG in Group D. Miyake et al. had proposed the discontinuity and incompleteness of virtual bronchoscopic (VB) bronchial wall, 28 and Rosell et al. suggested that bronchial wall reconstruction was not clear due to partial volume effect and noise. 29 As Asano et al. stated, it requires updates of VBN software and improvements of segmentation algorithms. 17 On the other hand, one foundation of VBN is a chest CT scan. 30 Adequate inspiration and stable end-inspiratory breath holding were more conducive to adequately dilating bronchi, avoiding CT image artifacts, and getting more bronchial branches. 22 Diez-Ferrer et al. performed chest CT scans for patients after positive airway pressure ventilation, and more VB bronchial branches could be segmented. 31 Furthermore, using chest CT scan data with thinner reconstruction slice thickness or larger reconstruction matrix, more bronchial branches,22,32 –34 longer bronchial length,22,33 more continuous or/and complete bronchial wall 34 were obtained. In this study, CT image reconstruction parameters have also been demonstrated to significantly affect automated navigation pathway planning success rate. To maximize segmentation performance and optimize the navigation capability of one VBN system, improving CT image quality and exploring optimal image reconstruction parameters for the VBN system may be crucial. The ratio of lesion size to the shortest linear distance from the VBN navigation endpoint >1.84 predicted that R-EBUS could locate PPNs, 35 and Kitamura et al. found that the distance from the VBN navigation endpoint to the edge of the lesion was a predictive factor for diagnostic yield of VBN-assisted TBB of PPLs, 36 those indicated the importance of the distance from the navigation endpoint to the lesion. DEC was significantly shorter in Group S, and this was consistent with the conclusion of the automatic navigation pathway planning success rate. Although MVG was not significantly different between the two groups, DEC was shorter in Group S, which indicates that the Synapse 3D system may intend to navigate the bronchoscope to more distal bronchi. As Kitamura et al. and Miyake et al. found, there may also be unsegmented bronchial branches on more distal bronchi segmented by Synapse 3D system.28,36
Decisive steps in diagnosing PPNs by TBB include accurate delivery of biopsy instruments to the lesion and obtaining satisfactory tissue samples. Although there was a statistical difference between Group S and Group D in terms of chest CT scan sequence, Part I has demonstrated that chest CT scan sequence was not the factor influencing automatic navigation pathway planning success rate. Therefore, baseline characteristics were well balanced between the two groups before and after PSM, and localization success rate and diagnostic yield were not significantly different.
The automated navigation pathway planning success rate was superior in Group S, while there was no significant difference in the localization success rate. It was possible that skilled respiratory interventional physicians and VBN-assisted TBB in combination with R-EBUS and/or X-ray fluoroscopy narrowed the difference above. The localization success rate was about 86%, while that was about 75% in a previous randomized controlled trial (RCT) on VBN combined with R-EBUS. 37 The difference may be attributed to improved skills of respiratory interventional physicians or/and enhanced performance of VBN systems. In another RCT on VBN combined with R-EBUS, the localization success rate reached 93%, 20 which may be due to the combination with X-ray fluoroscopy. Diagnostic yield was about 65% in both groups, which was inferior to that in RCT on VBN combined with R-EBUS. 37 Several studies have proved that diagnostic yield varies when different sampling methods and sequences are performed.26,38 However, in this study, sampling methods and sequences were determined by interventional physicians. Only one sampling method was performed for 20% of nodules in both groups and ⩽2 sampling methods were performed for 37.2% and 51.2% of nodules in Group S and Group D, respectively. Diagnostic yield for nodules in upper or lower lung lobes is inferior to that in the right middle lobe,27,37 and that for nodules in the peripheral 1/3 lung field is lower than that in the inner 2/3 lung field.15,25 In this study, more than 80% of nodules were located in the upper or lower lung lobes, and about 40% of nodules were located in the peripheral 1/3 lung field. These may be reasons for lower diagnostic yield.
Regarding cost-effectiveness, the Synapse 3D system (Version 4.4) costs about ¥ 800,000 and the DirectPath system (Version 2.0, Olympus, Japan) costs about ¥ 300,000 to purchase. Except for VBN, Synapse 3D enables airway analysis, pulmonary nodule quantitative analysis, emphysema analysis, bullae analysis, and pulmonary vessel analysis.
