Abstract
1. Rat livers were dissociated into their constituent cells by perfusion through the portal vein with a medium containing collagenase, and hepatocytes separated from non-parenchymal cells. 2. It is shown that the procedure described by Wisher & Evans [(1975) Biochem. J. 146, 375-388] for preparation of plasma membranes from liver tissue when applied to isolated hepatocytes also yielded subfractions of similar morphology and marker-enzyme distribution. 3. Thus the distribution of alkaline phosphodiesterase, 5'-nucleotidase and the basal and glucagon-stimulated adenylate cyclase among two 'light' vesicular and one 'heavy' junction-containing plasma-membrane subfractions paralleled that reported for tissue-derived plasma-membrane subfractions. 4. Increased recoveries and specific activities of plasma-membrane marker enzymes were obtained when soya-bean trypsin inhibitor was included in the collagenase-containing perfusion media used to dissociate the liver. 5. Polyacrylamide-gel-electrophoretic analysis of the corresponding plasma-membrane subfractions prepared from liver tissue and isolated hepatocytes were generally similar. 6. The results indicate that the functional polarity of the hepatocyte's plasma membrane is retained after tissue dissociation. The damage occurring to plasma-membrane ectoenzymes by the collagenase-perfusion procedure is discussed.
Full text
PDF

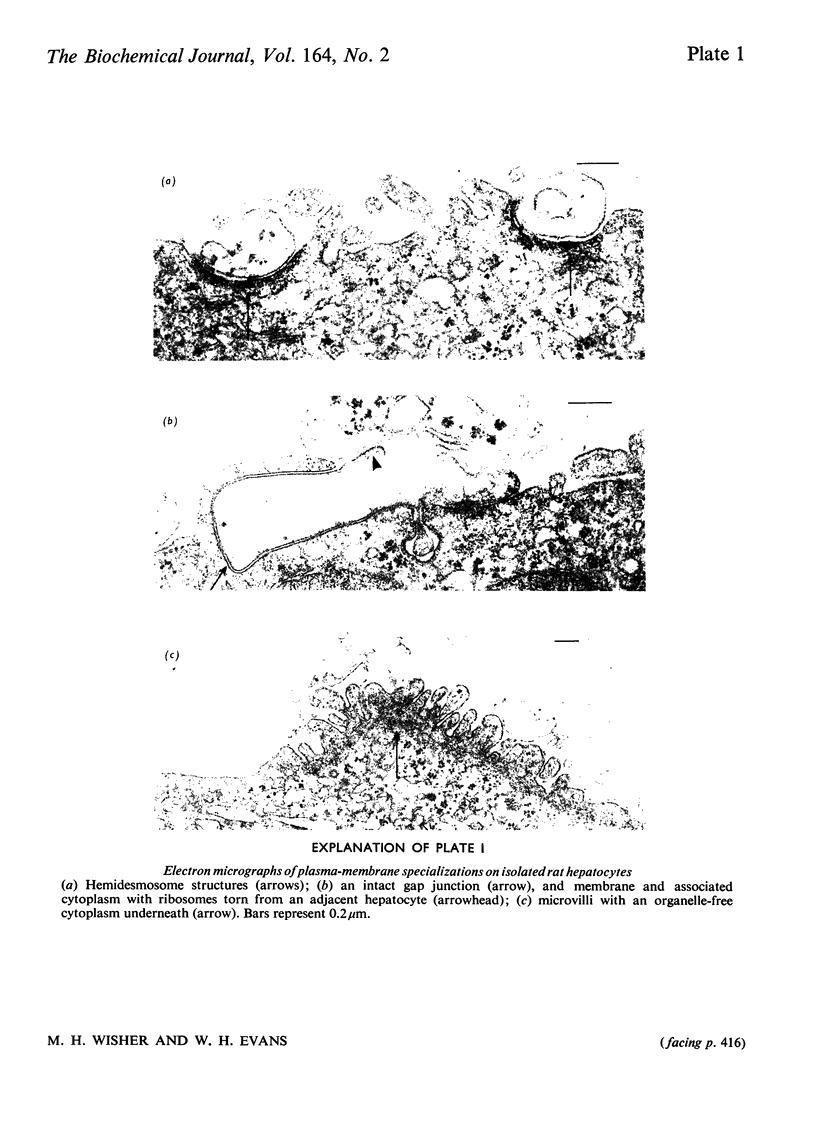
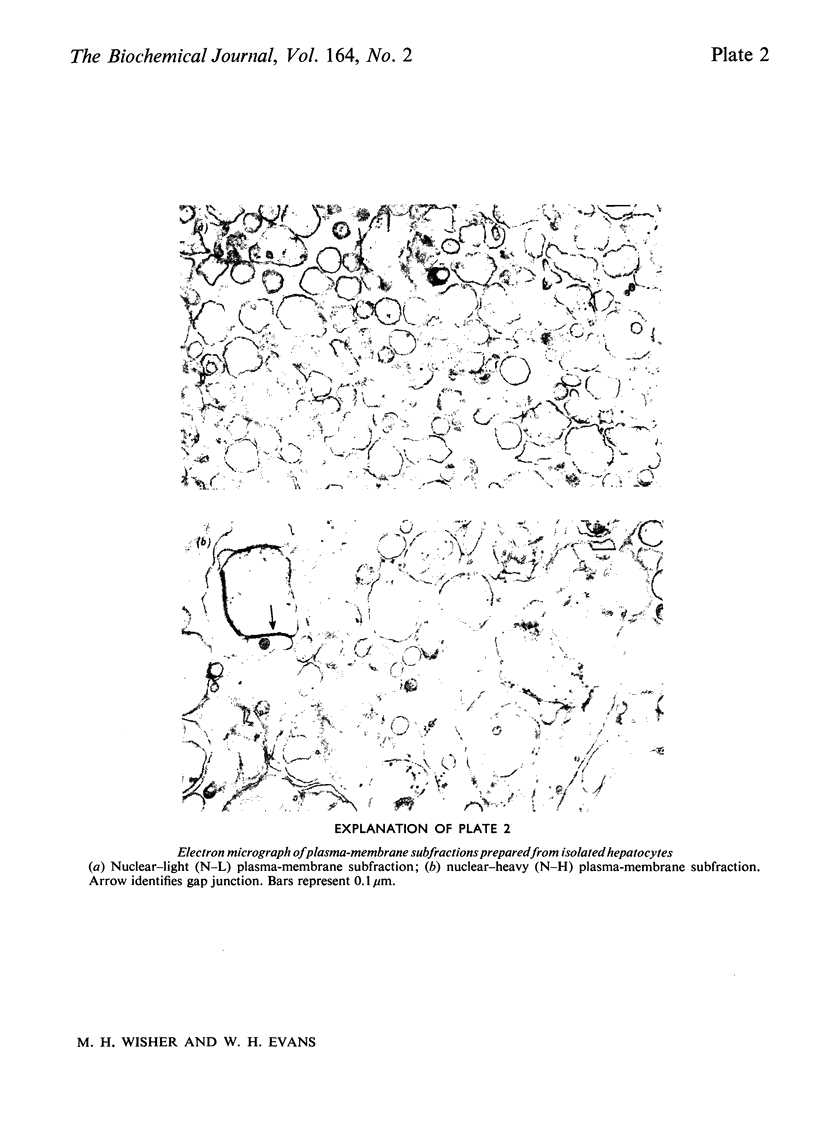
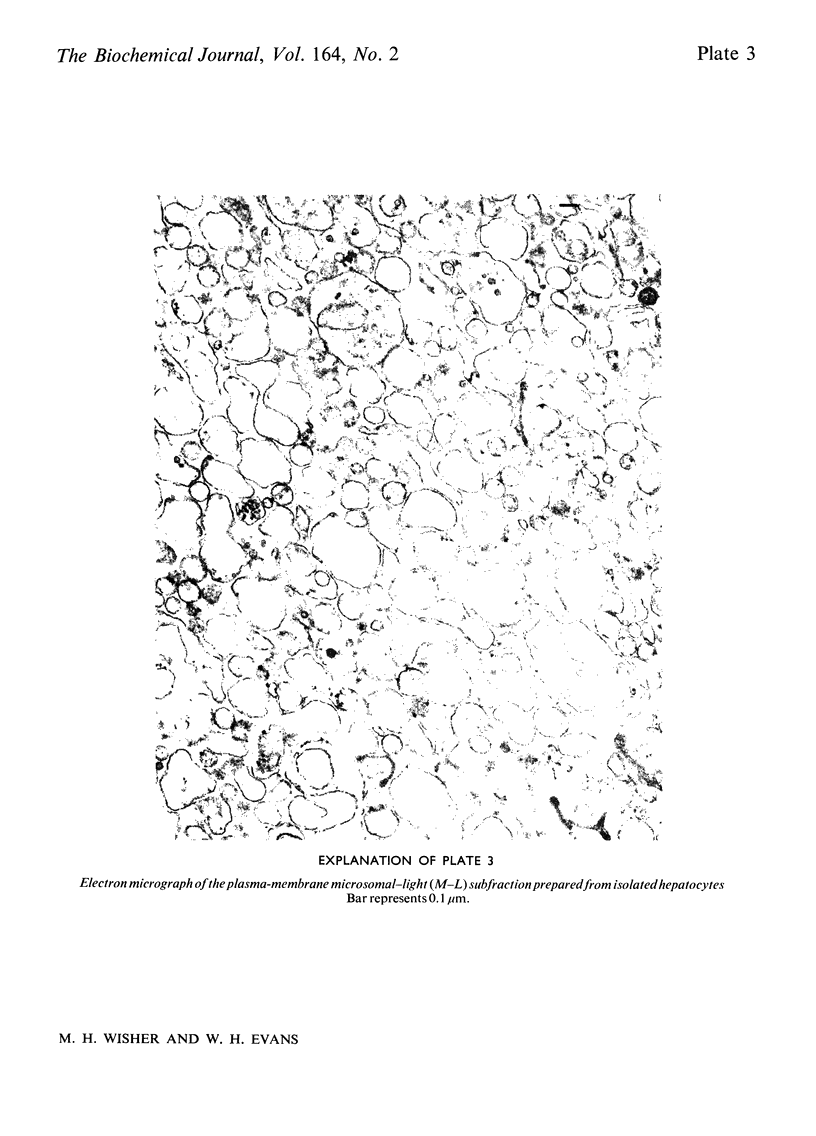

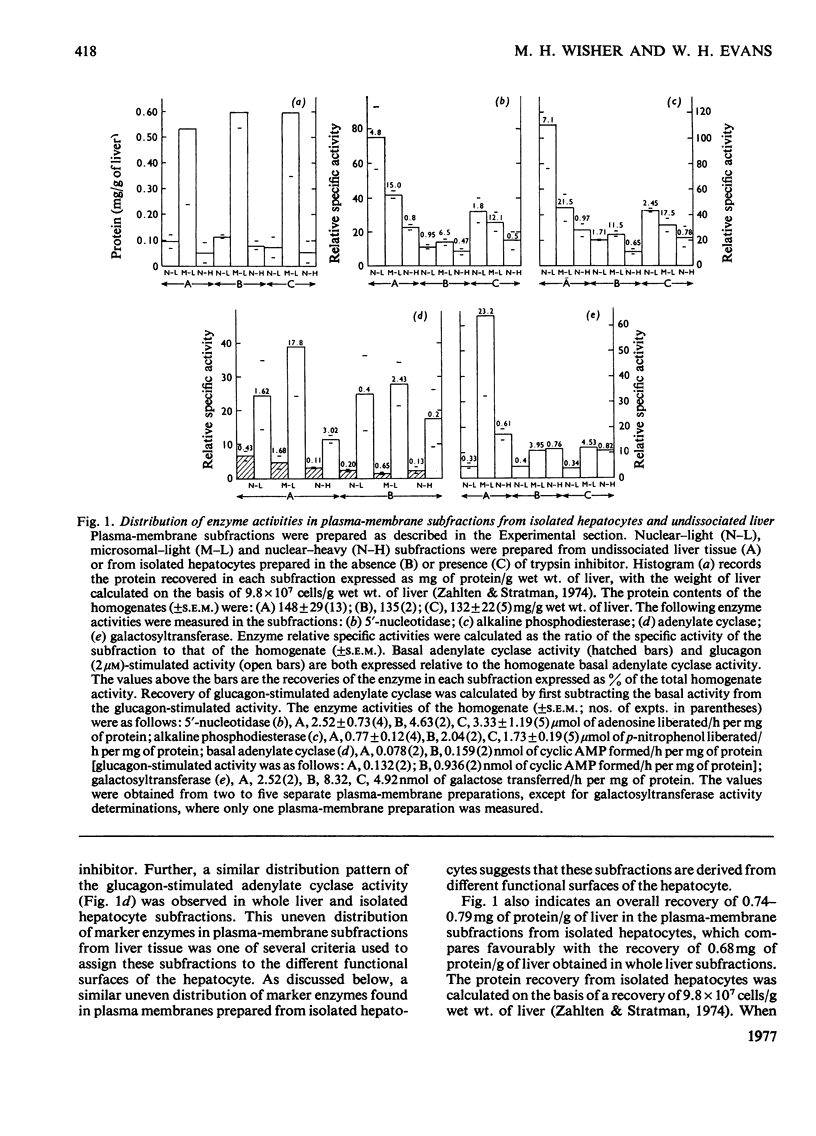
