Abstract
Objective
Dietary protein restriction induces adaptive changes in food preference, increasing protein consumption over carbohydrates or fat. We investigated whether motivation and reward signaling underpin these preferences.
Methods and Results
In an operant task, protein-restricted male mice responded more for liquid protein rewards, but not carbohydrate, fat, or sweet rewards compared to non-restricted mice. When the number of responses required to access protein reward varied, protein-restricted mice exhibited higher operant responses at moderate to high response requirements. The protein restriction-induced increase in operant responding for protein was absent in Fgf21-KO mice and mice with neuron-specific deletion of the FGF21 co-receptor beta-Klotho (KlbCam2ka). Fiber photometry recording of VTA dopamine neurons revealed that oral delivery of maltodextrin triggered a larger dopamine neuron activation than casein in control diet-fed mice, while casein triggered a larger activation in low-protein diet-fed mice. This restriction-induced shift in nutrient-specific VTA dopamine signaling was lost in Fgf21-KO mice.
Conclusion
These data suggest that the increased FGF21 during protein restriction acts in the brain to induce a protein-specific appetite by specifically enhancing the reward value of protein-containing foods and the motivation to consume them.
Keywords: FGF21, Dietary protein, Motivation, Food preference
Highlights
-
•
Protein restriction increases the motivation for protein but not other nutrients.
-
•
FGF21 signaling in the brain is required for this protein-specific motivation.
-
•
Protein restriction selectively increases the dopamine neuron response to protein.
-
•
FGF21 is required for low protein-induced shifts in dopamine neuron activity.
1. Introduction
It is well established that animals monitor their nutritional state, detecting and adaptively altering metabolism and feeding behavior in response to the restriction of energy, water, or sodium [[1], [2], [3], [4], [5], [6], [7]]. These adaptive responses generally involve neural mechanisms that increase the motivation to procure and consume the missing nutrient. Several groups, including our own, have recently focused on the possibility that the restriction of dietary protein intake also triggers adaptive responses, and protein-restricted rodents selectively shift nutrient preference such that they increase protein consumption relative to carbohydrate or fat [[8], [9], [10], [11]]. A handful of studies indicate that this ‘protein appetite’ is also associated with an increased motivation for protein, with protein-restricted rats or hamsters exhibiting increased responding for protein or protein-associated cues in an operant task [12,13] and increased activation of brain reward pathways in response to protein ingestion [14]. These data suggest that restricting access to dietary protein, or perhaps essential amino acids, induces a selective motivation for protein.
Here, we focus on the underlying mechanism driving this protein-specific shift in preference and motivation. For the past several years, we and others have demonstrated that the endocrine hormone Fibroblast Growth Factor 21 (FGF21) is robustly increased by low protein diets and required for animals to adaptively change food intake and nutrient preference during protein restriction [3,8,10,[15], [16], [17]]. We, therefore, tested whether FGF21 is also necessary for increased motivation for protein rewards in response to protein restriction. Our results demonstrate that protein-restricted mice exhibit a nutrient-specific increase in motivation for protein sources and that this increase in motivated behavior depends on FGF21 and its ability to signal in the brain. In addition, protein restriction enhances the activity of ventral tegmental area (VTA) dopamine neurons in response to oral protein delivery, and this nutrient-specific shift in dopamine neuron response is also FGF21-dependent. Together, these data suggest that FGF21 acts in the brain during protein restriction to selectively increase the responsivity of dopamine neurons to protein while also increasing the motivation to procure protein.
2. Materials and methods
2.1. Animals and diets
All animal-related procedures were approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee (IACUC) and were carried out in strict adherence to the guidelines and regulations set by the NIH Office of Laboratory Animal Welfare. Male C57BL/6J (WT) mice obtained from Jackson Laboratory were used in all experiments. Fgf21-KO mice on the B6 background were provided by Dr. Steven Kliewer [18] and bred in the homozygous state with C57BL/6J mice used as controls. Beta-Klotho floxed (Klblox/lox) mice were provided by Dr. Steven Kliewer [19,20] and crossed with Camk2a-Cre mice [21] to generate brain-specific Klb knockout (KlbCam2ka) mice. Th-Cre mice [22] were procured from the European Mouse Mutant Archive (B6.129X1-Thtm1(cre)Te/Kieg, EMMA #: EM_00254). Th-Cre was bred onto the Fgf21-KO background to allow viral targeting and fiber photometry recording from dopamine neurons in the absence of FGF21.
Mice were individually housed in a controlled environment with regulated temperature and humidity and maintained on a 12:12-hr light–dark cycle. Throughout the experiments, the mice had ad-libitum access to food and water unless stated otherwise. All experiments were conducted during the light cycle. The control and low protein (LP) diets were formulated and produced by Research Diets and designed to be isocaloric by adjusting the levels of protein and carbohydrates while keeping the fat content constant. The control diet consisted of 20% casein (by weight) as the primary source of protein, while the LP diet included 5% casein. Diet descriptions have been previously published [8,23,24].
2.2. Experiment 1: effects of protein restriction on the motivation for various nutrient rewards
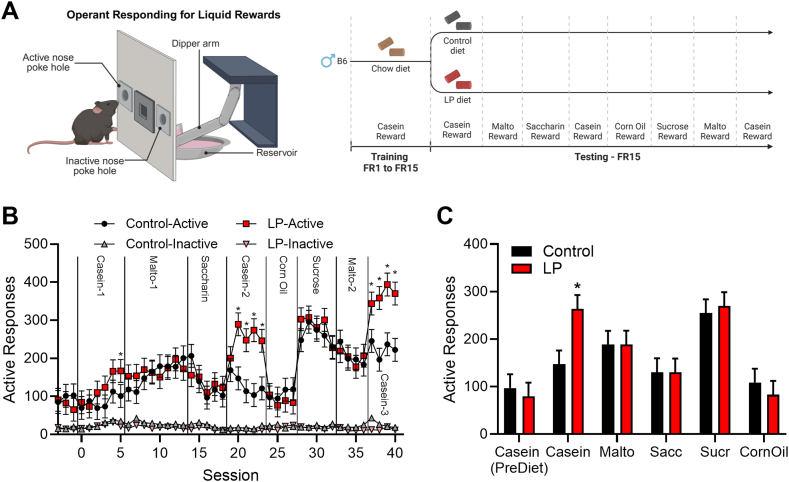
Experimental sessions were conducted in operant conditioning chambers (Med Associates, Inc.) equipped with two nose poke response devices, a house light for general illumination, and a switchable liquid dipper that provided access to 0.01 ml of an experimenter-made solution. Male C57BL/6J mice (n = 16) fed a chow diet were trained to nose poke for a 4% casein/0.2% saccharin solution by raising the dipper for 10 s each time the mouse made a nose poke response and providing response-independent dipper access at variable times with inter-delivery intervals averaging 6 min. Once a mouse made 10 responses within a single session, response-independent solution deliveries were discontinued, and only responses in the “active” nose poke hole (left/right assignment of the active nose poke hole was counterbalanced across mice) produced solution access. Once mice exhibited stable responding under a fixed ratio (FR) 1 schedule of reinforcement, the FR value was increased progressively to 15 (15 nose pokes = 1 reward) and held constant for the remainder of the experiment. At this point, mice were randomized to either control or LP diet (8/diet) for the remainder of the study (day 0), and across sessions, the liquid reward was altered between multiple nutrient sources as follows: 6 sessions of 4% casein/0.2% saccharin, 8 sessions of 4% maltodextrin/0.2% saccharin, 5 sessions of 0.2% saccharin, 5 sessions of 4% casein/0.2% saccharin, 4 sessions of corn oil, 5 sessions of 15% sucrose, 4 sessions of 4% maltodextrin/0.2% saccharin and 4 sessions of 4% casein/0.2% saccharin. Sessions occurred once per day and lasted 75 min.