Compared with other navigation bronchoscopy techniques, VBN is relatively easier to master. 39 However, beginners may lead to an unreasonable navigation pathway planned by the VBN system. 40 If the operator is not the pathway planner, it is necessary for the operator to confirm the navigation pathway before the procedure. It must also be acknowledged that VBN is not a real-time navigation like ENB and is unable to make the bronchoscope advance to increased angulation bronchi and maintain a static position like robotic-assisted bronchoscopy. Of note, novel bronchoscopic technologies, such as transbronchial lung cryobiopsy that can retrieve larger tissue samples with more preserved cellular architecture and fewer crush artifacts,41,42 and confocal laser endomicroscopy that enables higher resolution and real-time microscopic analysis of tissue architecture 43 have the potential to improve the detection of PPLs. However, the limitation of any single technique should be recognized. Therefore, integrating VBN and other available modalities should be the diagnosis of choice for PPLs.
Our study had some limitations. First, Part II was an exploratory study with a retrospective cohort design, the sample size was small and insufficient for subgroup analysis, and the evidence level may be inferior to RCT. Second, not all patients were ultimately diagnosed pathologically, and some were based on clinical follow-up data. In addition, a few cases had short follow-ups. Third, the study was conducted at a single center with experienced staff. Therefore, our results may be difficult to replicate in other centers.
Conclusion
In conclusion, the Synapse 3D system (Version 4.4) was superior in bronchial tree reconstruction quality and automated navigation pathway planning success rate. CT image reconstruction parameters and nodule diameter, bronchus sign, and distance from the hilum affected the automated navigation pathway planning success rate. DirectPath system (Version 2.0) was superior in navigation pathway planning duration. Localization success rate and diagnostic yield of VBN-assisted TBB showed no significant difference using the two VBN systems. Improvements in segmentation algorithms of VBN systems and using the most suitable chest CT scan data for them may be the breakthrough to improve the efficiency of VBN, especially for poor experienced interventional physicians.
Supplemental Material
Supplemental material, sj-pdf-1-tar-10.1177_17534666241307182 for Comparison of Synapse 3D system (Version 4.4) and DirectPath system (Version 2.0) in virtual bronchoscopic navigation application for peripheral pulmonary nodules by Xiang Li, Sen Tian, Yifei Zhang, Hui Chen, Yilin Chen, Qin Wang, Wei Zhang, Hui Shi, Haidong Huang, Xiaping Shen, Yao Fang, Lei Qu, Zhenhong Hu, Yuchao Dong and Chong Bai in Therapeutic Advances in Respiratory Disease
Acknowledgments
None.
Footnotes
ORCID iDs: Xiang Li  https://orcid.org/0009-0000-6182-161X
https://orcid.org/0009-0000-6182-161X
Sen Tian  https://orcid.org/0000-0002-6486-3142
https://orcid.org/0000-0002-6486-3142
Hui Shi  https://orcid.org/0009-0000-4497-4614
https://orcid.org/0009-0000-4497-4614
Chong Bai  https://orcid.org/0000-0003-2039-5751
https://orcid.org/0000-0003-2039-5751
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Xiang Li, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China; Department of Respiratory and Critical Care Medicine, General Hospital of Central Theater Command of the Chinese People’s Liberation Army, Wuhan, China.
Sen Tian, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China; Department of Respiratory and Critical Care Medicine, No. 906 Hospital of the Chinese People’s Liberation Army Joint Logistic Support Force, Ningbo, China.
Yifei Zhang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Hui Chen, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Yilin Chen, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Qin Wang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Wei Zhang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Hui Shi, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), No. 168 Changhai Road, Yangpu District, Shanghai, China.
Haidong Huang, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), No. 168 Changhai Road, Yangpu District, Shanghai, China.
Xiaping Shen, Department of Medical Imaging, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Yao Fang, Department of Respiratory and Critical Care Medicine, General Hospital of Central Theater Command of the Chinese People’s Liberation Army, Wuhan, China.
Lei Qu, Department of Respiratory and Critical Care Medicine, General Hospital of Central Theater Command of the Chinese People’s Liberation Army, Wuhan, China.
Zhenhong Hu, Department of Respiratory and Critical Care Medicine, General Hospital of Central Theater Command of the Chinese People’s Liberation Army, Wuhan, China.