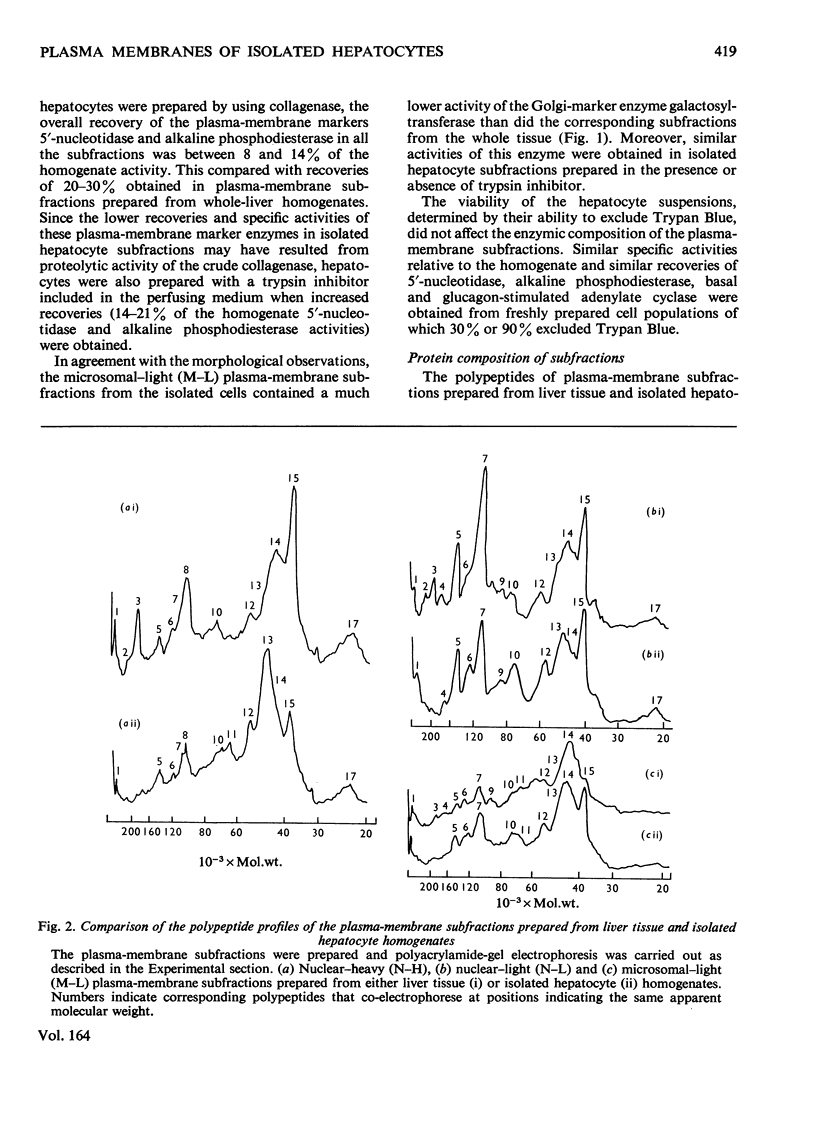



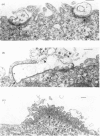
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwer M. S., Kroker R., Hegner D. Bile acids secretion and synthesis by isolated rat hepatocytes. Biochem Biophys Res Commun. 1975 May 19;64(2):603–609. doi: 10.1016/0006-291x(75)90364-2. [DOI] [PubMed] [Google Scholar]
- BIAVA C. G. STUDIES ON CHOLESTASIS. A RE-EVALUATION OF THE FINE STRUCTURE OF NORMAL HUMAN BILE CANALICULI. Lab Invest. 1964 Aug;13:840–864. [PubMed] [Google Scholar]
- Bergeron J. J., Ehrenreich J. H., Siekevitz P., Palade G. E. Golgi fractions prepared from rat liver homogenates. II. Biochemical characterization. J Cell Biol. 1973 Oct;59(1):73–88. doi: 10.1083/jcb.59.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff E., Wilkening J., Tran-Thi T. A., Decker K. Differentiation of the nucleotide pyrophosphatases of rat-liver plasma membranes and endoplasmic reticulum by enzymic iodination. Eur J Biochem. 1976 Feb 16;62(2):279–283. doi: 10.1111/j.1432-1033.1976.tb10158.x. [DOI] [PubMed] [Google Scholar]
- Carey F., Evans W. H. Identification of blood-sinusoidal plasma-membrane fractions from rat liver homogenates by radioiodinated-ligand binding. Biochem Soc Trans. 1977;5(1):103–104. doi: 10.1042/bst0050103. [DOI] [PubMed] [Google Scholar]
- Crane L. J., Miller D. L. Synthesis and secretion of fibrinogen and albumin by isolated rat hepatocytes. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1269–1277. doi: 10.1016/0006-291x(74)90335-0. [DOI] [PubMed] [Google Scholar]