2.3. Experiment 2: effect of protein restriction on motivation for protein in WT mice and mice lacking FGF21 signaling
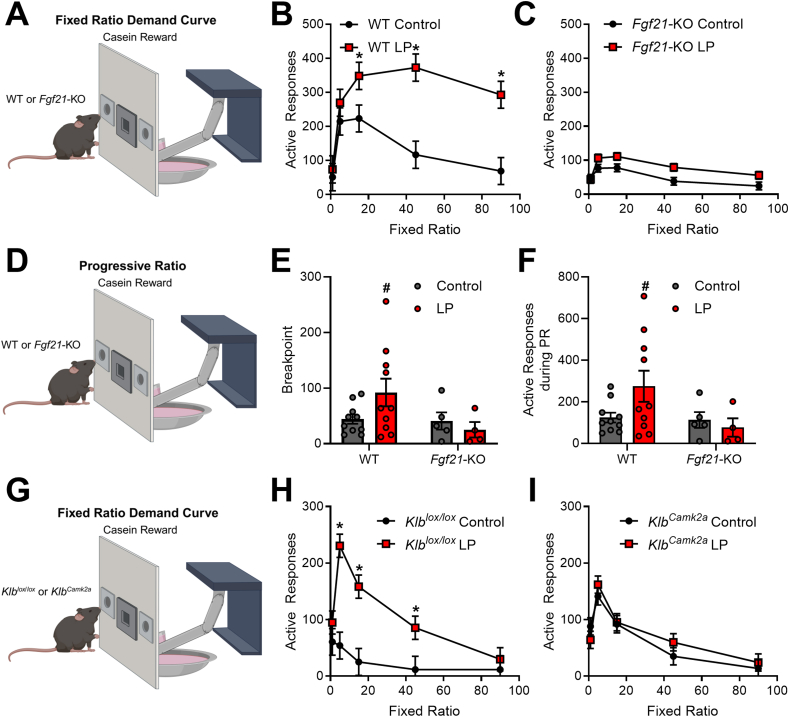
Training and experimental sessions were conducted using operant conditioning chambers. Male C57BL/6J (WT, n = 20) and Fgf21-KO (KO, n = 9) mice were randomly assigned to either control or LP diet for 10 days and subsequently trained to nose poke for 4% casein/0.2% saccharin solution by raising the dipper for 10 s each time the mouse made a nose poke response and providing response-independent dipper access at variable times with inter-delivery intervals averaging 6 min. Once a mouse made 10 responses within a single session, response-independent solution deliveries were discontinued, and only responses in the “active” nose poke hole (left/right assignment of the active nose poke hole was counterbalanced across mice) produced solution access. Once mice exhibited stable responding at FR 1, a demand assessment was conducted by varying the FR value in ascending order: 1, 5, 15, 45, and 90, with at least 5 individual sessions (120 min) at each FR value [25]. Following the sequence of FR values, mice were tested in two separate progressive ratio sessions (on separate days), in which the number of nose pokes required to elicit a reward progressively increased within the session [26], based on the following formula: Ratio Value = 5∗exp(Ratio Number∗0.2)-5. Progressive ratio (PR) sessions ended when mice failed to respond after 10 min (breakpoint) or after 120 min.
A separate study was conducted to replicate these findings in a group of brain-specific Klb knockout mice. Male Cre-negative Klblox/lox mice or Camk2-Cre positive littermates (KlbCamk2a) were randomized to control or LP diet, trained to nose poke for casein solution, and then tested over increasing FR values of 1, 5, 15, 45, and 90 (6–8 mice/genotype/diet).
2.4. Experiment 3: effect of protein restriction on preference and motivation for whey protein in WT and and Fgf21-KO mice
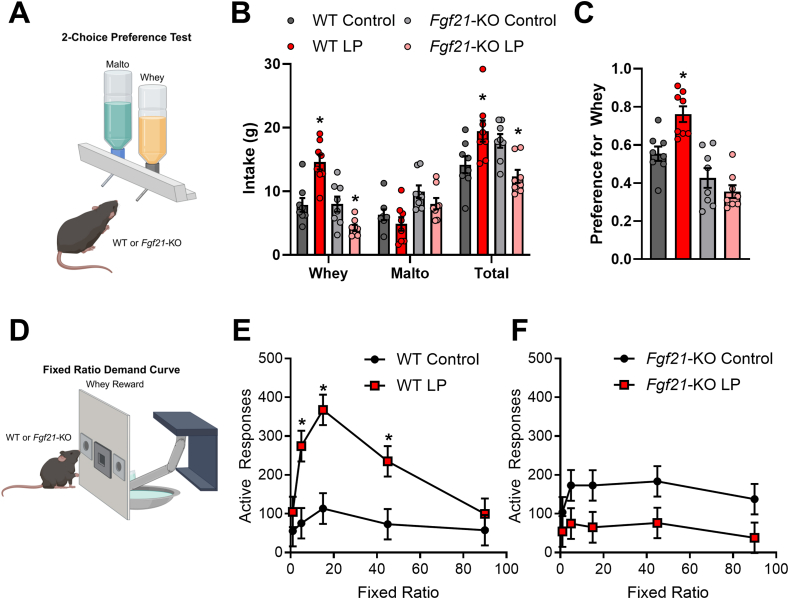
Male C57BL/6J and Fgf21-KO mice were placed on control or LP diet (8 mice/diet/genotype) for 7 days and were then offered two bottles containing either 4% whey/0.2% saccharin or 4% maltodextrin/0.2% saccharin for 3 days. Bottle locations were counterbalanced across mice and locations were swapped each day. Fluid consumption was measured daily and averaged across the 3-day experiment to provide average daily consumption. Preference for whey, calculated for each animal, was derived from average daily whey consumption divided by total consumption.
A separate group of male C57BL/6J and Fgf21-KO (KO) were placed on either control or LP diet (8 mice/diet/genotype) for 10 days and were trained to nose poke for 4% whey/0.2% saccharin by raising the dipper for 10 s each time the mouse made a nose poke response and providing response-independent dipper access at variable times with inter-delivery intervals averaging 6 min. Once a mouse made 10 responses within a single session, response-independent solution deliveries were discontinued, and only responses in the “active” nose poke hole (left/right assignment of the active nose poke hole was counterbalanced across mice) produced solution access. Once mice exhibited stable responding at FR 1, a demand assessment was conducted by varying the FR value in ascending order: 1, 5, 15, 45, and 90. However, this experiment was compressed into a shorter time window by providing mice with only a single session (120 min) at each FR.
2.5. Experiment 4: effect of protein restriction on VTA dopamine neuron activity in response to intraoral delivery of casein or maltodextrin
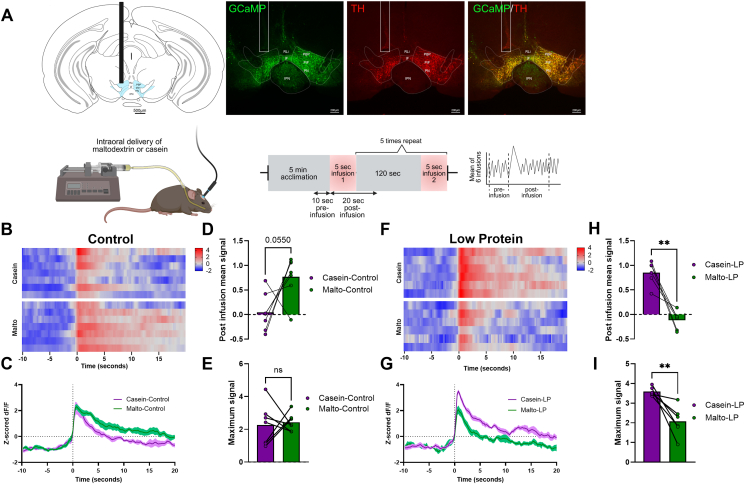
A Cre-dependent GCaMP6s virus (AAV-DJ-EF1a-DIO-GCaMP6; Stanford Gene Vector and Virus Core) was stereotaxically delivered to the VTA of male Th-Cre mice (n = 7), and a fiber optic cannula (Doric Lenses) was implanted at the injection site to record neural activity. Mice were given 3 weeks to recover, at which point fiber photometry recording (RZ10x Fiber Photometry Processor with integrated LUX LEDs and Photosensors; Tucker–Davis Technologies) of VTA dopamine neurons was conducted to confirm active GCaMP signal. After recovery, mice were placed on a control diet for 10 days. An intraoral cannula was then implanted, and mice were acclimated to intraoral water infusions. Mice were given a single overnight exposure to the solutions (4% casein/0.2% saccharin and 4% maltodextrin/0.2% saccharin) before the initiation of recordings. Neural activity in response to casein or maltodextrin was recorded during 15-minute sessions. Each session started with a 5-minute acclimation period, followed by six separate intraoral infusions at a rate of 4.8 μl/s for 5 s, with at least 120 s between infusions. VTA neural activity was recorded continuously throughout the session, but only 30-second segments, consisting of a 10-second pre-infusion period and a 20-second post-infusion period for each of the six infusions were analyzed. Casein and maltodextrin sessions were conducted on the same day approximately 5–6 h apart, with presentation order randomized for each animal. After recording under the control diet, all mice were switched to the LP diet for another 10 days, and the dopamine neuron activity during intraoral casein or maltodextrin infusion was again recorded as above. One mouse was excluded as the fiber optic cannula was dislodged during the LP diet phase, resulting in a sample size of n = 6 for LP diet experiment.