Yuchao Dong, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), Shanghai, China.
Chong Bai, Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Naval Medical University (Shanghai Changhai Hospital), No. 168 Changhai Road, Yangpu District, Shanghai, China.
Declarations
Ethics approval and consent to participate: This study was approved by the Institutional Review Board of the First Affiliated Hospital of Naval Medical University, Shanghai, China (IRB No. CHEC2022-034) and individual consent for this retrospective analysis was waived.
Consent for publication: Not applicable.
Author contributions: Xiang Li: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Sen Tian: Conceptualization; Data curation; Formal analysis; Methodology; Writing – review & editing.
Yifei Zhang: Data curation; Investigation; Resources; Writing – review & editing.
Hui Chen: Data curation; Investigation; Resources; Writing – review & editing.
Yilin Chen: Data curation; Investigation; Writing – review & editing.
Qin Wang: Investigation; Resources; Writing – review & editing.
Wei Zhang: Investigation; Resources; Writing – review & editing.
Hui Shi: Conceptualization; Data curation; Funding acquisition; Resources; Writing – review & editing.
Haidong Huang: Conceptualization; Data curation; Methodology; Resources; Writing – review & editing.
Xiaping Shen: Methodology; Resources; Writing – review & editing.
Yao Fang: Data curation; Formal analysis; Writing – review & editing.
Lei Qu: Data curation; Formal analysis; Writing – review & editing.
Zhenhong Hu: Data curation; Formal analysis; Writing – review & editing.
Yuchao Dong: Conceptualization; Resources; Writing – review & editing.
Chong Bai: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Resources; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural and Science Foundation of China (Grant number 82000102), the Collaborative Innovation Cluster Foundation of Shanghai Health Commission (Grant number 2020CXJQ03), and the National Natural and Science Foundation of China (Grant number 82270112).
The authors declare that there is no conflict of interest.
Availability of data and materials: Data are available on reasonable request from the corresponding author.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol 2016; 11: 39–51. [DOI] [PubMed] [Google Scholar]
- 3. Gould MK, Fletcher J, Iannettoni MD, et al. Evaluation of patients with pulmonary nodules: when is it lung cancer? ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007; 132: 108S–130S. [DOI] [PubMed] [Google Scholar]
- 4. Zhan P, Zhu QQ, Miu YY, et al. Comparison between endobronchial ultrasound-guided transbronchial biopsy and CT-guided transthoracic lung biopsy for the diagnosis of peripheral lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res 2017; 6: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han Y, Kim HJ, Kong KA, et al. Diagnosis of small pulmonary lesions by transbronchial lung biopsy with radial endobronchial ultrasound and virtual bronchoscopic navigation versus CT-guided transthoracic needle biopsy: a systematic review and meta-analysis. PLoS One 2018; 13: e191590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang C, Li X, Zhou Z, et al. Endobronchial ultrasonography with guide sheath versus computed tomography guided transthoracic needle biopsy for peripheral pulmonary lesions: a propensity score matched analysis. J Thorac Dis 2016; 8: 2758–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu Z, Tian S, Wang X, et al. Predictive value of the resistance of the probe to pass through the lesion in the diagnosis of peripheral pulmonary lesions using radial probe endobronchial ultrasound with a guide sheath. Front Oncol 2023; 13: 1168870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S, Kim N, Chung S, et al. Diagnostic accuracy and safety of electromagnetic navigation transthoracic needle biopsy under moderate sedation for the diagnosis of peripheral pulmonary lesions. Transl Lung Cancer Res 2023; 12: 1496–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huang H, Wu N, Tian S, et al. Application of bronchoscopy in the diagnosis