- Drochmans P., Wanson J. C., Mosselmans R. Isolation and subfractionation on ficoll gradients of adult rat hepatocytes. Size, morphology, and biochemical characteristics of cell fractions. J Cell Biol. 1975 Jul;66(1):1–22. doi: 10.1083/jcb.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EARL D. C., KORNER A. THE ISOLATION AND PROPERTIES OF CARDIAC RIBOSOMES AND POLYSOMES. Biochem J. 1965 Mar;94:721–734. doi: 10.1042/bj0940721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East A. G., Louis L. N., Hoffenberg R. Albumin synthesis by isolated rat liver cells. Exp Cell Res. 1973 Jan;76(1):41–46. doi: 10.1016/0014-4827(73)90416-3. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Gurd J. W. Properties of a 5'-nucleotidase purified from mouse liver plasma membranes. Biochem J. 1973 May;133(1):189–199. doi: 10.1042/bj1330189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H., Hood D. O., Gurd J. W. Purification and properties of a mouse liver plasma-membrane glycoprotein hydrolysing nucleotide pyrophosphate and phosphodiester bonds. Biochem J. 1973 Dec;135(4):819–826. doi: 10.1042/bj1350819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. H. Nucleotide pyrophosphatase, a sialoglycoprotein located on the hepatocyte surface. Nature. 1974 Aug 2;250(465):391–394. doi: 10.1038/250391a0. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969 Oct;43(1):59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIANETTO R., DE DUVE C. Tissue fractionation studies. 