For the analysis, custom MATLAB scripts were used to fit the GCaMP signal (465 nm) to the isosbestic control signal (405 nm), calculating dF/F. To establish a consistent baseline for evaluating signal changes, the mean baseline signal (average of the 10-second pre-infusion periods) was subtracted from all data points in the 30-second analysis window for each infusion. Then, a single curve representing the intraoral infusion-induced signal was generated by averaging baseline-adjusted signals of the six infusions per session per animal. Subsequently, Z-scores were calculated for each mouse to account for inter-animal variability in signal intensities. The mean used for the Z-score calculation was the mean signal across both maltodextrin and casein sessions for each mouse, and the standard deviation used for the Z-score calculation was the standard deviation across both maltodextrin and casein sessions for each mouse. Using these z-standardized values, the mean and maximum (peak) signal for the 20-second post-infusion period were calculated and compared.
To test whether LP-induced changes in dopamine neuron activity require FGF21, the Th-Cre allele was bred onto a homozygous Fgf21-KO background. Th-Cre;Fgf21-KO mice (n = 6) were then placed on control and LP diet, with the dopamine neuron response to intraoral delivery of casein and maltodextrin measured as described above.
2.6. Stereotaxic brain surgery and fiber optic cannula implantation
Mice were anesthetized using isoflurane (2–5%) and positioned under a stereotaxic instrument. Mice received 0.1 ml/100g body weight of 5 mg/ml carprofen subcutaneously preoperatively and 24 h after surgery. A midline incision was made on the skin, and the skull surface was leveled. The bregma landmark was identified and used to adjust the positioning. A 1 mm hole was drilled in the skull to accommodate the guide cannula (Plastics One), which was then lowered into the brain, precisely above the VTA target. A unilateral delivery of a recombinant Adeno-associated virus (rAAV) solution was performed, and the injection coordinates relative to bregma were as follows: anterior-posterior (AP) −3.3, medial-lateral (ML) +0.4, dorsal-ventral (DV) −4.4 and −4.2 mm for the two injections. Using an injector attached to a 1 μl Hamilton syringe, a total injection volume of 0.8 μl (0.4 μl for each injection) was infused at a rate of 0.1 μl/min. After the first injection, a 5-minute diffusion period was allowed before the guide cannula was raised to the higher site and the second injection was performed. Following the viral injections, a chronically implantable fiber optic cannula (Doric Lenses, MFC_400/430–0.48_4.3 mm_MF2.5_FLT) was implanted at a DV coordinate of −4.3 mm. The fiber optic cannula was secured using dental acrylic adhesive cement (C&B-Metabond). Following recovery, all mice were tested for signal strength and responsivity of neural activity before inclusion in the study. Following the experiments, mice were euthanized and immunohistochemistry was conducted to confirm appropriate placement of the fiber optic cannula.
2.7. Intraoral surgery and cannulation
Intraoral cannulas were placed under general anesthesia (2–5% isoflurane) and subcutaneous analgesia (carprofen) in the animal operating room at Pennington Biomedical Research Center, using a procedure modified from Stratford and Thompson [27]. A midline skin incision was made on the dorsal surface immediately caudal to the pinnae. A sterile stainless steel hypodermic tube was inserted through the incision and guided subcutaneously to the oral cavity lateral to the molars and an intraoral incision was made. A 6-cm length of flared polyethylene catheter with a small Teflon washer was inserted intraorally through the hypodermic tube and the tube was removed such that the Teflon washer rested flush against the inner buccal cavity just lateral to the first maxillary molar. The catheter was secured in place subcutaneously, the skin was closed using non-absorbable suture, and the catheter was flushed with sterile water. Following surgery, mice were provided 0.5 ml of warmed sterile saline subcutaneously and mashed food. Once fully recovered, mice were regularly infused with water via the intraoral cannula to ensure patency and acclimate the animal to intraoral fluid delivery.
2.8. Statistical analysis
Data were analyzed using the SAS version 10 software package (SAS Institute) via one-way, two-way, or repeated-measures ANOVA using the general linear model procedure. When experiment-wide tests were significant, post hoc comparisons were made using the LSMEANS statement with the PDIFF option and represent least significant differences tests for pre-planned comparisons. Fiber photometry data were analyzed with a two-tailed paired Student’s t-test to assess differences in mean signal and max signal before and after the low protein diet. All data are expressed as mean ± SEM, with probability values less than 0.05 considered statistically significant and less than 0.1 considered as trending toward statistical significance.
2.9. Audio/Visual Credits
Schematics in figures were created with BioRender.com.
3. Results
3.1. Protein-restricted mice exhibit a protein-specific increase in motivated behavior
Our prior work demonstrates that protein-restricted mice shift macronutrient preference, increasing the consumption of protein and reducing the consumption of carbohydrate in a two-choice test [8]. Prior work also indicates that protein restriction increases operant responding for protein rewards in rats, consistent with an increase in the motivation for protein [12]. We first tested whether this increase in protein motivation would translate to mice, and whether this increased motivation is protein-specific. Male C57BL/6J mice trained to nose poke for liquid rewards were transitioned from standard chow to a LP or isocaloric control diet and offered various liquid rewards that shifted between casein, maltodextrin, saccharin, sucrose, and corn oil. Mice exhibited a significant nutrient reward∗diet interaction (P = 0.03; Figure 1), with the LP group responding more strongly than control diet-fed mice when casein was the reward (P = 0.001). However, there was no difference in responding between control and LP-fed mice when any other nutrient served as the reward. Responding at the inactive nose poke was very low and did not differ between groups or across liquid rewards, indicating that active nose poke responding was controlled by liquid reward delivery and was not a random activity. In addition, the LP vs. control difference in responding for casein was relatively small in the first offering (<5 days of LP diet), but was much larger on subsequent exposures, presumably due to either the increased length of time on the LP diet or familiarity due to repeated exposures. Taken together, these data suggest that protein restriction specifically increases motivation for protein reward.
Figure 1.
Protein restricted mice selectively increase operant responding for protein. A. Male C57BL/6J mice were trained to nose poke for liquid casein rewards under a fixed ratio (FR) 1 schedule and the FR value was subsequently increased to FR15. Half the mice were then transitioned to a low protein (LP) diet and continued to respond for casein before being offered a variety of liquid nutrient rewards. LP mice significantly increased responding for casein, but not for any other nutrient reward B. Responses on active and inactive nose poke. C. Average active responses for each nutrient. ∗P < 0.05 vs respective control; 8 mice/diet.
3.2. Neuronal FGF21 action is required for LP-induced motivation for casein
To test whether FGF21 is required for this LP-induced increase in motivation for protein in male mice, we assessed the demand for protein by progressing control and LP diet-fed mice through an increasing sequence of fixed ratio values: 1, 5, 15, 45, and 90 (Figure 2A). Consistent with the data above, responding for casein was higher in WT mice on the LP diet compared to WT mice on the control diet at FR values 15, 45, and 90 (Figure 2B; Diet x FR P = 0.02). In contrast, responding was not significantly increased by LP diet in Fgf21-KO mice at any FR value (Figure 2C; Diet x FR P = 0.42). In behavioral economic terms, LP diet-fed mice exhibited greater defense of casein consumption than control diet-fed mice. Following the demand assessment, responding for casein was tested in a PR task (Figure 2D). In WT mice, the LP diet tended to increase breakpoint (Figure 2E; P = 0.084) and active responses (Figure 2F; P = 0.071) during the PR test. In contrast, there was no evidence of an increase in these endpoints in Fgf21-KO mice on the LP diet. Taken together, these data suggest that FGF21 is required for LP-induced increases in motivation for protein.
