Abstract
The European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with oncogene-addicted metastatic non-small-cell lung cancer (mNSCLC), published in January 2023, was modified according to previously established standard methodology, to produce the Pan-Asian adapted (PAGA) ESMO consensus guidelines for the management of Asian patients with oncogene-addicted mNSCLC. The adapted guidelines presented in this manuscript represent the consensus opinions reached by a panel of Asian experts in the treatment of patients with oncogene-addicted mNSCLC representing the oncological societies of China (CSCO), Indonesia (ISHMO), India (ISMPO), Japan (JSMO), Korea (KSMO), Malaysia (MOS), the Philippines (PSMO), Singapore (SSO), Taiwan (TOS) and Thailand (TSCO), co-ordinated by ESMO and the Korean Society for Medical Oncology (KSMO). The voting was based on scientific evidence and was independent of the current treatment practices, drug access restrictions and reimbursement decisions in the different regions of Asia. The latter are discussed separately in the manuscript. The aim is to provide guidance for the optimisation and harmonisation of the management of patients with oncogene-addicted mNSCLC across the different regions of Asia, drawing on the evidence provided by both Western and Asian trials, while respecting the differences in screening practices, molecular profiling and age and stage at presentation. Attention is drawn to the disparity in the drug approvals and reimbursement strategies between the different regions of Asia.
Key words: ESMO, guidelines, Pan-Asian, non-small cell lung cancer, treatment
Highlights
-
•
This article provides ESMO recommendations adapted for the treatment of oncogene-addicted mNSCLC in Asian patients.
-
•
It outlines the diagnosis/staging, management, treatment and follow-up of patients with oncogene-addicted mNSCLC.
-
•
The disparity in availability and reimbursement of certain tests and treatments between regions is discussed.
-
•
The aim is to encourage evidence-based medicine to improve the access of Asian patients to state-of-the-art cancer care.
Introduction
In 2020, lung cancer, with an estimated 1 796 144 deaths, was the leading cause of cancer-related deaths worldwide, accounting for 18.0% of all cancer-related deaths.1,2 The majority (1 122 517; 61.9%) of lung cancer-related deaths were in the continent of Asia and the four countries with the highest numbers of deaths from lung cancer were China (733 291 deaths, accounting for 39.7% of all deaths globally), the United States (138 225 deaths, 7.7%), Japan (83 369 deaths, 4.7%) and India (66 279 deaths, 3.7%).1 It is predicted that the number of global deaths from lung cancer will increase between 2022 and 2045 by 78.0% to 3.24 million deaths, with the continent of Asia predicted to see the second highest percentage rise in deaths (+89.4%; 2 163 499 deaths) after Africa (+122%; 100 951 deaths).3
The leading cause of lung cancer is tobacco smoking which, either first- or second-hand, has been predicted to be associated with 80%-90% of all lung cancer cases.4 In 2018, it was estimated that 26.6% (308 million) of Chinese adults were smokers (Smokers refers to tobacco smokers. Never smokers are people who have never smoked tobacco. Ever smokers are people who have smoked tobacco) and a further 732 million were exposed to second-hand smoke.4,5 Approximately half of all Chinese men smoke compared with 2.1% of women, and a similar pattern, with much higher percentages of men smoking than women, is seen throughout Asia.5,6 There is also a high prevalence of lung cancer in never smokers in East Asia, accounting for 39.7% of lung cancer cases in China, 38% in South Korea and 32.8% in Japan.7 The majority of these never-smoker lung cancer cases are in women and in 2011, the prevalence of lung cancer per 100 000 females in China was 21.3 cases compared with 16.4 and 11.4 for Germany and Italy, respectively, despite the higher (∼20%) prevalence of female adult smoking in these countries.7, 8, 9 Indoor cooking fumes and indoor coal burning may contribute to the incidence of lung cancer in never-smoking women in China.8,10 Other risk factors for lung cancer include diet, alcohol consumption, air pollution and occupational/environmental exposure, as well as infections such as tuberculosis.10,11
The dependency of tumour cells on a single oncogenic protein to maintain their malignant phenotype is known as ‘oncogene addiction’.12 Non-small-cell lung cancer (NSCLC) accounts for 85% of all lung cancer cases and has multiple oncogenic drivers. These include mutations in the epidermal growth factor receptor gene (EGFR; the most frequent aberration in Asian patients with NSCLC, and seen in 40%-60% of cases),13,14 the KRAS proto-oncogene, GTPase (KRAS mutated in 3.8%-8.0% of Asian cases of NSCLC)15 and the B-Raf proto-oncogene, serine/threonine kinase (BRAF mutated in 1.3% of Asian cases of NSCLC)16; mutations or amplifications in the erb-b2 receptor tyrosine kinase 2 [ERBB2, also known as the human epidermal growth factor 2 (HER2)] gene (mutated in 1%-4% of all NSCLC cases and is higher in Asian cases, and amplified in 2%-5% of all treatment-naïve cases)17, 18, 19; rearrangements involving the ALK receptor tyrosine kinase (ALK, 2.3%-6.7% of Asian cases of NSCLC),15 the ROS proto-oncogene 1, receptor tyrosine kinase (ROS1, 2.4% of all cases of NSCLC and overrepresented in Asian patients),20,21 the Ret proto-oncogene (RET, 1%-2% of all cases of NSCLC)22 and the neurotrophic receptor tyrosine kinase (NTRK) receptor genes, NTRK1, 2 and 3 (<1% of all cases of NSCLC)17; as well as structural rearrangements in the MET proto-oncogene, receptor tyrosine kinase (MET, the most frequent, which results in exon 14 skipping, seen in 2%-4% of all NSCLCs, or MET amplifications, seen in 1%-5% of all cases of NSCLC).23 The management and prognosis of NSCLC has been revolutionised through the identification of oncogenic drivers, such as those mentioned above, and the development of therapies that target them.24, 25, 26
NSCLC has two major subtypes: adenocarcinoma (ADC, accounting for 50% of all cases of NSCLC) and squamous-cell carcinoma (SCC, accounting for 30% of all cases of NSCLC).27,28 In a Korean study comparing never smokers with ever smokers, the ADC subtype was the predominant subtype in never smokers (89.8% of cases) and accounted for 44.9% of ever-smoker cases whereas the SCC subtype was seen in 3.5% of never-smoker cases and 41.9% of cases for ever smokers.9,29 While most oncogenic alterations are shared between the two subsets, there are differences in the prevalence. There is also a difference in the frequency of actionable alterations between the two subsets, for example, EGFR and KRAS mutations are comparatively enriched in ADC.30,31 In Asian populations with the ADC subtype, ∼80% will have targetable molecular alterations compared with 60% for Western populations.30 Targetable alterations are much lower in SCC, with a Dutch study finding that 77% of all SCCs did not have therapeutically relevant alterations.32 Similar rates were seen in an Indian analysis and comparison of SCC samples from Korean patients with those in The Cancer Genome Atlas database revealed a similar frequency in alterations in targetable genes.29,33 These findings suggest that, unlike ADC, there is no major difference in the rate of targetable mutations in SCC between Asian and Western patients.29,32
The most recent European Society for Medical Oncology (ESMO) Clinical Practice Guidelines for the diagnosis, treatment and follow-up of patients with oncogene-addicted metastatic NSCLC (mNSCLC) were published in January 2023.34 Therefore, a decision was taken by ESMO and the Korean Society for Medical Oncology (KSMO) that these latest ESMO guidelines should be adapted to provide updated Pan-Asian guidelines for the management and treatment of oncogene-addicted mNSCLC in patients of Asian ethnicity. This manuscript summarises the Pan-Asian adapted guidelines developed and agreed at a face-to-face working meeting that took place in Seoul on 27 April 2024, hosted by KSMO. Each recommendation is accompanied by the level of evidence (LoE), grade of recommendation (GoR) and, where applicable, ESMO-Magnitude of Clinical Benefit Scale (ESMO-MCBS) and ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) scores (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103996).35,36
Methodology
This Pan-Asian adaptation of the current ESMO Clinical Practice Guidelines34 was prepared in accordance with the principles of ESMO standard operating procedures (https://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology) and was a KSMO–ESMO initiative endorsed by the Chinese Society of Clinical Oncology (CSCO), the Indonesian Society of Hematology and Medical Oncology (ISHMO), the Indian Society of Medical and Paediatric Oncology (ISMPO), the Japanese Society of Medical Oncology (JSMO), the Malaysian Oncological Society (MOS), the Philippine Society of Medical Oncology (PSMO), the Singapore Society of Oncology (SSO), the Taiwan Oncology Society (TOS) and the Thai Society of Clinical Oncology (TSCO). An international panel of experts was selected from the KSMO (n = 6), the ESMO (n = 6 including the co-ordinator of the Pan-Asian Guideline adaptations, TY) and two experts from each of the nine other oncological societies. Only two of the six expert members from the KSMO (TMK and HRK) were allowed to vote on the recommendations together with the experts from each of the nine other Asian oncology societies (n = 20). All 20 Asian experts provided comments on the pre-meeting survey and one consensus response per society (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996). Only one voting member per Asian society was present at the face-to-face meeting. None of the additional KSMO non-voting members or experts and none of the ESMO members or experts were allowed to vote and were present in an advisory role only (see Supplementary Material: Methodology, available at https://doi.org/10.1016/j.esmoop.2024.103996). All the Asian experts eligible to vote (n = 20) approved the revised recommendations.
Results
A. Scientific adaptations of the ESMO recommendations
In the initial pre-meeting survey, the 20 voting Asian experts reported on the ‘acceptability’ of the 73 recommendations for the diagnosis, treatment and follow-up of patients with oncogene-addicted mNSCLC from the most recent ESMO Clinical Practice Guidelines34 (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996), in the five categories outlined in the text below and in Table 1. A lack of agreement in the pre-meeting survey was established for 37 recommendations, 27 of which were discussed at the face-to-face working meeting in Seoul to adapt the recently published ESMO Clinical Practice Guidelines. For ESMO ‘recommendations 1h, 3jj and 3kk’ there were discrepancies relating to their applicability in certain regions of Asia. Of these, ‘recommendations 1h and 3jj’ were not discussed at the face-to-face meeting (see details in Supplementary Material: Results and Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103996).
Table 1.
Summary of Asian consensus recommendations for the treatment of patients with oncogene-addicted mNSCLC
| Consensus | |
|---|---|
| 1. DIAGNOSIS, PATHOLOGY AND MOLECULAR BIOLOGY | |
| 1a. Adequate tissue material for histological diagnosis and molecular testing should be obtained to allow for individual treatment decisions [IV, A]. | 100% |
| 1b. Pathological diagnosis should be made according to the 2021 World Health Organization classification of lung tumours [IV, A]. | 100% |
| 1c. Specific subtyping of all NSCLCs is necessary for therapeutic decision making and should be carried out wherever possible. IHC stains should be used to reduce the NSCLC-not otherwise specified—rate to fewer than 10% of cases diagnosed [IV, A]. | 100% |
| 1d. The molecular tests below are recommended in patients with advanced non-squamous-cell carcinoma. They are not recommended in patients with a confident diagnosis of pure squamous-cell carcinoma, except in specific cases, e.g. young (<50 years) patients, never (<100 cigarettes in a lifetime)/former light smokers (<15 pack-years, all kinds of tobacco) or long-time ex-smokers (quit smoking >15 years ago, all kinds of tobacco) [IV, A]. In specific regions (e.g. India, Japan and China) with a high incidence of EGFR mutation in squamous-cell carcinomas, EGFR testing might also be performed regardless of smoking history [IV, B]. | 100% |
| 1e. EGFR mutation status should be determined [I, A]. The test methodology should provide adequate coverage of mutations in exons 18-21, including those associated with resistance to some therapies [III, A]. | 100% |
| 1f. The availability of TKIs effective against T790M-mutated recurrent disease makes T790M testing on disease relapse on first- or second-generation EGFR TKIs mandatory [I, A]. | 100% |
| 1g. Testing for ALK rearrangements should be carried out [I, A]. | 100% |
| 1h. Detection of the ALK translocation by FISH remains a standard, but IHC with high-performance ALK antibodies and validated assays may be used for screening [III, A] and have been accepted as an equivalent alternative to FISH for ALK testing. | 100% |
| 1i. Testing for ROS1 rearrangements should be carried out [II, A]. IHC may be used as a screening approach [IV, A]. Detection of a ROS1 translocation can be carried out or verified by FISH, NGS or reverse transcription PCR [IV, A]. | 100% |
| 1j. BRAF V600 mutation status testing should be carried out [II, A]. | 100% |
| 1k. Testing for NTRK rearrangements should be carried out [II, A]. Screening for NTRK rearrangements may use IHC or NGS, with appropriate testing follow-up to validate a positive result [II, A]. | 100% |
| 1l. Testing for MET exon 14 skipping mutations, MET amplifications, RET rearrangements, KRAS G12C mutations and HER2 mutations should be carried out [II, A]. | 100% |
| 1m. If available, multiplex platforms (NGS) for molecular testing are preferable [III, A]. | 100% |
| 1n. RNA-based NGS is preferred for identifying an expanding range of fusion genes [III, B]. Whichever testing modality is used, it is mandatory that adequate internal validation and quality control measures are in place and that laboratories participate in, and perform adequately in, external quality assurance schemes for each biomarker test [III, A]. | 100% |
| 1o. cfDNA (liquid biopsy) can be used to test for oncogenic drivers as well as resistance mutations, but all patients with a negative cfDNA blood test still require tissue biopsy [II, A]. | 100% |
| 2. STAGING AND RISK ASSESSMENT | |
| 2a. A complete history including a precise smoking history and comorbidities, weight loss, Eastern Cooperative Oncology Group performance status (ECOG PS) and physical examination must be recorded [IV, A]. | 100% |
| 2b. Laboratory standard tests including routine haematology, renal and hepatic functions and bone biochemistry tests are required. Other tests (e.g. lipid spectrum and creatine kinase levels) depend on toxicity of the targeted therapy that will be used [IV, A]. | 100% |
| 2c. An electrocardiogram is required if the targeted therapy can cause adverse cardiac events, including rhythmic modifications (e.g. long QT) [IV, A]. | 100% |
| 2d. Contrast-enhanced computed tomography (CT) scan of the chest and upper abdomen (including the liver and adrenal glands) should be carried out at diagnosis [IV, A]. | 100% |
| 2e. Imaging of the central nervous system (CNS) should be considered at diagnosis for all patients with metastatic disease [IV, B] and is required for patients with neurological symptoms or signs [IV, A]. If available, CNS imaging with gadolinium-enhanced magnetic resonance imaging (MRI) should be considered for all patients [IV, B]. | 100% |
| 2f. If bone metastases are clinically suspected, bone imaging is required [IV, B]. | 100% |
| 2g. Bone scintigraphy, ideally coupled with CT, can be used for detection of bone metastasis [IV, B]. [18F]2-fluoro-2-deoxy-D-glucose (FDG)-positron emission topography (PET)-CT is the most sensitive modality in detecting bone metastasis [III, B]. | 100% |
| 2h. FDG–PET–CT and brain imaging are recommended in patients suspected of having oligometastatic disease [IV, A]. In the presence of a solitary metastatic site on imaging studies, efforts might be considered to obtain a cytological or histological confirmation of stage IV disease [IV, B]. | 100% |
| 2i. For oligometastatic disease with the suspicion of mediastinal lymph node involvement, mediastinal disease should be pathologically proven if this potentially impacts the treatment plan [IV, A]. | 100% |
| 2j. NSCLC must be staged according to the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM (tumour–node–metastasis) 8th edition staging manual and must be grouped into the stage categories shown in Supplementary Tables S2 and S3, available at https://doi.org/10.1016/j.annonc.2022.12.009 [IV, A]. | 100% |
| 2k. Response evaluation is recommended after 8-12 weeks of treatment, using the same radiographic investigation that initially demonstrated the tumour lesions [IV, B]. Follow-up with a PET scan is not routinely recommended, due to its relatively low specificity despite a high sensitivity [IV, C]. | 100% |
| 2l. Measurements and response assessment should follow Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 [IV, A].115 The clinical relevance of RECIST in evaluating the response remains debatable as patients can derive benefit from continuing the same TKI after RECIST v1.1 progression [III, A]. | 100% |
| 3. MANAGEMENT OF ADVANCED AND METASTATIC DISEASE | |
| EGFR-mutated NSCLC | |
| 3a. All patients with a sensitising EGFR mutation should receive first-line EGFR TKIs irrespective of clinical parameters including PS, gender, tobacco exposure and histology [I, A]. | 100% |
| 3b. Third-generation EGFR TKIs (such as osimertinib) is the preferable first-line treatment option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R), especially for patients with CNS metastases [I, A; ESMO-Magnitude of Clinical Benefit (ESMO-MCBS) v1.1 score for osimertinib: 4; ESCAT: I-A]. | 100% |
| 3c. First- or second-generation EGFR TKIs (such as erlotinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], gefitinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], afatinib [I, B; ESMO-MCBS v1.1 score: 5; ESCAT: I-A] and dacomitinib [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]) are other first-line single-agent treatment options. | 100% |
| 3d. 3d-i. Another first-line option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R) is osimertinib combined with carboplatin-pemetrexed [I, A; ESMO-MCBS v1.1 score: 3; not EMA approved]. Alternatively, another option is 3d-ii or 3d-iii. 3d-ii. Another first-line option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R) is gefitinib combined with carboplatin-pemetrexed [I, B; not EMA approved]. 3d-iii. Another first-line option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R) is lazertinib combined with amivantamab [I, A; not EMA approved]. |
100% 100% 100% |
