Summary
Background
The advent of disease-modifying treatments (DMT) has changed natural history in 5q Spinal muscular atrophy (SMA). The aim of this study was to report survival and functional aspects in all the Italian type I children born since 2016.
Methods
The study included all symptomatic children with type I SMA born since January 1st, 2016, when DMTs became available in Italy. All the Italian SMA referral centers provided data on survival and motor, respiratory, and nutritional status. To compare survival rate pre and post DMTs approval, we also included similar data from SMA I patients born between January 1st, 2010, and December 31st, 2015. A two-proportion z-test was conducted to compare the two cohorts. The significance level was set at p < .05.
Findings
241 infants (98%) had type I SMA. Mean follow-up was 3.48 years (SD 2.33). Among type I patients, 42/241 did not survive (25 untreated), while 199 were alive at last follow-up (all treated; mean treatment age 0.6 years), with 25 needing >16 h/day ventilation or tracheostomy with continuous invasive ventilation. 130 of the 199 survivors (65%) achieved independent sitting, and 175 (87.9%) did not require tube feeding.
Interpretation
Our study provides a picture of the ‘new natural history’ of type I SMA, confirming the impact of the new therapies on the progression of type I with longer survival r and has better motor, respiratory and nutritional.
Funding
This research was partially funded by grants from the Italian Ministry of Health.
Keywords: Spinal muscular atrophy, Therapy, Natural history, Survival
Research in context.
Evidence before this study
Spinal muscular atrophy (SMA) is a progressive neurodegenerative disorder caused by mutations in the SMN1 gene on chromosome 5q. Natural history studies conducted before the availability of new treatments reported a very poor prognosis for type I SMA, with a survival rate of less than 8% at two years of age. The development of disease-modifying therapies has dramatically changed the disease course, leading to significant improvements in survival and motor, respiratory and bulbar function. We performed a MEDLINE search looking for all papers reporting clinical findings in type I infants treated with the available disease-modifying therapies since 2016, the year when the first studies on the therapies were published. While there were more than 200 studies providing important information on functional outcomes in clinical trials and in real-world settings, none of the previous studies report survival or long term follow up (>4 years) in large cohorts.
Added value of this study
This study provides a comprehensive analysis of survival and various functional outcomes for all type I SMA children born since 2016 within a network encompassing all Italian reference centers for SMA. Unlike previous studies, our study provides a broad overview of the impact of new therapies, examining not only survival rates but also the need for nutritional and ventilatory support. One of the key strengths of this cohort study is the absence of selection bias; as it includes data from all centers in Italy eligible to treat SMA. To our knowledge, this is the first and only systematic, long-term, national-level data collection that includes all type I SMA infants diagnosed since 2016, when the first therapies became available, without any selection criteria.
Implications of all the available evidence
These findings strongly support the transformative impact of new therapies on the survival of type I SMA infants, highlighting a new generation that is not only surviving longer but also experiencing significantly better motor, respiratory, and nutritional outcomes. Our findings also emphasize the critical importance of early diagnosis and treatment initiation, which are linked to improved survival and functional outcomes but also demonstrate that in some cases therapies may offer benefits even at more advanced stages of the disease. This could have important implications for clinical guidelines and policies on SMA screening and treatment access, reinforcing the need for early intervention while also supporting continued care for those diagnosed later.
Introduction
Spinal muscular atrophy (SMA) is a progressive neurodegenerative disorder caused by mutations in the survival motor neuron 1 (SMN1) gene.1 Historically 5q SMA was classified into three main types with pediatric onset. The most severe form, type I, was associated with early infantile onset (before six months) and inability to achieve the ability to sit unsupported. Within the SMA type I, at least 3 clinical subgroups can be distinguished based on the severity and time of onset of clinical signs: 1.1 or IA severe weakness from the moment of birth; 1.5 or IB onset of weakness after the neonatal period, but generally within 2 months; 1.9 or IC onset of weakness after the neonatal period with acquisition of head control.2
Recent natural history studies performed in the years before the advent of the new therapies reported that the survival at two years was less than 8%3,4 with a reported prevalence of 0.04–0.28/100000.5 These and other studies also report that for subjects surviving beyond the age of one year there is a nearly invariable need for permanent nutritional and respiratory support.4
The advent of disease-modifying treatments (DMTs) has radically changed the course of the type I SMA.5, 6, 7, 8, 9 Pivotal clinical trials have shown very high survival values at two-year in type I infants with two copies of SMN2 treated in the first six months of age.5,6,8 This result was associated with an improvement in motor performance: some newborns acquired the independent sitting position that had never been achieved in untreated newborns. These findings have been confirmed by real world data with cohort studies also reporting increased survival at the age of two years not only in the subjects receiving early treatment but also in infants with more advanced disease treated after the age of six months.6, 7, 8, 9, 10, 11, 12, 13 There is an increasing number of studies reporting longer follow up but so far there has been limited attempt to report long term information as part of a nationwide approach14, 15, 16 and to establish the new natural history of type I SMA taking into consideration the impact of the new therapies since they became standards of care.
The aim of this observational retrospective nationwide study was to report survival and a number of other functional aspects, including motor, respiratory and nutritional aspects, in all the symptomatic type I children born since January 1st, 2016 as part of the activities of a network including all the Italian reference centers for SMA.
Methods
Study design and participants
The study includes all the centres identified by the Italian government as referral centers for SMA throughout the whole nation. All centers have also been involved in a nation-based registry, International Spinal Muscular Atrophy Registry (iSMAR)17 originally including only 5 Italian academic centers in collaboration with UK and US networks, and subsequently including all the other Italian centers. This registry has a rigorous collection of data with structured electronic collection forms (eCRF) and regular training sessions of the evaluators to ensure quality control. This registry is a spin-off of a longstanding collaboration among the Italian SMA centers aimed at collecting natural history data in Italy since 2004. As part of this collaboration functional data and major milestones were regularly collected. ISMAr provided the opportunity to transfer data collected by the centers before the registry started into a more structured platform. As part of the activities of this collaboration, data on type I infants born before January 1st, 2016 were also available for comparison.
We identified all symptomatic Type I SMA subjects born since January 1st, 2016, the year the first therapy became available in Italy. All included infants had genetic confirmation of a SMN1 gene mutation and had severe hypotonia a and weakness and absence of reflexes at diagnosis with onset before the age of 6 months. Type 0 infants—those showing antenatal signs of the disease, such as reduced fetal movement observed between 30 and 36 weeks of gestation and born with contractures and severe clinical signs—were classified separately. We did not include asymptomatic patients identified through newborn screening that has recently become available in a restricted number of regions in Italy.
Ethics
Approval was granted by the Ethics Committee of Fondazione Policlinico Universitario Agostino Gemelli IRCCS (coordinating center) (Coordinating center approval number: 0030504/18) and by each ethic committee from all the other participating centers. Written informed consent was obtained in all participants/caregivers. This study was performed in line with the principles of the Declaration of Helsinki.
Procedures
Each centre was asked to provide details on whether the children were still surviving (or the age at death) or if they had more than 16 h/day of non-invasive ventilation (NIV) or tracheostomy, following the criteria used in clinical trials and real world data in treated patients and in a recent population based analysis study in untreated patients.18 In all patients tracheostomy was associated with continuous invasive ventilation and was always inserted according to care recommendations after failure of non-invasive ventilation or failed attempts to extubate. The centers were also asked to report subjects who had died and had not been inserted in the registry.