4. Comparative study of the binding of acid phosphatase, beta-glucuronidase and cathepsin by rat-liver particles. Biochem J. 1955 Mar;59(3):433–438. doi: 10.1042/bj0590433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurd J. W., Evans W. H. Distribution of liver plasma membrane 5' nucleotidase as indicated by its reaction with anti-plasma membrane serum. Arch Biochem Biophys. 1974 Sep;164(1):305–311. doi: 10.1016/0003-9861(74)90035-6. [DOI] [PubMed] [Google Scholar]
- HAMASHIMA Y., HARTER J. G., COONS A. H. THE LOCALIZATION OF ALBUMIN AND FIBRINOGEN IN HUMAN LIVER CELLS. J Cell Biol. 1964 Feb;20:271–279. doi: 10.1083/jcb.20.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch J. G., Fedorko M. E. Ultrastructure of human leukocytes after simultaneous fixation with glutaraldehyde and osmium tetroxide and "postfixation" in uranyl acetate. J Cell Biol. 1968 Sep;38(3):615–627. doi: 10.1083/jcb.38.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingebretsen W. R., Jr, Wagle S. R. A rapid method for the isolation of large quantities of rat liver parenchymal cells with high anabolic rates. Biochem Biophys Res Commun. 1972 Apr 28;47(2):403–410. doi: 10.1016/0006-291x(72)90728-0. [DOI] [PubMed] [Google Scholar]
- Ipata P. L. A coupled optical enzyme assay for 5'-nucleotidase. Anal Biochem. 1967 Jul;20(1):30–36. doi: 10.1016/0003-2697(67)90261-8. [DOI] [PubMed] [Google Scholar]
- Iype P. T., Bhargava P. M., Tasker A. D. Some aspects of the chemical and cellular composition of adult rat liver. Exp Cell Res. 1965 Nov;40(2):233–251. doi: 10.1016/0014-4827(65)90257-0. [DOI] [PubMed] [Google Scholar]
