Abstract
Anaphylaxis can induce life-threatening coagulopathy by releasing various mediators from activated mast cells. These mediators directly affect coagulation and fibrinolytic pathways, increasing the bleeding risk. Diagnosis and management of anaphylaxis-induced coagulopathy remain challenging. We report a unique case of a 44-year-old man with undiagnosed systemic mastocytosis who developed peanut-induced anaphylactic shock, resulting in cardiac arrest. Laboratory tests revealed elevated serum tryptase and severe coagulopathy. Thromboelastography, a point-of-care viscoelastic monitoring (VEM) test identified the presence of heparin-like anticoagulants within minutes. Bone marrow examination subsequently confirmed isolated mastocytosis. This case highlights the potential of VEM for rapid diagnosis and management of coagulopathy in patients with anaphylaxis, potentially aiding in the identification of mast cell degranulation in undifferentiated shock. We suggest that VEM should be considered in the investigation of patients with suspected anaphylaxis-induced coagulopathy.
Keywords: Case report, Anaphylaxis, Heparin, Heparin-like mediator, Coagulopathy, Systemic mastocytosis, Viscoelastic monitoring, Thromboelastography
Introduction
Anaphylaxis, a potentially life-threatening immune response, is characterised by the rapid release of a multitude of mediators from activated mast cells and basophils. These mediators can directly influence the coagulation cascade and fibrinolysis,1 potentially inducing severe coagulopathy. Viscoelastic monitoring (VEM) techniques such as thromboelastography allow real-time, dynamic assessment of clot formation and fibrinolysis.2 The viscoelastic assessment of coagulation is more comprehensive than traditional tests. In anaesthetised patients, VEM has demonstrated increased fibrinolysis during anaphylaxis.3 However, its use in demonstrating the release of heparin-like anticoagulants during anaphylaxis has not previously been reported.
Case presentation
A man in his mid-40 s with a history of peanut allergy but no other known medical conditions developed anaphylaxis following accidental peanut exposure. This rapidly progressed to cardiac arrest within minutes. He received prompt treatment with intramuscular epinephrine auto-injector (0.3 mg), cardiopulmonary resuscitation and supportive therapy (chlorpheniramine 10 mg, hydrocortisone 100 mg and 500 mL colloid).
Upon arrival at the hospital the patient was alert and haemodynamically stable, but was agitated and combative. He required sedation, endotracheal intubation and mechanical ventilation.
Serum tryptase levels, measured approximately 1 hour after anaphylaxis onset (t0; Table 1) were significantly elevated (>200 µg/L), confirming the diagnosis. 5 hours later, a routine coagulation profile revealed unexpectedly profound abnormalities (Table 1). Prothrombin time (PT), activated partial thromboplastin time (aPTT) and thrombin time (TT) were all markedly prolonged (>240 s). Except for mildly elevated alkaline phosphatase activity (433 IU/L), liver enzyme activities, bilirubin and albumin were within their reference ranges.
Table 1.
Results of key investigations including the coagulation profile, haemoglobin, platelets, and tryptase.
| Time after presentation to hospital (hours) | 0 | 5 | 8 | 20 | 54 | 78 | 102 | 144 | 160 | 214 |
|---|---|---|---|---|---|---|---|---|---|---|
| Days | 0 | 0.21 | 0.33 | 0.83 | 2.25 | 3.25 | 4.25 | 6 | 6.67 | 8.92 |
| PT (13–16 s) | >250 | 22.9 | 16.9 | 14.6 | 14.8 | 14.1 | 14.2 | 14.5 | 14.4 | |
| aPTT (26–36 s) | >240 | >240 | 51.2 | 34.8 | 38.5 | 38.0 | 35.3 | 30.9 | 38.2 | |
| TT (16–1 9 s) | >240 | >240 | 30.4 | |||||||
| RT (16–22 s) | 55.5 | 43.5 | 24.8 | |||||||
| Fibrinogen (1.5–4.0 g/L) | 1.0 | 1.1 | 2.2 | |||||||
| D-dimer (<500 µg/L) | 590 | |||||||||
| Anti-Xa (U/mL) | 0.70 | 0.20 | 0.1 | |||||||
| Factor IX (50–200 IU/dL) | 73 | |||||||||
| Factor VIII (50–200 IU/dL) | 216 | |||||||||
| Platelets (150–450 × 109/L) | 464 | 263 | 309 | 313 | 509 | |||||
| Haemoglobin (13–17 g/dL) | 12.4 | 13 | 9.8 | 8.3 | 7.6 | 7.6 | ||||
| Creatinine (60–90 µmol/L) | 215 | 231 | 214 | 215 | 235 | 237 | 174 | 185 | 170 | 161 |
| Troponin (0–0.04 µg/L) | 0.1 | |||||||||
| Creatine kinase (24–195 IU/L) | 1,089 | 1,440 | 1,518 | |||||||
| C reactive protein (0–8 mg/L) | 0.7 | >160 | >160 | >160 | 150 | 116 | ||||
| Tryptase (0–16 µg/L) | >200 | 38 | 76 |
PT, prothrombin time; aPTT, activated partial thromboplastin time; TT, thrombin time; RT, reptilase time.