Details on the achievement of major milestone such as sitting or walking or on the need for respiratory or nutritional support (gastrostomy tube (GTube) or nasogastric tube (NGT)) were also collected. In all patients tube feeding was always inserted according to care recommendations, i.e., after clinical or videfluoroscopy evidence of dysphagia or failure to thrive.
Information on current pharmacological treatments with DMTs and recent possible changes were also collected.
Statistical analysis
To allow for survival rate comparison pre and post DMTs approval, we also included data on the age at death and age at initiation of NIV >16 h/day or tracheostomy insertion in SMA I patients born between January 1, 2010, and December 31, 2015. A two-proportion z-test was conducted to compare the proportions of infants who passed away and those surviving with NIV >16 h/day or tracheostomy with continuous invasive ventilation between the two cohorts (2010–2015 vs. 2016 onward). The significance level was set at p < .05.
As part of our registry, in order to avoid duplicates, a system allowing to generate unique global identifier numbers is provided to each center and, the results from each center are centrally reviewed to check for possible duplicates.
Participants characteristics were described as proportions (percentages) for categorical variables and means with standard deviation (SD) for continuous variables.
Role of the funding source
This research was partially funded by grants from the Italian Ministry of Health (MCP: GR-2018-12365706, GC: GR-2021-12374579, EM: RF-2019-12370334 and PNRR-MR1-2022-12376937).
Funders had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Results
The cohort of infants born since January 1st, 2016 includes 247 subjects currently or previously followed in the 33 participating centers. Each of these infants was diagnosed after symptomatic presentation to the clinic and none were identified by newborn screening or enrolled in this study in a pre-symptomatic state. Of the 247, 241 (98%) were classified as type 1, six (2%) as type 0.
SMN2 copies were available in 242 of the 247 (98%). Out of the 242 individuals, 7 (2.89%) had one SMN2 copy, 212 (87.60%) had two, 22 (9.09%) had three, and 1 (0.41%) had four or more SMN2 copies. The mean length of follow up was 3.48 (SD 2.33), ranging between 0.10 and 8.26 years.
Type 0
Of the six infants with SMA 0 born after 2016, only one—treated with nusinersen at 30 days of life—was still alive at the last follow-up. This infant required over 16 h of daily non-invasive ventilation (NIV) and had a gastrostomy tube (GTube) placed, but did not reach any motor milestones, such as head control, independent sitting, standing, or walking.
The other five infants did not survive. Three had a tracheostomy with continuous invasive ventilation, while two were on NIV for 16 h a day. Only one of these infants received nusinersen at 15 days of life, while the families of the other four chose not to pursue DMTs.
Type I
Fig. 1 reports details of survival status in the whole cohort and in the SMA type I infants. Fig. 2 reports details of survival status in the type I infants subdivided by year of birth and treatment status.
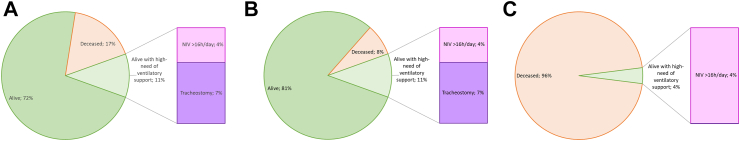
Fig. 1.
Percentage of survival status in SMA I. Key to figure: Panel A = whole cohort of SMA I, Panel B = whole cohort of treated SMA I, Panel C = whole cohort of untreated SMA I. NIV = non invasive ventilation, Tracheostomy = tracheostomy with continuous invasive ventilation.
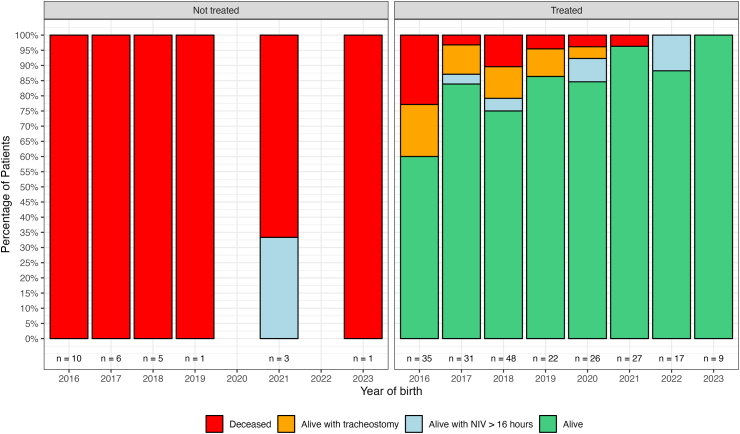
Fig. 2.
SMA I percentage of survival status subdivided by year of birth and treatment status. Key to figure: NIV = non invasive ventilation, Tracheostomy = tracheostomy with continuous invasive ventilation.
Out of the 241 type I infants born after 2016, 199 were still alive at the last follow-up, with a mean age of 4.04 years (SD = 2.13), ranging from 0.12 to 8.26 years. Of the 42 who did not survive 17 received DMTs, while 25 did not undergo any DMT treatment. In all patients who did not survive, the cause of death was related to respiratory failure. Table 1 shows the details of the number of SMA I infants born for each year subdivided by age at treatment (if any).
Table 1.
Details of the number of SMA I infants born for each year subdivided by age at treatment (if any).