| 3e. EGFR TKIs combined with anti-angiogenic therapy are additional first-line treatment options, including erlotinib-bevacizumab [I, B; ESMO-MCBS v1.1 score: 2; ESCAT: I-A; EMA approved, not FDA approved] or erlotinib-ramucirumab [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]. | 100% |
| 3f. Considering toxicity, cost increases with adding additional treatments and patient inconvenience, single-agent EGFR TKIs are still a standard first-line treatment [I, A; ESCAT: I-A]. | 100% |
| 3g. Afatinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-B] or osimertinib [III, B; ESCAT: I, B] is a recommended treatment option for patients with a major uncommon, non-exon 20 insertion, sensitising EGFR mutation. | 100% |
| 3h. Patients who have moderate radiological progression with ongoing clinical benefit may continue with EGFR TKIs [III, A]. | 100% |
| 3i. Upon resistance to first-line first- or second-generation EGFR TKIs, patients should be tested for the presence of the EGFR exon 20 T790M mutation from plasma cfDNA and/or tumour re-biopsy [I, A]. | 100% |
| 3j. Patients with T790M-positive resistance should receive third-generation EGFR TKIs (such as osimertinib) [I, A; ESMO-MCBS v 1.1 score: 4; ESCAT: I-A] as second-line therapy, whereas T790M-negative resistance might be treated with platinum-based ChT [III, A]. Third-generation EGFR TKIs (such as osimertinib) can be considered in the case of brain-only progression [III, B]. | 100% |
| 3k. Genomic analysis by plasma- or tissue-based NGS might be considered for a patient who develops resistance to a third-generation TKI (such as osimertinib) [III, C]. | 100% |
| 3l. Platinum plus pemetrexed ChT combined with amivantamab is the SoC upon progression on osimertinib [I, A]. Platinum doublet ChT remains an option [III, B]. Clinical trial enrolment is encouraged, especially if a targetable resistance mechanism is identified [III, B]. | 100% |
| 3m. The combination of platinum plus paclitaxel/pemetrexed ChT with an anti-PD-(L)1 and bevacizumab/biosimilar may be considered as a treatment option for patients following EGFR TKI failure, no contraindication for ICIs and anti-angiogenic agent [III, B; for carboplatin-paclitaxel-atezolizumab-bevacizumab ESMO-MCBS v1.1 score: 3]. | 100% |
| 3n. Single-agent ICIs may be considered as a treatment option only after progression on EGFR TKIs and ChT [IV, C]. | 100% |
| ALK-rearranged NSCLC | |
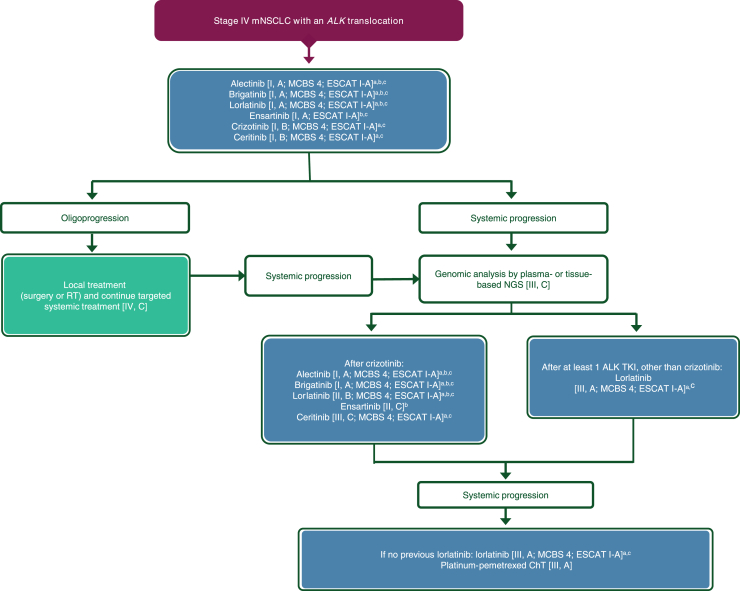
| 3o. Patients should be treated in the first-line setting with alectinib, brigatinib, ensartinib or lorlatinib [I, A; ESMO-MCBS v1.1 score: 4; ESCAT: I-A]. These options are preferred over crizotinib or ceritinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A]. | 100% |
| 3p. Alectinib [I, A; ESMO-MCBS score: 4; ESCAT: I-A] or brigatinib [I, A; ESMO-MCBS score: 4; ESCAT: I-A] is recommended in patients who progress on treatment with, or are intolerant to, crizotinib. | 100% |
| 3q. Lorlatinib [II, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], ceritinib [III, C; ESMO- MCBS v1.1 score: 4; ESCAT: I-A] and ensartinib [II, C] represent additional treatment options at crizotinib resistance. | 100% |
| 3r. In patients who progress after a second-generation ALK TKI, the newer-generation ALK inhibitor lorlatinib is an option [III, A; ESMO-MCBS v1.1 score: 4; ESCAT: I-A]. | 100% |
| 3s. Following progression on lorlatinib, ChT with a platinum-pemetrexed-based combination is recommended [III, A]. | 100% |
| 3t. Genomic analysis by plasma- or tissue-based NGS might be considered for a patient who develops resistance to a second- or third-generation ALK TKI [III, C]. | 100% |
| Treatment of ROS1-rearranged NSCLC | |
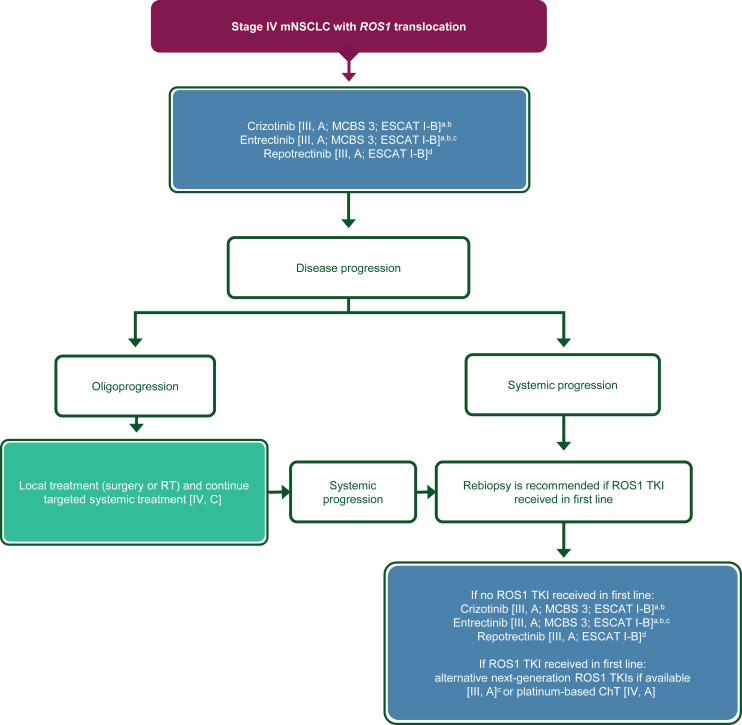
| 3u. Crizotinib or entrectinib is recommended in the first-line setting [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B]. | 100% |
| 3v. Entrectinib, if available, is preferred over crizotinib in patients with brain metastases [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B]. | 100% |
| 3w. Repotrectinib, if available, is an option in the first-line setting but is not EMA approved [III, A; ESCAT: I-B]. | 100% |
| 3x. If patients have received crizotinib in the first-line setting, they may be offered a newer-generation TKI if available [III, A] (no EMA approval) or platinum-based ChT in the second-line setting [IV, A]. | 100% |
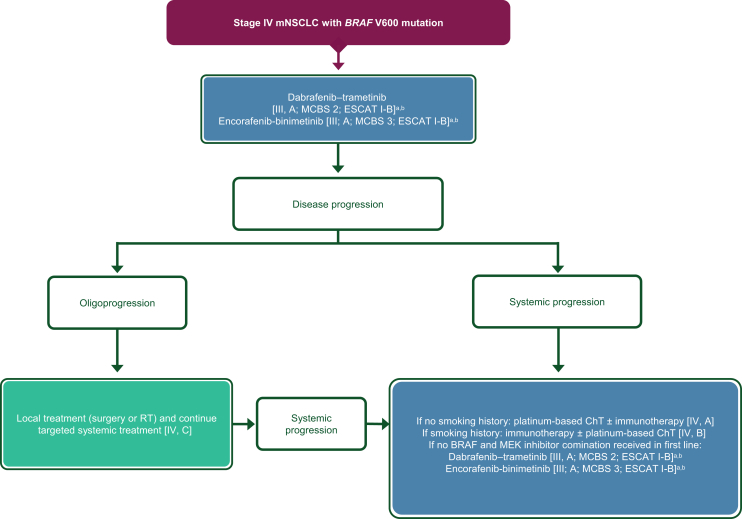
| BRAF mutations | |
| 3y. BRAF-MEK inhibition using dabrafenib-trametinib is recommended [III, A; ESMO-MCBS v1.1 score: 2; ESCAT: I-B]. Another option is encorafenib-binimetinib [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B]. | 100% |
| 3z. If patients have received BRAF-MEK inhibition in the first-line setting, they may be offered platinum-based ChT with or without immunotherapy in the second-line setting, regardless of their smoking history [IV, A]. For patients with a smoking history, immunotherapy with or without ChT should be considered as per the ESMO CPG on non-oncogene-addicted mNSCLC [IV, B].99 | 100% |
| RET fusions | |
| 3aa. Treatment with selpercatinib is recommended as first-line therapy for patients with RET fusion-positive NSCLC [I, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-C]. | 100% |
| Other oncogenic drivers for which targeted therapy is available | |
| 3bb. Platinum-doublet ChT with or without ICIs is recommended as first-line therapy for patients with a MET amplification orHER2 mutation [IV, B]. | 100% |
| 3cc. Capmatinib and tepotinib in first line [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B; FDA approved, not EMA approved] or in second line [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B] are recommended in patients with a MET exon 14 skipping mutation. Savolitinib may also be an option [III, A; ESCAT: I-B; not EMA or FDA approved]. | 100% |
| 3dd. If patients have received a MET-specific inhibitor in the first-line setting, they may be offered platinum-based ChT with or without immunotherapy in the second-line setting, regardless of smoking history [IV, A]. For patients with a smoking history, immunotherapy with or without ChT should be considered as per the ESMO CPG on non-oncogene-addicted mNSCLC [IV, B].99 | 100% |
| 3ee. In patients with HER2 exon 20 mutations, trastuzumab-deruxtecan, if available, is recommended for patients following prior first-line therapy [III, B; ESCAT: II-B]. | 100% |
| 3ff. Larotrectinib and entrectinib are recommended for patients with NSCLC and an NTRK gene fusion and who have no satisfactory treatment options [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-C]. | 100% |
| 3gg. For KRAS G12C-mutated NSCLC, it is recommended to follow the first-line treatment algorithms in the ESMO CPG on non-oncogene-addicted mNSCLC [III, A].99 | 100% |
| 3hh. Platinum-doublet ChT can be given to patients with KRAS G12C-mutated NSCLC and progression on first-line ICI monotherapy [III, A]. | 100% |
| 3ii. Sotorasib is recommended for treatment of KRAS G12C-mutated NSCLC failing prior therapy [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-B]. | 100% |
| 3jj. Adagrasib is recommended for treatment of KRAS G12C-mutated NSCLC failing prior therapy [III, B; ESMO-MCBS v1.1 score: 2; ESCAT: I-B; FDA approved, not EMA approved]. | 100% |
| 3kk. Amivantamab combined with platinum-based ChT is recommended for the treatment of EGFR exon 20 insertion-mutated NSCLC in the first-line setting [I, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B] or in the second- or later-line settings [III, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-B]. | 100% |
| 3ll. Amivantamab monotherapy is an option after platinum-based ChT failure for the treatment of EGFR exon insertion-mutated NSCLC not previously exposed to amivantamab [II, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-B] | 100% |
| Special populations | |
| 3mm. TKIs should be given to patients with PS ≥ 2 and an oncogenic driver [III, A]. | 100% |
| 3nn. TKIs should be given to elderly patients [II, A]. | 100% |
| 3oo. Patients with oligometastatic disease at diagnosis may experience long-term PFS following systemic therapy and LAT (high-dose RT or surgery) [II, B], but due to limited evidence, inclusion in clinical trials is preferred. | 100% |
| 3pp. Patients with advanced NSCLC and a driver mutation, with oligoprogression while on molecular targeted therapy, may benefit from LAT (high-dose RT or surgery) including improved long-term disease-free survival, but data are limited and inclusion in clinical trials is preferred. | 100% |
| 4. FOLLOW-UP, LONG-TERM IMPLICATIONS AND SURVIVORSHIP | |
| 4a. Follow-up every 8-12 weeks should be carried out if there is an option for a next line of therapy [IV, A]. | 100% |
| 4b. Psychosocial support should be offered if needed [IV, A]. | 100% |
| 4c. Smoking cessation should be encouraged [IV, A]. | 100% |
| Palliative care in stage IV | |
| 4d. Early palliative care intervention is recommended, in parallel with standard oncological care [I, A]. | 100% |
AJCC, American Joint Committee on Cancer; ALK, ALK tyrosine kinase receptor; BRAF, B-Raf proto-oncogene serine/threonine kinase; cfDNA, circulating free DNA; ChT, chemotherapy; CNS, central nervous system; CPG, Clinical Practice Guidelines; CT, computed tomography; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets; ESMO, European Society for Medical Oncology; ESMO-MCBS; ESMO-Magnitude of Clinical Benefit Scale; FDA, US Food and Drug Administration; FDG, [18F]2-fluoro-2-deoxy-D-glucose; HER2, human epidermal growth factor receptor 2; ICI, immune checkpoint inhibitor; IHC, immunohistochemistry; KRAS, KRAS proto-oncogene, GTPase; LAT, local ablative therapy; MET, MET proto-oncogene, receptor tyrosine kinase; mNSCLC, metastatic non-small-cell lung cancer; NGS, next-generation sequencing; NSCLC, non-small-cell lung cancer; NTRK, neurotrophic tyrosine receptor kinase; PET, positron emission topography; PS, performance status; RECIST v1.1; Response Evaluation Criteria in Solid Tumours, version 1.1; RET, ret proto-oncogene; ROS1, ROS proto-oncogene 1, receptor tyrosine kinase; RT, radiotherapy; SoC, standard of care; TKIs, tyrosine kinase inhibitors; TNM, tumour–node–metastasis; UICC, Union for International Cancer Control.
CSCO, Chinese Society of Clinical Oncology; ESMO, European Society for Medical Oncology; ISHMO, Indonesian Society of Haematology and Medical Oncology; ISMPO, Indian Society of Medical and Paediatric Oncology; JSMO, Japanese Society of Medical Oncology; KSMO, Korean Society for Medical Oncology; MOS, Malaysian Oncological Society; PSMO, Philippine Society of Medical Oncology; SSO, Singapore Society of Oncology; TOS, Taiwan Oncology Society; TSCO, Thai Society of Clinical Oncology.
1. Diagnosis, pathology and molecular biology—recommendations 1a-f
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 1a-c, f-h, and j-o’ (Table 1), without change, and, following discussion at the face-to face meeting, the revised ‘recommendations 1d, e and i’.
Although EGFR mutations are more frequent in SCC in non-smokers than smokers,29,37,38 and are more common in females than males with SCC,38 discussion concerning ESMO ‘recommendation 1d’ focused around the high incidence of EGFR mutations in SCC found in certain regions of Asia and in Asian patients with SCC compared with Caucasian patients with SCC.29 For example, in one Chinese study of 163 pure SCC cases, EGFR mutations were identified in 18% of cases, significantly higher than in western countries where EGFR mutations occur in <5% of SCC cases.39,40 As a result, it was agreed to modify the text of the original ESMO ‘recommendation 1d’ to include a sentence suggesting EGFR testing might be performed for patients with SCC regardless of their smoking history in those regions where the incidence of EGFR-mutated SCC is high, as shown in the bold text below and in Table 1, to read as follows (100% consensus):
-
1d.
The molecular tests below are recommended in patients with advanced non-squamous-cell carcinoma.They arenot recommended in patients with a confident diagnosis ofpuresquamous-cell carcinoma, except inspecificcases, e.g. young (<50 years) patients, never (<100 cigarettes in a lifetime)/former light smokers (<15 pack-years, all kinds of tobacco) or long-time ex-smokers (quit smoking >15 years ago, all kinds of tobacco) [IV, A].In specific regions (e.g. India, Japan and China) with a high incidence of EGFR mutation in squamous-cell carcinoma, EGFR testing might also be performed regardless of smoking history [IV, B; consensus = 100%].
In the case of the original ESMO ‘recommendation 1e’ (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) it was felt that the second sentence regarding minimum testing was unnecessary. There was, however, some discussion around applicability and minimal testing where resources or material are limited. These will be discussed in part B of these guidelines below which covers their applicability in the different regions of Asia. Thus, the text was modified by removing the second sentence and as per the bold text below and in Table 1, to read as follows (100% consensus):
-
1e.
EGFR mutation status should be determined [I, A].Thetest methodology should provide adequate coverage of mutations in exons 18-21, including those associated with resistance to some therapies [III, A; consensus = 100%].
For the original ESMO ‘recommendation 1i’, which suggests using FISH for the detection of ROS1 translocations, with the possibility of using immunohistochemistry as a screening approach (see Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996), there was discussion regarding the fact that other assays can be used, such as next-generation sequencing (NGS) and RT–PCR. It was agreed (100% consensus) that a sentence should be appended to the original ESMO recommendation outlining the recommended assays for the detection of ROS1 translocations, as per the bold text below and in Table 1, to read as follows:
-
1i.
Testing for ROS1 rearrangements should be carried out [II, A]. IHC may be used as a screening approach [IV, A].Detection of a ROS1 translocation can be performed or verified by FISH, NGS or reverse transcription PCR [IV, A; consensus = 100%].
2. Staging and risk assessment—recommendations 2a-l
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 2a-b and 2d-g’ (Table 1), without change, and following discussion the revised ‘recommendations 2c, h and i’.
For the original ESMO ‘recommendation 2c’, it was pointed out that although most targeted therapies can affect QT interval, serious adverse events (SAEs) from prolonged QT are a very infrequent occurrence. However, the Pan-Asian panel of experts agreed that the use of electrocardiograms should be used to monitor the activity of the heart if a targeted therapy is known to cause adverse cardiac events. Thus ESMO ‘recommendation 2c’ was agreed without modification (100% consensus) to read as follows:
-
2c.
An electrocardiogram is required if the targeted therapy can cause adverse cardiac events, including rhythmic modifications (e.g. long QT) [IV, A; consensus = 100%].
While all the Pan-Asian panel of experts agreed that 18-fluoro-deoxyglucose positron emission tomography computed tomography (FDG–PET–CT) and brain imaging should be carried out for patients with oligometastatic disease which is the subject of the original ESMO ‘recommendation 2h’, there was some discussion around the second sentence and the GoR of the recommendation which reads as follows:
In the presence of a solitary metastatic site on imaging studies, efforts should be made to obtain a cytological or histological confirmation of stage IV disease [IV, A].
It was felt that, where possible, a biopsy for cytological and histological purposes should be taken in patients with suspected oligometastatic disease, but this is not always possible. As a result, the GoR was downgraded from ‘A’ to ‘B’, ‘strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended’, and the wording of the recommendation modified as per the text in bold below and in Table 1 (100% consensus), to read as follows:
-
2h.
FDG–PET–CT and brain imaging are recommended in patients suspectedof havingoligometastatic disease [IV, A]. In the presence of a solitary metastatic site on imaging studies, effortsmight be consideredto obtain a cytological or histological confirmation of stage IV disease [IV,B; consensus = 100%].
The Pan-Asian panel of experts agreed that, in the case of oligometastatic disease, the pathological evaluation of suspected mediastinal disease should only be carried out if the findings would likely affect the treatment options for the patient, i.e. in the case of N0 with suspicion of mediastinal disease that might change a radiation target volume. However, it was felt that clarification was needed regarding what was meant in the original ESMO ‘recommendation 2i’ by ‘mediastinal disease’. Therefore, for clarification, the text was modified, as below in bold and in Table 1 (100% consensus), to read:
-
2i.
For oligometastatic diseasewith the suspicion of mediastinal lymph node involvement, mediastinal disease should be pathologically proven if this potentially impacts the treatment plan [IV, A; consensus = 100%].
3. Management of advanced and metastatic disease—recommendations 3a-pp
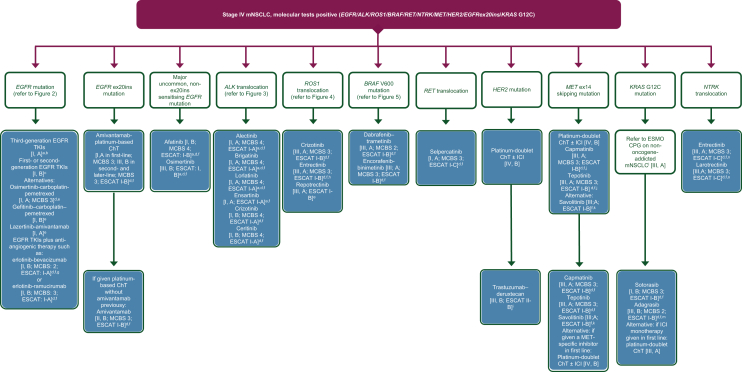
Figure 1 shows an algorithm for the treatment of stage IV NSCLC following positive findings on molecular tests adapted from the original ESMO Clinical Practice Guidelines34 and based on the discussions described below.
Figure 1.
Treatment algorithm for stage IV mNSCLC after positive findings on molecular tests. Purple: general categories or stratification; blue: systemic anticancer therapy. ChT, chemotherapy; CPG, Clinical Practice Guideline; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of molecular Targets; FDA, Food and Drug Administration; ICI, immune checkpoint inhibitor; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; TKI, tyrosine kinase inhibitor. aPreferred option(s). bAn example of an approved third-generation TKI is osimertinib [I, A; ESMO-Magnitude of Clinical Benefit (ESMO-MCBS) v1.1 score: 4; ESCAT: I-A]. Lazertinib is another third-generation EGFR TKI that has been approved in Korea for the first-line treatment of patients with EGFR mutations. cExamples of approved first- and second-generation TKIs include erlotinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], gefitinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], afatinib [I, B; ESMO-MCBS v1.1 score: 5; ESCAT: I-A] and dacomitinib [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]. dESMO-MCBS v1.1 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35eNot EMA approved. fESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34gEMA approved, not FDA approved. hPreferred over crizotinib in patients with brain metastases. iFDA approved; application for EMA approval withdrawn by the manufacturer. jFDA approved; not EMA approved in first line. kNot EMA or FDA approved. lA parallel ESMO CPG on non-oncogene-addicted mNSCLC is available at: https://www.esmo.org/guidelines/guidelines-by-topic/lung-and-chest-tumours.99mFDA approved; not EMA approved. nIf the patient has not been treated previously with a medicine that works in the same way as entrectinib. oFor patients who have no satisfactory alternative treatments.