and treatment of peripheral pulmonary lesions in China: a national cross-sectional study. J Cancer 2023; 14: 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giri M, Puri A, Wang T, Huang G, Guo S. Virtual bronchoscopic navigation versus non-virtual bronchoscopic navigation assisted bronchoscopy for the diagnosis of peripheral pulmonary lesions: a systematic review and meta-analysis. Ther Adv Respir Dis 2021; 15: 1631370920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kawakita N, Takizawa H, Toba H, et al. Cone-beam computed tomography versus computed tomography-guided ultrathin bronchoscopic diagnosis for peripheral pulmonary lesions: a propensity score-matched analysis. Respirology 2021; 26: 477–484. [DOI] [PubMed] [Google Scholar]
- 12. Yutaka Y, Sato T, Isowa M, et al. Electromagnetic navigation bronchoscopy versus virtual bronchoscopy navigation for improving the diagnosis of peripheral lung lesions: analysis of the predictors of successful diagnosis. Surg Today 2022; 52: 923–930. [DOI] [PubMed] [Google Scholar]
- 13. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet (London, England) 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 14. Huang Y, Zhong X, Li J, et al. Initiatory use of virtual bronchoscopic navigation software: DirectPath. Int J Respir 2015; 35: 600–605. [Google Scholar]
- 15. Diez-Ferrer M, Morales A, Tebe C, et al. Ultrathin bronchoscopy with and without virtual bronchoscopic navigation: influence of segmentation on diagnostic yield. Respiration 2019; 97: 252–258. [DOI] [PubMed] [Google Scholar]
- 16. Kurimoto N, Miyazawa T, Okimasa S, et al. Endobronchial ultrasonography using a guide sheath increases the ability to diagnose peripheral pulmonary lesions endoscopically. Chest 2004; 126: 959–965. [DOI] [PubMed] [Google Scholar]
- 17. Asano F, Eberhardt R, Herth FJ. Virtual bronchoscopic navigation for peripheral pulmonary lesions. Respiration 2014; 88: 430–440. [DOI] [PubMed] [Google Scholar]
- 18. Zheng X, Zhong C, Xie F, et al. Virtual bronchoscopic navigation and endobronchial ultrasound with a guide sheath without fluoroscopy for diagnosing peripheral pulmonary lesions with a bronchus leading to or adjacent to the lesion: a randomized non-inferiority trial. Respirol 2023; 28: 389–398. [DOI] [PubMed] [Google Scholar]
- 19. Tachihara M, Ishida T, Kanazawa K, et al. A virtual bronchoscopic navigation system under X-ray fluoroscopy for transbronchial diagnosis of small peripheral pulmonary lesions. Lung Cancer 2007; 57: 322–327. [DOI] [PubMed] [Google Scholar]
- 20. Ishida T, Asano F, Yamazaki K, et al. Virtual bronchoscopic navigation combined with endobronchial ultrasound to diagnose small peripheral pulmonary lesions: a randomised trial. Thorax 2011; 66: 1072–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia Y, Zhai B, He T, et al. Effect of new model-based iterative reconstruction on quantitative analysis of airway tree by computer-aided detection software in chest computed tomography. J Comput Assist Tomogr 2021; 45: 166–170. [DOI] [PubMed] [Google Scholar]
- 22. Nardelli P, Khan KA, Corvo A, et al. Optimizing parameters of an open-source airway segmentation algorithm using different CT images. Biomed Eng Online 2015; 14: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Baaklini WA, Reinoso MA, Gorin AB, et al. Diagnostic yield of fiberoptic bronchoscopy in evaluating solitary pulmonary nodules. Chest 2000; 117: 1049–1054. [DOI] [PubMed] [Google Scholar]
- 24. Oki M, Saka H, Asano F, et al. Use of an ultrathin vs thin bronchoscope for peripheral pulmonary lesions: a randomized trial. Chest 2019; 156: 954–964. [DOI] [PubMed] [Google Scholar]
- 25. Kim SH, Kim J, Pak K, et al. Ultrathin bronchoscopy for the diagnosis of peripheral pulmonary lesions: a meta-analysis. Respiration 2023; 102: 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsumoto Y, Nakai T, Tanaka M, et al. Diagnostic outcomes and safety of cryobiopsy added to conventional sampling methods: an observational study. Chest 2021; 160: 1890–1901. [DOI] [PubMed] [Google Scholar]