| Year of birth | Age at treatment | Age at diagnosisa | Infants who died and age at deathb | Infants survived and age at last follow upb | Duration of follow upc | Tracheod | NIV ≥ 16 h/dayd | Gtubed | Nusinersend | Risdiplamd | Onasemnogene abeparvovecd | Switchd | Addd |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2016 | <6 months (N = 9) | 0.4 (0.3) 0.3 (0.2, 1.1) 0 (0%) |
3 (33.3%) 1.4 (0.6) 1.2 (1.0, 2.0) |
6 (66.7%) 7.2 (0.2) 7.2 (7.0, 7.4) |
5.1 (3.2) 7.1 (0.4, 7.4) |
0 (0%) | 1 (11.1%) | 4 (44.4%) | 7 (77.8%) | 0 (0%) | 0 (0%) | 2 (22.2%) | 0 (0%) |
| 6–12 months (N = 21) | 0.5 (0.2) 0.4 (0.2, 0.8) 2 (9.5%) |
3 (14.3%) 1.0 (0.3) 1.1 (0.7, 1.2) |
18 (85.7%) 6.6 (1.7) 7.1 (2.2, 8.3) |
5.8 (2.6) 7.1 (0.6, 8.3) |
4 (19.0%) | 0 (0%) | 9 (42.9%) | 13 (61.9%) | 4 (19.0%) | 0 (0%) | 4 (19.0%) | 0 (0%) | |
| >12 months (N = 5) | 1.0 (1.1) 0.5 (0.3, 3.0) 0 (0%) |
2 (40.0%) 7.0 (0.1) 7.0 (6.9, 7.1) |
3 (60.0%) 6.4 (1.6) 7.1 (4.6, 7.5) |
6.2 (1.3) 6.5 (4.6, 7.5) |
3 (60.0%) | 1 (20.0%) | 3 (60.0%) | 2 (40.0%) | 0 (0%) | 0 (0%) | 3 (60.0%) | 0 (0%) | |
| Not treated (N = 10) | 0.1 (0.1) 0.1 (0.0, 0.4) 0 (0%) |
10 (100%) 1.1 (1.4) 0.5 (0.2, 5.0) |
0 (0%) | 1.0 (1.4) 0.6 (0.2, 5.0) |
1 (10.0%) | 0 (0%) | 1 (10.0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2017 | <6 months (N = 9) | 0.3 (0.1) 0.2 (0.2, 0.4) 0 (0%) |
0 (0%) | 9 (100%) 6.2 (0.8) 6.5 (4.4, 7.1) |
6.2 (0.8) 6.5 (4.4, 7.1) |
0 (0%) | 1 (11.1%) | 4 (44.4%) | 2 (22.2%) | 6 (66.7%) | 0 (0%) | 1 (11.1%) | 0 (0%) |
| 6–12 months (N = 15) | 0.5 (0.2) 0.5 (0.2, 1.1) 0 (0%) |
0 (0%) | 15 (100%) 5.4 (1.6) 6.1 (2.2, 7.1) |
5.4 (1.6) 6.1 (2.2, 7.1) |
3 (20.0%) | 0 (0%) | 4 (26.7%) | 3 (20.0%) | 3 (20.0%) | 0 (0%) | 9 (60.0%) | 0 (0%) | |
| >12 months (N = 7) | 0.6 (0.3) 0.7 (0.2, 1.0) 0 (0%) |
1 (14.3%) 3.1 (NA) 3.1 (3.1, 3.1) |
6 (85.7%) 4.1 (2.1) 4.4 (1.3, 6.4) |
4.0 (2.0) 3.2 (1.3, 6.4) |
0 (0%) | 1 (14.3%) | 4 (57.1%) | 3 (42.9%) | 2 (28.6%) | 0 (0%) | 1 (14.3%) | 1 (14.3%) | |
| Not treated (N = 6) | 0.4 (0.5) 0.3 (0.0, 1.3) 0 (0%) |
6 (100%) 0.7 (0.4) 0.7 (0.2, 1.2) |
0 (0%) | 0.7 (0.4) 0.7 (0.2, 1.2) |
1 (16.7%) | 0 (0%) | 1 (16.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2018 | <6 months (N = 36) | 0.2 (0.1) 0.2 (0.0, 0.4) 1 (2.8%) |
3 (8.3%) 2.4 (3.0) 0.9 (0.5, 5.9) |
33 (91.7%) 5.1 (1.1) 5.4 (1.2, 6.4) |
4.9 (1.5) 5.4 (0.5, 6.4) |
4 (11.1%) | 2 (5.6%) | 14 (38.9%) | 7 (19.4%) | 2 (5.6%) | 10 (27.8%) | 9 (25.0%) | 8 (22.2%) |
| 6–12 months (N = 9) | 0.6 (0.2) 0.6 (0.2, 0.9) 0 (0%) |
2 (22.2%) 1.0 (0.3) 1.0 (0.8, 1.2) |
7 (77.8%) 4.8 (1.7) 5.4 (1.1, 5.7) |
3.9 (2.3) 5.2 (0.8, 5.7) |
1 (11.1%) | 0 (0%) | 2 (22.2%) | 5 (55.6%) | 1 (11.1%) | 0 (0%) | 3 (33.3%) | 0 (0%) | |
| >12 months (N = 3) | 0.4 (0.4) 0.4 (0.2, 0.7) 1 (33.3%) |
0 (0%) | 3 (100%) 5.1 (0.1) 5.1 (5.0, 5.2) |
5.1 (0.1) 5.1 (5.0, 5.2) |
0 (0%) | 0 (0%) | 2 (66.7%) | 1 (33.3%) | 0 (0%) | 1 (33.3%) | 1 (33.3%) | 0 (0%) | |
| Not treated (N = 5) | 0.2 (0.1) 0.1 (0.1, 0.3) 1 (20.0%) |
5 (100%) 0.5 (0.2) 0.5 (0.2, 0.7) |
0 (0%) | 0.5 (0.2) 0.5 (0.2, 0.7) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2019 | <6 months (N = 17) | 0.2 (0.1) 0.2 (0.0, 0.4) 1 (5.9%) |
0 (0%) | 17 (100%) 4.2 (0.7) 4.3 (2.6, 5.1) |
4.2 (0.7) 4.3 (2.6, 5.1) |
2 (11.8%) | 0 (0%) | 10 (58.8%) | 5 (29.4%) | 0 (0%) | 1 (5.9%) | 11 (64.7%) | 0 (0%) |
| 6–12 months (N = 3) | 0.6 (0.2) 0.5 (0.5, 0.9) 0 (0%) |
1 (33.3%) 3.1 (NA) 3.1 (3.1, 3.1) |
2 (66.7%) 4.8 (0.5) 4.8 (4.4, 5.1) |
4.2 (1.1) 4.4 (3.0, 5.1) |
0 (0%) | 0 (0%) | 2 (66.7%) | 2 (66.7%) | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | |
| >12 months (N = 2) | 0.9 (0.5) 0.9 (0.6, 1.2) 0 (0%) |
0 (0%) | 2 (100%) 4.5 (0.8) 4.5 (3.9, 5.0) |
4.5 (0.8) 4.5 (3.9, 5.0) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (50.0%) | 1 (50.0%) | |
| Not treated (N = 1) | 0.3 (NA) 0.3 (0.3, 0.3) 0 (0%) |
1 (100%) Missing |
0 (0%) | 0.3 (NA) 0.3 (0.3, 0.3) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2020 | <6 months (N = 18) | 0.2 (0.1) 0.2 (0.0, 0.4) 1 (5.6%) |
1 (5.6%) 0.8 (NA) 0.8 (0.8, 0.8) |
17 (94.4%) 2.9 (0.8) 3.3 (1.2, 3.8) |