EGFR-mutated NSCLC
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 3a, 3f, 3h-i’ and, following discussion, the revised ‘recommendations 3b-e, 3g, 3j-3n’ (Table 1).
The global randomised phase III FLAURA trial compared the third-generation EGFR tyrosine kinase inhibitors (TKI) osimertinib to either of the first-generation EGF TKIs gefitinib or erlotinib in 556 patients with previously untreated mNSCLC harbouring a classical activating EGFR (exon 19 deletion or exon 21 L858R mutation) mutation. The median overall survival (OS) was superior for the osimertinib group (38.6 months) compared with the comparator group [31.8 months; hazard ratio (HR of death 0.80, 95% confidence interval (CI) 0.64-1.00, P = 0.046], and grade ≥3 adverse events (AEs) were 42% in the osimertinib group compared with 47% in the comparator group.41 Two national subset analyses of the FLAURA trial, from China and Japan, reported improved progression-free survival (PFS) for the osimertinib groups compared with the comparator group (17.8 months compared with 9.8 months, respectively, for the Chinese subset; HR 0.56, 95% CI 0.37-0.85; and 19.1 months compared with 13.8 months, respectively, for the Japanese subset; HR 0.61, 95% CI 0.38-0.99).42,43 For the Chinese subset, the median OS for osimertinib was 33.1 months compared with 25.7 months for the comparator first-generation EGFR TKIs (HR 0.85, 95% CI 0.56-1.29, nominal P = 0.442). The Pan-Asian panel of experts agreed with the ESMO ‘recommendation 3b’ that third-generation EGFR TKIs, such as osimertinib, should be the preferred first-line option for the treatment of patients with classical activating EGFR mutations, especially for patients with central nervous system (CNS) metastases. However, there are other third-generation TKIs in development for the treatment of EGFR-mutated NSCLC.44 An example is the EGFR TKI lazertinib which has been approved in Korea for the treatment of patients with NSCLC harbouring EGFR T790M mutations that have previously received EGFR TKI therapy,45 based on the results of a phase I/II study in 78 patients with activating EGFR aberrations (exon 19 deletion, L858R mutation or T790M mutation), which had an overall response rate (ORR) of 57.9%, disease control rate (DCR) of 89.5% and a median PFS of 11.0 months.46 Furthermore, in the global, randomised phase III LASER301 trial which compared lazertinib with gefitinib in 393 untreated patients with locally advanced or metastatic NSCLC harbouring a classical EGFR mutation, lazertinib had a significantly longer median PFS (20.6 months compared with 9.7 months for gefitinib; HR 0.45, 95% CI 0.34-0.58, P < 0.001).47 Although there were no significant differences in the ORR (76.0% versus 76.1%, respectively) or DCR (both 93.9%) between the two groups, the median duration of response (DOR) was longer in the lazertinib group than the gefitinib group (19.4 months versus 8.3 months, respectively).47 Thus, it was felt that it was inadvisable to specifically name one third-generation TKI. As a result, ESMO ‘recommendation 3b’ was modified as per the text in bold below and in Table 1 (100% consensus) to read:
-
3b.
Third-generation EGFR TKIs (such as osimertinib) arethe preferable first-line treatment option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R), especially for patients with CNS metastases [I, A; ESMO-Magnitude of Clinical Benefit (ESMO-MCBS) v1.1 scorefor osimertinib: 4; ESCAT: I-A; consensus = 100%].
Although third-generation EGFR TKIs are the preferred first-line treatment for patients with classical activating EGFR-mutated mNSCLC, first- and second-generation EGFR TKIs have proven efficacy in these patients and are another first-line treatment option (ESMO ‘recommendation 3c’) and may be more widely available due to differences in reimbursement policies and regional approvals (see the applicability section B below). Again, as with ‘recommendation 3b’, instead of naming specific agents, it was deemed preferable to have more generic wording around the generation and class of targeted therapy. As a result, ESMO ‘recommendation 3c’ was modified as per the text in bold below and in Table 1 (100% consensus) to read:
-
3c.
First- or second-generation EGFR TKIs (such aserlotinib[I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], gefitinib[I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], afatinib[I, B; ESMO-MCBS v1.1 score: 5; ESCAT: I-A]and dacomitinib[I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]) are other first-line single-agent treatment options[consensus = 100%].
However, there was a great deal of discussion around ESMO ‘recommendation 3d’ and alternative first-line treatment options for patients with mNSCLC that have classical activating EGFR mutations. The first-line use of the first-generation EGFR TKI gefitinib combined with carboplatin and pemetrexed is supported by an Indian randomised phase III trial in patients with advanced NSCLC harbouring an EGFR-sensitising mutation who were scheduled to receive first-line palliative therapy. In this study, 350 patients were randomised to receive either gefitinib alone or in combination with carboplatin-pemetrexed. The addition of the chemotherapy (ChT) produced a significantly longer estimated median PFS (16 months) compared with the monotherapy arm’s median PFS (8 months; HR 0.51, 95% CI 0.39-0.66, P < 0.001).48 With a median follow-up time of 17 months, the estimated median OS was not reached for patients treated with gefitinib-carboplatin-pemetrexed compared with an estimated median OS of 17 months for those patients receiving gefitinib alone (unadjusted HR 0.45, 95% CI 0.31-0.65, P < 0.001) and the estimated 18-month OS rates were 74.3% and 48.7%.48 The Japanese phase III NEJ009 study compared gefitinib alone with gefitinib plus carboplatin-pemetrexed in 345 previously untreated patients with EGFR-mutated NSCLC and reported an ORR of 84% for patients in the combination arm compared with 67% for patients in the gefitinib monotherapy arm (P < 0.001) and an improved median PFS for patients in the combination arm (20.9 versus 11.2 months) with an HR of 0.49 (95% CI 0.39-0.62, P < 0.001).49 However, although the initial report of the study also found an improvement in OS for patients treated with gefitinib-carboplatin-pemetrexed compared with gefitinib alone, this was not the case when the updated results were published with an updated median OS of 49.0 months for the combination arm and 38.5 months for the gefitinib arm (HR 0.82, 95% CI 0.64-1.06, P = 0.127).49,50 Consistent across both trials was that patients in the combination arm had a higher percentage of grade ≥3 treatment-related toxicities. In the Indian study, grade ≥3 toxicities were reported in 49.4% of patients treated with gefitinib alone and 75% of patients treated with gefitinib-carboplatin-pemetrexed (P < 0.001) with clinically relevant serious toxicities of 50.6% and 58.2% (P < 0.001), respectively. The primary causes of excess toxicity in the combination arm were myelosuppression, nephrotoxicity and hypokalaemia, and 16.7% of patients discontinued pemetrexed in the combination arm as a result of toxicities compared with 1.1% of patients in the monotherapy arm.48 In the Japanese study, the rate of grade ≥3 treatment-related AEs (TRAEs) was lower in the monotherapy group (31.0%) compared with the combination group (65.3%), with neutropenia, anaemia and thrombocytopenia being more common in the combination group, and the rate of treatment discontinuation due to AEs was 10.7% in the combination group compared with 9.9% in the monotherapy group.49 The results of these studies support the use of gefitinib in combination with carboplatin-pemetrexed and the Pan-Asian panel of experts agreed with its inclusion as another first-line option for the treatment of advanced EGFR-mutated NSCLC.
Other first-line options were also discussed. The first of these was based on the international phase III FLAURA-2 trial in patients with classical activating EGFR-mutated advanced NSCLC where 557 patients were randomised to receive either the third-generation EGFR TKI osimertinib alone or in combination with pemetrexed plus either carboplatin or cisplatin.51 The addition of ChT to osimertinib led to an improved ORR of 83% compared with 76% for patients in the osimertinib monotherapy arm. The median PFS was significantly longer for patients in the combination arm (25.5 months) compared with those in the monotherapy arm (16.7 months; HR 0.62, 95% CI 0.48-0.80, P < 0.001). The respective PFS rates at 24 months were 57% and 41%. Furthermore, in all subgroup analyses by ethnicity, estimates of PFS were more favourable, including among Chinese patients (HR of progression 0.49, 95% CI 0.30-0.81), non-Chinese Asian patients (HR of progression 0.76, 95% CI 0.53-1.09) and non-Asian patients (HR of progression 0.55, 95% CI 0.37-0.83), for patients treated with osimertinib plus ChT compared with patients treated with osimertinib alone.51 In a second interim analysis of the updated OS data (41% data maturity), a trend benefit was reported for OS with the median OS not reached for the combination and 36.7 months for osimertinib monotherapy (OS HR 0.75, 95% CI 0.57-0.97).52 In this analysis, the post-progression analysis showed a benefit for the addition of ChT to osimertinib across the prespecified endpoints of time to first subsequent treatment (HR 0.73, 95% CI 0.56-0.94), time to progression on second-line therapy (HR 0.70, 95% CI 0.52-0.93) and time to second subsequent treatment (HR 0.69, 95% CI 0.57-0.97).52 Both grade ≥3 AEs and SAEs were higher in patients treated with osimertinib-carboplatin-pemetrexed (64% and 38%, respectively) compared with patients treated with osimertinib alone (27% and 19%, respectively) and the most common AEs in the combination group were haematologic, including anaemia and neutropenia. AEs led to discontinuation of osimertinib in 11% of patients in the combination arm compared with 6% of patients in the monotherapy arm. Fatal AEs considered possibly related to study treatment were reported in five patients in the combination arm and one patient in the osimertinib monotherapy arm.51 Another alternative first-line option for the treatment of patients with classical activating EGFR mutations that was discussed was the combination of the third-generation TKI lazertinib with the EGFR-MET bispecific antibody amivantamab. This combination was assessed in the global phase III MARIPOSA trial where 1074 patients with treatment-naïve EGFR-mutated advanced NSCLC were randomised 2 : 2 : 1 to receive either lazertinib plus amivantamab or osimertinib or lazertinib alone.53 At a median follow-up of 22.0 months, the median PFS was 23.7 months for patients in the lazertinib-amivantamab arm compared with 16.6 months for those patients in the osimertinib arm (HR 0.70, 95% CI 0.58-0.85, P < 0.001) and, although obtained from an unstratified proportional hazards model which should not be used to infer definitive treatment effects, in a subgroup analysis, estimates of PFS were more favourable for Asian patients treated with lazertinib plus amivantamab compared with osimertinib (HR 0.67, 95% CI 0.52-0.86) as well as for patients with an EGFR exon 19 deletion (HR 0.65, 95% CI 0.51-0.85).53 With a median follow-up of 31.1 months, an updated analysis requested by health authorities found 44% of patients in the lazertinib-amivantamab arm were still receiving treatment compared with 34% in the osimertinib arm.54 The initial subgroup analysis found PFS estimates were more favourable for patients with brain metastases (HR 0.69, 95% CI 0.53-0.92), while the later updated analysis reported a nonsignificant trend for longer median intracranial PFS for patients in the lazertinib-amivantamab group compared with those in the osimertinib group (24.9 months versus 22.2 months, respectively; HR 0.82, 95% CI 0.82-1.09, nominal P = 0.165).53,54 In a secondary analysis of biomarkers of high-risk disease, lazertinib plus amivantamab treatment significantly improved the median PFS compared with osimertinib treatment across all high-risk subgroups analysed including among patients with TP53 co-mutations (HR 0.65, 95% CI 0.48-0.86, P = 0.003) and in patients with liver metastases at baseline (HR 0.58, 95% CI 0.37-0.91, P = 0.017).55 An interim OS analysis found that, in the lazertinib plus amivantamab group, 82% and 74% of patients were alive at 18 and 24 months, respectively, compared with 79% and 69%, respectively, in the osimertinib group and while not formally tested, the updated analysis found that the median OS was not estimable in the lazertinib-amivantamab cohort compared with 37.3 months in the osimertinib cohort (HR 0.77, 95% CI 0.61-0.96, P = 0.019).53,54 Although the ORR for the lazertinib-amivantamab group (86%) was similar to that of the osimertinib group (85%), the median DOR for responders was higher in the lazertinib-amivantamab group (25.8 months) compared with the osimertinib group (16.8 months).53,56 Furthermore, the median PFS after subsequent therapy (PFS2) for patients treated with lazertinib-amivantamab was not estimable compared with 32.4 months for patients treated with osimertinib (HR 0.73, 95% CI 0.59-0.91, P = 0.004). A higher percentage of patients experienced grade ≥3 or higher AEs in the amivantamab-lazertinib group (75%) compared with the osimertinib group (43%). Serious AEs were also higher in the amivantamab-lazertinib group (49%) compared with the osimertinib group (33%). The most common grade ≥3 TRAEs across the study were rash and paronychia and the combination of lazertinib plus amivantamab had higher rates of EGFR- and MET-related AEs including hypoalbuminaemia and peripheral oedema, as well as venous thromboembolism compared with osimertinib, and the rate of discontinuation of all study treatments in the combination arm due to TRAEs was 10%.53 As a result of these studies, ESMO ‘recommendation 3d’ was split into three (‘recommendations 3d-i-iii’), including the original ESMO ‘recommendation 3d’ (renumbered ‘recommendation 3d-ii’) and two newly proposed recommendations ‘recommendations 3d-i and 3d-ii’. The discussion then turned to the LoE and GoR for the new recommendations. It was agreed that the LoE for each was I, ‘Evidence from at least one large randomised, controlled trial of good methodological quality (low potential for bias) or meta-analyses of well-conducted randomised trials without heterogeneity’ (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103996, which describes the evaluation behind LoE and GoR scores). For the GoR, the Pan-Asian panel of experts had to weigh up the strong evidence of efficacy with the disadvantages of the AEs and toxicity associated with each treatment regimen. It was agreed that the original ‘recommendation 3d’, gefitinib-carboplatin-pemetrexed, should remain with a GoR of ‘B’, ‘strong or moderate evidence for efficacy but with a limited clinical benefit, generally recommended’. For the two new recommendations, it was decided that, although osimertinib-carboplatin-pemetrexed and lazertinib-amivantamab have associated toxicity profiles which need to be carefully monitored for and managed, the efficacy, as measured by the greatly improved PFS for both regimens, was sufficient to warrant a GoR of ‘A’, ‘strong evidence for efficacy with a substantial clinical benefit, strongly recommended’. This was agreed with 100% consensus by the Pan-Asian panel of experts and ‘recommendation 3d’ was split into three, to read, with changes shown in bold below and in Table 1, as follows:
-
3d-i
Another first-line optionfor patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R) is osimertinib combined with carboplatin-pemetrexed [I, A; ESMO-MCBS v1.1 score: 3; not EMA approved]. Alternatively, another option is 3d-ii or 3d-iii.
-
3d-ii
Another first-line option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R)is gefitinib combined with carboplatin-pemetrexed [I, B; not EMA approved].
-
3d-iii
3d-iii Another first-line option for patients with a classical activating EGFR mutation (exon 19 deletion or exon 21 L858R) is lazertinib combined with amivantamab [I, A; not EMA approved].
The combination of anti-angiogenic therapies in combination with EGFR TKIs in previously untreated patients has been assessed in several clinical trials. The Japanese NEJ026 randomised phase III trial assessed the first-generation EGFR TKI erlotinib alone or in combination with the anti-angiogenic agent bevacizumab in 228 chemotherapy-naïve patients with advanced NSCLC harbouring an EGFR mutation. In the interim analysis, the median PFS for patients in the erlotinib plus bevacizumab group was 16.9 months compared with 13.3 months for patients in the erlotinib monotherapy group (HR 0.605, 95% CI 0.417-0.877, P = 0.016).57 With a median follow-up of 39.2 months, there was, however, no significant difference between the two groups for median OS (50.7 months for the combination group compared with 46.2 months for the monotherapy group; HR 1.007, 95% CI 0.681-1.490, P = 0.97).58 An improved PFS was also reported in the Chinese ARTEMIS-CTONG1509 randomised phase III trial which compared the first-generation EGFR TKI erlotinib alone or in combination with the anti-angiogenic agent bevacizumab in 311 untreated patients with advanced NSCLC where, for patients in the erlotinib-bevacizumab arm, the median PFS was 17.9 months compared with 11.2 months for patients in the erlotinib monotherapy arm (HR 0.55, 95% CI 0.41-0.73, P < 0.001). The reported OS data remained immature with 172/311 (55%) events recorded but there was no significant difference between the median OS for the combination arm (36.2 months) and the monotherapy arm (31.6 months; HR 0.92, 95% CI 0.69-1.23, P = 0.581).59 Consistent across both trials were the increased number of grade ≥3 AEs seen in the combination arm, with nine SAEs seen in the combination arm of the Japanese trial compared with five in the monotherapy arm,57 and, in the Chinese trial, 54.8% of patients in the bevacizumab plus erlotinib arm had grade ≥3 TRAEs compared with 26.1% of patients in the erlotinib monotherapy arm.59 Erlotinib has also been assessed in combination with the anti-vascular endothelial growth factor (VEGF)2 antibody ramucirumab or placebo in the randomised phase III RELAY trial in 449 previously untreated patients with EGFR-mutated metastatic disease.60 The median PFS was 19.4 months in the erlotinib-ramucirumab cohort and 12.4 months in the erlotinib-placebo cohort (stratified HR 0.59, 95% CI 0.46-0.76, P < 0.0001). Similar PFS results were seen in an East Asian subset analysis of this trial, with a median PFS of 19.4 months for erlotinib-ramucirumab compared with 12.5 months for erlotinib-placebo (HR 0.63, 95% CI 0.485-0.833, P = 0.0009) and the respective 1-year PFS rates were 72.4% compared with 52.2%.61 For the Southeast Asian patients, grade ≥3 TEAEs were more common in the erlotinib plus ramucirumab arm (70.7% of patients) compared with the erlotinib plus placebo arm (49.4% of patients).61 Although there was an increase in the toxicity with the addition of the anti-angiogenic agents bevacizumab or ramucirumab to erlotinib, the Pan-Asian panel of experts agreed, without modification, ESMO ‘recommendation 3e’ (100% consensus), which reads:
-
3e.
EGFR TKIs combined with anti-angiogenic therapy are additional first-line treatment options, including erlotinib-bevacizumab [I, B; ESMO-MCBS v1.1 score: 2; ESCAT: I-A; EMA approved, not FDA approved] or erlotinib-ramucirumab [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A; consensus = 100%].
There was some discussion around the efficacy of afatinib and osimertinib and the treatment of patients with uncommon non-exon 20 insertion sensitising EGFR mutations. Although it was accepted that osimertinib is better for treating brain metastases, there is currently no evidence regarding any differences in the efficacy between the two agents. In the Japanese non-randomised phase II UNICORN trial, osimertinib was assessed in previously untreated patients with NSCLC harbouring uncommon EGFR mutations.62 Of the 40 patients, half had G719X mutations and a quarter had S768I mutations in the EGFR gene. The ORR was 55.0%, the DCR was 90.0% and the median DOR was 22.7 months. The median OS was not reached and with a median follow-up time of 12.7 months, the median PFS was 9.4 months. Analysis based on whether the patients had solitary or compound uncommon EGFR mutations found that both the ORR (45.5% and 66.7%, respectively) and median PFS (5.4 months and 9.8 months, respectively) were shorter for patients whose tumours had solitary EGFR mutations. Patients with solitary EGFR mutations also had a shorter median DOR and median OS (22.7 months and 23.0 months, respectively) compared with patients with compound EGFR mutations (not reached for both).62 The Japanese randomised phase III ACHILLES/TORG1834 trial compared afatinib with pemetrexed in combination with either cisplatin or carboplatin in treatment-naïve NSCLC with a sensitising uncommon/compound EGFR mutation without an exon 20 insertion or de novo EGFR T90M mutation.63 A total of 109 patients were randomised 2 : 1 to receive afatinib or platinum-containing ChT followed by pemetrexed maintenance therapy. There was no significant difference in ORR between the two groups (61.4% for the afatinib group compared with 47.1% for the ChT group; P = 0.2069) but, with a median follow-up time of 12.5 months, the median PFS was 10.6 months for patients treated with afatinib compared with 5.7 months for patients treated with ChT (HR 0.422, 95% CI 0.256-0.694, P = 0.0007).63 The panel of Pan-Asian experts agreed that the LoE for the use of osimertinib for the treatment of patients with NSCLC harbouring uncommon EGFR mutations should remain ‘III’ but, because of the size of the ACHILLES/TORG1834, the LoE for afatinib should be changed to ‘I’. As a result, the text for ESMO ‘recommendation 3g’ was modified, as per the text in bold below and in Table 1 (100% consensus), to read as follows:
-
3g.