Figure 2.
FGF21 signaling in the brain is required for LP-induced increases in operant responding for protein. A. Male C57BL6 and Fgf21-KO mice were placed on a control or low protein (LP) diet and trained to nosepoke for casein. B. Operant responding for casein in a fixed ratio demand curve in C57BL6 mice (WT; 10 mice/diet). C. Operant responding for casein in a fixed ratio demand curve in Fgf21-KO mice (KO; 4–5 mice/diet). D. Male C57BL6 and Fgf21-KO mice were placed on a control or LP diet and trained to nosepoke for casein. E. Progressive ratio breakpoint in WT and Fgf21-KO mice. F. Total active responses during a progressive ratio task in WT and Fgf21-KO mice. G. Male Klblox/lox and Klblox/lox;Cam2ka-Cre (KlbCamk2a) were placed on a control or LP diet and trained to nosepoke for casein. H. Operant responding for casein in a fixed ratio demand curve in Klb-floxed control (6–8 mice/diet). I. Operant responding for casein in a fixed ratio demand curve in brain-specific Klb knockout mice (KlbCamk2a; 8 mice/diet).∗P < 0.05; #P < 0.10 vs respective control.
Our prior work suggests that LP-induced changes in food intake and macronutrient preference are largely driven by FGF21 signaling directly within the brain [8]. To test whether LP-induced protein motivation also requires brain FGF21 action, mice bearing neuron-specific deletion of Klb (KlbCamk2a) or their floxed littermate controls (Klblox/lox) were tested using the same demand assessment described above (Figure 2G). In Klblox/lox mice, the LP diet again increased responding for casein (Figure 2H, Diet x FR P = 0.0038). However, there was no effect of LP diet on responding in KlbCamk2a mice (Figure 2I; Diet x FR P = 0.535). Taken together, these data indicate that LP-induced increases in motivation for casein require FGF21 signaling in the brain.
3.3. Protein restriction increases the preference and motivation for whey protein
All of the work described above utilized casein as the protein source, and thus the observed behaviors may be driven by sensory properties unique to casein. We therefore tested whether LP-induced changes in motivation translate to other protein sources, using whey as the protein source. We first used a 24-hr two-bottle choice model to test the consumption of 4% whey solution vs. 4% maltodextrin (Figure 3A). Comparing male WT vs Fgf21-KO mice, we observed a significant interaction between diet and genotype for whey consumption, total liquid consumption, and whey preference (Figure 3B, C; all Ps < 0.01), with WT mice on LP diet consuming more whey (P < 0.001) and showing higher preference (P = 0.0015) relative to control diet-fed mice. In contrast, LP did not increase whey intake and preference in Fgf21-KO mice.
Figure 3.
Protein restriction increases preference and motivation for whey protein in WT but not Fgf21-KO mice. A. Male mice were placed on a control or low protein (LP) diet for 7 days, and were then offered the choice beween 4% whey and 4% maltodextrin solutions in the home cage over three 24-hr periods. B. Average 24hr consumption of whey and malto. C. Preference ratio for whey (whey consumption divided by total consumption). D. Male mice on control or LP diet were trained to nosepoke for whey in a rapid demand curve assessment. E. Operant responding for whey in a fixed ratio demand curve in C57BL/6J mice (WT). F. Operant responding for whey in a fixed ratio demand curve in Fgf21-KO mice (KO). ∗P < 0.05 vs respective control; 8 mice/group).
We then moved to an operant responding paradigm similar to that described above, except that after mice were trained to respond at FR1, only one operant session occurred at each FR value (Figure 3D). In this more rapid protocol, we observed a significant interaction between diet and genotype (P = 0.046). WT mice on LP diet responded for whey more strongly than control mice at FR5, FR15, and F45 (P < 0.01, Figure 3E). This LP-induced increase in responding was absent in Fgf21-KO mice, who tended (P ≥ 0.06) to reduce responding for whey when on LP diet (Figure 3F). Taken together, these data reinforce the concept that protein restriction significantly increases the preference for protein relative to carbohydrate as well as the motivation to procure protein. This LP-induced effect manifests across different protein sources and requires FGF21 signaling.
3.4. FGF21 is required for nutrient-specific shifts in VTA dopamine neuron activity in protein-restricted animals
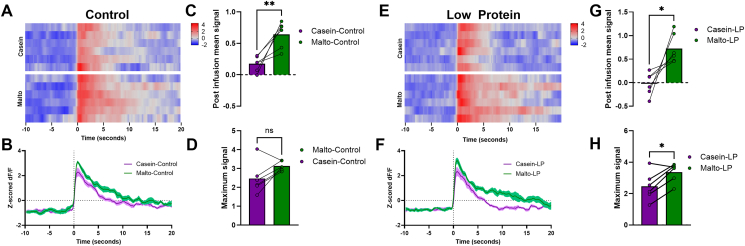
Dopamine neurons within the VTA are closely linked to reward and motivation, and recent work in rats suggests that protein restriction enhances protein-induced activation of unidentified VTA neurons [14]. We, therefore, sought to use mice to specifically test whether dopamine neurons within the VTA respond to the delivery of protein within the oral cavity, and if this response is enhanced by protein restriction. To test this question, Th-Cre mice were used for fiber photometry recording of VTA neurons in response to the intraoral delivery of casein or maltodextrin (Figure 4A). We tested the relative strength of casein vs. maltodextrin-induced neural activity to assess macronutrient-specific differences as compared to broader nutrient-agnostic effects. When male WT mice consuming the control diet were tested, intraoral delivery of maltodextrin or casein both produced distinct increases in VTA dopamine neuron activity (Figure 4B, C). However, the mean signal strength following maltodextrin infusion tended to be larger than the mean signal following casein infusion (P = 0.055, Figure 4D), though this difference did not reach statistical significance. There was no difference in the maximum (peak) signal (Figure 4E) between maltodextrin and casein in the 20 s post-infusion.
Figure 4.
Protein restriction induces a macronutrient-dependent shift in VTA-dopamine neuron activity. A. (Top left) Schematic illustration showing the placement of a fiber optic cannula targeting the lateral VTA, specifically the parabrachial pigmented nucleus (PBP), parainterfascicular nucleus (PIF), and paranigral nucleus (PN); (Top right) IHC images of GCaMP6 and TH; (Bottom left) schematic of the experimental setup; (Bottom right) fiber photometry recording protocol B. Heat map of neuron activity in individual animals on Control diet, with each row representing z-scored dF/F signal from a single animal. C. Average change in neuronal activity in response to nutrient infusion in Control-fed animals. Solid line indicates mean and shaded area represents SEM. D. Mean signal during the 20 s post intraoral infusion on control diet. E. Maximum signal during the 20 s post intraoral infusion on control diet. F. Heat map of neuron activity in individual animals on LP diet, with each row representing z-scored dF/F signal from a single animal. G. Average change in neuronal activity in LP-fed animals. Solid line indicates mean and shaded area represents SEM. H. Mean signal during the 20 s post intraoral infusion on LP diet. I. Maximum signal during the 20 s post intraoral infusion on LP diet. ∗P < 0.05, ∗∗P < 0.01 vs. respective control; 6 mice/group. Modified schematic of mouse brain atlas adapted from Paxinos and Franklin [66].
Interestingly, these macronutrient-induced neuron responses reversed when mice were fed the LP diet. In this case, casein induced a larger increase compared to maltodextrin (Figure 4F, G), resulting in a larger mean (Figure 4H; P < 0.01) and maximum (Figure 4I; P < 0.01) signal compared to maltodextrin in the 20 s post intraoral infusion. Collectively, these data indicate that LP shifts the relative strength of the VTA dopamine neuron response, increasing the size of the casein vs maltodextrin-induced dopamine response.