2.8 (0.9) 3.3 (0.8, 3.8) |
1 (5.6%) | 2 (11.1%) | 6 (33.3%) | 2 (11.1%) | 1 (5.6%) | 1 (5.6%) | 12 (66.7%) | 2 (11.1%) |
| 6–12 months (N = 6) | 0.5 (0.3) 0.5 (0.0, 0.8) 0 (0%) |
0 (0%) | 6 (100%) 2.9 (1.2) 3.0 (0.7, 4.0) |
2.9 (1.2) 3.0 (0.7, 4.0) |
0 (0%) | 1 (16.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) | 4 (66.7%) | 1 (16.7%) | |
| >12 months (N = 2) | 0.4 (0.4) 0.4 (0.1, 0.8) 0 (0%) |
0 (0%) | 2 (100%) 3.2 (0.1) 3.2 (3.2, 3.2) |
3.2 (0.1) 3.2 (3.2, 3.2) |
0 (0%) | 0 (0%) | 1 (50.0%) | 0 (0%) | 1 (50.0%) | 1 (50.0%) | 0 (0%) | 0 (0%) | |
| 2021 | <6 months (N = 20) | 0.2 (0.1) 0.2 (0.0, 0.4) 0 (0%) |
1 (5.0%) 2.4 (NA) 2.4 (2.4, 2.4) |
19 (95.0%) 2.1 (0.5) 2.0 (1.2, 3.1) |
2.1 (0.5) 2.1 (1.2, 3.1) |
0 (0%) | 0 (0%) | 3 (15.0%) | 2 (10.0%) | 0 (0%) | 11 (55.0%) | 4 (20.0%) | 3 (15.0%) |
| 6–12 months (N = 6) | 0.6 (0.1) 0.6 (0.5, 0.8) 0 (0%) |
0 (0%) | 6 (100%) 1.8 (0.8) 1.6 (1.0, 3.0) |
1.8 (0.8) 1.6 (1.0, 3.0) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (66.7%) | 2 (33.3%) | 0 (0%) | |
| >12 months (N = 1) | 0.3 (NA) 0.3 (0.3, 0.3) 0 (0%) |
0 (0%) | 1 (100%) 2.5 (NA) 2.5 (2.5, 2.5) |
2.5 (NA) 2.5 (2.5, 2.5) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) | |
| Not treated (N = 3) | 0.5 (NA) 0.5 (0.5, 0.5) 2 (66.7%) |
2 (66.7%) 0.5 (0.4) 0.5 (0.2, 0.8) |
1 (33.3%) 0.8 (NA) 0.8 (0.8, 0.8) |
0.6 (0.4) 0.8 (0.2, 0.8) |
0 (0%) | 1 (33.3%) | 1 (33.3%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 2022 | <6 months (N = 14) | 0.2 (0.1) 0.2 (0.0, 0.4) 0 (0%) |
0 (0%) | 14 (100%) 1.3 (0.4) 1.4 (0.6, 2.1) |
1.3 (0.4) 1.4 (0.6, 2.1) |
0 (0%) | 2 (14.3%) | 3 (21.4%) | 2 (14.3%) | 0 (0%) | 6 (42.9%) | 2 (14.3%) | 4 (28.6%) |
| 6–12 months (N = 3) | 0.7 (0.2) 0.8 (0.5, 0.8) 0 (0%) |
0 (0%) | 3 (100%) 1.7 (0.5) 1.7 (1.3, 2.2) |
1.7 (0.5) 1.7 (1.3, 2.2) |
0 (0%) | 0 (0%) | 0 (0%) | 1 (33.3%) | 0 (0%) | 2 (66.7%) | 0 (0%) | 0 (0%) | |
| 2023 | <6 months (N = 9) | 0.1 (0.2) 0.1 (−0.2, 0.3) 0 (0%) |
0 (0%) | 9 (100%) 0.7 (0.3) 0.8 (0.1, 1.1) |
0.7 (0.3) 0.8 (0.1, 1.1) |
0 (0%) | 0 (0%) | 2 (22.2%) | 1 (11.1%) | 1 (11.1%) | 4 (44.4%) | 3 (33.3%) | 0 (0%) |
| Not treated (N = 1) | 0.1 (NA) 0.1 (0.1, 0.1) 0 (0%) |
1 (100%) 0.1 (NA) 0.1 (0.1, 0.1) |
0 (0%) | 0.1 (NA) 0.1 (0.1, 0.1) |
0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
| Overall | <6 months (N = 132) | 0.2 (0.1) 0.2 (−0.2, 1.1) 3 (2.3%) |
8 (6.1%) 1.8 (1.8) 1.1 (0.5, 5.9) |
124 (93.9%) 3.7 (2.0) 3.7 (0.1, 7.4) |
3.5 (2.0) 3.4 (0.1, 7.4) |
7 (5.3%) | 8 (6.1%) | 46 (34.8%) | 28 (21.2%) | 10 (7.6%) | 33 (25.0%) | 44 (33.3%) | 17 (12.9%) |
| 6–12 months (N = 63) | 0.5 (0.2) 0.5 (0.0, 1.1) 2 (3.2%) |
6 (9.5%) 1.3 (0.9) 1.1 (0.7, 3.1) |
57 (90.5%) 4.9 (2.3) 5.6 (0.7, 8.3) |
4.5 (2.4) 5.2 (0.6, 8.3) |
8 (12.7%) | 1 (1.6%) | 17 (27.0%) | 24 (38.1%) | 8 (12.7%) | 7 (11.1%) | 23 (36.5%) | 1 (1.6%) | |
| >12 months (N = 20) | 0.7 (0.6) 0.6 (0.1, 3.0) 1 (5.0%) |
3 (15.0%) 5.7 (2.3) 6.9 (3.1, 7.1) |
17 (85.0%) 4.5 (1.8) 5.0 (1.3, 7.5) |
4.6 (1.7) 5.0 (1.3, 7.5) |
3 (15.0%) | 2 (10.0%) | 10 (50.0%) | 6 (30.0%) | 3 (15.0%) | 3 (15.0%) | 6 (30.0%) | 2 (10.0%) | |
| Not treated (N = 26) | 0.2 (0.3) 0.1 (0.0, 1.3) 3 (11.5%) |
25 (96.2%) 0.8 (1.0) 0.5 (0.1, 5.0) |
1 (3.8%) 0.8 (NA) 0.8 (0.8, 0.8) |
0.7 (0.9) 0.5 (0.1, 5.0) |
2 (7.7%) | 1 (3.8%) | 3 (11.5%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
Key to table: All calculations regarding age or follow-up duration are provided in years.
Switch = subjects who went from nusinersen to risdiplam or Onasemnogene abeparvovec or from risdiplam to nusinersen or Onasemnogene abeparvovec; Add = subjects who went from Onasemnogene abeparvovec to risdiplam and/or nusinesen; NIV = non invasive ventilation, GTube = gastrostomy tube, Tracheo = tracheostomy with continuous invasive ventilation.
Mean (SD), Median [Range], Missing (%).
n (%), Mean (SD), Median [Range].
Mean (SD), Median [Range].
n (%)—all data are presented as nusinersen-only, risdiplam-only, onasemnogene-only or switch.
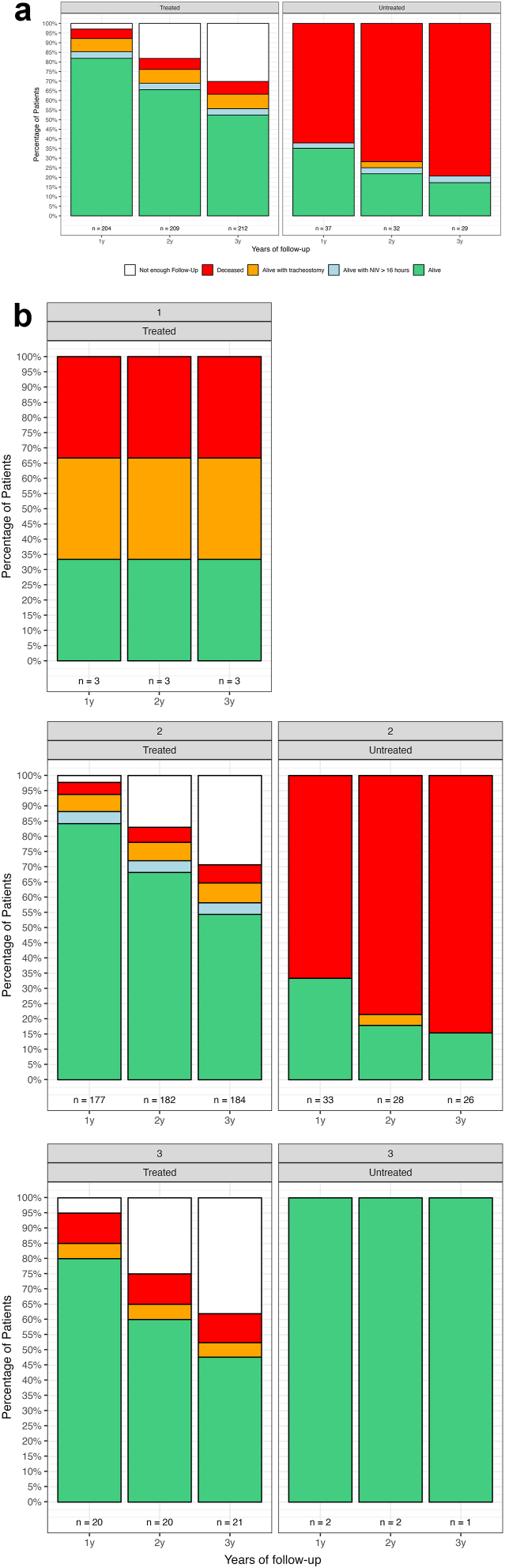
Fig. 3 shows percentage of survival status subdivided by years of follow-up and SMN2 copy number.