Afatinib[I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-B]or osimertinib [III, B; ESCAT: I, B] is a recommended treatment option for patients with a major uncommon, non-exon 20 insertion, sensitising EGFR mutation [consensus = 100%].
Third-generation EGFR TKIs, such as osimertinib, are designed to inhibit EGFR TKI-sensitising and EGFR T790M resistance mutations which can arise in 50% of patients who progress on earlier-generation TKIs.64,65 The randomised phase III AURA-3 trial compared osimertinib with platinum-based therapy plus pemetrexed in 419 patients with NSCLC harbouring EGFR T790M mutations that had progressed on a first-line EGFR TKI.66 With a median follow-up time of 8.3 months, the median PFS for patients in the osimertinib cohort was 10.1 months compared with 4.4 months for patients in the ChT cohort (HR after adjustment for Asian or non-Asian ethnicity 0.30, 95% CI 0.23-0.41, P < 0.001). The estimated PFS at both 6 and 12 months was greater for patients in the osimertinib cohort (69% and 44%, respectively) compared with the ChT cohort (37% and 10%, respectively). The ORR was also significantly better in the osimertinib cohort (71%) compared with the ChT cohort (31%; odds ratio 5.39, 95% CI 3.47-8.48, P < 0.001).67 Furthermore, in subgroup analysis, Asian patients (HR 0.32, 95% CI 0.24-0.44) and patients with CNS metastases (HR 0.32, 95% CI 0.21-0.49) were found to have a lower risk of progression on osimertinib compared with platinum-based therapy plus pemetrexed.67 The Pan-Asian panel of experts agreed with original ESMO ‘recommendation 3j’, but in keeping with previous amendments amended the wording to include any approved third-generation TKI for the treatment of EGFR T790M-mutated NSCLC and, based on the results for CNS metastases reported in the AURA-3 trial, chose to add a statement recommending that third-generation TKIs can be considered for cases of brain-only progression. The modified ESMO ‘recommendation 3j’, with changes shown in bold below and in Table 1 (100% consensus), reads:
-
3j.
Patients with T790M-positive resistance should receivethird-generation EGFR TKIs (such asosimertinib)[I, A; ESMO-MCBS v 1.1 score: 4; ESCAT: I-A]as second-line therapy, whereas T790M-negative resistancemightbe treated with platinum-based ChT [III, A].Third-generation EGFR TKIs (such as osimertinib) can be considered in the case of brain-only progression [III, B; consensus = 100%].
The discussion for ESMO ‘recommendation 3k’ and the use of genomic analysis following the acquisition of resistance to osimertinib and other third-generation TKIs focused on how this might be of benefit, especially because in many regions of Asia there are no approved therapies following failure of treatment with third-generation TKIs. Also, with NGS-based tests not reimbursed in many regions of Asia (for details see section B on the applicability of the recommendations below), it is likely that genomic analysis will have to be paid for in full by many patients. It was suggested, however, that as well as providing further insight into the mechanisms that drive resistance to third-generation EGFR TKIs, performing genomic analyses of patients’ samples following progression on third-generation EGFR TKIs might identify other mutations which could be therapeutically targeted. Indeed, several studies have identified mechanisms of resistance to both first- and second-line osimertinib, including targetable genomic aberrations such as MET amplification and mutations, HER2 amplification and mutations, NTRK fusions, RET fusion, ALK fusion, as well as mutations in genes of the RAS-MAP kinase pathway.64 As a result of these discussions, the text of ESMO ‘recommendation 3k’ was modified to align with the GoR which remained ‘C’, ‘Insufficient evidence for efficacy or benefit does not outweigh the risk of the disadvantages (adverse events, costs, …) optional’ (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103996), with changes shown in bold below and in Table 1 (100% consensus), to read:
-
3k.
Genomic analysis byplasma- or tissue-basedNGSmight be considered fora patient who develops resistance to a third-generation TKI(such as osimertinib)[III, C; consensus = 100%].
Although in the results of the survey (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) all of the Pan-Asian panel of experts agreed with ESMO ‘recommendation 3l’ which reads:
-
3l.
Platinum-doublet ChT is the SoC upon progression on osimertinib [III, A]. Clinical trial enrolment is encouraged, especially if a targetable resistance mechanism is identified [III, B],
their opinions given before the results of the randomised phase III MARIPOSA-2 trial were published. This trial compared amivantamab plus carboplatin-pemetrexed in combination with or without lazertinib with carboplatin-pemetrexed alone in 657 patients with EGFR-mutated NSCLC who had progressed on prior osimertinib treatment.68 The median PFS for patients treated with ChT alone was 4.2 months compared with 6.3 months for patients treated with amivantamab plus ChT (HR compared with ChT alone 0.48, 95% CI 0.36-0.64, P < 0.001) and 8.3 months for patients treated with amivantamab plus lazertinib and ChT (HR compared with ChT alone 0.44, 95% CI 0.35-0.56, P < 0.001). These findings were confirmed for Asian patients in an analysis of predefined subgroups, where the risk of disease progression or death for Asian patients treated with amivantamab plus ChT (HR 0.58, 95% CI 0.39-0.85) or amivantamab plus lazertinib and ChT (HR 0.51, 95% CI 0.37-0.71) was lower than for Asian patients treated with ChT alone.68 However, while grade ≥3 TEAEs and SAEs for patients in the ChT-alone arm were 48% and 20%, respectively, they were higher in both the amivantamab plus ChT arm (72% and 32% of patients, respectively) and the amivantamab plus lazertinib and ChT arm (92% and 52%), the latter of which necessitated a regimen change due to toxicity.68 Based on these findings, it was agreed that the combination of amivantamab plus carboplatin-pemetrexed should be the preferred standard of care (SoC) following progression on osimertinib treatment. It was recognised that platinum-based doublet regimens are still an option, particularly in those regions of Asia where amivantamab is not approved or available (see section B on the applicability of the recommendations below), but, because of the inclusion of amivantamab plus carboplatin-pemetrexed, the GoR for the use of platinum-doublet ChT in this setting was downgraded to ‘B’. Furthermore, with several studies with therapeutic strategies designed to overcome resistance to osimertinib and other third-generation EGFR TKIs,64 patients should be encouraged to enrol on to a relevant clinical trial. As a result, ESMO ‘recommendation 3l’ was modified, as per the text in bold below and in Table 1 (100% consensus) to read:
-
3l.
Platinumplus pemetrexedChTcombined with amivantamabis the SoC upon progression on osimertinib[I, A].Platinum-doublet ChT remains an option[III,B]. Clinical trial enrolment is encouraged, especially if a targetable resistance mechanism is identified [III, B; consensus = 100%].
There was a great deal of discussion around the use of the anti-programmed death-ligand 1 (PD-L1) antibody atezolizumab plus the anti-VEGF antibody bevacizumab in combination with carboplatin-paclitaxel for the treatment of patients who have progressed on EGFR TKIs. The Chinese randomised phase III Impower151 trial compared bevacizumab-carboplatin-pemetrexed with and without atezolizumab as a first-line treatment for 305 chemotherapy-naïve patients with metastatic non-squamous NSCLC.69 With a median follow-up of 14 months, the investigator-assessed PFS was 9.5 months for patients in the atezolizumab-bevacizumab-carboplatin-pemetrexed arm compared with 7.1 months for the bevacizumab-carboplatin-pemetrexed arm (stratified HR 0.84, 95% CI 0.65-1.09, P = 0.1838) and in a subgroup analysis, the PFS was similar between the arms for the EGFR-mutated/ALK-altered subgroup (10.4 compared with 7.0 months, respectively). The respective all-case AEs, grade 3-4 AEs and grade 5 AEs occurred in 99.3%, 66.4% and 5.9% of patients treated with atezolizumab-bevacizumab-carboplatin-pemetrexed compared with 100%, 61.4% and 6.5% of patients treated with bevacizumab-carboplatin-pemetrexed.69 In the Korean randomised phase III ATTLAS trial, atezolizumab plus bevacizumab and carboplatin-pemetrexed was compared with pemetrexed plus either carboplatin or cisplatin followed by pemetrexed maintenance in 228 patients with EGFR- or ALK-mutated NSCLC.70 Patients were randomised 2 : 1 into the atezolizumab-bevacizumab-ChT and ChT arms and the respective ORRs were 69.5% compared with 41.9% (P < 0.001). With a median follow-up time of 26.1 months, the median PFS was 8.48 months for patients treated with atezolizumab-bevacizumab-carboplatin-pemetrexed compared with 5.62 months for patients treated with doublet ChT (HR 0.62, 95% CI 0.45-0.86, P = 0.004). Both the median DOR (7.10 months for the atezolizumab-bevacizumab-carboplatin-pemetrexed arm compared with 7.06 months for the ChT-alone arm; HR 0.80, 95% CI 0.49-1.30, P = 0.345) and median OS (20.63 months for the atezolizumab-bevacizumab-carboplatin-pemetrexed arm compared with 20.27 months for the ChT-alone arm; HR 1.01, 95% CI 0.69-1.46, P = 0.975) were similar between the two cohorts. In a subgroup analysis, patients with prior first- or second-generation EGFR TKI treatment in the atezolizumab-bevacizumab-carboplatin-pemetrexed arm had a significantly longer median PFS than patients in the ChT arm (11.10 months compared with 5.62 months; HR 0.46, 95% CI 0.29-0.73, P < 0.001) although there was no significant difference in OS (28.91 months compared with 24.44 months, respectively; HR 1.23, 95% CI 0.68-2.20, P = 0.493). There was a higher incidence of both any-grade and grade ≥3 TRAEs in the atezolizumab-bevacizumab-carboplatin-pemetrexed arm (96.7% and 35.1%, respectively) compared with the ChT-alone arm (75.7% and 14.9%, respectively).70 In the Chinese randomised phase III ORIENT-31 trial, 476 patients with EGFR-mutated non-squamous NSCLC that had progressed on prior EGFR TKI therapy were randomised 1 : 1 : 1 to receive either the cisplatin-pemetrexed or the anti-programmed cell death protein 1 (PD-1) antibody sintilimab plus cisplatin-pemetrexed alone or in combination with the bevacizumab biosimilar IBI305. With median follow-up durations of 14.4 months for the ChT-alone group, 15.1 months for the sintilimab plus ChT group and 12.9 months for the sintilimab plus ChT and IBI305 group, the respective median PFS were 4.3 months compared with 5.5 months (HR compared with chemotherapy alone 0.72, 95% CI 0.55-0.94, two-sided P = 0.016) and 7.2 months (HR compared with chemotherapy alone 0.51, 95% CI 0.39-0.67, two-sided P < 0.0001).71 The median OS was 19.2 months for patients treated with ChT alone compared with 20.5 months for patients treated with sintilimab-cisplatin-pemetrexed and 21.1 months for patients treated with sintilimab-IBI305-carboplatin-pemetrexed. Grade ≥3 TRAEs occurred in 49% of patients in the ChT-alone arm, 41% of patients in the sintilimab-cisplatin-pemetrexed arm and 56% of patients in the sintilimab-IBI305-carboplatin-pemetrexed arm.71 Based on these results, the Pan-Asian panel of experts agreed to modify the ESMO ‘recommendation 3m’ to include bevacizumab biosimilars and other anti-PD-1 or anti-PD-L1 antibodies that have been approved for the treatment of NSCLC. The original recommendation had a statement that patients should have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 and have no prior immune checkpoint inhibitor (ICI) treatment but it was felt, for consistency, that this should be removed to give a modified ‘recommendation 3m’, with the changes shown in bold text below and in Table 1 (100% consensus):
-
3m.
The combination ofplatinum plus paclitaxel/pemetrexed ChT with an anti-PD-(L)1 and bevacizumab/biosimilarmay be considered as a treatment option for patientsfollowingEGFR TKI failure, no contraindication for ICIs and anti-angiogenic agent [III, B;for carboplatin-paclitaxel-atezolizumab-bevacizumabESMO-MCBS v1.1 score: 3; consensus = 100%].
Members of four of the Asian oncology societies disagreed with the use of single-agent ICIs for the treatment of patients who have progressed following EGFR TKIs and ChT, because they felt that most data showed no benefit of ICI monotherapy in patients with EGFR-mutated NSCLC and that there are risks of hyper progression. In a Taiwanese retrospective study of 74 patients with stage IIIB/IV NSCLC who were treated with nivolumab or pembrolizumab monotherapy, EGFR mutation was associated with a poorer response to treatment in a multivariate analysis (adjusted odds ratio 0.09, 95% CI 0.01-0.93, P = 0.043).72 Although there was a shorter PFS for patients with EGFR-mutated NSCLC compared with patients with wild-type EGFR NSCLC (1.3 months for EGFR-mutated NSCLC compared with 2.8 months EGFR wild-type NSCLC, respectively), this was not significant in multivariate analysis (HR 1.26, 95% CI 0.61-2.60, P = 0.534). There was also no significant difference in OS in univariate analysis (HR 1.07, 95% CI 0.50-2.26, P = 0.867).72 These findings for the impact of EGFR mutation status on OS were not confirmed in the 5-year results of the phase I KEYNOTE-001 study, which investigated pembrolizumab for patients with advanced NSCLC including 74 previously treated patients with EGFR-mutated tumours. In a subgroup analysis, it was found that the median OS was shorter for patients with EGFR-mutated NSCLC compared with EGFR wild-type NSCLC (6.0 months compared with 11.9 months) and associated with a lower 5-year OS rate (7.9% for EGFR-mutated compared with 16.4% for EGFR wild-type NSCLC).73 In a Chinese retrospective study of 99 patients assessing real-world evidence for the use of ICIs in patients with EGFR-mutated NSCLC who had developed resistance to EGFR TKIs, 20 patients were treated with ICI monotherapy. For these patients, the DCR was 40.0% which was significantly lower than for patients receiving ICI in combination with other agents (72.15%; P = 0.007). Patients treated with ICI monotherapy also had a significantly shorter median PFS (3.0 months; HR 0.54, 95% CI 0.32-0.92, log-rank P = 0.020) and median OS (7.4 months; HR 0.46, 95% CI 0.26-0.83, log-rank P = 0.009) compared with patients treated with an ICI in combination with other agents (5.2 months and 19.0 months, respectively).74 In the large, international retrospective IMMUNOTARGET study of 551 patients receiving ICI monotherapy for advanced NSCLC with at least one oncogenic driver alteration, 125 patients had EGFR mutations. The median PFS for the EGFR subgroup was 2.1 months which was lower than for the entire patient cohort (2.8 months; 95% CI 2.5-3.1) although PD-L1 positivity was significantly correlated with a longer PFS (2.8 months for patients with PD-L1-positive EGFR-mutated disease compared with 1.7 months for those with PD-L1-negative EGFR-mutated disease; P < 0.01). The ORR was 12.2% and the median OS was 10.0 months for patients with EGFR-mutated NSCLC.75 Results from a meta-analysis comparing outcomes for patients treated with ICIs compared with those treated with docetaxel found that although overall patients achieved an improved OS when treated with ICIs, this was not the case for patients with EGFR mutations who showed no significant difference in OS whether they were treated with ICI monotherapy or docetaxel (HR 1.05, 95% CI 0.70-1.55, P < 0.81, treatment–mutation interaction P = 0.03).76 Based on these results and because the GoR for ESMO ‘recommendation 3n’ is ‘C’, ‘insufficient evidence for efficacy or benefit does not outweigh the risk of the disadvantages (adverse events, costs, …) optional’, the Pan-Asian panel of experts accepted and agreed without modification with ESMO ‘recommendation 3n’ (100% consensus) which reads:
-
3n.
Single-agent ICIs may be considered as a treatment option only after progression on EGFR TKIs and ChT [IV, C; consensus = 100%].
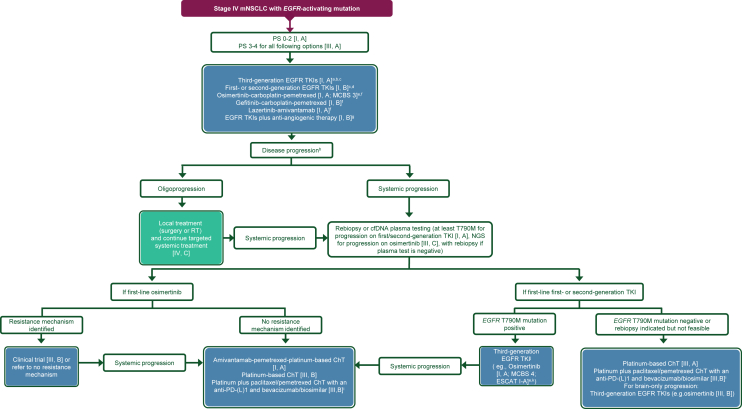
Figure 2 shows the treatment algorithm for the treatment of patients with stage IV NSCLC with an EGFR-activating mutation.
Figure 2.
Treatment algorithm for stage IV mNSCLC with EGFR-activating mutation. Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management. cfDNA, cell-free DNA; ChT, chemotherapy; CPG, Clinical Practice Guideline; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of Molecular Targets; FDA, Food and Drug Administration; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; NGS, next-generation sequencing; PD-L1, programmed death-ligand 1; PS, performance status; RT, radiotherapy; TKI, tyrosine kinase inhibitor. aPreferred option(s). bAn example of an approved third-generation TKI is osimertinib [I, A; ESMO-Magnitude of Clinical Benefit (ESMO-MCBS) v1.1 score: 4; ESCAT: I-A]. Lazertinib is another third-generation EGFR TKI that has been approved in Korea for the first-line treatment of patients with EGFR mutations. cRecommended treatment option for patients with a major uncommon, non-exon 20 insertion, sensitising EGFR mutation afatinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-B] or osimertinib [III, B; ESCAT: I, B]. In China, sunvozertinib is approved for the treatment of patients with advanced or mNSCLC with an EGFR exon 20 insertion. dExamples of approved first- and second-generation TKIs include erlotinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], gefitinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], afatinib [I, B; ESMO-MCBS v1.1 score: 5; ESCAT: I-A] and dacomitinib [I, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]. eESMO-MCBS v1.1 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35 fNot EMA approved. gESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34hEMA approved, not FDA approved. iPatients who have moderate radiological progression with ongoing clinical benefit may continue with EGFR TKIs [III, A]. jAn example of amivantamab-anti-PD-(L)1-bevacizumab-pemetrexed-platinum ChT is carboplatin-paclitaxel-atezolizumab-bevacizumab [III, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-A]. kLazertinib is a third-generation EGFR TKI that has been approved in Korea for the treatment of patients with EGFR T790M mutations who have failed prior EGFR TKI therapy.
ALK-rearranged NSCLC
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 3r-s’, without change and, following discussion, revised ‘recommendations 3o-q’ (Table 1). Original ESMO ‘recommendation 3t’ (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) was deleted. A new recommendation numbered ‘recommendation 3t’ was proposed and agreed upon (100% consensus).
Several studies have demonstrated that the second- and third-generation ALK TKIs, alectinib, brigatinib and lorlatinib, have superior efficacy over the first-generation ALK TKIs, crizotinib and ceritinib, for the treatment of ALK TKI-naïve patients with ALK-positive NSCLC.77, 78, 79, 80, 81 Included in these is the updated 5-year follow-up results of the randomised phase III CROWN study which investigated lorlatinib compared with crizotinib in patients with ALK-positive NSCLC where the median PFS for patients in the lorlatinib group had not been reached compared with a median PFS of 9.1 months for those patients in the crizotinib group (HR 0.19, 95% CI 0.13-0.27). Furthermore, among patients with brain metastases at baseline, the 5-year PFS rate was higher for the lorlatinib group (63%) compared with the crizotinib group (10%; HR 0.24, 95% CI 0.16-1.36) and the median time to intracranial progression was not reached in the lorlatinib group compared with 16.4 months in the crizotinib group (HR 0.06, 95% CI 0.03-0.12). Discussion for ‘ESMO recommendation 3o’ turned to the second-generation ALK TKI ensartinib and the results of the randomised phase III eXalt3 trial. In this study, ensartinib was compared with crizotinib in 290 patients with advanced ALK-positive NSCLC who have received no prior ALK TKI therapy and up to one prior chemotherapy regimen. For patients treated with ensartinib, the median PFS was 25.8 months which was significantly longer than the median PFS of 12.7 months for patients treated with crizotinib (HR 0.51, 95% CI 0.35-0.72, log-rank P < 0.001). Furthermore, for patients with brain metastases, the intracranial response rate was 63.6% for patients in the ensartinib arm compared with 21.1% for patients in the crizotinib arm.82 Based on these findings, the Pan-Asian panel of experts agreed to modify ESMO ‘recommendation 3o’ to include ensartinib (100% consensus), to read as shown in bold below and in Table 1:
-
3o.