Considering FGF21’s role as a signal of the protein-restricted state, we then tested whether this LP-induced shift in VTA dopamine neuron activity requires FGF21. To address this question, the Th-Cre allele was crossed onto the Fgf21-KO background, and these Th-Cre;Fgf21-KO mice were used to test nutrient-induced activation of VTA dopamine neurons via fiber photometry as described above (Figure 5A). In the absence of FGF21, we again observed readily apparent increases in VTA dopamine neuron activity in response to the oral delivery of either casein or maltodextrin. Maltodextrin produced a relatively larger dopamine activation as compared to casein (Figure 5B, C), with a significantly larger mean signal in the 20-second post-infusion period (Figure 5D; P < 0.01). However, the LP-induced shift in the relative strength of these responses was absent in Th-Cre;Fgf21-KO mice, such that maltodextrin continued to produce a relatively larger increase in mean dopamine neuron activity when mice consumed LP diet (Figure 5G,H, 5I, P < 0.05). Taken together, these data suggest that FGF21 is essential for macronutrient-specific shifts in dopamine neuron activity in protein-restricted animals.
Figure 5.
Deletion of FGF21 blocks the effects of protein restriction on VTA-dopamine neuron activity. Male Th-Cre; Fgf21-KO mice on Control diet were used to assess fiber photometry recording of TH-positive neurons in the VTA in response to intraoral delivery of either 4% casein or 4% maltodextrin solutions. Mice were then placed on LP diet for 10 days and the recording was repeated. A. Heat map of neuron activity in individual animals, with each row representing z-scored dF/F signal from a single animal. B. Average change in neuronal activity on control diet. Solid line indicates mean and shaded area represents SEM. C. Mean signal during the 20 s post intraoral infusion on control diet. D. Maximum signal during the 20 s post intraoral infusion on control diet. E. Heat map of neuron activity in individual animals, with each row representing z-scored dF/F signal from a single animal. F. Average change in neuronal activity on LP diet. Solid line indicates mean and shaded area represents SEM. G. Mean signal during the 20 s post intraoral infusion on LP diet. H. Maximum signal during the 20 s post intraoral infusion on LP diet. ∗P < 0.05, ∗∗P < 0.01 vs. respective control; 6 mice/group.
4. Discussion
Prior work has established that protein restriction alters macronutrient intake [9,28,29]. Protein-restricted mice increase total food intake if maintained exclusively on a low protein diet, but selectively shift their preference towards protein-rich foods if given a choice between various nutritional options [[8], [9], [10], [11],15]. In addition, these changes in protein intake require FGF21 signaling in the brain, as the deletion of either FGF21 from the whole animal or deletion of its receptor in neurons blocks these adaptive changes in food intake in response to protein restriction [8,15,23]. Here, we focus on the mechanisms that drive these FGF21-dependent changes in macronutrient preference, focusing specifically on motivation, reward, and dopamine signaling.
Motivation drives food intake in various need states, and therefore one would predict that a protein-restricted state would increase the value of protein and lead animals to work harder to obtain protein-rich rewards [30]. Although there has been very little work related to protein-specific motivation, prior work has shown that protein restriction increases operant responding for protein in both golden hamsters [13] and rats [12]. Therefore, we sought to extend these data to mice, enabling the use of mouse genetics to test the role of FGF21 in driving these changes in motivation during protein restriction.
We first sought to establish a model to assess motivation for liquid rewards in mice, as liquid rewards provide a means to easily test various nutrients in discrete amounts over time. We observed clear and significant increases in operant responding for casein in LP-fed mice as compared to control mice, but interestingly there was no LP effect for any other nutrient tested, including maltodextrin, sucrose, corn oil, and saccharin. While these rewards were not precisely balanced for caloric content or palatability, the results provide strong evidence that the LP-induced increase in motivation is nutrient-specific and that the hyperphagia observed in animals maintained exclusively on a low protein diet is driven by a specific motivation to consume protein [17]. Indeed, our prior data indicate that protein-restricted mice do not increase maltodextrin consumption when it is offered alone, nor do they consistently increase total food intake if they can choose between high and low protein diets [8]. These observations are consistent with the concept of protein leveraging, which predicts that animals prioritize protein over energy if they cannot select between foods to balance their energy vs. protein needs [17,31,32].
Interestingly, we did not observe any difference in operant responding for sucrose, despite evidence that FGF21 inhibits sweet intake [33,34]. Based on this prior work and the fact that the LP diet induces a large increase in FGF21 [8,15,23], we anticipated that mice on LP might reduce operant responding relative to control mice when sucrose or saccharin was the reward. It seems possible that LP-induced FGF21 signaling drives different mechanisms than exogenous FGF21 administration, or that other pathways activated in the low protein state serve to blunt the effects of FGF21 on sweet intake. Interestingly, recent work indicates that a low-protein meal does not alter the consumption of a sweet dessert but does increase the consumption of a high-protein dessert [35], while the well-established effects of carbohydrate meals to drive FGF21 were blocked by relatively small amounts of added protein [36]. Recent work also indicates that protein-restricted mice exhibit decreased consumption of sucrose, decreased sucrose-induced conditioned place preference, and decreased dopamine release in the nucleus accumbens [37]. It is unclear why protein-restricted mice did not show a reduction in operant responding for sucrose in this study, and more focused work on protein restriction and its effects on motivation for sweet rewards would be necessary to resolve this question.
Having established a model in which protein-restricted mice exhibit a clear increase in motivation for protein, we next focused on the mechanisms that might drive this protein-specific motivation. As described above, our prior work indicates that FGF21 is essential for LP-induced hyperphagia in mice on single-choice diets [15,23], as well as LP-induced changes in protein vs carbohydrate preference in two-choice models [8]. We, therefore, tested whether FGF21 is also required for protein restriction to increase motivation for casein, using a fixed ratio demand curve assessment in which the effort to procure casein increased with time. We observed that the LP diet markedly increased operant responding for casein in WT mice, suggesting an increase in motivation for protein. In contrast, this LP effect was completely lost in Fgf21-KO mice. This demand curve approach was replicated in a different line of mice bearing brain-specific deletion of the FGF21 co-receptor Klb (KlbCamk2a-Cre), and again we observed that the LP diet increased operant responding for casein in the floxed controls, while this LP-effect was completely lost in the brain-specific Klb knockouts. Of note, the WT mice and Klblox/lox mice exhibit different demand curves despite similar background genetics, with WT mice exhibiting more active nose-poke responses during high FR sessions. The reason for this discrepancy is currently unclear but may be due to the time between experiments and variations in personnel, resulting in slight discrepancies in training or animal handling that are difficult to eliminate during behavioral tests. Therefore, the primary focus is the effect of diet within each experimental group, and the current data, generated from two separate genetic lines, support the overarching hypothesis that protein restriction increases the motivation for casein and that this increased motivation requires intact FGF21 signaling.
LP-induced changes in casein consumption or motivation might be driven by some property inherent to casein that is unrelated to its nutritional value. To confirm that protein restriction broadly increased consumption and motivation for protein, we replicated our core observations using whey, since whey protein contains a different amino acid composition and digestibility profile compared to casein [[38], [39], [40]]. We first tested whether protein restriction altered whey consumption or preference in a 2-choice paradigm with maltodextrin. Just as with casein [8,11], mice on LP diet significantly increased their consumption and preference for whey vs. maltodextrin. Importantly, this shift in macronutrient preference was lost in Fgf21-KO mice, again demonstrating FGF21’s importance in mediating these behavioral responses. We then tested whether protein restriction altered motivation for whey, using a rapid demand curve operant paradigm. Here again, mice on LP diet increased responding for whey in a manner analogous to mice responding for casein, and again this LP-induced increase in responding was lost in Fgf21-KO mice. Taken together with the operant data in Figure 1, Figure 2, these data indicate that the LP-induced increase in consumption, preference, and motivation for casein extends to other protein sources, but not to other macronutrients. However, while whey and casein are different proteins, they are both balanced, animal-based proteins. Therefore, it remains possible that other protein sources, particularly those with poorly balanced amino acid profiles, might produce different outcomes. Previous studies have shown varying levels of preference for various protein sources in protein-restricted rats [41], while animals also detect specific amino acid deficits in food and select diets to balance and replete the deficiency [[42], [43], [44]]. Nevertheless, the current work is consistent with the hypothesis that protein-restricted mice manifest a ‘protein appetite,’ with protein restriction causing animals to specifically seek and consume protein without increasing the consumption of other foods. The changes in feeding behavior driven by protein restriction are fundamentally different from the effects of energy restriction or general dietary (food) restriction, which promote hyperphagia and increased motivation to procure food in general. Finally, and importantly, these data again support the essential role of FGF21 in mediating these protein-specific changes in motivation and consumption.