- Jeejeebhoy K. N., Ho J., Greenberg G. R., Phillips M. J., Bruce-Robertson A., Sodtke U. Albumin, fibrinogen and transferrin synthesis in isolated rat hepatocyte suspensions. A model for the study of plasma protein synthesis. Biochem J. 1975 Jan;146(1):141–155. doi: 10.1042/bj1460141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER L. L., BLY C. G., WATSON M. L., BALE W. F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951 Nov;94(5):431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. J., Oda M., Edwards V. D., Greenberg G. R., Jeejeebhoy K. N. Ultrastructural and functional studies of cultured hepatocytes. Lab Invest. 1974 Nov;31(5):533–542. [PubMed] [Google Scholar]
- Schnaitman C., Erwin V. G., Greenawalt J. W. The submitochondrial localization of monoamine oxidase. An enzymatic marker for the outer membrane of rat liver mitochondria. J Cell Biol. 1967 Mar;32(3):719–735. doi: 10.1083/jcb.32.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solyom A., Lauter C. J., Trams E. G. Plasma membranes from isolated liver cells. Biochim Biophys Acta. 1972 Aug 9;274(2):631–637. doi: 10.1016/0005-2736(72)90211-8. [DOI] [PubMed] [Google Scholar]
- Sweat F. W., Hupka A. Adenyl cyclase in hepatic parenchymal and reticuloendothelial cells. Biochem Biophys Res Commun. 1971 Sep 17;44(6):1436–1442. doi: 10.1016/s0006-291x(71)80246-2. [DOI] [PubMed] [Google Scholar]