Patients should be treated in the first-line setting with alectinib, brigatinib, ensartinibor lorlatinib [I, A; ESMO-MCBS v1.1 score: 4; ESCAT: I-A]. These options are preferred over crizotinib or ceritinib [I, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A; consensus = 100%].
The European randomised phase III ALUR study compared the second-generation ALK inhibitor alectinib with pemetrexed or docetaxel in 107 patients with ALK-positive NSCLC who had progressed on prior platinum-based doublet ChT and crizotinib treatment.83 The median investigator-assessed PFS was 9.6 months for patients in the alectinib arm compared with 1.4 months in the ChT arm (HR 0.15, 95% CI 0.08-0.29, P < 0.001). For patients with measurable CNS disease, those treated with alectinib had a significantly higher CNS ORR than those treated with ChT (54.2% compared with 0%, respectively; P < 0.001). Because of these results, the Pan-Asian panel of experts agreed with the use of alectinib for the treatment of patients with ALK-positive NSCLC who have progressed on or were intolerant to crizotinib treatment. They also felt that brigatinib should be included in this recommendation based on the results of the randomised phase III ALTA-3 trial comparing brigatinib with alectinib in 248 ALK-positive NSCLC patients who have progressed on crizotinib treatment.84 The median PFS for patients in the brigatinib arm was 19.3 months compared with 19.2 months for patients in the alectinib arm (HR 0.97, 95% CI 0.66-1.42, P = 0.8672) and the respective ORRs were 52% and 61%. Forty-four percent of patients in the brigatinib group had grade ≥3 TRAEs compared with 18% of patients in the alectinib group. The OS data were immature at the time of reporting but as a result of the PFS results, the Pan-Asian panel of experts agreed to modify ESMO ‘recommendation 3p’ to include brigatinib (100% consensus), to read as shown in bold below and in Table 1:
-
3p.
Alectinib[I, A; ESMO-MCBS score: 4; ESCAT: I-A] or brigatinib [I, A; ESMO-MCBS score: 4; ESCAT: I-A]is recommended in patients who progress on treatment with, or are intolerant to, crizotinib [consensus = 100%].
There was a great deal of discussion around the GoR for the use of lorlatinib, ceritinib or ensartinib as further options to treat crizotinib-resistant NSCLC. In the original ESMO ‘recommendation 3q’ the three newer-generation ALK inhibitors all had a GoR of ‘A’, ‘strong evidence for efficacy with a substantial clinical benefit, strongly recommended’ which was questioned. Lorlatinib was assessed in a phase II clinical trial in patients with advanced ALK-positive NSCLC which included 59 patients who had received prior treatment with crizotinib either with or without chemotherapy.85 The ORR was 73%, and the median PFS was 11.1 months and results were similar irrespective of the ALK mutation status.86 SAEs occurred in 7% of patients of which 3% discontinued treatment.85 In a Chinese phase II trial assessing lorlatinib in previously treated patients with ALK-positive NSCLC, one cohort, consisting of 67 patients, had been treated with prior crizotinib with an ORR of 70.1% (one-sided P < 0.0001, based on exact test for null hypothesis ORR ≤ 30%) and, with a median follow-up time of 11.0 months the median PFS was not reached with the probability of being event free at 12 months calculated to be 66.4%. Furthermore, of 36 patients with brain metastases, 29 (80.6%) had an intracranial response. Grade 3-4 all-causality AEs occurred in 54.1% of patients and grade 5 AEs were reported for 7.3% of patients although none were considered treatment related.87 In the Chinese phase I/II ASCEND-6 study, lorlatinib was assessed in 103 patients with ALK-rearranged NSCLC that had previously been treated with crizotinib.88 The ORR was 41.7% and, with a median follow-up time of 34 months, the median PFS was 7.2 months and the median OS was 17.5 months. Grade 3-4 AEs were reported for 65% of patients and SAEs occurred in 35% of patients. Seventeen of the 23 on-treatment deaths were due to study indication. In a Chinese phase II study ensartinib was assessed in 160 patients with ALK-positive crizotinib-resistant NSCLC, and 52% of patients in the full analysis set (147 patients) had an objective response, and 70% of 40 patients with measurable brain metastases had an intracranial objective response.89 Updated 5-year results for this study reported a median OS of 53.2 months.90 Finally, in a Chinese retrospective study comparing the efficacy of different sequential treatments following crizotinib progression in 128 patients with ALK-positive NSCLC, it was found that patients treated with second-generation ALK inhibitors had a superior median OS (58.5 months) compared with patients who received other systemic therapies (33.0 months; P < 0.001) with patients who received sequential lorlatinib found to have a significantly longer median OS (114.0 months) compared with patients treated with second-generation ALK TKIs (58.5 months; P = 0.020).91 As a result of these studies and their findings, it was agreed to give lorlatinib a GoR of ‘B’, with a GoR of ‘C’ for ceritinib and ensartinib. Also, as a result of the inclusion of brigatinib in the modified ‘recommendation 3p’ above, it was removed from ‘recommendation 3q’. Thus, ESMO ‘recommendation 3q’ was modified, as per the text in bold below and in Table 1 (100% consensus), to read:
-
3q.
Lorlatinib [II, B; ESMO-MCBS v1.1 score: 4; ESCAT: I-A], ceritinib [III, C; ESMO-MCBS v1.1 score: 4; ESCAT: I-A] and ensartinib [II,C]represent additional treatment options for patients with crizotinib resistance[consensus = 100%].
The use of atezolizumab-bevacizumab-paclitaxel-carboplatin following progression on lorlatinib was the subject of ESMO ‘recommendation 3t’ and the discussion centred on the Chinese randomised phase III IMpower151 trial that compared bevacizumab-carboplatin-pemetrexed with and without atezolizumab as a first-line treatment in 305 chemotherapy-naïve patients with metastatic non-squamous NSCLC.69 Although a numerical increase was observed with the addition of atezolizumab, there was no significant increase in the median investigator-assessed PFS (7.1 months for the without-atezolizumab group compared with 9.5 months for the with-atezolizumab group; stratified HR 0.84, 95% CI 0.65-1.09, P = 0.1838) and the PFS in the EGFR/ALK-positive subgroup was similar for the two groups (7.0 months for the without-atezolizumab group compared with 10.4 months for the atezolizumab group). As a result, the trial missed its primary PFS endpoint and because of this as well as the small number of patients with an ALK rearrangement (1.3%), the Pan-Asian panel of experts decided to delete original ESMO ‘recommendation 3t’ (100% consensus) which read:
-
3t.
Following progression on lorlatinib, atezolizumab-bevacizumab-paclitaxel-carboplatin can be considered [III, B; ESMO-MCBS v1.1 score: 3],
the Pan-Asian panel of experts felt that the ESMO recommendations were missing advice on genomic testing for patients who develop resistance to second- or third-generation ALK TKIs as is recommended for those patients who progress on third-generation EGFR TKIs (‘recommendation 3k’) and, as such, a new recommendation was proposed and agreed upon (Table 1; 100% consensus) which reads:
-
3t.
Genomic analysis by plasma- or tissue-based NGS might be considered for a patient who develops resistance to a second- or third-generation ALK TKI [III, C; consensus = 100%].
Figure 3 shows the treatment algorithm for the treatment of patients with ALK-positive stage IV NSCLC.
Figure 3.
Treatment algorithm for stage IV mNSCLC with ALK translocation. Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management. ALK, anaplastic lymphoma kinase; ChT, chemotherapy; CPG, Clinical Practice Guideline; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of Molecular Targets; FDA, Food and Drug Administration; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; NGS, next-generation sequencing; RT, radiotherapy; TKI, tyrosine kinase inhibitor. aESMO-MCBS v1.1 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35bPreferred option. cESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34
ROS1-rearranged NSCLC
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 3u-x’ (Table 1), without change. However, since the face-to-face meeting, results from the phase I/II TRIDENT-1 trial assessing the ROS1 TKI, repotrectinib, in ROS1 fusion-positive NSCLC were published, which led to a retrospective amendment to ESMO ‘recommendation 3w’.92
The TRIDENT-1 trial included 56 patients who were chemotherapy-naïve but had received prior treatment with at least one ROS1 TKI and 71 patients who had not previously been treated with a ROS1 TKI.92 For those patients who had received prior ROS1 TKI therapy, the ORR was 38% with a median DOR and median PFS of 14.8 months and 9.0 months, respectively.92 Of relevance for ESMO ‘recommendation 3w’, the ORR for patients who had not previously received a ROS1 TKI was 79% with a median DOR and median PFS of 34.1 months and 35.7 months, respectively. The most common any-grade TRAEs were dizziness, dysgeusia and paresthesia. Grade ≥3 TRAEs were experienced by 29% of all patients who received the recommended phase II dose, the most common of which were dizziness and increased blood creatine kinase levels.92 Based on these findings, the Food and Drug Administration (FDA) approved repotrectinib for the treatment of ROS1-positive NSCLC.93 As a result of both the data from the TRIDENT-1 study and the subsequent FDA approval, it was retrospectively agreed by the Pan-Asian panel of experts that the GoR for ESMO ‘recommendation 3w’, which was B, should be upgraded to A as is shown below and in Table 1 (100% consensus) and reads:
-
3w.
Repotrectinib, if available, is an option in the first-line setting but is not EMA approved [III, A; ESCAT: I-B; consensus = 100%].
Figure 4 shows the treatment algorithm for the treatment of patients with ROS1-rearranged stage IV NSCLC.
Figure 4.
Treatment algorithm for stage IV mNSCLC with ROS1 translocation. Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management. ChT, chemotherapy; CPG, Clinical Practice Guideline; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of Molecular Targets; FDA, Food and Drug Administration; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; RT, radiotherapy; TKI, tyrosine kinase inhibitor. aESMO-MCBS v1.1111 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35bESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG guideline authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34cPreferred over crizotinib in patients with brain metastases. dNot EMA approved.
BRAF-mutated NSCLC
Following discussion, the revised ‘recommendation 3z’ was accepted completely (100% consensus). ESMO ‘recommendation 3y’ was retrospectively revised and accepted completely (100% consensus) (Table 1).
Although the Pan-Asian panel of experts unanimously agreed with ESMO ‘recommendation 3y’ in the survey (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) which, as a result, was not discussed at the face-to-face meeting, it was later suggested that the combination of encorafenib-binimetinib should be included based on the single-arm phase II PHAROS trial in BRAF V600E-mutated mNSCLC.94 For treatment-naïve and previously treated patients, the respective ORRs were 75% and 46%, and the respective DORs were 40.0 months and 16.7 months. The median OS was not estimable for treatment-naïve patients and was 27.7 months for previously treated patients.95 Any-grade TRAEs were seen in 94% of patients, with grade 3 and grade 4 TRAEs seen in 38% and 3% of patients, respectively. The most common grade 3 TRAEs were aspartate transferase increased (7%) and alanine transferase increased (5%) and the grade 4 TRAEs were colitis, disseminated intravascular coagulation, increased gamma-glutamyl transferase and hyponatraemia.94 Furthermore, after the face-to-face meeting, a second phase II study (IFCT-1904) investigating encorafenib plus binimetinib in BRAF V600E-mutant NSCLC reported an ORR of 66.7%, a DCR of 85.7% and a median PFS of 11.1 months.96 It was thus retrospectively agreed to include encorafenib-binimetinib as a treatment option for patients with BRAF V600E-mutated mNSCLC and ESMO ‘recommendation 3y’ was amended to read as follows (100% consensus):
-
3y.
BRAF-MEK inhibition using dabrafenib-trametinib is recommended [III, A; ESMO-MCBS v1.1 score: 2; ESCAT: I-B].Another option is encorafenib-binimetinib [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B; consensus = 100%].
In the international retrospective IMMUNOTARGET study of 551 patients receiving ICI monotherapy for advanced NSCLC with at least one oncogenic driver alteration, 43 patients had BRAF-mutated disease [of which 17 (39.5%) had BRAF V600E mutations].75 The median PFS was 3.1 months for the BRAF-mutated subgroup and the 12-month PFS for this subgroup was 18.0 months and the ORR was 24.3%. There was, however, no subgroup analysis based on smoking history.75 In a Japanese retrospective study which investigated the impact of smoking history on the effectiveness of ICI therapy in 487 patients with NSCLC, it was discovered that there was no significant difference between non-smokers and smokers with respect to the ORR (63% compared with 51%, respectively; P = 0.43) and median PFS (10.2 months compared with 9.2 months, respectively; P = 0.81), and in a multivariate analysis of patients who received ICI combination therapy there was no significant association between PFS (HR 1.31, 95% CI 0.70-2.45, P = 0.40) or OS (HR 0.40, 95% CI 0.14-1.13, P = 0.083) and non-smoker status.97 Further analysis based on whether patients were treated with ICI as a monotherapy or in combination did reveal differences, however. For non-smokers treated with ICI monotherapy, there was a significantly lower ORR (10% for non-smokers compared with 26% for smokers; P = 0.002), shorter median PFS (1.8 months for non-smokers compared with 3.8 months for smokers; P < 0.001) and shorter median OS (8.0 months for non-smokers compared with 15.4 months for smokers; P = 0.026). However, for non-smokers treated with ICI combinations there was a significantly longer median OS compared with smokers (not reached compared with 26.3 months; P = 0.045), although there was no statistical significance between the two groups for ORR and median PFS.97 In a systematic review and meta-analysis of 12 randomised clinical trials involving 6497 patients with NSCLC, no significant differences were found in ICI efficacy, as measured by OS between current/former smokers and never smokers (interaction HR 0.77, 95% CI 0.69-0.86, I2 25, P = 0.21).98 It was thus agreed that reference to smoking in the first sentence of the original ESMO ‘recommendation z’ (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) should be omitted (100% consensus) so that the new recommendation reads (Table 1):
-
3z.
If patients have received BRAF-MEK inhibition in the first-line setting, they may be offered platinum-based ChT with or without immunotherapy in the second-line setting, regardless of their smoking history[IV, A]. For patients with a smoking history, immunotherapy with or without ChT should be considered as per the ESMO clinical practice guideline (CPG) on non-oncogene-addicted mNSCLC [IV, B; consensus = 100%].99
Figure 5 shows the treatment algorithm for the treatment of patients with stage IV NSCLC with a BRAF V600 mutation.
Figure 5.
Treatment algorithm for stage IV mNSCLC with BRAF V600 mutation. Purple: general categories or stratification; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management. ChT, chemotherapy, CPG, Clinical Practice Guideline; EMA, European Medicines Agency; ESCAT, ESMO Scale for Clinical Actionability of Molecular Targets; FDA, Food and Drug Administration; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mNSCLC, metastatic non-small-cell lung cancer; RT, radiotherapy. aESMO-MCBS v1.1111 was used to calculate scores for new therapies/indications approved by the EMA or FDA. The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-evaluation-forms).35bESCAT scores apply to alterations from genomic-driven analyses only.36 These scores were defined by the ESMO CPG authors and validated by the ESMO Translational Research and Precision Medicine Working Group.34
RET fusions
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 3cc, 3ff-gg, 3ii-jj’ without change and, following discussion, the revised ‘recommendations 3aa-bb, 3dd, 3hh and 3kk’ (Table 1). ESMO ‘recommendation 3cc’ was retrospectively revised following the meeting and accepted completely (100% consensus). The original ESMO ‘recommendation 3ee’ had a statement that trastuzumab deruxtecan was not approved by the EMA for HER2-positive mNSCLC but this has subsequently changed and as a result, the statement has been removed. The Pan-Asian panel of experts agreed to delete the original ESMO ‘recommendation 3ll’ (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996). A new recommendation numbered ‘recommendation 3ll’ was proposed and agreed (100% consensus).
Original ESMO ‘recommendation 3aa’ recommended the use of either selpercatinib or pralsetinib for the treatment of RET fusion-positive NSCLC. However, although pralsetinib has been granted approval by the EMA for the first-line treatment of patients with advanced RET fusion-positive NSCLC and by the FDA for the treatment of adult patients with mNSCLC harbouring a RET fusion,100,101 its future availability remains uncertain. Thus, it was agreed to remove pralsetinib from ESMO ‘recommendation 3aa’. The discussion moved on to selpercatinib which was assessed in the international randomised phase III LIBRETTO-431 trial in previously untreated patients with RET fusion-positive NSCLC in which 212 patients were randomised to receive either selpercatinib or platinum-based ChT with or without pembrolizumab.102 Patients treated with selpercatinib had a longer median PFS (24.8 months) compared with those treated with pembrolizumab-ChT (11.2 months; HR 0.46, 95% CI 0.31-0.70, P < 0.001), a greater ORR (84% compared with 65%, respectively) and longer median DOR (24.2 months compared with 11.5 months, respectively). As a result, it was agreed that the LoE for selpercatinib should be increased from ‘III’ to ‘I’ and ESMO ‘recommendation 3aa’ was modified, with the change shown in bold below and in Table 1 (100% consensus), to read:
-
3aa.
Treatment with selpercatinib is recommended as first-line therapy for patients with RET fusion-positive NSCLC [I, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-C; consensus = 100%].
Other oncogenic drivers for which targeted therapy is available
Discussion concerning ESMO ‘recommendation 3bb’ focused on whether a platinum-containing doublet ChT regimen with or without ICI was the best option for patients with an NTRK gene fusion or an EGFR exon 20 mutation. It was felt that NTRK inhibitors such as larotrectinib and entrectinib are a better first-line option for the treatment of patients with NTRK gene fusions.17 The randomised phase III PAPILLON trial compared the anti-EGFR antibody amivantamab plus ChT with ChT alone in patients with EGFR exon 20 insertions.103 The interim results are discussed in detail for the original ESMO ‘recommendation 3kk’ below, but this study found that amivantamab plus ChT was superior to ChT alone.103 Based on these findings it was agreed that amivantamab plus ChT was a better option for patients with NSCLC harbouring EGFR exon 20 insertions. As a result of this discussion, mention of NTRK gene fusions and EGFR exon 20 insertion mutations was removed from ‘recommendation 3bb’ which now reads as follows (100% consensus):
-
3bb.
Platinum-doublet ChT with or without ICIs is recommended as first-line therapy for patients with a MET amplificationorHER2 mutation [IV, B; consensus = 100%].
Although all the Pan-Asian panel of experts agreed with ESMO ‘recommendation 3cc’ in the initial survey (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996) and, as a result, it was not discussed at the face-to-face meeting, it was suggested retrospectively that as savolitinib has been approved for the treatment of patients with mNSCLC with MET exon 14 skipping alterations by China’s National Medical Products Administration (NSMPA) it should also be included.104 Approval was based on results from the single-arm confirmatory phase IIIb trial in 87 treatment-naïve and 79 previously treated Chinese patients with locally advanced or metastatic MET exon 14-mutated NSCLC which showed an ORR of 62.1% and 39.2% for the treatment-naïve and previously treated patients, respectively. The respective DCRs were 92.0% and 92.4%. Grade ≥3 TEAEs were seen in 60.2% of patients, with hepatic function abnormal (16.9%), alanine aminotransferase increased (14.5%) and aspartate aminotransferase increased (14.5%) being the most common.105,106 As a result of these findings and the approval of savolitinib by China’s NSMPA, it was agreed to retrospectively amend ESMO ‘recommendation 3cc’ to read as follows (100% consensus):
-
3cc.
Capmatinib and tepotinib in first line [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B; FDA approved, not EMA approved] or in second line [III, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B] are recommended in patients with a MET exon 14 skipping mutation.Savolitinib may also be an option [III, A; ESCAT: I-B; not EMA or FDA approved; consensus = 100%].