Because these data demonstrate that protein restriction leads to an FGF21-dependent increase in the rewarding value of protein, we next focused on the neural mechanisms mediating this behavior. The homeostatic detection and defense against nutrient restriction is mediated by multiple brain areas, including those classically associated with reward [45,46]. The mesolimbic dopamine system is particularly linked with reward behavior, including the response to food and food rewards [[47], [48], [49], [50], [51]]. Indeed, neurons in the VTA were activated by protein intake in rats, and this activation was enhanced by protein restriction [14]. Similarly, meal-induced cFos in the nucleus accumbens was enhanced in protein-restricted rats consuming a high-protein meal [52]. Similar to observations in rodents, findings in humans also suggest that brain reward responses adapt to the body’s protein status, further emphasizing the potential for nutrient-specific modulation of reward pathways. Functional MRI studies have revealed that brain activity in response to protein intake involves multiple regions. In individuals with low protein levels, there was heightened activity in reward-related regions, including the orbitofrontal cortex and striatum, when exposed to food cues, compared to individuals with higher protein levels [53]. Conversely, after consuming a protein-rich meal, reduced activation was observed in the hippocampus, amygdala, anterior cingulate, and parahippocampal areas when participants viewed food images [54].
Considering the importance of the reward system to feeding behavior, we used mouse genetics to target VTA dopamine neurons (via Th-Cre) and test their activity in the absence of FGF21 (via Fgf21-KO mice). Delivery of either casein or maltodextrin into the oral cavity produced a clear, temporally discrete increase in VTA-dopamine neuron activity. In mice consuming a control diet, the relative response of VTA dopamine neurons to intraoral maltodextrin was slightly but consistently larger than the response to intraoral casein. These effects are consistent with our observation that control-fed mice prefer maltodextrin to casein in a 2-choice preference test [8]. Conversely, in mice consuming the LP diet, casein produced a larger activation of dopamine neurons compared to maltodextrin, again consistent with the fact that LP-fed mice prefer casein. Thus, these data suggest that protein restriction induces macronutrient-specific shifts in the response of the reward system to orally delivered nutrients. Finally, we point out that this comparison of casein to maltodextrin-induced signal is critical for disentangling macronutrient-specific effects from broad, nutrient-independent changes. Both casein and maltodextrin induce VTA dopamine signaling and are readily consumed, regardless of whether the animal is on the control or LP diet. However, protein-restricted mice exhibit a selective shift in the relative value of these two macronutrients, such that carbohydrate is more valued in control animals and protein is more valued in protein-restricted animals. These behavioral changes are mirrored by changes in the relative strength of dopamine neuron activation following oral delivery.
Finally, we tested whether these diet-induced shifts in macronutrient-induced dopamine neuron activity were dependent on FGF21, using Th-Cre;Fgf21-KO to selectively record from VTA dopamine neurons in the absence of FGF21. We again observed that VTA dopamine activity was increased by the intraoral infusion of both maltodextrin and casein, but the strength of the casein vs. maltodextrin-induced signal did not shift when Th-Cre;Fgf21-KO mice were fed LP. As such, not only do Fgf21-KO mice not change their preference or motivation for protein on a LP diet, but the dopamine neuron response to specific macronutrients also does not shift. Therefore, these data are consistent with the work above and collectively support a model in which FGF21 acts in the brain to promote protein intake by altering the reward system response to nutrient ingestion to enhance the value of protein.
While our work broadly supports a model in which FGF21 acts in the brain to increase protein motivation, there are several caveats to consider with this work. First, where and how FGF21 acts to alter neural activity in the VTA is unclear. Available evidence suggests that Klb is not expressed within the VTA [19,55], and thus it is likely that these effects are mediated indirectly in response to FGF21 action in upstream brain areas. Second, although it is well established that VTA dopamine neurons play a critical role in motivation and learning, our data do not definitively demonstrate that changes in dopamine neuron activity drive the changes in operant responding observed during protein restriction. Indeed, it is somewhat simplistic to view dopamine neuron activation as strictly encoding reward, as dopamine neurons respond to various stimuli, including aversive stimuli. Third, despite specific expression of TH in dopamine neurons [56], we acknowledge that this TH-Cre strategy inevitably targets a small number of non-dopaminergic neurons [57,58]. However, this non-specificity is primarily detected in areas medial (rostral linear nucleus and interfascicular nucleus) or outside the VTA (interpeduncular nucleus), which are not targeted in our study. Instead, our fiber optic cannula targeted the lateral VTA (parabrachial pigmented nucleus, parainterfascicular nucleus, paranigral nucleus), where there is a high degree of specificity in the TH-Cre model. While no animal model is perfect, because of the selectivity in the targeted areas, we are confident that our work primarily measures dopamine neuron activity in the lateral VTA. Fourth, while our Fgf21-KO mice are on the C57BL/6J background, they are not littermates to the B6 controls used in this study (Figure 2), and thus subtle genetic differences could exist. Finally, an important limitation of this study is the use of only male mice. Although recent data suggest that both males and females respond similarly in terms of protein preference under protein restriction [59] and that FGF21 increases dietary protein consumption in female mice [60], we acknowledge that females may be less sensitive to the metabolic effects of protein or amino acid restriction [[61], [62], [63], [64]]. Therefore, sex differences may exist in the level of operant responses and reward-evoked responses in the mesolimbic circuit [65].
5. Conclusion
These data indicate that protein restriction induces a macronutrient-specific increase in motivation for protein. Protein-restricted mice will work harder for multiple protein sources in an operant task, but they do not work harder for carbohydrate, sweet, or fat. In addition, these behavioral changes are accompanied by changes in the mesolimbic reward system, with protein restriction altering the dopamine neuron response to nutrient ingestion in a macronutrient-specific fashion. Finally, both the behavioral and cellular manifestations of protein reward are dependent on FGF21, and most likely its ability to signal in the brain. As such, these data provide convincing evidence that FGF21 is an endocrine signal of protein restriction that acts in the brain to specifically enhance the reward value of protein-containing foods and promote their consumption. FGF21 thus provides a compelling mechanism to explain how animals within complex nutritional landscapes effectively balance the physiological need for protein vs. other macro and micronutrients.
CRediT authorship contribution statement
Md Shahjalal H. Khan: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Sora Q. Kim: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Robert C. Ross: Methodology, Investigation. Florina Corpodean: Methodology, Investigation. Redin A. Spann: Writing – review & editing, Writing – original draft, Investigation. Diana A. Albarado: Investigation. Sun O. Fernandez-Kim: Investigation. Blaise Clarke: Investigation. Hans-Rudolf Berthoud: Writing – review & editing, Visualization, Methodology. Heike Münzberg: Writing – review & editing, Methodology. David H. McDougal: Writing – original draft, Investigation. Yanlin He: Writing – review & editing, Methodology. Sangho Yu: Writing – original draft, Visualization, Methodology, Investigation. Vance L. Albaugh: Writing – review & editing, Visualization, Methodology, Investigation. Paul L. Soto: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Conceptualization. Christopher D. Morrison: Writing – review & editing, Writing – original draft, Visualization, Methodology, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Christopher Morrison reports financial support was provided by National Institutes of Health. Sora Kim reports financial support was provided by National Institutes of Health. Redin Spann reports was provided by National Institutes of Health. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank the leadership and staff of the PBRC Comparative Biology Core, Animal Metabolism and Behavior Core, Cell Biology and Bioimaging Core, and Genomics Core Facility for their skillful assistance and excellent technical support.
Funding sources
This work was supported by the National Institutes of Health (NIH) R01DK123083 and S10OD023703 to C.D.M. SQK was supported by T32DK064584. RAS was supported by F32DK130544. This project used facilities within the Animal Metabolism & Behavior Core, Genomics Core, and Cell Biology and Bioimaging Core at PBRC that are supported in part by NIH center awards P20GM135002 and P30DK072476, as well as an NIH equipment award S10OD023703.