Fig. 3.
SMA I percentage of survival status subdivided by years of follow-up. Key to figure: The bars represent the total number of patients at each year of follow-up, regardless of their survival or treatment status. The “n” values include all subjects, and treatment status can change over time, as some patients started treatment later during the follow-up period. Panel A: percentage of survival status subdivided by years of follow-up; Panel B: percentage of survival status subdivided by years of follow-up and SMN2 copy number. The individual with 4+ SMN2 copies did not reach 1 year of follow-up, therefore was excluded from the figure. NIV = non invasive ventilation, Tracheostomy = tracheostomy with continuous invasive ventilation.
Among the 199 surviving type I subjects, 16 have a tracheostomy with continuous invasive ventilation and nine require more than 16 h of daily NIV. No additional patient was ever decannulated or weaned off NIV>16 h/day. Only one of them was untreated (Table 2).
Table 2.
SMA I population characteristics subdivided in alive subjects at last follow-up, alive subjects living with a NIV > 16 h/day or a tracheostomy with continuous invasive ventilation at last follow-up, deceased subjects at last follow-up.
| Alive subjects | ||
|---|---|---|
| N of DMTs treated infants (n = 174) | N of DMTs untreated infants (n = 0) | |
| SMA type | ||
| I | 174 (100%) | 0 (0%) |
| Sex | ||
| Female | 88 (50.6%) | |
| Male | 86 (49.4%) | |
| Age at genetic diagnosis (years) | ||
| Mean (SD) | 0.3 (0.2) | |
| Median [Range] | 0.3 (−0.2, 1.2) | |
| Missing (%) | 3 (1.7%) | |
| Age at last follow-up (years) | ||
| Mean (SD) | 3.9 (2.1) | |
| Median [Range] | 3.9 (0.1, 8.3) | |
| Age at treatment initiation (years) | ||
| Mean (SD) | 0.5 (0.6) | |
| Median [Range] | 0.4 (0.0, 4.5) | |
| SMN2 copy number | ||
| 1 | 1 (0.6%) | |
| 2 | 152 (87.4%) | |
| 3 | 19 (10.9%) | |
| 4+ | 1 (0.6%) | |
| Unknown | 1 (0.6%) | |
| Respiratory support | ||
| Spontaneous breathing | 47 (27.0%) | |
| Non-invasive ventilation > 16 h/day | 127 (73.0%) | |
| Nutritional support | ||
| Oral fed | 124 (71.3%) | |
| NGT | 2 (1.1%) | |
| NGT + oral fed | 1 (0.6%) | |
| G tube | 43 (24.7%) | |
| G tube + oral fed | 4 (2.3%) | |
| Motor milestones | ||
| None | 26 (14.9%) | |
| Head control | 148 (85.1%) | |
| Sitting | 128 (73.6%) | |
| Standing | 21 (12.1%) | |
| Walking | 3 (1.72%) | |
| Alive subjects living with niv > 16 h or tracheostomy with continuous invasive ventilation | ||
|---|---|---|
| N of DMTs treated infants (n = 24) | N of DMTs untreated infants (n = 1) | |
| SMA type | ||
| I | 24 (96.0%) | 1 (100%) |
| Sex | ||
| Female | 9 (37.5%) | 0 (0%) |
| Male | 16 (62.5%) | 1 (100%) |
| Age at genetic diagnosis (years) | ||
| Mean (SD) | 0.4 (0.6) | NA |
| Median [Range] | 0.2 (0.1, 3.0) | NA |
| Missing (%) | 1 (4.2%) | 1 (100%) |
| Age at last follow-up (years) | ||
| Mean (SD) | 5.0 (2.1) | 0.8 (NA) |
| Median [Range] | 5.5 (0.6, 7.7) | 0.8 (0.8, 0.8) |
| Age at treatment initiation (years) | ||
| Mean (SD) | 0.7 (0.9) | – |
| Median [Range] | 0.4 (0.1, 3.7) | |
| SMN2 copy number | ||
| 1 | 1 (4.2%) | 0 (0%) |
| 2 | 20 (83.3%) | 0 (0%) |
| 3 | 1 (4.2%) | 0 (0%) |
| 4+ | 0 (0%) | 0 (0%) |
| Unknown | 2 (8.3%) | 1 (100%) |
| Respiratory support | ||
| Tracheostomy with continuous invasive ventilation | 16 (66.7%) | 0 (0%) |
| Non invasive ventilation > 16 h/day | 8 (33.3%) | 1 (100%) |
| Nutritional support | ||
| Oral fed | 1 (4.2%) | 0 (0%) |
| NGT | 5 (20.8%) | 0 (0%) |
| G tube | 18 (75.0%) | 1 (100%) |
| Motor milestones | ||
| None | 16 (66.7.0%) | 1 (100%) |
| Head control | 8 (33.3%) | 0 (0%) |
| Sitting | 5 (20.8%) | 0 (0%) |
| Deceased subjects | ||
|---|---|---|
| N of DMTs treated infants (n = 17) | N of DMTs untreated infants (n = 25) | |
| SMA type | ||
| I | 17 (94.4%) | 25 (86.2%) |
| Sex | ||
| Female | 7 (41.2%) | 12 (48.0%) |
| Male | 10 (58.8%) | 13 (52.0%) |
| Age at genetic diagnosis (years) | ||
| Mean (SD) | 0.4 (0.3) | 0.2 (0.3) |
| Median [Range] | 0.4 (0.0, 1.1) | 0.1 (0.0, 1.3) |
| Missing (%) | 2 (11.8%) | 2 (8.0%) |
| Age at last follow-up (years) | ||
| Mean (SD) | 2.0 (2.0) | 0.7 (0.9) |
| Median [Range] | 1.0 (0.4, 6.5) | 0.5 (0.1, 5.0) |
| Age at treatment initiation (years) | ||
| Mean (SD) | 0.9 (1.3) | - |
| Median [Range] | 0.5 (0.0, 5.3) | |
| SMN2 copy number | ||
| 1 | 1 (5.9%) | 0 (0%) |
| 2 | 14 (82.4%) | 24 (96.0%) |
| 3 | 2 (11.8%) | 0 (0%) |
| 4+ | 0 (0%) | 0 (0%) |
| Unknown | 0 (0%) | 1 (4.0%) |
| Respiratory support | ||
| Spontaneous breathing | 7 (41.2%) | 23 (92.0%) |
| Non-invasive ventilation > 16 h/day | 9 (52.9%) | 0 (0%) |
| Invasive ventilation | 1 (5.9%) | 2 (8.0%) |
| Nutritional support | ||
| Oral fed | 4 (23.5%) | 2 (8.0%) |
| NGT | 9 (52.9%) | 21 (84.0%) |
| NGT + oral fed | 0 (0%) | 1 (4.0%) |
| G tube | 4 (23.5%) | 1 (4.0%) |
| Motor milestones | ||
| None | 12 (70.6%) | 17 (68.0%) |
| Head control | 5 (29.4%) | 8 (32.0%) |
| Sitting | 2 (11.8%) | 0 (0%) |
| Standing | 0% | 0% |
| Walking | 0% | 0% |
Among the 199 surviving type I subjects, 19 have a GTube and five require NGT. Only one of them was untreated (Table 2).