- Touster O., Aronson N. N., Jr, Dulaney J. T., Hendrickson H. Isolation of rat liver plasma membranes. Use of nucleotide pyrophosphatase and phosphodiesterase I as marker enzymes. J Cell Biol. 1970 Dec;47(3):604–618. doi: 10.1083/jcb.47.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagle S. R., Hofmann F., Decker K. Studies on urea synthesis, insulin degradation and phagocytosis by isolated rat Kupffer cells. Biochem Biophys Res Commun. 1976 Sep 20;72(2):448–455. doi: 10.1016/s0006-291x(76)80063-0. [DOI] [PubMed] [Google Scholar]
- Weigand K., Otto I. Secretion of serum albumin by enzymatically isolated rat liver cells. FEBS Lett. 1974 Sep 15;46(1):127–129. doi: 10.1016/0014-5793(74)80350-9. [DOI] [PubMed] [Google Scholar]
- Wincek T. J., Hupka A. L., Sweat F. W. Stimulation of adenylate cyclase from isolated hepatocytes and Kupffer cells. J Biol Chem. 1975 Nov 25;250(22):8863–8873. [PubMed] [Google Scholar]
- Wisher M. H., Evans W. H. Functional polarity of the rat hepatocyte surface membrane. Isolation and characterization of plasma-membrane subfractions from the blood-sinusoidal, bile-Canalicular and contiguous surfaces of the hepatocyte. Biochem J. 1975 Feb;146(2):375–388. doi: 10.1042/bj1460375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright J. D., Green C. The role of the plasma membrane in fatty acid uptake by rat liver parenchymal cells. Biochem J. 1971 Aug;123(5):837–844. doi: 10.1042/bj1230837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W. The isolation of hormone-sensitive rat hepatocytes by a modified enzymatic technique. Arch Biochem Biophys. 1974 Aug;163(2):600–608. doi: 10.1016/0003-9861(74)90519-0. [DOI] [PubMed] [Google Scholar]