Contributor Information
Paul L. Soto, Email: Soto1@lsu.edu.
Christopher D. Morrison, Email: Christopher.Morrison@pbrc.edu.
Data availability
Data will be made available on request.
References
- 1.Berthoud H.R., Morrison C.D., Ackroff K., Sclafani A. Learning of food preferences: mechanisms and implications for obesity & metabolic diseases. Int J Obes. 2021;45(10):2156–2168. doi: 10.1038/s41366-021-00894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud H.R., Munzberg H., Richards B.K., Morrison C.D. Neural and metabolic regulation of macronutrient intake and selection. Proc Nutr Soc. 2012;71(3):390–400. doi: 10.1017/S0029665112000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hill C.M., Qualls-Creekmore E., Berthoud H.R., Soto P., Yu S., McDougal D.H., et al. FGF21 and the physiological regulation of macronutrient preference. Endocrinology. 2020;161(3):bqaa019. doi: 10.1210/endocr/bqaa019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berridge K.C., Flynn F.W., Schulkin J., Grill H.J. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98(4):652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 5.Geerling J.C., Loewy A.D. Central regulation of sodium appetite. Exp Physiol. 2008;93(2):177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- 6.Krause E.G., Sakai R.R. Richter and sodium appetite: from adrenalectomy to molecular biology. Appetite. 2007;49(2):353–367. doi: 10.1016/j.appet.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuda T., Hiyama T.Y., Niimura F., Matsusaka T., Fukamizu A., Kobayashi K., et al. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nat Neurosci. 2017;20(2):230–241. doi: 10.1038/nn.4463. [DOI] [PubMed] [Google Scholar]
- 8.Hill C.M., Laeger T., Dehner M., Albarado D.C., Clarke B., Wanders D., et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019;27(10):2934–2947 e2933. doi: 10.1016/j.celrep.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan M.S., Spann R.A., Munzberg H., Yu S., Albaugh V.L., He Y., et al. Protein appetite at the interface between nutrient sensing and physiological homeostasis. Nutrients. 2021;13(11) doi: 10.3390/nu13114103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larson K.R., Chaffin A.T., Goodson M.L., Fang Y., Ryan K.K. Fibroblast growth factor-21 controls dietary protein intake in male mice. Endocrinology. 2019;160(5):1069–1080. doi: 10.1210/en.2018-01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murphy M., Peters K.Z., Denton B.S., Lee K.A., Chadchankar H., McCutcheon J.E. Restriction of dietary protein leads to conditioned protein preference and elevated palatability of protein-containing food in rats. Physiol Behav. 2018;184:235–241. doi: 10.1016/j.physbeh.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiacchierini G., Naneix F., Apergis-Schoute J., McCutcheon J.E. Restriction of dietary protein in rats increases progressive-ratio motivation for protein. Physiol Behav. 2022;254 doi: 10.1016/j.physbeh.2022.113877. [DOI] [PubMed] [Google Scholar]
- 13.DiBattista D. Operant responding for dietary protein in the golden hamster (Mesocricetus auratus) Physiol Behav. 1999;67(1):95–98. doi: 10.1016/s0031-9384(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 14.Chiacchierini G., Naneix F., Peters K.Z., Apergis-Schoute J., Snoeren E.M.S., McCutcheon J.E. Protein appetite drives macronutrient-related differences in ventral tegmental area neural activity. J Neurosci. 2021;41(23):5080–5092. doi: 10.1523/JNEUROSCI.3082-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laeger T., Henagan T.M., Albarado D.C., Redman L.M., Bray G.A., Noland R.C., et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124(9):3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solon-Biet S.M., Cogger V.C., Pulpitel T., Heblinski M., Wahl D., McMahon A.C., et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metabol. 2016;24(4):555–565. doi: 10.1016/j.cmet.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Solon-Biet S.M., Clark X., Bell-Anderson K., Rusu P.M., Perks R., Freire T., et al. Toward reconciling the roles of FGF21 in protein appetite, sweet preference, and energy expenditure. Cell Rep. 2023;42(12) doi: 10.1016/j.celrep.2023.113536. [DOI] [PubMed] [Google Scholar]
- 18.Potthoff M.J., Inagaki T., Satapati S., Ding X., He T., Goetz R., et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106(26):10853–10858. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bookout A.L., de Groot M.H., Owen B.M., Lee S., Gautron L., Lawrence H.L., et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med. 2013;19(9):1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ding X., Boney-Montoya J., Owen B.M., Bookout A.L., Coate K.C., Mangelsdorf D.J., et al. betaKlotho is required for fibroblast growth factor 21 effects on growth and metabolism. Cell Metabol. 2012;16(3):387–393. doi: 10.1016/j.cmet.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casanova E., Fehsenfeld S., Mantamadiotis T., Lemberger T., Greiner E., Stewart A.F., et al. A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis. 2001;31(1):37–42. doi: 10.1002/gene.1078. [DOI] [PubMed] [Google Scholar]
- 22.Lindeberg J., Usoskin D., Bengtsson H., Gustafsson A., Kylberg A., Soderstrom S., et al. Transgenic expression of Cre recombinase from the tyrosine hydroxylase locus. Genesis. 2004;40(2):67–73. doi: 10.1002/gene.20065. [DOI] [PubMed] [Google Scholar]
- 23.Laeger T., Albarado D.C., Burke S.J., Trosclair L., Hedgepeth J.W., Berthoud H.R., et al. Metabolic responses to dietary protein restriction require an increase in FGF21 that is delayed by the absence of GCN2. Cell Rep. 2016;16(3):707–716. doi: 10.1016/j.celrep.2016.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill C.M., Laeger T., Albarado D.C., McDougal D.H., Berthoud H.-R., Münzberg H., et al. Low protein-induced increases in FGF21 drive UCP1-dependent metabolic but not thermoregulatory endpoints. Sci Rep. 2017;7(1):8209. doi: 10.1038/s41598-017-07498-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hursh S.R. Behavioral economics. J Exp Anal Behav. 1984;42(3):435–452. doi: 10.1901/jeab.1984.42-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134(3483):943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 27.Stratford J.M., Thompson J.A. MSG-evoked c-fos activity in the nucleus of the solitary tract is dependent upon fluid delivery and stimulation parameters. Chem Senses. 2016;41(3):211–220. doi: 10.1093/chemse/bjv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill C.M., Berthoud H.R., Munzberg H., Morrison C.D. Homeostatic sensing of dietary protein restriction: a case for FGF21. Front Neuroendocrinol. 2018;51:125–131. doi: 10.1016/j.yfrne.2018.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrison C.D., Reed S.D., Henagan T.M. Homeostatic regulation of protein intake: in search of a mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302(8):R917–R928. doi: 10.1152/ajpregu.00609.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tome D., Chaumontet C., Even P.C., Darcel N., Azzout-Marniche D. Protein status modulates the rewarding value of foods and meals to maintain an adequate protein intake. Physiol Behav. 2019;206:7–12. doi: 10.1016/j.physbeh.2019.03.012. [DOI] [PubMed] [Google Scholar]
- 31.Raubenheimer D., Simpson S.J. Protein appetite as an integrator in the obesity system: the protein leverage hypothesis. Philos Trans R Soc Lond B Biol Sci. 2023;378(1888) doi: 10.1098/rstb.2022.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raubenheimer D., Simpson S.J. Protein leverage: theoretical foundations and ten points of clarification. Obesity. 2019;27(8):1225–1238. doi: 10.1002/oby.22531. [DOI] [PubMed] [Google Scholar]
