Summary
Cell cycle progression requires periodic gene expression through splicing control. However, the splicing factor that directly controls this cell cycle-dependent splicing remains unknown. Cell cycle-dependent expression of the AURKB (aurora kinase B) gene is essential for chromosome segregation and cytokinesis. We previously reported that RNPS1 is essential to maintain precise splicing in AURKB intron 5. Here we show that RNPS1 plays this role in PSAP complex with PNN and SAP18, but not ASAP complex with ACIN1 and SAP18. Whole-transcriptome sequencing of RNPS1- and PNN-deficient cells indicated that RNPS1, either alone or as PSAP complex, is an essential splicing factor for a subset of introns. Remarkably, protein expression of RNPS1, but not PNN, is coordinated with cyclical splicing in PSAP-controlled introns including AURKB intron 5. The ubiquitin-proteasome pathway is involved in the periodic decrease of RNPS1 protein level. RNPS1 is a key factor that controls periodic splicing during the cell cycle.
Subject areas: Cell biology, Cellular physiology, Molecular biology, Properties of biomolecules
Graphical abstract
Highlights
-
•
The AURKB gene encodes a protein kinase essential for cytokinesis and cell cycle
-
•
PSAP complex (RNPS1, PNN, and SAP18) is required for precise splicing in AURKB intron 5
-
•
RNPS1 protein in PSAP complex controls periodic splicing during the cell cycle
-
•
Cyclical decrease of RNPS1 protein is mediated by the ubiquitin-proteasome pathway
Cell biology; Cellular physiology; Molecular biology; Properties of biomolecules.
Introduction
Human pre-mRNA splicing takes place in the huge multimolecular complex, termed spliceosome, that is a highly organized dynamic ribonucleoprotein machine composed of five snRNPs and numerous proteins (reviewed in Will and Lührmann1 and Kastner et al.2). Amid the panoply of proteins in this spliceosome, it is amazing to observe that each individual protein factor often drastically changes the outcome of splicing in a precisely regulated manner.
We originally identified human RNPS1 (RNA binding protein with serine-rich domain 1) as a general splicing activator in vitro.3 We next found that RNPS1 exists in the spliceosome and regulates a variety of alternative splicing events, often collaborating with other splicing regulators including SRSF11 (p54), TRA2B (hTra2β), and PNN (PININ).4,5 RNPS1 was also identified as a peripheral factor of the exon junction complex (EJC) formed on spliced mRNA (reviewed in Le Hir et al.6 and Schlautmann and Gehring7).
ACIN1 (ACINUS) and PNN are scaffold proteins and form two alternative ternary complexes with RNPS1 and SAP18, termed ASAP and PSAP, respectively.8,9 ACIN1 is involved in apoptosis and regulation of splicing and transcription10,11,12 and PNN was originally identified as a desmosome-associated protein but also function as a regulator of alternative splicing.13,14
Since ACIN1 bridges the ASAP complex to the EJC core14 and PNN in PSAP complex is structurally related to ACIN1,9 both ASAP and PSAP complexes must interact with the EJC. Recent transcriptome-wide analysis indicated that the ASAP and PSAP complexes regulate distinct alternative splicing either in EJC core-dependent or -independent manner.14 On the other hand, the EJC core alone, without any peripheral factors repress aberrant re-splicing on mature mRNA in cancer cells, implicating an important role in terminating splicing.15 Many lines of evidence support that ASAP and PSAP complexes, with or without EJC core, are general regulators of splicing (reviewed in Schlautmann and Gehring7). However, their individual biological and physiological significance remains to be investigated.
We have been studying splicing regulation involved in mitotic cell-cycle progression using the AURKB (aurora kinase B) gene as a model. The AURKB gene encodes a serine/threonine protein kinase that is essential in chromosome segregation and cytokinesis during the cell cycle (reviewed in Krenn and Musacchio16). We previously showed that RNPS1, recruited by the EJC core, is critical for efficient and faithful splicing in mitotic cell cycle-related genes including AURKB.17 Indeed, RNPS1 depletion causes a reduction of the AURKB protein due to multiple aberrant splicing, resulting in abnormal multinucleation phenotype that is fully rescued by ectopic expression of AURKB.18 Regarding the mechanism of action, we found that RNPS1 protein binds directly to a specific element in exon 5 upstream of the authentic 5′ splice site, and this binding is critical to prevent multiple aberrant splicing using a common pseudo 5′ splice site.18 Here we demonstrate that RNPS1 plays a role as the PSAP component in preventing aberrant splicing of AURKB pre-mRNA.