Although the Pan-Asian panel of experts all agreed with the original ESMO ‘recommendation 3dd’ (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103996), they felt that the wording should be modified for consistency with ‘recommendation 3z’ above with changes shown in bold below and in Table 1, to read as follows (100% consensus):
-
3dd.
Ifpatients have received a MET-specific inhibitorin the first-linesetting,they may be offeredplatinum-basedChT with or withoutimmunotherapy in the second-line setting, regardless of smoking history [IV, A]. For patients with a smoking history, immunotherapy with or without ChT should be considered as per the ESMO CPG on non-oncogene-addicted mNSCLC [IV, B; consensus = 100%].99
The use of platinum-doublet ChT as an option for treating patients with KRAS G12C-mutated NSCLC who have progressed on first-line ICI monotherapy was widely discussed for ESMO ‘recommendation 3hh’. Many members of the Pan-Asian panel of experts explained that the KRAS G12C inhibitors sotorasib or adagrasib, which are both approved for the second- and later-line treatment of patients with locally advanced or metastatic KRAS G12C-mutated NSCLC107,108 and are covered in ESMO ‘recommendations 3ii and 3jj’, are their preferred options for this indication but agreed, without modification, with ‘recommendation 3hh’ (100% consensus) which reads:
-
3hh.
Platinum-doublet ChT can be given to patients with KRAS G12C-mutated NSCLC and progression on first-line ICI monotherapy [III, A; consensus = 100%].
As mentioned above, the combination of amivantamab with ChT was assessed in the randomised phase III PAPILLON trial versus ChT alone in 308 treatment-naïve patients with EGFR exon 20 insertion mutations.103 A significantly longer median PFS was reported for the amivantamab plus ChT group (11.4 months) compared with the ChT-alone group (6.7 months; HR 0.40, 95% CI 0.30-0.53, P < 0.001), and the PFS at 18 months was 31% for patients in the amivantamab plus ChT group compared with 3% for patients in the ChT-alone group. Furthermore, the ORR was significantly higher for patients in the amivantamab plus ChT group (73%) compared with patients in the ChT-alone group (47%; rate ratio 1.50, 95% CI 1.32-1.68, P < 0.001).103 As a result of these findings, amivantamab plus carboplatin-pemetrexed has been granted EMA and FDA approval for the first-line treatment of patients with advanced or metastatic NSCLC with EGFR exon 20 insertion mutations.109,110 The Pan-Asian panel of experts agreed that amivantamab plus ChT should be included as a first-line option in ‘recommendation 3kk’ and the wording was modified, as per the text in bold below and in Table 1 (100% consensus) to read:
-
3kk.
Amivantamabcombined with platinum-based ChTis recommended forthetreatment of EGFR exon 20 insertion-mutated NSCLCin the first-line setting [I, A; ESMO-MCBS v1.1 score: 3; ESCAT: I-B] or in the second- or later-line settings[III, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-B].
In the phase I CHRYSALIS study, which investigated the use of amivantamab for the treatment of 81 patients with EGFR exon 20-mutated NSCLC who had progressed on prior platinum-based ChT, the ORR was 40% with a median DOR of 11.1 months and the median PFS was 8.3 months.111 Based on these data and the subsequent EMA and FDA approval that was granted for the use of amivantamab monotherapy to treat patients with advanced or metastatic NSCLC harbouring an EGFR exon 20 insertion mutation who had progressed on or after platinum-based chemotherapy,109,112 a new recommendation was proposed and agreed upon by the Pan-Asian panel of experts (100% consensus) which reads:
-
3ll.
Amivantamab monotherapy is an option after platinum-based ChT failure for the treatment of EGFR exon insertion-mutated NSCLC not previously exposed to amivantamab [II, B; ESMO-MCBS v1.1 score: 3; ESCAT: I-B; consensus = 100%].
The novel EGFR TKI mobocertinib has been withdrawn from the market because the phase III EXCLAIM-2 trial comparing mobocertinib with platinum-based chemotherapy in the first-line treatment for patients with NSCLC harbouring EGFR exon 20 insertion mutations failed to meet its primary PFS endpoint.113,114 As a result, the Pan-Asian panel of experts agreed (100% consensus) to delete the original ESMO ‘recommendation 3ll’ which read:
-
3ll.
Mobocertinib can be given as treatment of EGFR exon 20 insertion-mutated NSCLC failing prior therapy [III, C; ESMO-MCBS v1.1 score: 2; ESCAT: I-B; FDA approved, not EMA approved].
Follow-up, long-term implications and survivorship—recommendations 4a-d
The Pan-Asian panel of experts agreed with and accepted completely (100% consensus) the original ESMO recommendations, ‘recommendations 4a-d’ (Table 1), without change.
B. Applicability of the recommendations
Following the face-to-face meeting in Seoul, the Pan-Asian panel of experts agreed and accepted completely (100% consensus) the revised ESMO recommendations above for the diagnosis, treatment and follow-up of oncogene-addicted mNSCLC in patients of Asian ethnicity (Table 1). However, the applicability of each of the guideline recommendations is impacted by the individual drug and testing approvals and reimbursement policies for each region. The drug and treatment availability for the regions represented by the 10 participating Asian oncological societies is summarised in Supplementary Table S3, available at https://doi.org/10.1016/j.esmoop.2024.103996, and individually for each region in Supplementary Tables S4-S13, available at https://doi.org/10.1016/j.esmoop.2024.103996.
CSCO
The health care system in China is based on the horizontal coverage of the two basic medical insurance systems for urban employees and residents in urban and rural areas and it is supplemented by the vertical extension of six medical insurance policy systems, including accrued Medicare insurance, supplementary medical insurance, serious disease insurance, long-term care insurance, medical assistance and medical insurance for poverty alleviation. For biomarker-related diagnostic testing, 5% of patients will not receive any reimbursement and there is provincial variation regarding reimbursement of NGS panel testing with some covering around 90% of the cost whereas others do not provide any reimbursement. Although liquid biopsy testing is approved, it is not reimbursed. In China, 5.4% of patients have no insurance to cover drug costs and will pay entirely ‘out of pocket’. 26.3% of patients have employers/social insurance to help cover drug costs and the remaining 68.3% of patients have private insurance. In China, most targeted agents for the treatment of mNSCLC are approved, with the exception of the ROS1 inhibitor repotrectinib, the MET inhibitor capmatinib, the NTRK inhibitor larotrectinib, the anti-EGFR antibody amivantamab as well as the KRAS G12C inhibitors sotorasib and adagrasib. For those agents that are approved for the treatment of mNSCLC, there is no reimbursement for drugs targeting RET fusions, MET inhibitors or the anti-HER2 drug conjugate trastuzumab deruxtecan. For the remaining drugs, patients would usually pay 10%-30% of the costs. In China, sunvozertinib is approved for the treatment of patients with advanced NSCLC or mNSCLC with an EGFR exon 20 insertion and who have progressed on, or are intolerant to, platinum-based chemotherapy. The ALK inhibitor ensartinib is approved by the Chinese National Medical Products Administration (NMPA) for the treatment of patients with ALK-positive advanced NSCLC or mNSCLC who have progressed on, or are intolerant to, crizotinib treatment (Supplementary Table S4, available at https://doi.org/10.1016/j.esmoop.2024.103996). It is estimated that it takes about 3 years for drugs to be approved in NMPA following EMA or FDA approval, but once approved by the NMPA not all drugs will be reimbursed. One of the biggest limiting factors to accessing new treatments in China is that new drugs can only be applied from phase II clinical trials after completion of phase I clinical trials. The safety and effectiveness of new biomarker-related diagnostic tests is one of the limiting factors to accessing them.
ISHMO
The Jaminan Kesehatan Nasional (JKN), Indonesian Health Care System covers ∼95.75% of health expenses in Indonesia. The remaining health expenses are covered by private insurance and/or paid independently by the patient. The cancer drug reimbursement system for the JKN insurance is carried out based on the criteria listed in the National Drug Formulary (FORNAS). With regard to biomarker-related diagnostic tests for NSCLC, tests such as NGS panel testing and liquid biopsies are not covered by the JKN, but the JKN fully covers EGFR mutation tests for exon 18-21. ALK tests using IHC are not covered by the JKN but partial reimbursement is available from a pharmaceutical company. For the treatment of NSCLC, the JKN only covers the use of first-generation EGFR TKIs and afatinib, as well as the ALK inhibitor alectinib (Supplementary Table S5, available at https://doi.org/10.1016/j.esmoop.2024.103996). Drugs that have not been approved in Indonesia such as ROS1 inhibitors, trastuzumab deruxtecan and amivantamab can be accessed through the Special Access Scheme which can take 6-8 weeks after approval. There is limited integrated national data on the health expenses of NSCLC drugs in Indonesia. Data on TKI drugs usage in the Dharmais National Cancer Center found that 95% of costs were covered by the JKN and 5% by private insurance and/or out-of-pocket payments. In the JKN national health insurance, there is no regulation of partial coverage (co-payment) for cancer drugs that are restricted in the National Standard of Medication List (FORNAS) meaning patients have to pay for access to restricted cancer drugs or, based on the recommendations given by the third party via a patient assistance program (PAP), they may be subsidised by pharmaceutical companies. The role of the third party is to examine the patients’ financial capabilities. The PAP covers at least 30%-67% of the total cancer drug expenses depending on the type of TKI drugs. The average time from EMA/FDA approval to national approval in Indonesia is ∼3-4 years, depending on when the multinational innovator drug companies register the drugs in Indonesia. Once approved, the new drug can be made available immediately to patients, depending on the drug company’s supply chain. However, new drug reimbursement in the JKN will require it to be reviewed and listed in the FORNAS with considerations to be met including cost-effectiveness and budget impact. The biggest limiting factors to accessing the new treatment include the time taken for registration and approval of the new drug by the Badan Pengawas Obat dan Makanan (BPOM, the Indonesian Food and Drug Supervisory Agency), as well as the relatively high costs of new medications and the limited access to clinical trials in Indonesia. On the other hand, the biggest limiting factors to accessing new biomarker-related diagnostic tests and tools are the limited number of laboratories and human resources to carry out the tests, financial constraints due to the relatively high costs, as well as availability and regulations for registering commercial reagents for new biomarker tests.
ISMPO
In India, health care is provided by a central government scheme (the Ayushman scheme). There are also individual insurance schemes although many cover only basic health care services. Fifty percent of patients will have to pay entirely ‘out of pocket’ for biomarker-related diagnostic tests with only 5% of patients receiving reimbursement. For drug costs, many insurance schemes only cover basic health care services and 50% of patients pay entirely ‘out of pocket’ for their treatment. Five percent of patients’ drug costs are covered by private insurance and 3% by employers/social insurance. With the exception of first-generation EGFR TKIs, 30% of the costs of which are reimbursed, the costs of all other targeted treatments for mNSCLC are not reimbursed. Many targeted agents for the treatment of mNSCLC are not currently approved in India, including ROS1 inhibitors, RET fusion inhibitors and NTRK inhibitors although they are available in private practices in India (Supplementary Table S6, available at https://doi.org/10.1016/j.esmoop.2024.103996). Approval for new drugs in India can take from months to years after EMA or FDA approval has been given but once approved, a drug can be made available to patients within months. Cost is the biggest limiting factor to accessing new treatments. It is also the biggest limiting factor to accessing new biomarker-related diagnostic tests and tools along with access to the technology.
JSMO
Japan’s health care system is characterised by universal coverage, provided through a mandatory health insurance scheme that covers all residents. The system is funded by a combination of government subsidies, contributions from employers and employees and ‘out-of-pocket’ payments, with a strong emphasis on preventive care and equal access to treatment for all citizens. In Japan, reimbursement for health care costs is staggered depending on age, with adults aged under 70 required to pay for 30% of their treatment costs, those aged 70-74 pay 20% and those 75 and older, 10%. This applies to all treatment costs and diagnostic tests, including NGS testing and liquid biopsies when an adequate tumour sample is not available. Single and multiplex tests (approved multiplex tests include the NGS tests Oncomine DxTT and Lung Cancer Compact panel, and the PCR-based Amoy9in) are used differently for the diagnosis of NSCLC, depending on the situation and institution in Japan. Many of the targeted therapies for the treatment of mNSCLC are approved, although trastuzumab deruxtecan, NTRK inhibitors and sotorasib are approved for second-line treatment (Supplementary Table S7, available at https://doi.org/10.1016/j.esmoop.2024.103996). An application for amivantamab is currently being evaluated in Japan. It may take from several months to years for the Pharmaceuticals and Medical Devices Agency (PDMA) of Japan to assess the efficacy and safety of new drugs although there are some drugs that are approved first in Japan before anywhere else in the world. Once approved, it can take between 60 and 90 days for drugs to become available. The biggest limiting factor to accessing both new treatments and biomarker-related diagnostic tests is the delay in development and approval timing in Japan due to the lack of development in Japan. This can cause a drug lag which can be overcome or minimised if Japan is included in global drug development (i.e. as a part of the worldwide development).
KSMO
The health security system in Korea has two components: mandatory National Health Insurance (NHI) and medical aid. The major sources of NHI funding include contributions from those who are insured and government subsidies. The medical aid program is a form of public assistance that uses government subsidies to provide low-income groups with health care services and, usually, the patient only bears 5% of the cost for medications that are approved and reimbursed under the NHI. The NHI system provides health care coverage to all citizens, including for biomarker diagnostic tests, although some patients have to pay entirely ‘out of pocket’ for biomarker-matched drug costs. Most institutions in Korea carry out single-gene tests at the first diagnosis, although upfront NGS testing is increasing to minimise tissue loss in certain cases. NGS testing is approved and 50% reimbursed in NSCLC adenocarcinoma at the time point of diagnosis and disease progression during treatment. Multiplexed NGS liquid biopsies are only approved as laboratory-developed tests in Korea although the Cobas plasma EGFR test has been approved and 95% of the cost is reimbursed. Drugs for most classes of targeted therapy have been approved for the treatment of patients with mNSCLC in Korea. Lazertinib is approved in Korea for the first-line treatment of patients with EGFR-mutated tumours or in the second line for patients with EGFR T790M-mutated lung cancer. Although it varies, it can take ∼1 year for approval to be granted once EMA or FDA approval has been given and, once approved, it can take a few months for drugs to be made available in Korea. The biggest limiting factor to accessing new treatments in Korea is the cost and unmet medical needs.
MOS
Malaysia operates a two-tier health care system consisting of both a government-based universal health care system and a co-existing private health care system. Across the health care systems, mNSCLC is primarily treated by clinical oncologists, with a small fraction of patients treated by medical oncologists or chest physicians. Drugs in the reimbursed Ministry of Health formulary (also known as the Bluebook) are fully subsidised but each public hospital has a limited quantity allocated to it. Biomarker-related diagnostic tests, including liquid biopsies, with the exception of EGFR PCR testing and immunohistochemistry for ALK, are not reimbursed in Malaysia although pharmaceutical companies do provide partial support to cover the costs of NGS and full support for PD-L1 testing (Supplementary Table S9, available at https://doi.org/10.1016/j.esmoop.2024.103996). NGS testing may also be available to patients enrolled in clinical trials. There is no cost to patients for reimbursed cancer drugs in Malaysia. Beyond the reimbursed drug list, patients may access cancer drugs via out-of-pocket payment (40%), private insurance (40%) or state funding (20%). State funding is exclusively available to civil servants or pensioners. Most classes of targeted therapies for the treatment of mNSCLC are approved in Malaysia except lazertinib, ensartinib, RET fusion inhibitors and trastuzumab deruxtecan, the latter of which has been withdrawn from the market. Osimertinib is only reimbursed in the second-line setting for patients with EGFR T790M mutations. It takes ∼2 years for drug approval in Malaysia following EMA or FDA approval. Once approved, it can take ∼3 months for drugs to become available. The biggest limiting factors to accessing new drugs are the high costs of treatment and the long delay in local drug approval and reimbursement. For new biomarker-related tests, the biggest factors limiting access are high costs, the long turnaround time because most tests are conducted externally and the lack of development in the public health care system.
PSMO
In the Philippines, health care support is limited, and the majority of patients have to pay entirely ‘out of pocket’ for biomarker-related diagnostic testing (99%) and drug costs (97%). There is strong competition between companies for diagnostic tests because approval is not required in the Philippines; this has helped to bring down costs but has come at the expense of accuracy due to a lack of quality assurance. Only 3% of patients have insurance, as covered by private (2%) or employers/social (1%) insurance. Most (>50%) of the patients decide not to proceed with NGS panel tests because of ‘out-of-pocket’ costs and, although EGFR and ALK testing are covered (liquid biopsy EGFR testing is fully subsidised by the pharmaceutical industry), most patients choose not to do the test because of the high drug costs. Furthermore, for patients who have actionable driver mutations, >50% of patients opt to receive chemotherapy as they will not be able to afford the recommended oral TKI since the cost of chemotherapy is partly covered by government health insurance while the cost of targeted therapy is not. Most classes of targeted therapies for the treatment of mNSCLC are not approved in the Philippines with only EGFR TKIs, including osimertinib but not dacomitinib, and early and newer-generation ALK inhibitors approved (Supplementary Table S10, available at https://doi.org/10.1016/j.esmoop.2024.103996). The usual patient’s ‘out-of-pocket’ contribution for a reimbursed cancer drug ranges from 75% to 100%. In the Philippines it can take between 6 and 12 months for drug approval once it has been approved by the EMA or FDA and it can take 3-6 months once approval has been given for drugs to become available. The biggest limiting factor to accessing new treatments in the Philippines are cost, local approval and testing. Cost is also the biggest limiting factor to accessing new biomarker-related tests.
SSO
The health care system of Singapore uses a co-payment model where approved drugs on the Cancer Drug List can be paid for out of national insurance and pension savings. Additional costs are covered on a means-tested basis which means ‘out-of-pocket’ payments for reimbursed cancer drugs vary from patient to patient. Although biomarker-related diagnostic tests are typically not reimbursed, such diagnostics may be reimbursed if carried out in an inpatient setting. Although all patients’ drug costs are covered, to some extent, by national insurance and pension savings, 30%-50% of patients also have private health care insurance to mitigate costs of treatment. All classes of targeted therapy for the treatment of oncogene-driven mNSCLC are approved in Singapore with the exception of RET fusion inhibitors, although selpercatinib approval is expected soon (Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2024.103996). Following EMA or FDA approval, it can typically take between 1 and 2 years for approval to be granted in Singapore and a further 6 months, once approval has been given, for the drugs to be available. The time to approval is one of the biggest limiting factors for access to new treatments in Singapore. Cost is also a factor that can limit access to new treatments as well access to new biomarker-related diagnostic tests.
TOS
Taiwan has a single-payer NHI system, providing comprehensive coverage for its citizens. The NHI is funded through premiums, government contributions and co-payments, ensuring universal access to health care services and fostering a competitive health care market. This means that the costs for all reimbursed cancer drugs are usually covered, and most diagnostic tests are either partially or fully covered; this includes NGS panel tests (partially reimbursed) which are typically provided following testing for EGFR if the result comes back as wild-type. There is no reimbursement for liquid biopsy tests. Most drug costs are covered by NHI (90%) with the remaining 10% being covered either by private insurance (5%) or, for those without insurance, by the patients themselves (5%). All classes of targeted therapy for the treatment of mNSCLC are approved in Taiwan, with the exception of the ROS1 inhibitor repotrectinib and KRAS G12C inhibitor adagrasib (Supplementary Table S12, available at https://doi.org/10.1016/j.esmoop.2024.103996). The average time for a drug to be approved in Taiwan following EMA or FDA approval is 9-18 months. After a drug receives regulatory approval for clinical use, it can be available for use in patients within a few months. However, the reimbursement phase, where the drug’s cost is covered by health insurance, often involves a more prolonged process. This phase may require additional assessments, negotiations on pricing and the establishment of guidelines for the drug’s reimbursement, contributing to a timeline of 1-2 years or even longer before widespread coverage is achieved. Cost is the biggest limiting factor to accessing new treatments and accessing new biomarker-related diagnostic tests because if the cost is too high it becomes difficult for health insurance to cover the expenses.