- 27. Asano F, Shinagawa N, Ishida T, et al. Virtual bronchoscopic navigation improves the diagnostic yield of radial-endobronchial ultrasound for peripheral pulmonary lesions with involved bronchi on CT. Intern Med 2015; 54: 1021–1025. [DOI] [PubMed] [Google Scholar]
- 28. Miyake K, Morimura O, Inoue T, et al. The direct oblique method: a new gold standard for bronchoscopic navigation that is superior to automatic methods. J Bronchology Interv Pulmonol 2018; 25: 305–314. [DOI] [PubMed] [Google Scholar]
- 29. Rosell J, Cabras P. A three-stage method for the 3D reconstruction of the tracheobronchial tree from CT scans. Comput Med Imaging Graph 2013; 37: 430–437. [DOI] [PubMed] [Google Scholar]
- 30. Asano F, Matsuno Y, Shinagawa N, et al. A virtual bronchoscopic navigation system for pulmonary peripheral lesions. Chest 2006; 130: 559–566. [DOI] [PubMed] [Google Scholar]
- 31. Diez-Ferrer M, Gil D, Tebe C, et al. Positive airway pressure to enhance computed tomography imaging for airway segmentation for virtual bronchoscopic navigation. Respiration 2018; 96: 525–534. [DOI] [PubMed] [Google Scholar]
- 32. Adachi T, Machida H, Nishikawa M, et al. Improved delineation of CT virtual bronchoscopy by ultrahigh-resolution CT: comparison among different reconstruction parameters. Jpn J Radiol 2020; 38: 884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morita Y, Yamashiro T, Tsuchiya N, et al. Automatic bronchial segmentation on ultra-HRCT scans: advantage of the 1024-matrix size with 0.25-mm slice thickness reconstruction. Jpn J Radiol 2020; 38: 953–959. [DOI] [PubMed] [Google Scholar]
- 34. Bartlett DJ, Koo CW, Bartholmai BJ, et al. High-resolution chest computed tomography imaging of the lungs: impact of 1024 matrix reconstruction and photon-counting detector computed tomography. Invest Radiol 2019; 54: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen H, Yu Y, Yu X, et al. An innovative method: predicting the visibility of radial endobronchial ultrasound for peripheral pulmonary nodules by virtual bronchoscopic navigation. Technol Cancer Res Treat 2022; 21: 2081083618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kitamura A, Tomishima Y, Imai R, et al. Findings of virtual bronchoscopic navigation can predict the diagnostic rate of primary lung cancer by bronchoscopy in patients with peripheral lung lesions. Bmc Pulm Med 2022; 22: 270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bo L, Li C, Pan L, et al. Diagnosing a solitary pulmonary nodule using multiple bronchoscopic guided technologies: a prospective randomized study. Lung Cancer 2019; 129: 48–54. [DOI] [PubMed] [Google Scholar]
- 38. Mondoni M, Rinaldo RF, Carlucci P, et al. Bronchoscopic sampling techniques in the era of technological bronchoscopy. Pulmonology 2022; 28: 461–471. [DOI] [PubMed] [Google Scholar]
- 39. Fielding D, Oki M. Technologies for targeting the peripheral pulmonary nodule including robotics. Respirology 2020; 25: 914–923. [DOI] [PubMed] [Google Scholar]
- 40. Ishiwata T, Gregor A, Inage T, et al. Bronchoscopic navigation and tissue diagnosis. Gen Thorac Cardiovasc Surg 2020; 68: 672–678. [DOI] [PubMed] [Google Scholar]
- 41. Tang Y, Tian S, Chen H, et al. Transbronchial lung cryobiopsy for peripheral pulmonary lesions. a narrative review. Pulmonology 2024; 30(5): 475–484. [DOI] [PubMed] [Google Scholar]
- 42. Kim SH, Mok J, Kim S, et al. Clinical outcomes of transbronchial cryobiopsy using a 1.1-mm diameter cryoprobe for peripheral lung lesions: a prospective pilot study. Respir Med 2023; 217: 107338. [DOI] [PubMed] [Google Scholar]
- 43. Tian S, Huang H, Zhang Y, et al. The role of confocal laser endomicroscopy in pulmonary medicine. Eur Respir Rev 2023; 32: 220185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-tar-10.1177_17534666241307182 for Comparison of Synapse 3D system (Version 4.4) and DirectPath system (Version 2.0) in virtual bronchoscopic navigation application for peripheral pulmonary nodules by Xiang Li, Sen Tian, Yifei Zhang, Hui Chen, Yilin Chen, Qin Wang, Wei Zhang, Hui Shi, Haidong Huang, Xiaping Shen, Yao Fang, Lei Qu, Zhenhong Hu, Yuchao Dong and Chong Bai in Therapeutic Advances in Respiratory Disease