Among the 199 surviving subjects, 26 (25 treated and one not treated) did not achieve any motor milestones such as head control, sitting independently, standing, or walking. Of the remaining 173 subjects, 135 gained head control, 130 of these 135 gained the ability to sit independently, 21 of these 135 gained the ability to stand, and three of these 135 gained the ability to walk. All subjects who gained the ability to stand or walk did not require a tracheostomy with continuous invasive ventilation or more than 16 h/day of NIV (Table 2). Supplementary Table S1 shows SMA I percentage of motor milestones acquired subdivided by smn2 copy number and treatment status.
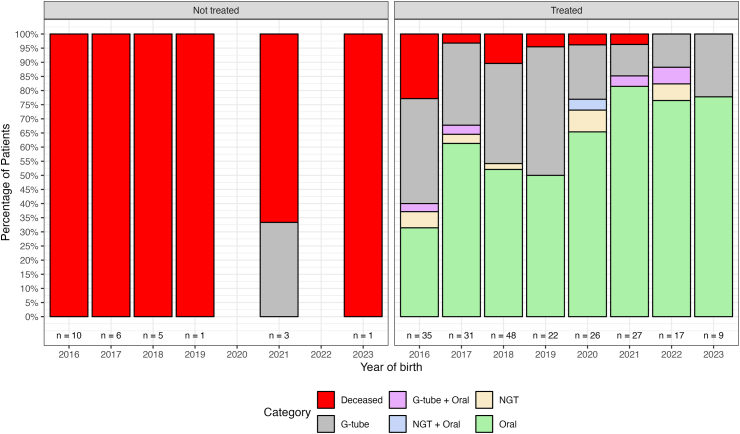
Fig. 4 reports the percentage of nutritional status in the SMA I subjects subdivided by year of birth and treatment status.
Fig. 4.
SMA I percentage of nutritional status subdivided by year of birth and treatment status. Key to figure: NIV = non invasive ventilation, G-tube = gastrostomy tube, NGT = Nasogastric tube, Tracheo = tracheostomy with continuous invasive ventilation.
Survival rate pre and post DMTs
Of the 241 type I infants born after 2016, 42 (17%) passed away (mean age: 1.28 years (SD = 1.70)), while another 25 (10%) are surviving with NIV >16 h/day or a tracheostomy with continuous invasive ventilation.
Of the 140 type I infants born between January 1st 2010, and December 31st 2015, 97 (69%) passed away at a mean age of 1.12 years (SD = 1.80), while another 40 (29%) are surviving with NIV > 16 h/day or a tracheostomy with continuous invasive ventilation.
Comparing the two cohorts of treated infants born after January 1, 2016 and untreated patients born before 2016, the z-test for proportions shows a significant difference (z = −10.14, p < 0.001) in the proportion of infants who passed away, indicating a significantly lower rate in the post-2016 cohort. A significant difference is also observed (z = −4.55, p < 0.001) in the proportion of infants requiring NIV >16 h/day or tracheostomy with continuous invasive ventilation, favoring the post-2016 group. Additionally, the cumulative z-test (z = −13.22, p < 0.001) demonstrates a markedly lower combined proportion of infants passing away or requiring NIV >16 h/day or a tracheostomy with continuous invasive ventilation in the post-2016 cohort (27% vs. 97%). These results hold even when untreated patients born after 2016 are included in the post-2016 cohort.
Discussion
Our results, obtained as part of a nationwide study, include 247 infants with early onset SMA (241 with type I) born or referred to one of the participating centers since 2016, the year when the first therapy became available. As expected, the number of type 0 infants was very small (2.48%), and with the exception of one who required 24/7 ventilation, none survived beyond the age of 3 months, regardless of whether they had been treated or not. The type I cohort also included a number of patients (10%) whose families refused this option and opted for palliative care. In the near totality of cases, especially in the first years after the treatments became available, the families justified their choice fearing that the long term results available at that time were not enough or that the expected results (increased survival, sitting) would not be compatible with long term quality of life.
In type I infants the overall survival in the infants born after 2016 was 80%. If we exclude the small number of infants who did not receive treatment, survival in the treated type I infants was around 90%. Not surprisingly these values are significantly different from those found in untreated patients born before 2016 followed in our centers. Even if the p-values should be interpreted with caution because the statistical comparisons presented was descriptive, the results are clearly in favor of DMT treated infants.
There was also a small number of infants requiring tracheostomy with continuous invasive ventilation. It is of note that the tracheostomies were more often performed between 2016 and 2020 and much less after that. Our experience is that in the first years after treatment became available tracheostomy was often performed following the criteria used in the pretreatment era, i.e., following any severe respiratory event requiring intubation as these were considered the initial sign of an inevitable progressive severe respiratory deterioration that was unlikely to recover.19 With increasing evidence of infants treated with DMTs who could be extubated and transferred to NIV, there has been a change in attitude with tracheostomy being considered only in the infants in whom consecutive attempts to transfer to NIV failed.20
The number of treated infants requiring NIV >16 h/day was also relatively small while the number of night-time NIV was still very high, this probably related to the care recommendations suggesting using it as a prophylactic measure also in the absence of abnormal sleep studies.
It is of interest that the percentage of event free survival (i.e., surviving with no tracheostomy or NIV>16 h/day) was in line with the range reported in the first clinical trials,21, 22, 23 even if the results are not easily comparable because of different inclusion criteria. On one hand our real world cohort had theoretically a lower chance to see an increase in survival after treatment as, at variance with the clinical trials, a significant number of infants were treated after the age of 6 months, and also included those with respiratory and bulbar involvement that were excluded from clinical trials. Furthermore, while in clinical trials event free survival was assessed at the end of the trial duration, between 18 and 24 months, the 80% survival in our type I cohort the mean age of follow up was 3.48 years (longest follow up at 8.26 years). In 10 of the treated patients with fatal events or need for tracheostomy with continuous invasive ventilation or >16 h/day ventilation, these occurred after the age of two years. On the other hand, our cohort also included infants with three or more SMN2 copies, who are more likely to have better outcome, that were not included in the pivotal trials. Even when focusing on the infants with two SMN2 copies (87% of our type I cohort) the event free survival in treated type I infants was around 82% at last follow up and, only for the ones who reached at least two years of follow-up, 65%. Further studies, assessing prospectively.