It was reported that SR protein kinase CLK1 (CDC-like kinase 1) controls many periodically spliced pre-mRNAs, including AURKB, during the cell cycle.19 However, splicing factors were not known to be directly involved in cyclical splicing. We have discovered that the protein level of RNPS1 is coordinated with cell cycle-dependent periodic splicing of AURKB pre-mRNA. Here we propose an important function of RNPS1 in PSAP complex, as a critical modulator that temporally controls periodic splicing observed during cell cycle.
Results
PSAP promotes efficient and precise splicing of AURKB pre-mRNA
Previously, we reported that siRNA-mediated knockdown of RNPS1 induced aberrant splicing in the AURKB intron 5 and MDM2 intron 10 (Figure S1A), and we identified the functional RNPS1 binding site upstream of the AURKB intron 5.18 On the other hand, our previous yeast two-hybrid screening revealed that RNPS1 can bind to ACIN1, PNN, GPATCH8, and LUC7L3 (hLuc7A).4 This observation is consistent with the finding that ACIN1 and PNN form the ASAP and PSAP complex, respectively, with the common components RNPS1 and SAP18.8,9
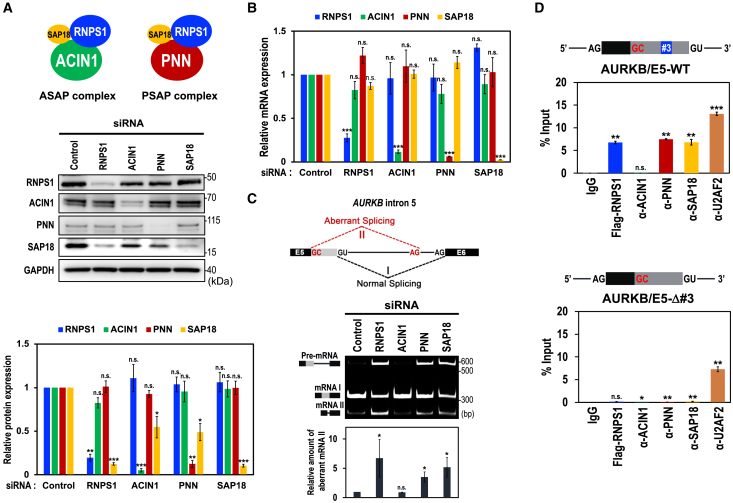
We then performed knockdown of all the components of the PSAP and ASAP complexes (RNPS1, ACIN1, PNN, and SAP18) in HeLa cells. We confirmed that each siRNA led to effective depletion of the target mRNA and the protein product (Figures 1A and 1B). Notably, the knockdown of RNPS1, ACIN1, and PNN also caused co-depletion of SAP18 protein (Figure 1A); however, SAP18 mRNA levels were barely changed (Figure 1B), indicating that SAP18 protein is stabilized in either ASAP or PSAP complex. The co-depletion of SAP18 protein was also observed by the knockdown of RNPS1, ACIN1, and PNN in HEK293 cells (Figure S2A; cf. Figure S2B).
Figure 1.
PSAP component binds to a specific site that promotes normal splicing of the AURKB pre-mRNA
(A) Schematic structures of ASAP and PSAP complexes (upper scheme). siRNA-mediated depletion of the indicated ASAP/PSAP components in HeLa cells were analyzed by Western blotting with indicated antibodies (middle panel). Individual bands on the Western blots were quantified and the relative values were standardized to that in the control siRNA (lower graph). Means ± standard errors (SE) are given for three independent experiments and Welch’s t-test values were calculated (∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, n.s. p > 0.05). See also Figure S2A.
(B) The mRNA levels of ASAP/PSAP components in (A) were analyzed by RT–qPCR using specific primer sets. See (A) for the statistical analysis. See also Figure S2B.
(C) Splicing defect and aberrant splicing in the endogenous AURKB gene, induced by siRNA-mediated depletion of PSAP proteins, were detected by RT–PCR, visualized by PAGE, and individual bands on the PAGE gel were quantified. The ratios of the aberrantly spliced mRNA value (II) to the sum of the spliced mRNAs value (I + II) were standardized to that of the control siRNA and plotted. See (A) for the statistical analysis. See also Figures S1 and S3.