TSCO
In Thailand 5% of patients have private insurance and 30% of patients have employers/social insurance, but patients do not pay for all their treatment costs. There are three major reimbursement schemes: universal coverage, social security and government officer (CSMBS). These differ in terms of reimbursement for the treatment of advanced NSCLC in Thailand, with greater reimbursement for treatment for those covered by CSMBS, which covers all single and double ChT treatments but pemetrexed is allowed for second- or later-line treatment only. Universal coverage and social security do not cover pemetrexed or vinorelbine. The CSMBS scheme also covers the costs of osimertinib (for patients with EGFR T790M-mutated NSCLC), ALK inhibitors (ceritinib and brigatinib only) and atezolizumab (in the second-line setting), together with pembrolizumab (in the first-line setting for tumours with ≥50% PD-L1 expression). For reimbursed cancer drugs, which include the EGFR TKIs, erlotinib and osimertinib (second-line with reimbursement through the CSMBS), and the ALK inhibitors ceritinib and brigatinib (first-line), all drug costs are covered. All classes of targeted therapy for mNSCLC are approved in Thailand, but most patients have to pay the full costs of the drugs with no reimbursement. In Thailand, approval for new drugs can take 12-24 months following EMA or FDA approval and, once approved, it can take a further 6-12 months for the drugs to become available for patients. The costs and reimbursement are the biggest limiting factors to accessing new drugs and new biomarker-related diagnostic tests.
Conclusions
The results of the voting by the Asian experts both before and after the face-to-face meeting in Seoul showed 73.8% concordance with the ESMO recommendations for the treatment of patients with oncogene-addicted NSCLC (Supplementary Table S2 and Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103996). These recommendations therefore constitute the consensus clinical practice guidelines for the treatment of patients with oncogene-addicted mNSCLC in Asia. The variations in the availability for the patients of diagnostic testing, drugs and therefore treatment possibilities, between the different regions represented, reflect the differences in the organisation of their health care systems and their reimbursement strategies, and will have a significant impact on the implementation of the scientific recommendations in certain regions. Thus, policy initiatives are advised, based on this guideline document, in order to improve the access of all oncogene-addicted mNSCLC patients across all the Asian regions.
Acknowledgements
The authors would like to thank the leaders of ESMO and KSMO for their support in facilitating this face-to-face meeting. They would also like to thank Klizia Marinoni and Fiona Perdomo from the Scientific and Medical Division of ESMO for the project management and scientific coordination, Akhil Kapoor of ESMO Leaders Generation Programme for his participation, Zarina Othman and Aries Low from the ESMO Singapore Office for the logistical support and Minhee Kim of KSMO for the logistic organisation of the face-to-face meeting of experts. Nicola Latino, ESMO Head MSD of Scientific Affairs, is acknowledged for her contribution in confirming the ESMO-MCBS scores. Svetlana Jezdic, ESMO Medical Affairs, is acknowledged for her contribution in confirming the ESCAT scores. Anne Kinsella and Nick Davies of Cancer Communications and Consultancy Ltd, Cheshire, UK are acknowledged for their contribution to the preparation of the manuscript.
Funding
All costs relating to this consensus conference were covered by the ESMO and KSMO from central dedicated funds (no grant number is applicable). There was no external funding of the event or the manuscript production.
Disclosure
SHL declares speaker’s engagement from Amgen, AstraZeneca/MedImmune, Eli Lilly, Merck Sharp & Dohme, Roche and Yuhan; advisory board/expert testimony role from Abion, AstraZeneca/MedImmune, BeiGene, BMS/Ono, Daiichi Sankyo, Eli Lilly, IMBdx, ImmuneOncia, Janssen, Merck (German), Merck Sharp & Dohme, Novartis, Pfizer, Roche and Takeda; institutional research grant from AstraZeneca, Lunit and Merck Sharp & Dohme. JM declares speaker’s engagement from AstraZeneca, Boehringer Ingelheim, Bristol-Meyers Squibb, MSD, Novartis and Roche; expert testimony role from AstraZeneca, Boehringer Ingelheim, MSD and Novartis; travel grant from Ipsen. TMK declares consultancy/advisory board role for AstraZeneca, Boryng, Daiichi Sankyo, Janssen, Novartis, Regeneron, Samsun Bioepis and Takeda; non-renumerated PI role from AbbVie, Amgen, AstraZeneca/MedImmune, Bayer, BeiGene, BeyondBio Inc., Black Diamond Therapeutics, Blueprint Medicines Corporation, Boehringer Ingelheim, Boryung, Dizal Pharmaceutical, Fore Biotherapeutics, Genmab, Hanmi, Incyte Corporation, Janssen, Merk Serono, Merck Sharp & Dohme, Novartis, RAPT Therapeutics, Regeneron, Roche/Genentech, Sanofi and Takeda. HRK declares an advisory board or consultation role from Bayer, AstraZeneca, Bristol Myers Squibb, Takeda and Yhan; research funding from the Yonsi Lee Youn Jae Fellowship; honoraria from AstraZeneca, Bristol Myers Squibb and Roche/Genentech; stock ownership in Bridgebio Therapeutics. CZ declares speaker’s engagement from Alice, AnHeart, Boehringer Ingelheim, C-Stone, Eli Lily, Hengrui, Innovent Biologics, LUYE Pharma, MSD, Qilu Pharma, Roche, Sanofi and TopAlliance Biosciences Inc.; advisory board role from Amoy Diagnostics. SAK declares speaker’s engagement from APL, AstraZeneca, Pfizer and Roche; transportation and accommodation from Roche. KP declares advisory board role from Merck and Novartis; funding as local PI from Alkem, Johnson and Johnson and Roche. HH declares speaker’s engagement from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Merck Biopharma, MSD, Novartis Pharmaceuticals, Ono Pharmaceuticals, Pfizer and Takeda Pharmaceutical; advisory board/steering committee role from AstraZeneca, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical and Pfizer; institutional funding/research grant from Bayer Yakuhin, Chugai Pharmaceutical, Clinical Research Support Center Kyushu, Comprehensive Support Project for Oncological Research of Breast Cancer (CSPOR-BC), Eisai, Japan Clinical Cancer Research Organization, Japanese Gastric Cancer Association, Kyowa Kirin, Mebix, Medical Research Support, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Otsuka Pharmaceutical, Shionogi, SRL Medisearch, Thoracic Oncology Research Group (TORG) and West Japan Oncology Group (WJOG); royalties from Sysmex; non-renumerated member role from Japanese Society of Medical Oncology and West Japan Oncology Group. DDWL declares speaker’s engagement from AstraZeneca, Ipsen, MSD, Pfizer, Roche, Takeda and Zuellig Pharma; advisory board role from Eisai; funding as local PI from BeiGene. MSI declares speaker’s engagement from AstraZeneca, MSD Philippines, Pfizer Philippines and Zuellig Pharma Philippines; advisory board role from AstraZeneca, MSD Philippines, Roche Philippines and Pfizer Philippines; funding as coordinating PI/local PI from AstraZeneca, MSD and Roche. JCHY declares speaker’s engagement from AstraZeneca, Boehringer Ingelheim and Novartis; advisory board/steering committee role from Amgen, Arrivant, AstraZeneca, Bayer, Blueprint Medicine, BMS, Boehringer Ingelheim, Cullinan, Daiichi Sankyo, Eli Lilly, Esai, Gilead, GSK, InHeart Therapeutics, Ipsen, Janssen, Merck, Merck Serono, MSD, Novartis, Numab, Omega Therapeutics, Ono Pharmaceuticals, Pfizer, Puma Pharmaceuticals, Regeneron, Roche/Genentech, Sanofi, Taiho, Takeda Oncology and Yuhan Pharmaceuticals; funding as coordinating PI from AstraZeneca, Dizal Pharmaceutical and MSD; non-renumerated member role from ASCO and IASLC. TR declares speaker’s engagement from Amgen, AstraZeneca, BMS, MSD, Novartis, Pfizer and Roche; advisory board/steering committee role from Amgen, AstraZeneca, BMS, MSD, Novartis, Pfizer, Roche and Yuhan; funding as local PI from AstraZeneca, MSD, Novartis and Roche. VS declares speaker’s engagement from AstraZeneca, Eisai, MSD, Novartis, Roche and Takeda; funding as local PI from AstraZeneca, Daiichi, MSD, Novartis, Regeneron and Takeda. CEW declares speaker’s engagement from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai, Eli Lilly & Company, Guardant Health, Johnson & Johnson, MSD, Novartis, Pfizer, Roche and Takeda; non-renumerated PI role from Chugai and Daiichi Sankyo; product samples (non-renumerated) from Novartis and Roche. YA declares speaker’s engagement from AstraZeneca and Roche. MS declares speaker’s engagement from Hi Esai, Pfizer and Vexxa; funding as local PI from AstraZeneca; non-renumerated member of American Society of Clinical Oncology (ASCO) and Philippine Society of Medical Oncology (PSMO). MT declares non-renumerated leadership role from Malaysian Oncological Society (MOS). HM declares speaker’s engagement from AstraZeneca, Chugai Pharmaceutical, Merck Sharp and Dohme and Takeda Pharmaceuticals. VN declares funding as local PI from Amgen, AstraZeneca and Sanofi India; Institutional funding from Dr Reddy's Laboratories and Intas Pharmaceuticals. LZ declares institutional research grant from AstraZeneca, BMS and Roche; funding as trial chair from AstraZeneca, China Shiyao Pharma, Hansoh Pharma, Henrui Pharm, Kelun Pharm, Novartis and QiLu Pharm. ES declares speaker’s engagement from Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Cor2Ed, Daiichi Sankyo, Elsevier, Imedex, Merck, MSD, Novartis, Peervoice, Prova Education, Servier, Suzhou Liangihui Network Technology Company Ltd and TouchIME; expert testimony/advisory role from AbbVie, Astellas, AstraZeneca, Bristol Meyers Squibb, My Personal Therapeutics, Roche, Servier, Viracta and Zymeworks; institutional research grant from AstraZeneca, Bristol Meyers Squibb; funding as coordinating/local PI from Amgen, AstraZeneca, Basilea, Daiichi Sankyo, Merus, Mirati, MSD and Roche; Trial Steering Committee (TSC) for Amgen; independent data monitoring Committee (IDMC) role from BeiGene, Everest Clinical Research, Jazz Pharmaceuticals and Zymeworks; EORTC GI clinical trials group role; non-renumerated leadership role from UK & Ireland Oesophagogastric Group (UKIOG). TY declares speaker’s engagement from Bayer Yakuhin, Chugai Pharmaceutical, Merck Biopharma, MSD KK, Ono Pharmaceutical and Takeda Pharmaceutical; consultancy role from Sumitomo Corp; institutional research grant from Amgen KK, Bristol-Myers Squibb K.K., Chugai Pharmaceutical, Daiichi Sankyo, Eisai, FALCO Biosystems, Genomedia Inc., Molecular Health GmbH, MSD KK, Medical & Biological Laboratories Co., Merus N.V., Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer Japan, Roche Diagnostics, Sanofi K.K., Sysmex Corp, Taiho Pharmaceutical and Takeda Pharmaceuticals. JOP declares advisory board role from Adicet Bio, AstraZeneca, BMS (Celgene), ImmuneOncia, MediRama, MedPacto, Merck, Merck Sereno and Servier; research grant from ABL Bio, BMS (Celgene) Eutilex, MedPacto and Servier; travel support from Minneamrita Therapeutics LLC. GP declares employment by ESMO; non-renumerated membership of ASCO, Hellenic Cooperative Oncology Group (HeCOG) and Hellenic Society of Medical Oncology. SPe declares speaker’s engagement from AstraZeneca, BMS, Boehringer Ingelheim, Ecancer, Eli Lilly, Fishawack, Illumina, Imedex, Medscape, Mirati, MSD, Novartis, OncologyEducation, PER, Pfizer, PRIME, RMEI, Roche/Genentech, RTP, Sanofi and Takeda; advisory board/steering committee role from AbbVie, Amgen, Arcus, AstraZeneca, Bayer, BeiGene, Bio Invent, Biocartis, Blueprint Medicines, BMS, Boehringer Ingelheim, Daiichi Sankyo, Debiopharm, Eli Lilly, F-Star, Foundation Medicine, Genzyme, Gilhead, GlaxoSmithKline, Hutchmed, Illumina, Incyte, IQVIA, iTheos, Janssen, Merck Serono, Mirati, MSD, Novartis, Novocure, Pfizer, PharmaMar, Phosplatin Therapeutics, Regeneron, Sanofi, Seattle Genetics, Takeda and Vaccibody; funding as coordinating PI from AstraZeneca; funding as trial chair from GSK; associate editor/deputy editor role from Elsevier member of board of directors of Galenica. JBA declares a non-renumerate PI role from Boryung and MSD. SPo declares speaker’s engagement from Medscape, Novocure, PharmaMar and VJ Oncology; advisory board/expert testimony role from Amgen, AnHeart, Arcus Biosciences, AstraZeneca, Bayer, Blueprint, BMS, Boehringer Ingelheim, Daiichi Sankyo, Ellipses, EQRx, Gilead, GlaxoSmithKline, Guardant Health, IO Biotech, Janssen, Lilly, Merck Serono, Mirati, MSD, Novartis, Pfizer, Roche, Sanofi, Takeda and Turning Point Therapeutics; institutional research grant from Guardant Health; funding as a coordinating PI/ local PI/sub-investigator from Amgen, Ariad, AstraZeneca, Blueprint, BMS, Boehringer Ingelheim, Celgene, Daiichi Sankyo, GlaxoSmithKline, Janssen, Lilly, MSD, Roche, Takeda, Trizel and Turning Point Therapeutics; non-renumerated steering committee/advisory role from ALK Positive UK, British Thoracic Oncology Group, International Association for the Study of Lung Cancer, Lung Cancer Europe and Ruth Strauss Foundation; non-renumerated role from ESMO Thoracic Faculty and Foundation Council Member of the European Thoracic Oncology Platform; non-renumerated member of the board of directors for the Mesothelioma Applied Research Foundation. All other authors have declared no conflicts of interest.
Supplementary data
References
- 1.Li C., Lei S., Ding L., et al. Global burden and trends of lung cancer incidence and mortality. Chin Med J (Engl) 2023;136(13):1583–1590. doi: 10.1097/CM9.0000000000002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J., Ervik M., Lam F., et al. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. 2024. https://gco.iarc.who.int/today Available at.
- 4.Chen P., Liu Y., Wen Y., et al. Non-small cell lung cancer in China. Cancer Commun (Lond) 2022;42(10):937–970. doi: 10.1002/cac2.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao S., Li N., Wang S., et al. Lung cancer in People's Republic of China. J Thorac Oncol. 2020;15(10):1567–1576. doi: 10.1016/j.jtho.2020.04.028. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization WHO Report on the Global Tobacco Epidemic, 2019. 2019. https://apps.who.int/iris/bitstream/handle/10665/326043/9789241516204-eng.pdf?ua=9789241516201 Available at.
- 7.Zhou F., Zhou C. Lung cancer in never smokers-the East Asian experience. Transl Lung Cancer Res. 2018;7(4):450–463. doi: 10.21037/tlcr.2018.05.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou W., Christiani D.C. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho J., Choi S.M., Lee J., et al. Proportion and clinical features of never-smokers with non-small cell lung cancer. Chin J Cancer. 2017;36(1):20. doi: 10.1186/s40880-017-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam W.K. Lung cancer in Asian women-the environment and genes. Respirology. 2005;10(4):408–417. doi: 10.1111/j.1440-1843.2005.00723.x. [DOI] [PubMed] [Google Scholar]
- 11.Molina J.R., Yang P., Cassivi S.D., et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pagliarini R., Shao W., Sellers W.R. Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO Rep. 2015;16(3):280–296. doi: 10.15252/embr.201439949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melosky B., Kambartel K., Hantschel M., et al. Worldwide prevalence of epidermal growth factor receptor mutations in non-small cell lung cancer: a meta-analysis. Mol Diagn Ther. 2022;26(1):7–18. doi: 10.1007/s40291-021-00563-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan D.S., Yom S.S., Tsao M.S., et al. The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: status in 2016. J Thorac Oncol. 2016;11(7):946–963. doi: 10.1016/j.jtho.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 15.Schabath M.B., Cress D., Munoz-Antonia T. Racial and ethnic differences in the epidemiology and genomics of lung cancer. Cancer Control. 2016;23(4):338–346. doi: 10.1177/107327481602300405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roviello G., D'Angelo A., Sirico M., et al. Advances in anti-BRAF therapies for lung cancer. Invest New Drugs. 2021;39(3):879–890. doi: 10.1007/s10637-021-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu F., Wei Y., Zhang H., et al. NTRK fusion in non-small cell lung cancer: diagnosis, therapy, and TRK inhibitor resistance. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.864666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shigematsu H., Takahashi T., Nomura M., et al. Somatic mutations of the HER2 kinase domain in lung adenocarcinomas. Cancer Res. 2005;65(5):1642–1646. doi: 10.1158/0008-5472.CAN-04-4235. [DOI] [PubMed] [Google Scholar]
- 19.Shih J.Y. ERBB2 amplification in NSCLC: how many faces? J Thorac Oncol. 2024;19(5):668–670. doi: 10.1016/j.jtho.2024.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Bergethon K., Shaw A.T., Ou S.H., et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Q., Zhan P., Zhang X., et al. Clinicopathologic characteristics of patients with ROS1 fusion gene in non-small cell lung cancer: a meta-analysis. Transl Lung Cancer Res. 2015;4(3):300–309. doi: 10.3978/j.issn.2218-6751.2015.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascetta P., Sforza V., Manzo A., et al. RET inhibitors in non-small-cell lung cancer. Cancers (Basel) 2021;13(17):4415. doi: 10.3390/cancers13174415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Remon J., Hendriks L.E.L., Mountzios G., et al. MET alterations in NSCLC - current perspectives and future challenges. J Thorac Oncol. 2023;18(4):419–435. doi: 10.1016/j.jtho.2022.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Ferrara M.G., Di Noia V., D'Argento E., et al. Oncogene-addicted non-small-cell lung cancer: treatment opportunities and future perspectives. Cancers (Basel) 2020;12(5):1196. doi: 10.3390/cancers12051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Jager V.D., Timens W., Bayle A., et al. Developments in predictive biomarker testing and targeted therapy in advanced stage non-small cell lung cancer and their application across European countries. Lancet Reg Health Eur. 2024;38 doi: 10.1016/j.lanepe.2024.100838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jager V.D., Timens W., Bayle A., et al. Future perspective for the application of predictive biomarker testing in advanced stage non-small cell lung cancer. Lancet Regional Health Eur. 2024;38 doi: 10.1016/j.lanepe.2024.100839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicholson A.G., Tsao M.S., Beasley M.B., et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17(3):362–387. doi: 10.1016/j.jtho.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Moreno P., Brambilla E., Thomas R., et al. Squamous cell carcinoma of the lung: molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18(9):2443–2451. doi: 10.1158/1078-0432.CCR-11-2370. [DOI] [PubMed] [Google Scholar]
- 29.Joshi A., Mishra R., Desai S., et al. Molecular characterization of lung squamous cell carcinoma tumors reveals therapeutically relevant alterations. Oncotarget. 2021;12(6):578–588. doi: 10.18632/oncotarget.27905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedlaender A., Perol M., Banna G.L., et al. Oncogenic alterations in advanced NSCLC: a molecular super-highway. Biomark Res. 2024;12(1):24. doi: 10.1186/s40364-024-00566-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heist R.S., Sequist L.V., Engelman J.A. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012;7(5):924–933. doi: 10.1097/JTO.0b013e31824cc334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steeghs E.M.P., Groen H.J.M., Schuuring E., et al. Mutation-tailored treatment selection in non-small cell lung cancer patients in daily clinical practice. Lung Cancer. 2022;167:87–97. doi: 10.1016/j.lungcan.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Kim Y., Hammerman P.S., Kim J., et al. Integrative and comparative genomic analysis of lung squamous cell carcinomas in East Asian patients. J Clin Oncol. 2013;32(2):121–128. doi: 10.1200/JCO.2013.50.8556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hendriks L.E., Kerr K.M., Menis J., et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):339–357. doi: 10.1016/j.annonc.2022.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Cherny N.I., Dafni U., Bogaerts J., et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017;28(10):2340–2366. doi: 10.1093/annonc/mdx310. [DOI] [PubMed] [Google Scholar]
- 36.Mateo J., Chakravarty D., Dienstmann R., et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT) Ann Oncol. 2018;29(9):1895–1902. doi: 10.1093/annonc/mdy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.An S.J., Chen Z.H., Su J., et al. Identification of enriched driver gene alterations in subgroups of non-small cell lung cancer patients based on histology and smoking status. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0040109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q., Zhu L., Zhang J. Epidermal growth factor receptor gene mutation status in pure squamous-cell lung cancer in Chinese patients. BMC Cancer. 2015;15:88. doi: 10.1186/s12885-015-1056-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giannini R., Lupi C., Sensi E., et al. EGFR and KRAS mutational analysis in a large series of Italian non-small cell lung cancer patients: 2,387 cases from a single center. Oncol Rep. 2016;36(2):1166–1172. doi: 10.3892/or.2016.4874. [DOI] [PubMed] [Google Scholar]
- 40.Lindeman N.I., Cagle P.T., Beasley M.B., et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. J Thorac Oncol. 2013;8(7):823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramalingam S.S., Vansteenkiste J., Planchard D., et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 42.Cheng Y., He Y., Li W., et al. Osimertinib versus comparator EGFR TKI as first-line treatment for EGFR-mutated advanced NSCLC: FLAURA China, a randomized study. Target Oncol. 2021;16(2):165–176. doi: 10.1007/s11523-021-00794-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohe Y., Imamura F., Nogami N., et al. Osimertinib versus standard-of-care EGFR-TKI as first-line treatment for EGFRm advanced NSCLC: FLAURA Japanese subset. Jpn J Clin Oncol. 2019;49(1):29–36. doi: 10.1093/jjco/hyy179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Attili I., Corvaja C., Spitaleri G., et al. New generations of tyrosine kinase inhibitors in treating NSCLC with oncogene addiction: strengths and limitations. Cancers (Basel) 2023;15(20):5079. doi: 10.3390/cancers15205079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dhillon S. Lazertinib: first approval. Drugs. 2021;81(9):1107–1113. doi: 10.1007/s40265-021-01533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee KH, Ahn M-J, Han J-Y, et al. Efficacy and safety of lazertinib 240 mg as the clinical dose in patients with EGFR T790M mutant NSCLC: data from a phase I/II study. J Clin Oncol. 38(suppl 15):9572-9572.