These findings therefore support the impact of DMTs on survival as also highlighted by the comparison with the cohort followed in the same centers in the years immediately preceding the availability of the DMTs. The much higher survival in our cohort treated with DMTs cannot be ascribed to possible concomitant improvements in standards of care. In the few infants born after 2016 in whom family opted for palliative care, who received similar standards of care to the treated ones, the survival was similar to the one reported before 2016.
We also observed a reduction in the number of infants requiring tube feeding. Until the arrival of new treatments GTube was often inserted at the first signs of dysphagia or failure to thrive and in some countries was performed even before their occurrence as it was seen as an inevitable choice. These recommendations were partly still followed in the first few years after therapies became available but, as reported for tracheostomies, there has been an overall reduction in the number of GTube inserted as a result of a change in the clinicians’ attitude to insert tube feeding. In many centers there is a wait and see attitude when a single episode of aspiration occurring during an acute event is followed by complete recovery. The change in attitude is also secondary to the increasing evidence that a number of subjects with GTube were able to regain at least partially the ability to eat by mouth. Both respiratory and bulbar findings in our study highlight the need for prospective systematic studies assessing how both monitoring and care of these aspects have changed following the advent of the new therapies. These studies would also provide details on how different aspects of care including sleep monitoring, reasons for ventilatory support starting and continuing, video fluoroscopy results, may different among centers in the same country and across different countries.
When motor milestones were examined, we observed that 57% of the infants achieved head control. Before therapies this was only achieved in a small proportion of type I infants. More importantly over 53% achieved the ability to sit independently that was previously never achieved in type I infants. A small proportion achieved standing and walking. Not surprisingly, most of the infants who did not achieve head control or sitting had been treated after the first year of life, when they already had very severe clinical signs. This however did not hold true for all the older or more severe cases, as 8 of weakest patients, including 3 with tracheostomy with continuous invasive ventilation, achieved some milestones.
This study was not designed to evaluate possible differences among treatments as the drugs became available at different times, with significant differences on the duration of follow up among treatments. The analysis was also complicated by the fact that, as recently reported, a number of patients, originally on nusinersen, switched to gene therapy when this became available and therefore we had a limited number of naïve patients receiving gene therapy only.24 There is also limited experience with risdiplam as, until recently this could only be administered in infants older than 2 months as part of a compassionate use program only for children who could not be treated with nusinersen. Further follow up will allow to obtain long term data for infants treated with the individual drugs and to establish possible differences between individual therapies and possible combinations or associations that are becoming increasingly frequent.
Because of the limited access to newborn screening in Italy, we also had limited information on infants identified by newborn screening and treated with DMTs as neonates. They all survive and have a different profile of improved survival and function from the symptomatic cohort described here (data not shown) and will require a separate report once neonatal screening will be more widely implemented and characterized.25, 26, 27
In conclusion our results provide an overview of the ‘new natural history’ of type I SMA and draw a picture of a new generation of type I infants that is surviving longer and has better motor, respiratory and nutritional outcome. Our findings, collected in all the patients diagnosed in Italy in the last years are therefore, unlike clinical trials that were performed in selected cohorts, representative of the whole Italian population without any selection or possible bias.
Work is in progress to obtain additional information regarding other aspects of function or other features, not previously reported in untreated subjects, such as cognitive and neuropsychological aspects of type I SMA, scoliosis and kyphosis that were not systematically measured in all the centers.
Contributors
Maria Carmela Pera, Giorgia Coratti, Marika Pane, Valeria Ada Sansone, Roberto De Sanctis, Richard Finkel, Eugenio Mercuri designed and conceptualized study; verified and analyzed the data; drafted the manuscript for intellectual content. Riccardo Masson, Adele D’Amico, Michela Catteruccia, Caterina Agosto, Antonio Varone, Claudio Bruno, Sonia Messina, Federica Ricci, Irene Bruno, Elena Procopio, Antonella Pini, Sabrina Siliquini, Riccardo Zanin, Emilio Albamonte, Angela Berardinelli, Chiara Mastella, Giovanni Baranello, Stefano Carlo Previtali, Antonio Trabacca, Chiara Bravetti, Delio Gagliardi, Massimiliano Filosto had a major role in the acquisition of data; revised the manuscript for intellectual content. All authors read and approved the final version of the manuscript.
Data sharing statement
Individual partecipant data that underlie the results reported in this article will be shared, after deidentification beginnig 3 months and ending 5 years following article publication, with Researchers who provide a methodologically sound proposal. Proposal should be directed to eugeniomaria.mercuri@unicatt.it; to gain access, data requestors will need to sign a data access agreement.
Declaration of interests
Pera MC reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Coratti G reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Pane M reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Masson R reports personal fees from ROCHE, PTC and PFIZER outside the submitted work.
Sansone V reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
D’Amico A reports personal fees from BIOGEN S.R.L., ROCHE, GENZYME and NOVARTIS outside the submitted work.
Catteruccia M has nothing to disclose.
Agosto C has nothing to disclose.
Varone A has nothing to disclose.
Bruno C report personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Messina S report personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Ricci F report personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS, PTC, SANOFI, PFIZER outside the submitted work.
Bruno I reports personal fees from BIOGEN S.R.L., CHIESI, ASTRAZENECA, INOZYME, NOVARTIS outside the submitted work.
Procopio E has nothing to disclose.
Pini A report personal fees from BIOGEN S.R.L., ROCHE and NOVARTIS outside the submitted work.
Siliquini S has nothing to disclose.
Zanin R has nothing to disclose.
Albamonte E reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Berardinelli A has nothing to disclose.
Mastella C has nothing to disclose.
Baranello G reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Previtali SC reports personal fees from LSC Lifescience, Esperare Foundation, Alia Therapeutics, Argenx, Wave outside the submitted work.
Trabacca A has nothing to disclose.
Bravetti C has nothing to disclose.
Gagliardi D has nothing to disclose.
Filosto M reports personal fees from Sanofi, Reata/Biogen, Amicus.
De Sanctis R reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS outside the submitted work.
Finkel R reports personal fees from BIOGEN S.R.L., ROCHE, NOVARTIS, SCHOLAR ROCK, outside the submitted work.
Mercuri E reports personal fees from BIOGEN S.R.L., ROCHE, AVEXIS, SCHOLAR ROCK and NOVARTIS outside the submitted work.
Acknowledgements
EM is funded by grant from the Italian Ministry of Health (RF-2019- 12370334; PNRR-MR1-2022-12376937). MCP is funded by grant from the Italian Ministry of Health (GR-2018-12365706). GC is funded by grant from the Italian Ministry of Health (GR-2021-12374579). We acknowledge the contribution of Biogen, Novartis and Roche to the Italian SMA registry.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102967.