- 33.von Holstein-Rathlou S., BonDurant L.D., Peltekian L., Naber M.C., Yin T.C., Claflin K.E., et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metabol. 2016;23(2):335–343. doi: 10.1016/j.cmet.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talukdar S., Owen B.M., Song P., Hernandez G., Zhang Y., Zhou Y., et al. FGF21 regulates sweet and alcohol preference. Cell Metabol. 2016;23(2):344–349. doi: 10.1016/j.cmet.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C.T., Larson K.R., Sims L.C., Ryan K.K. Dietary protein restriction modulates ‘dessert’ intake after a meal, via fibroblast growth factor 21 (FGF21) Physiol Behav. 2023;272 doi: 10.1016/j.physbeh.2023.114368. [DOI] [PubMed] [Google Scholar]
- 36.Ramne S., Duizer L., Nielsen M.S., Jorgensen N.R., Svenningsen J.S., Grarup N., et al. Meal sugar-protein balance determines postprandial FGF21 response in humans. Am J Physiol Endocrinol Metab. 2023;325(5):E491–E499. doi: 10.1152/ajpendo.00241.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C.T., Magana D.G., Roshgadol J., Tian L., Ryan K.K. Dietary protein restriction diminishes sucrose reward and reduces sucrose-evoked mesolimbic dopamine signaling. Appetite. 2024;203 doi: 10.1016/j.appet.2024.107673. [DOI] [PubMed] [Google Scholar]
- 38.Boirie Y., Dangin M., Gachon P., Vasson M.P., Maubois J.L., Beaufrere B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Proc Natl Acad Sci U S A. 1997;94(26):14930–14935. doi: 10.1073/pnas.94.26.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dangin M., Boirie Y., Garcia-Rodenas C., Gachon P., Fauquant J., Callier P., et al. The digestion rate of protein is an independent regulating factor of postprandial protein retention. Am J Physiol Endocrinol Metab. 2001;280(2):E340–E348. doi: 10.1152/ajpendo.2001.280.2.E340. [DOI] [PubMed] [Google Scholar]
- 40.Zaman K., Mun H.C., Solon-Biet S.M., Senior A.M., Raubenheimer D., Simpson S.J., et al. Mice regulate dietary amino acid balance and energy intake by selecting between complementary protein sources. J Nutr. 2024;154(6):1766–1780. doi: 10.1016/j.tjnut.2024.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Deutsch J.A., Moore B.O., Heinrichs S.C. Unlearned specific appetite for protein. Physiol Behav. 1989;46(4):619–624. doi: 10.1016/0031-9384(89)90341-7. [DOI] [PubMed] [Google Scholar]
- 42.Ettle T., Roth F.X. Dietary preferences for feeds varying in threonine concentration by the piglet. Physiol Behav. 2005;85(3):289–295. doi: 10.1016/j.physbeh.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 43.Henry Y. Self-selection of lysine by growing pigs: choice combinations between deficient or suboptimal and adequate or superoptimal dietary levels according to sex. Reprod Nutr Dev. 1993;33(6):489–502. doi: 10.1051/rnd:19930601. [DOI] [PubMed] [Google Scholar]
- 44.Gietzen D.W., Aja S.M. The brain’s response to an essential amino acid-deficient diet and the circuitous route to a better meal. Mol Neurobiol. 2012;46(2):332–348. doi: 10.1007/s12035-012-8283-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison C.D., Berthoud H.R. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65(12 Pt 1):517–534. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- 46.Berthoud H.R., Munzberg H., Morrison C.D. Blaming the brain for obesity: integration of hedonic and homeostatic mechanisms. Gastroenterology. 2017;152(7):1728–1738. doi: 10.1053/j.gastro.2016.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise R.A. Catecholamine theories of reward: a critical review. Brain Res. 1978;152(2):215–247. doi: 10.1016/0006-8993(78)90253-6. [DOI] [PubMed] [Google Scholar]
- 48.Wightman R.M., Robinson D.L. Transient changes in mesolimbic dopamine and their association with ‘reward’. J Neurochem. 2002;82(4):721–735. doi: 10.1046/j.1471-4159.2002.01005.x. [DOI] [PubMed] [Google Scholar]
- 49.Wise R.A. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 50.Geisler C.E., Hayes M.R. Metabolic hormone action in the VTA: reward-directed behavior and mechanistic insights. Physiol Behav. 2023;268 doi: 10.1016/j.physbeh.2023.114236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu T.M., McCutcheon J.E., Roitman M.F. Parallels and overlap: the integration of homeostatic signals by mesolimbic dopamine neurons. Front Psychiatr. 2018;9:410. doi: 10.3389/fpsyt.2018.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaumontet C., Recio I., Fromentin G., Benoit S., Piedcoq J., Darcel N., et al. The protein status of rats affects the rewarding value of meals due to their protein content. J Nutr. 2018;148(6):989–998. doi: 10.1093/jn/nxy060. [DOI] [PubMed] [Google Scholar]
- 53.Griffioen-Roose S., Smeets P.A., van den Heuvel E., Boesveldt S., Finlayson G., de Graaf C. Human protein status modulates brain reward responses to food cues. Am J Clin Nutr. 2014;100(1):113–122. doi: 10.3945/ajcn.113.079392. [DOI] [PubMed] [Google Scholar]
- 54.Leidy H.J., Lepping R.J., Savage C.R., Harris C.T. Neural responses to visual food stimuli after a normal vs. higher protein breakfast in breakfast-skipping teens: a pilot fMRI study. Obesity. 2011;19(10):2019–2025. doi: 10.1038/oby.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hultman K., Scarlett J.M., Baquero A.F., Cornea A., Zhang Y., Salinas C.B.G., et al. The central fibroblast growth factor receptor/beta klotho system: comprehensive mapping in Mus musculus and comparisons to nonhuman primate and human samples using an automated in situ hybridization platform. J Comp Neurol. 2019;527(12):2069–2085. doi: 10.1002/cne.24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales M., Margolis E.B. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci. 2017;18(2):73–85. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 57.Lammel S., Steinberg E.E., Foldy C., Wall N.R., Beier K., Luo L., et al. Diversity of transgenic mouse models for selective targeting of midbrain dopamine neurons. Neuron. 2015;85(2):429–438. doi: 10.1016/j.neuron.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stuber G.D., Stamatakis A.M., Kantak P.A. Considerations when using cre-driver rodent lines for studying ventral tegmental area circuitry. Neuron. 2015;85(2):439–445. doi: 10.1016/j.neuron.2014.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volcko K.L., McCutcheon J.E. Protein preference and elevated plasma FGF21 induced by dietary protein restriction is similar in both male and female mice. Physiol Behav. 2022;257:113994. doi: 10.1016/j.physbeh.2022.113994. [DOI] [PubMed] [Google Scholar]
- 60.Wu C.T., Larson K.R., Goodson M.L., Ryan K.K. Fibroblast growth factor 21 and dietary macronutrient intake in female mice. Physiol Behav. 2022;257 doi: 10.1016/j.physbeh.2022.113995. [DOI] [PubMed] [Google Scholar]
- 61.Larson K.R., Russo K.A., Fang Y., Mohajerani N., Goodson M.L., Ryan K.K. Sex differences in the hormonal and metabolic response to dietary protein dilution. Endocrinology. 2017;158(10):3477–3487. doi: 10.1210/en.2017-00331. [DOI] [PubMed] [Google Scholar]
- 62.Richardson N.E., Konon E.N., Schuster H.S., Mitchell A.T., Boyle C., Rodgers A.C., et al. Lifelong restriction of dietary branched-chain amino acids has sex-specific benefits for frailty and lifespan in mice. Nat Aging. 2021;1(1):73–86. doi: 10.1038/s43587-020-00006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Green C.L., Pak H.H., Richardson N.E., Flores V., Yu D., Tomasiewicz J.L., et al. Sex and genetic background define the metabolic, physiologic, and molecular response to protein restriction. Cell Metabol. 2022;34(2):209–226 e205. doi: 10.1016/j.cmet.2021.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu D., Yang S.E., Miller B.R., Wisinski J.A., Sherman D.S., Brinkman J.A., et al. Short-term methionine deprivation improves metabolic health via sexually dimorphic, mTORC1-independent mechanisms. FASEB J. 2018;32(6):3471–3482. doi: 10.1096/fj.201701211R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lefner M.J., Dejeux M.I., Wanat M.J. Sex differences in behavioral responding and dopamine release during pavlovian learning. eNeuro. 2022;9(2) doi: 10.1523/ENEURO.0050-22.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paxinos G., Franklin K.B. Academic Press; 2019. Paxinos and Franklin’s the mouse brain in stereotaxic coordinates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.