- 47.Cho B.C., Ahn M.J., Kang J.H., et al. Lazertinib versus gefitinib as first-line treatment in patients with EGFR-mutated advanced non-small-cell lung cancer: results from LASER301. J Clin Oncol. 2023;41(26):4208–4217. doi: 10.1200/JCO.23.00515. [DOI] [PubMed] [Google Scholar]
- 48.Noronha V., Patil V.M., Joshi A., et al. Gefitinib versus gefitinib plus pemetrexed and carboplatin chemotherapy in EGFR-mutated lung cancer. J Clin Oncol. 2020;38(2):124–136. doi: 10.1200/JCO.19.01154. [DOI] [PubMed] [Google Scholar]
- 49.Hosomi Y., Morita S., Sugawara S., et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38(2):115–123. doi: 10.1200/JCO.19.01488. [DOI] [PubMed] [Google Scholar]
- 50.Miyauchi E., Morita S., Nakamura A., et al. Updated analysis of NEJ009: gefitinib-alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated EGFR. J Clin Oncol. 2022;40(31):3587–3592. doi: 10.1200/JCO.21.02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Planchard D., Janne P.A., Cheng Y., et al. Osimertinib with or without chemotherapy in EGFR-mutated advanced NSCLC. N Engl J Med. 2023;389(21):1935–1948. doi: 10.1056/NEJMoa2306434. [DOI] [PubMed] [Google Scholar]
- 52.Valdiviezo N., Okamoto I., Hughes B.G.M., et al. 4O first-line (1L) osimertinib (osi) ±platinum-pemetrexed in EGFR-mutated (EGFRm) advanced NSCLC: FLAURA2 post-progression outcomes. ESMO Open. 2024;9:102583. [Google Scholar]
- 53.Cho B.C., Lu S., Felip E., et al. Amivantamab plus lazertinib in previously untreated EGFR-mutated advanced NSCLC. N Engl J Med. 2024:1486–1498. doi: 10.1056/NEJMoa2403614. [DOI] [PubMed] [Google Scholar]
- 54.Gadgeel S., Cho B.C., Lu S., et al. Paper presented at the IASLC 2024 World Conference on Lung Cancer; San Diego, CA: September 7-10, 2024. Amivantamab plus lazertinib vs osimertinib in first-line EGFR-mutant advanced NSCLC: longer follow-up of the MARIPOSA study. [Google Scholar]
- 55.Felip E., Cho B.C., Gutiérrez V., et al. Amivantamab plus lazertinib vs osimertinib in first-line EGFR-mutant advanced non-small cell lung cancer (NSCLC) with biomarkers of high-risk disease: a secondary analysis from the phase 3 MARIPOSA study. J Clin Oncol. 2024;42(suppl 16) doi: 10.1016/j.annonc.2024.05.541. 8504-8504. [DOI] [PubMed] [Google Scholar]
- 56.Cho B.C., Felip E., Spira A.I., et al. LBA14 Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann Oncol. 2023;34:S1306. [Google Scholar]
- 57.Saito H., Fukuhara T., Furuya N., et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20(5):625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 58.Kawashima Y., Fukuhara T., Saito H., et al. Bevacizumab plus erlotinib versus erlotinib alone in Japanese patients with advanced, metastatic, EGFR-mutant non-small-cell lung cancer (NEJ026): overall survival analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Respir Med. 2022;10(1):72–82. doi: 10.1016/S2213-2600(21)00166-1. [DOI] [PubMed] [Google Scholar]
- 59.Zhou Q., Xu C.R., Cheng Y., et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): a multicenter phase 3 study. Cancer Cell. 2021;39(9):1279–1291.e1273. doi: 10.1016/j.ccell.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Nakagawa K., Garon E.B., Seto T., et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 61.Nishio M., Seto T., Reck M., et al. Ramucirumab or placebo plus erlotinib in EGFR-mutated, metastatic non-small-cell lung cancer: East Asian subset of RELAY. Cancer Sci. 2020;111(12):4510–4525. doi: 10.1111/cas.14655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okuma Y., Kubota K., Shimokawa M., et al. First-line osimertinib for previously untreated patients with NSCLC and uncommon EGFR mutations: the UNICORN phase 2 nonrandomized clinical trial. JAMA Oncol. 2024;10(1):43–51. doi: 10.1001/jamaoncol.2023.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miura S., Tanaka H., Misumi T., et al. LBA66 Afatinib versus chemotherapy for treatment-naïve non-small cell lung cancer with a sensitizing uncommon epidermal growth factor receptor mutation: a phase III study (ACHILLES/TORG1834) Ann Oncol. 2023;34:S1310–S1311. [Google Scholar]
- 64.Leonetti A., Sharma S., Minari R., et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019;121(9):725–737. doi: 10.1038/s41416-019-0573-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Z.F., Ren S.X., Li W., et al. Frequency of the acquired resistant mutation T790 M in non-small cell lung cancer patients with active exon 19Del and exon 21 L858R: a systematic review and meta-analysis. BMC Cancer. 2018;18(1):148. doi: 10.1186/s12885-018-4075-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mok T.S., Wu Y.L., Ahn M.J., et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahn M.J., Han J.Y., Kim D.W., et al. Osimertinib in patients with T790M-positive advanced non-small cell lung cancer: Korean subgroup analysis from phase II studies. Cancer Res Treat. 2020;52(1):284–291. doi: 10.4143/crt.2019.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Passaro A., Wang J., Wang Y., et al. Amivantamab plus chemotherapy with and without lazertinib in EGFR-mutant advanced NSCLC after disease progression on osimertinib: primary results from the phase III MARIPOSA-2 study. Ann Oncol. 2024;35(1):77–90. doi: 10.1016/j.annonc.2023.10.117. [DOI] [PubMed] [Google Scholar]
- 69.Zhou C., Dong X., Chen G., et al. OA09.06 IMpower151: phase III study of atezolizumab + bevacizumab + chemotherapy in 1L metastatic nonsquamous NSCLC. J Thoracic Oncol. 2023;18(11):S64–S65. [Google Scholar]
- 70.Park S., Kim T.M., Han J.Y., et al. Phase III, randomized study of atezolizumab plus bevacizumab and chemotherapy in patients with EGFR- or ALK-mutated non-small-cell lung cancer (ATTLAS, KCSG-LU19-04) J Clin Oncol. 2024;42(11):1241–1251. doi: 10.1200/JCO.23.01891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lu S., Wu L., Jian H., et al. Sintilimab plus chemotherapy for patients with EGFR-mutated non-squamous non-small-cell lung cancer with disease progression after EGFR tyrosine-kinase inhibitor therapy (ORIENT-31): second interim analysis from a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Respir Med. 2023;11(7):624–636. doi: 10.1016/S2213-2600(23)00135-2. [DOI] [PubMed] [Google Scholar]
- 72.Lin S.Y., Yang C.Y., Liao B.C., et al. Tumor PD-L1 expression and clinical outcomes in advanced-stage non-small cell lung cancer patients treated with nivolumab or pembrolizumab: real-world data in Taiwan. J Cancer. 2018;9(10):1813–1820. doi: 10.7150/jca.24985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garon E.B., Hellmann M.D., Rizvi N.A., et al. Five-year overall survival for patients with advanced non‒small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol. 2019;37(28):2518–2527. doi: 10.1200/JCO.19.00934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu J., Huang D., Wang Y., et al. The efficacy of immune checkpoint inhibitors in advanced EGFR-Mutated non-small cell lung cancer after resistance to EGFR-TKIs: real-world evidence from a multicenter retrospective study. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.975246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mazieres J., Drilon A., Lusque A., et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30(8):1321–1328. doi: 10.1093/annonc/mdz167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee C.K., Man J., Lord S., et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12(2):403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 77.Solomon B.J., Bauer T.M., Mok T.S.K., et al. Efficacy and safety of first-line lorlatinib versus crizotinib in patients with advanced, ALK-positive non-small-cell lung cancer: updated analysis of data from the phase 3, randomised, open-label CROWN study. Lancet Respir Med. 2023;11(4):354–366. doi: 10.1016/S2213-2600(22)00437-4. [DOI] [PubMed] [Google Scholar]
- 78.Peters S., Camidge D.R., Shaw A.T., et al. Alectinib versus crizotinib in untreated ALK-positive non-small-cell lung cancer. N Engl J Med. 2017;377(9):829–838. doi: 10.1056/NEJMoa1704795. [DOI] [PubMed] [Google Scholar]
- 79.Ahn M.J., Kim H.R., Yang J.C.H., et al. Efficacy and safety of brigatinib compared with crizotinib in Asian vs. Non-Asian patients with locally advanced or metastatic alk-inhibitor-naive ALK+ non-small cell lung cancer: final results from the phase III ALTA-1L study. Clin Lung Cancer. 2022;23(8):720–730. doi: 10.1016/j.cllc.2022.07.008. [DOI] [PubMed] [Google Scholar]
- 80.Camidge D.R., Kim H.R., Ahn M.J., et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 2020;38(31):3592–3603. doi: 10.1200/JCO.20.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Solomon B.J., Liu G., Felip E., et al. Lorlatinib versus crizotinib in patients with advanced ALK-positive non-small cell lung cancer: 5-year outcomes from the phase III CROWN study. J Clin Oncol. 2024;42:3400–3409. doi: 10.1200/JCO.24.00581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Horn L., Wang Z., Wu G., et al. Ensartinib vs crizotinib for patients with anaplastic lymphoma kinase-positive non-small cell lung cancer: a randomized clinical trial. JAMA Oncol. 2021;7(11):1617–1625. doi: 10.1001/jamaoncol.2021.3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Novello S., Mazieres J., Oh I.J., et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018;29(6):1409–1416. doi: 10.1093/annonc/mdy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang J.C., Liu G., Lu S., et al. Brigatinib versus alectinib in ALK-positive NSCLC after disease progression on crizotinib: results of phase 3 ALTA-3 trial. J Thorac Oncol. 2023;18(12):1743–1755. doi: 10.1016/j.jtho.2023.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Solomon B.J., Besse B., Bauer T.M., et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018;19(12):1654–1667. doi: 10.1016/S1470-2045(18)30649-1. [DOI] [PubMed] [Google Scholar]
- 86.Shaw A.T., Solomon B.J., Besse B., et al. Alk resistance mutations and efficacy of lorlatinib in advanced anaplastic lymphoma kinase-positive non-small-cell lung cancer. J Clin Oncol. 2019;37(16):1370–1379. doi: 10.1200/JCO.18.02236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lu S., Zhou Q., Liu X., et al. Lorlatinib for previously treated ALK-positive advanced NSCLC: primary efficacy and safety from a phase 2 study in People's Republic of China. J Thorac Oncol. 2022;17(6):816–826. doi: 10.1016/j.jtho.2022.02.014. [DOI] [PubMed] [Google Scholar]
- 88.Wu Y.-L., Shi Y., Tan D.S.W., et al. Phase 1/2 study of ceritinib in Chinese patients with advanced anaplastic lymphoma kinase-rearranged non-small cell lung cancer previously treated with crizotinib: results from ASCEND-6. Lung Cancer. 2020;150:240–246. doi: 10.1016/j.lungcan.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y., Zhou J., Zhou J., et al. Efficacy, safety, and biomarker analysis of ensartinib in crizotinib-resistant, ALK-positive non-small-cell lung cancer: a multicentre, phase 2 trial. Lancet Respir Med. 2020;8(1):45–53. doi: 10.1016/S2213-2600(19)30252-8. [DOI] [PubMed] [Google Scholar]
- 90.Zheng J., Wang T., Yang Y., et al. Updated overall survival and circulating tumor DNA analysis of ensartinib for crizotinib-refractory ALK-positive NSCLC from a phase II study. Cancer Commun (Lond) 2024;44(4):455–468. doi: 10.1002/cac2.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma X., Yang S., Zhang K., et al. Efficacy of different sequential patterns after crizotinib progression in advanced anaplastic lymphoma kinase-positive non-small cell lung cancer. Thoracic Cancer. 2022;13(12):1788–1794. doi: 10.1111/1759-7714.14455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Drilon A., Camidge D.R., Lin J.J., et al. Repotrectinib in ROS1 fusion-positive non-small-cell lung cancer. N Engl J Med. 2024;390(2):118–131. doi: 10.1056/NEJMoa2302299. [DOI] [PubMed] [Google Scholar]
- 93.U.S. Food and Drink Administration FDA Approves Repotrectinib for ROS1-Positive Non-Small Cell Lung Cancer. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-repotrectinib-ros1-positive-non-small-cell-lung-cancer Available at.
- 94.Riely G.J., Smit E.F., Ahn M.J., et al. Phase II, open-label study of encorafenib plus binimetinib in patients with BRAF(V600)-mutant metastatic non-small-cell lung cancer. J Clin Oncol. 2023;41(21):3700–3711. doi: 10.1200/JCO.23.00774. [DOI] [PubMed] [Google Scholar]
- 95.Riely G.J., Ahn M.J., Clarke J., et al. LBA56 Updated efficacy and safety from the phase II PHAROS study of encorafenib plus binimetinib in patients with BRAF V600E-mutant metastatic NSCLC (mNSCLC) Ann Oncol. 2024;35:S1246–S1247. [Google Scholar]
- 96.Planchard D., Mazieres J., Mascaux C., et al. 1259MO Encorafenib plus binimetinib in patients (pts) with previously untreated BRAF V600E-mutant advanced non-small cell lung cancer (NSCLC): an open-label, multicenter phase II trial (IFCT-1904 ENCO-BRAF) Ann Oncol. 2024;35:S806–S807. [Google Scholar]
- 97.Nakahama K., Izumi M., Yoshimoto N., et al. Influence of smoking history on the effectiveness of immune-checkpoint inhibitor therapy for non-small cell lung cancer: analysis of real-world data. Anticancer Res. 2023;43(5):2185–2197. doi: 10.21873/anticanres.16381. [DOI] [PubMed] [Google Scholar]
- 98.Chen D.L., Li Q.Y., Tan Q.Y. Smoking history and the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2021;13(1):220–231. doi: 10.21037/jtd-20-1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hendriks L.E., Kerr K.M., Menis J., et al. Non-oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(4):358–376. doi: 10.1016/j.annonc.2022.12.013. [DOI] [PubMed] [Google Scholar]
- 100.U.S. Food and Drink Administration FDA Approves Pralsetinib for Non-Small Cell Lung Cancer with RET Gene Fusions. 2023. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pralsetinib-non-small-cell-lung-cancer-ret-gene-fusions Available at.
- 101.European Medicines Agency An Overview of Gavreto and Why It Is Authorised in the EU. 2021. https://www.ema.europa.eu/en/documents/overview/gavreto-epar-medicine-overview_en.pdf Available at.
- 102.Zhou C., Solomon B., Loong H.H., et al. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023;389(20):1839–1850. doi: 10.1056/NEJMoa2309457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhou C., Tang K.J., Cho B.C., et al. Amivantamab plus chemotherapy in NSCLC with EGFR exon 20 insertions. N Engl J Med. 2023;389(22):2039–2051. doi: 10.1056/NEJMoa2306441. [DOI] [PubMed] [Google Scholar]
- 104.Markham A. Savolitinib: first approval. Drugs. 2021;81(14):1665–1670. doi: 10.1007/s40265-021-01584-0. [DOI] [PubMed] [Google Scholar]
- 105.Lu S., Guo Q., Yang N., et al. 1MO A phase IIIb study of savolitinib in patients with locally advanced or metastatic NSCLC harboring MET exon 14 mutation. ESMO Open. 2024;9:102580. [Google Scholar]
- 106.Yu Y., Guo Q., Zhang Y., et al. Savolitinib in patients in China with locally advanced or metastatic treatment-naive non-small-cell lung cancer harbouring MET exon 14 skipping mutations: results from a single-arm, multicohort, multicentre, open-label, phase 3b confirmatory study. Lancet Respir Med. 2024 doi: 10.1016/s2213-2600(24)00211-x. [DOI] [PubMed] [Google Scholar]
- 107.U.S. Food and Drink Administration FDA Grants Accelerated Approval to Sotorasib for KRAS G12C Mutated NSCLC. 2021. Available at https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-sotorasib-kras-g12c-mutated-nsclc.
- 108.U.S. Food and Drink Administration FDA Grants Accelerated Approval to Adagrasib for KRAS G12C-Mutated NSCLC. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-adagrasib-kras-g12c-mutated-nsclc Available at.
- 109.ESMO New Indication Concerns Combination Therapy with Carboplatin and Pemetrexed for the First-Line Treatment of Adult Patients with Advanced NSCLC with Activating EGFR Exon 20 Insertion Mutations. 2024. https://www.esmo.org/oncology-news/ema-recommends-extension-of-indications-for-amivantamab Available at.
- 110.U.S. Food and Drug Administration FDA Approves Amivantamab-vmjw for EGFR Exon 20 Insertion-Mutated Non-Small Cell Lung Cancer Indications. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-amivantamab-vmjw-egfr-exon-20-insertion-mutated-non-small-cell-lung-cancer-indications Available at.
- 111.Park K., Haura E.B., Leighl N.B., et al. Amivantamab in EGFR exon 20 insertion-mutated non-small-cell lung cancer progressing on platinum chemotherapy: initial results from the CHRYSALIS phase I study. J Clin Oncol. 2021;39(30):3391–3402. doi: 10.1200/JCO.21.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.U.S. Food and Drug Administration FDA Grants Accelerated Approval to Amivantamab-vmjw for Metastatic Non-Small Cell Lung Cancer. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-amivantamab-vmjw-metastatic-non-small-cell-lung-cancer Available at.
- 113.Hanley M.J., Camidge D.R., Fram R.J., et al. Mobocertinib: mechanism of action, clinical, and translational science. Clin Transl Sci. 2024;17(3) doi: 10.1111/cts.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jänne P.A., Wang B.C., Cho B.C., et al. 507O EXCLAIM-2: phase III trial of first-line (1L) mobocertinib versus platinum-based chemotherapy in patients (pts) with epidermal growth factor receptor (EGFR) exon 20 insertion (ex20ins)+ locally advanced/metastatic NSCLC. Ann Oncol. 2023;34:S1663–S1664. [Google Scholar]
- 115.Eisenhauer E.A., Therasse P., Bogaerts J., et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.