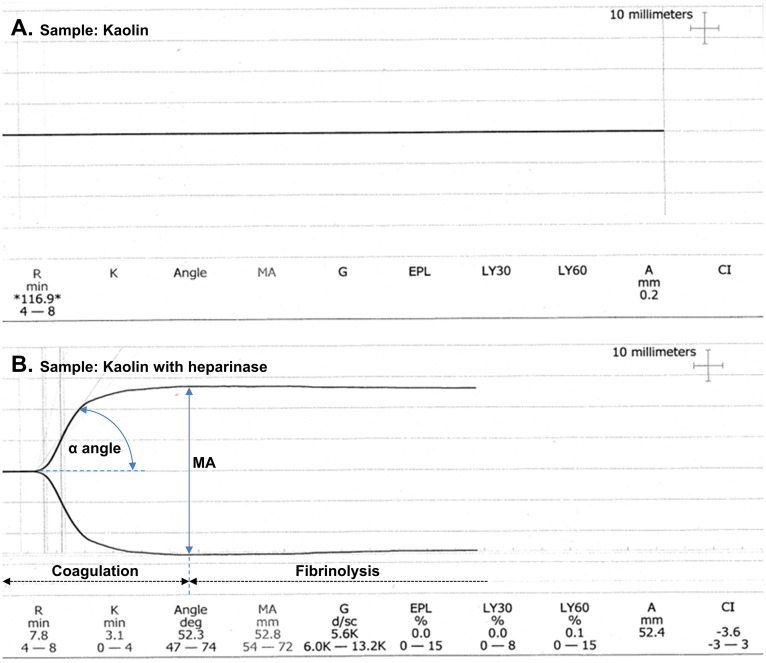
Repeat coagulation testing on a fresh sample (Table 1) showed some improvement in PT, but the aPTT remained significantly prolonged. To further investigate the coagulopathy, thromboelastography was performed (Fig. 1). The unheparinased kaolin sample (Fig. 1A) did not clot, suggesting severe coagulopathy. Notably, although the patient had not received any exogenous heparin, the heparinased kaolin sample (Fig. 1B) demonstrated near-normal clot formation. This finding strongly suggested the presence of a heparin-like anticoagulant in the patient's blood. The coagulopathy normalised spontaneously within 30 h of presentation.
Fig. 1.
Thromboelastography findings in a patient with mastocytosis and peanut-induced anaphylaxis. The reaction time (R time) or clotting time indicates when the thromboelastograph's amplitude is 2 mm. The clot kinetics time (K time) is measured at a predefined clot strength (thromboelastograph amplitude of 20 mm). The α angle represents the speed of fibrin formation and cross-linkage. It is measured from the horizontal axis to a line tangential to the points at which the R and K times are measured. The maximal amplitude (MA) represents the maximum attainable clot strength. The G-value is a measure of clot strength and the coagulation index (CI) reflects the linear relationship between the R time, K time, α angle, and MA. Fibrinolysis (lysis; LY) is measured at 30 min (LY30) and 60 min (LY60) after the MA and is presented as the percentage return of the MA to baseline. The estimated percent lysis (EPL) is the percentage change of MA over time. Panel A: Thromboelastography performed on a plain kaolin sample demonstrates markedly prolonged R time (>116 min), indicating the absence of clot formation within the observation timeframe. Panel B: Thromboelastography performed on a kaolin sample treated with heparinase shows significant improvement in clot formation compared to panel A. The R time, K time and α angle are within their respective reference ranges. The MA, G-value and CI are slightly low, suggesting potential contributions from other anticoagulants besides the heparin-like substance. Fibrinolysis parameters (EPL, LY30 and LY60) are within the reference ranges indicating minimal fibrinolytic activity. Interpretation: the substantial improvement in clot formation with heparinase treatment strongly suggests the presence of heparin-like anticoagulants released during mast cell degranulation in the context of anaphylaxis. However, the possibility of additional contributing factors cannot be excluded as heparinase treatment did not completely normalise the thromboelastography trace.