Contributor Information
Eugenio Mercuri, Email: eugeniomaria.mercuri@unicatt.it.
the Italian SMA type I Study Group:
Alice Gardani, Maria Antonella Costantino, Ilaria Bitetti, Matteo Tuana Franguel, Maria Sframeli, Andrea Magnolato, Myriam Rausa, Elena Pagliaccia, Mirea Negri, Cesare Del Monaco, Beatrice Berti, Daniela Leone, Concetta Palermo, Enrico Bertini, Antonella Longo, Claudia Dosi, Sara Carnicella, Simone Morando, Noemi Brolatti, Alessandra Vento, Ilaria Cavallina, Roberta Ferrante, Laura Bernasconi, Marco Piastra, Orazio Genovese, Nicola Forcina, Francesca Benedetti, Simona Damioli, Lavinia Fanelli, Giulia Stanca, Giulia Norcia, Matteo Sacchini, Chiara Ticci, and Elena Briganti
Appendix ASupplementary data
References
- 1.D'Amico A., Mercuri E., Tiziano F.D., Bertini E. Spinal muscular atrophy. Orphanet J Rare Dis. 2011;6:71. doi: 10.1186/1750-1172-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mercuri E., Lucibello S., Perulli M., et al. Longitudinal natural history of type I spinal muscular atrophy: a critical review. Orphanet J Rare Dis. 2020;15(1):84. doi: 10.1186/s13023-020-01356-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel R.S., McDermott M.P., Kaufmann P., et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolb S.J., Coffey C.S., Yankey J.W., et al. Natural history of infantile-onset spinal muscular atrophy. Ann Neurol. 2017;82(6):883–891. doi: 10.1002/ana.25101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verhaart I.E.C., Robertson A., Wilson I.J., et al. Prevalence, incidence and carrier frequency of 5q-linked spinal muscular atrophy - a literature review. Orphanet J Rare Dis. 2017;12(1):124. doi: 10.1186/s13023-017-0671-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bitetti I., Lanzara V., Margiotta G., Varone A. Onasemnogene abeparvovec gene replacement therapy for the treatment of spinal muscular atrophy: a real-world observational study. Gene Ther. 2023;30(7–8):592–597. doi: 10.1038/s41434-022-00341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pane M., Coratti G., Sansone V.A., et al. Type I spinal muscular atrophy patients treated with nusinersen: 4-year follow-up of motor, respiratory and bulbar function. Eur J Neurol. 2023;30(6):1755–1763. doi: 10.1111/ene.15768. [DOI] [PubMed] [Google Scholar]
- 8.Weiss C., Ziegler A., Becker L.L., et al. Gene replacement therapy with onasemnogene abeparvovec in children with spinal muscular atrophy aged 24 months or younger and bodyweight up to 15 kg: an observational cohort study. Lancet Child Adolesc Health. 2022;6(1):17–27. doi: 10.1016/S2352-4642(21)00287-X. [DOI] [PubMed] [Google Scholar]
- 9.Pechmann A., Behrens M., Dornbrack K., et al. Effect of nusinersen on motor, respiratory and bulbar function in early-onset spinal muscular atrophy. Brain. 2022;146(2):668–677. doi: 10.1093/brain/awac252. [DOI] [PubMed] [Google Scholar]
- 10.Erdos J., Wild C. Mid- and long-term (at least 12 months) follow-up of patients with spinal muscular atrophy (SMA) treated with nusinersen, onasemnogene abeparvovec, risdiplam or combination therapies: a systematic review of real-world study data. Eur J Paediatr Neurol. 2022;39:1–10. doi: 10.1016/j.ejpn.2022.04.006. [DOI] [PubMed] [Google Scholar]
- 11.Weststrate H., Stimpson G., Thomas L., et al. Evolution of bulbar function in spinal muscular atrophy type 1 treated with nusinersen. Dev Med Child Neurol. 2022;64(7):907–914. doi: 10.1111/dmcn.15171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D'Silva A.M., Holland S., Kariyawasam D., et al. Onasemnogene abeparvovec in spinal muscular atrophy: an Australian experience of safety and efficacy. Ann Clin Transl Neurol. 2022;9(3):339–350. doi: 10.1002/acn3.51519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modrzejewska S., Kotulska K., Kopyta I., et al. Nusinersen treatment of Spinal Muscular Atrophy Type 1 - results of expanded access programme in Poland. Neurol Neurochir Pol. 2021;55(3):289–294. doi: 10.5603/PJNNS.a2021.0020. [DOI] [PubMed] [Google Scholar]
- 14.Audic F., Dubois S.M., Durigneux J., et al. Effect of nusinersen after 3 years of treatment in 57 young children with SMA in terms of SMN2 copy number or type. Arch Pediatr. 2024;31(2):117–123. doi: 10.1016/j.arcped.2023.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Cho J., Lee J., Kim J., et al. Nusinersen demonstrates effectiveness in treating spinal muscular atrophy: findings from a three-year nationwide study in Korea. Front Neurol. 2023;14 doi: 10.3389/fneur.2023.1294028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheijmans F.E.V., Cuppen I., van Eijk R.P.A., et al. Population-based assessment of nusinersen efficacy in children with spinal muscular atrophy: a 3-year follow-up study. Brain Commun. 2022;4(6) doi: 10.1093/braincomms/fcac269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mercuri E., Finkel R., Scoto M., et al. Development of an academic disease registry for spinal muscular atrophy. Neuromuscul Disord. 2019;29(10):794–799. doi: 10.1016/j.nmd.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 18.Wijngaarde C.A., Stam M., Otto L.A.M., et al. Population-based analysis of survival in spinal muscular atrophy. Neurology. 2020;94(15):e1634–e1644. doi: 10.1212/WNL.0000000000009248. [DOI] [PubMed] [Google Scholar]
- 19.Finkel R.S., Mercuri E., Meyer O.H., et al. Diagnosis and management of spinal muscular atrophy: Part 2: pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul Disord. 2017;28(3):197–207. doi: 10.1016/j.nmd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Sansone V.A., Pirola A., Albamonte E., et al. Respiratory needs in patients with type 1 spinal muscular atrophy treated with nusinersen. J Pediatr. 2020;219:223–228.e4. doi: 10.1016/j.jpeds.2019.12.047. [DOI] [PubMed] [Google Scholar]
- 21.Finkel R.S., Mercuri E., Darras B.T., et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N Engl J Med. 2017;377(18):1723–1732. doi: 10.1056/NEJMoa1702752. [DOI] [PubMed] [Google Scholar]
- 22.Day J.W., Finkel R.S., Chiriboga C.A., et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 23.Darras B.T., Masson R., Mazurkiewicz-Beldzinska M., et al. Risdiplam-treated infants with type 1 spinal muscular atrophy versus historical controls. N Engl J Med. 2021;385(5):427–435. doi: 10.1056/NEJMoa2102047. [DOI] [PubMed] [Google Scholar]
- 24.Pane M., Berti B., Capasso A., et al. Onasemnogene abeparvovec in spinal muscular atrophy: predictors of efficacy and safety in naive patients with spinal muscular atrophy and following switch from other therapies. EClinicalMedicine. 2023;59 doi: 10.1016/j.eclinm.2023.101997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crawford T.O., Swoboda K.J., De Vivo D.C., et al. Continued benefit of nusinersen initiated in the presymptomatic stage of spinal muscular atrophy: 5-year update of the NURTURE study. Muscle Nerve. 2023;68(2):157–170. doi: 10.1002/mus.27853. [DOI] [PubMed] [Google Scholar]
- 26.Mercuri E., Muntoni F., Baranello G., et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy type 1 (STR1VE-EU): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(10):832–841. doi: 10.1016/S1474-4422(21)00251-9. [DOI] [PubMed] [Google Scholar]
- 27.Strauss K.A., Farrar M.A., Muntoni F., et al. Onasemnogene abeparvovec for presymptomatic infants with two copies of SMN2 at risk for spinal muscular atrophy type 1: the Phase III SPR1NT trial. Nat Med. 2022;28(7):1381–1389. doi: 10.1038/s41591-022-01866-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.