During hospitalisation, the patient's haemoglobin gradually decreased, reaching 7.6 g/dL by day 5. Computed tomography revealed cerebrovascular ischaemia, a renal capsular haematoma and two renal masses (diagnosed radiologically as renal cell carcinoma). As the coagulopathy had resolved spontaneously, only two units of packed red blood cells were transfused. The patient remained haemodynamically stable with no evidence of further bleeding.
Bone marrow aspirate and trephine demonstrated normocellular marrow with a significant infiltration of 5–10% mast cells enveloping blood vessels. These mast cells displayed characteristic purple granules on Giemsa staining and positive immunostaining for C-kit and mast cell tryptase, confirming the diagnosis of isolated mastocytosis.
The patient made a full recovery of neurological function and remained stable with no evidence of further bleeding, thrombosis or allergic response. 12 days after admission he was discharged home with advice to avoid known allergens, precipitants of mast cell activation, an Epipen and outpatient follow-up with urology, nephrology, immunology and haematology.
Discussion
This case describes severe coagulopathy in the setting of anaphylactic shock triggered by peanut exposure in a sensitised patient with previously undiagnosed mastocytosis. The clinical course was complicated by significant haemorrhage, cerebrovascular ischaemia and type 2 myocardial infarction.
The patient did not receive exogenous heparin. The normalisation of the thromboelastogram with heparinase (Fig. 1) strongly suggests the presence of an endogenous heparin-like anticoagulant, likely released during mast cell degranulation in the setting of mastocytosis. This is further supported by the spontaneous resolution of the coagulopathy within 30 h.
Although the platelet count and peripheral blood film were normal, the low fibrinogen level suggests additional mechanisms. Mast cell degranulation can release tissue plasminogen activator, contributing to hypofibrinogenaemia.1 Tryptase can also directly activate the single-chain urinary-type plasminogen activator, further degrading fibrinogen and other coagulation factors.4,5 This hyperfibrinolysis likely contributed to the prolonged clotting times observed on thromboelastography and the low fibrinogen level.
Both heparin-like substances and tryptase are present in mast cells.1 The severe coagulopathy was likely due to the release of a heparin-like anticoagulant, tryptase and other modulators of the coagulation cascade upon immunoglobulin E-mediated anaphylaxis triggered by peanut exposure in the context of underlying mastocytosis.
This case highlights the potential for anaphylaxis to induce both thrombophilia and bleeding disorders, a phenomenon rarely described in the literature.3,4,6,7 The use of plasma heparin levels (ie anti-factor Xa activity) to screen patients for mast cell activation syndrome (MCAS) has been explored.8 Vysniauskaite et al8 found elevated anti-factor Xa activity in 41% of patients with MCAS, suggesting a correlation with mast cell activity. They therefore suggested that the measurement of anti-factor Xa activity could be used to identify the presence of pathologically irritable mast cells.8 However, such assays are expensive, time consuming and require laboratory analysis.
The use of VEM to screen for the release of endogenous heparin-like anticoagulants has not previously been reported. This case demonstrates the potential of thromboelastography for rapid near-patient identification of endogenous heparin-like anticoagulant release (within 10 min) and increased fibrinolysis (within 30 min).
Traditional coagulation tests, such as PT and aPTT, offer static snapshots of coagulopathy at specific time points. This limits their ability to detect subtle abnormalities or dynamic changes in the coagulation cascade. In contrast, VEM provides a more comprehensive assessment. By measuring clot formation time, clot strength and fibrinolysis rate, VEM provides a dynamic picture of the coagulation process, allowing more timely and accurate diagnosis and management.
Healthcare providers should be aware of the potential for anaphylaxis to cause coagulopathy independent of dilution or disseminated intravascular coagulation. We, therefore, recommend routine coagulation assessment in all patients diagnosed with or suspected of having anaphylaxis. Given the increasing availability of VEM, its use as a rapid bedside test should be considered when investigating patients with undifferentiated shock. Beyond its established role in managing major haemorrhage, VEM could reveal endogenous heparin release and/or severe fibrinolysis suggestive of mast cell degranulation. Further studies are needed to determine the prevalence of coagulopathy in patients with allergic reactions and anaphylaxis. Such data could define the sensitivity and specificity of VEM as a screening tool for mast cell degranulation in undifferentiated shock.
Conclusions
Thromboelastography, a cost-effective, convenient and increasingly available point-of-care test, should be considered for rapid identification of coagulopathy within minutes in patients suspected of severe allergies or anaphylaxis.
Patient consent
The patient provided verbal informed consent for the publication of this manuscript. As the patient was a person with low literacy skills, the content of the case report and the consent form were read to the patient, and their understanding and agreement were confirmed. A statement acknowledging the provision of this verbal consent was signed by the consultant physician responsible for the patient's care.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Rajkumar Rajendram: Conceived and designed the study, Collected and analyzed patient data, Wrote the first draft of the manuscript, Developed figures and tables, Contributed to manuscript revision, Provided overall intellectual leadership and supervision. Abdul Hadi Al-Qahtani: Provided clinical expertise and guidance, Reviewed and edited the manuscript, figures and tables, Provided critical review and feedback. Farrukh Sheikh: Provided clinical expertise and guidance, Reviewed and edited the manuscript, figures and tables, Provided critical review and feedback. All authors: Read and approved the final manuscript, Agree to be accountable for the content of the work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Crivellato E., Beltrami C.A., Mallardi F., Ribatti D. The mast cell; an active participant or an innocent bystander. Histol Histopathol. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- 2.Tyler P.D., Yang L.M., Snider S.B., Lerner A.B., Aird W.C., Shapiro N.I. New uses for thromboelastography and other forms of viscoelastic monitoring in the emergency department: a narrative review. Ann Emerg Med. 2021;77:357–366. doi: 10.1016/j.annemergmed.2020.07.026. [DOI] [PubMed] [Google Scholar]
- 3.De Souza R.L., Short T., Warman G.R., Maclennan N., Young Y. Anaphylaxis with associated fibrinolysis, reversed with tranexamic acid and demonstrated by thrombelastography. Anaesth Intens Care. 2004;32:580–587. doi: 10.1177/0310057X0403200419. [DOI] [PubMed] [Google Scholar]
- 4.Lombardini C., Helia R.E., Boehlen F., Merlani P. “Heparinization” and hyperfibrinogenolysis by wasp sting. Am J Emerg Med. 2009;27:1176.e1–1176.e11763. doi: 10.1016/j.ajem.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Thomas V.A., Wheeless C.J., Stack M.S., Johnson D.A. Human mast cell tryptase fibrinogenolysis: kinetics, anticoagulation mechanism, and cell adhesion disruption. Biochemistry. 1998;37:2291–2298. doi: 10.1021/bi972119z. [DOI] [PubMed] [Google Scholar]
- 6.Mazzi G., Raineri A., Lacava E., De Roia D., Santarossa L., Orazi B.M. Primary hyperfibrinogenolysis in a patient with anaphylactic shock. Haematologica. 1994;79:283–285. [PubMed] [Google Scholar]
- 7.Wang J.L., Shen E.Y., Ho M.Y. Isolated prolongation of activated partial thromboplastin time following wasp sting. Acta Paediatr Taiwan. 2005;46:164–165. [PubMed] [Google Scholar]
- 8.Vysniauskaite M., Hertfelder H.J., Oldenburg J., et al. Determination of plasma heparin level improves identification of systemic mast cell activation disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124912. [DOI] [PMC free article] [PubMed] [Google Scholar]