(D) RNA immunoprecipitation assay was performed using HeLa cells co-transfected with Flag-RNPS1 expression plasmids and the indicated reporter AURKB-Exon 5 mini-genes. After immunoprecipitation using the indicated antibodies, immunoprecipitated RNAs were quantified by RT–qPCR using specific primers. See (A) for the statistical analysis.
Then, we examined whether these PSAP- and ASAP-components operate together with RNPS1 to ensure precise splicing in the AURKB intron 5 (Figure 1C) and the MDM2 intron 10 (Figure S1B). We found that the knockdown of PNN and SAP18, as well as RNPS1, generated unspliced pre-mRNA and induced aberrant splicing at expense of normal mRNA, but knockdown of ACIN1 did not. This confirms that the PSAP, but not ASAP, plays a role to restore the normal splicing in AURKB intron 5. The knockdown of other RNPS1-binding proteins, GPATCH8 and LUC7L3, barely affected in normal splicing (I) of the AURKB intron 5 and MDM2 intron 10 (Figure S1B).
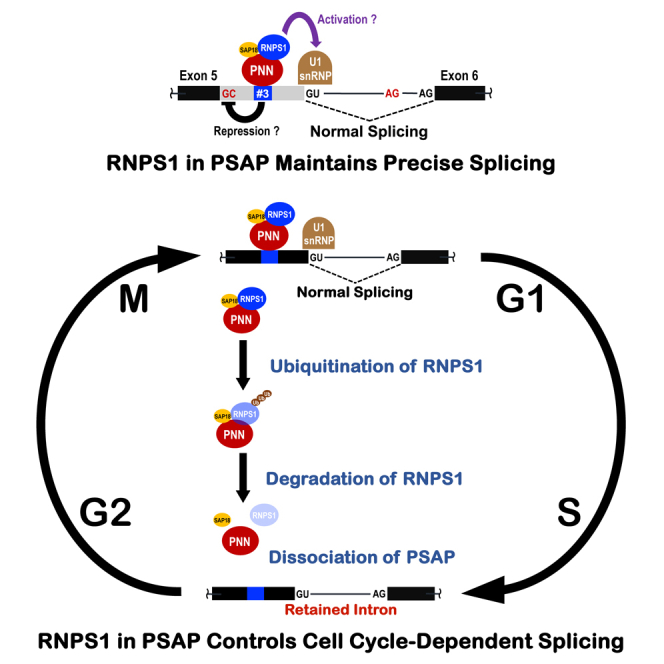
Previously, we demonstrated that RNPS1 binds to a specific cis-acting element (termed #3) located upstream of the authentic 5′ splice site of intron 5 (Figure 1D, upper scheme).18 We thus examined whether the PSAP complex binds to this cis-acting element by RNA immunoprecipitation experiments using antibodies against each of the PSAP components (Figure 1D, lower graphs). As we expected, RNPS1, PNN, and SAP18 bound to the AURKB/E5-WT, but ACIN1 did not. Notably, deletion of this cis-acting element “#3” greatly reduced the binding of the PSAP components. We conclude that PSAP binding to the specific element “#3” is critical for efficient and precise splicing in the AURKB intron 5.
PSAP either represses the pseudo 5′ splice site or activates the authentic 5′ splice site in AURKB pre-mRNA
Since the “#3” element is located between the upstream pseudo 5′ splice site and the downstream authentic 5′ splice site, we postulated two conceivable mechanisms leading to the normal splicing; either (1) PSAP represses the pseudo 5′ splice site, and/or (2) PSAP activates the authentic 5′ splice site (Figure S3A). To examine distance-dependent effects of PSAP binding, such as interaction with and/or steric hindrance to the U1 snRNP, we increased the distance of the “#3” element from either the authentic 5′ splice site or the pseudo 5′ splice site (Figure S3B).
These two variant AURKB pre-mRNAs, as well as the original AURKB pre-mRNAs, had equivalent repression of the normal 5′ splice site by either RNPS1-knockdown or PNN-knockdown (Figure S3C), suggesting that both postulated mechanisms (see aforementioned text) are involved. Several previous studies indeed support both possibilities (see Discussion).
Individual subsets of introns are spliced out by RNPS1, PNN and PSAP
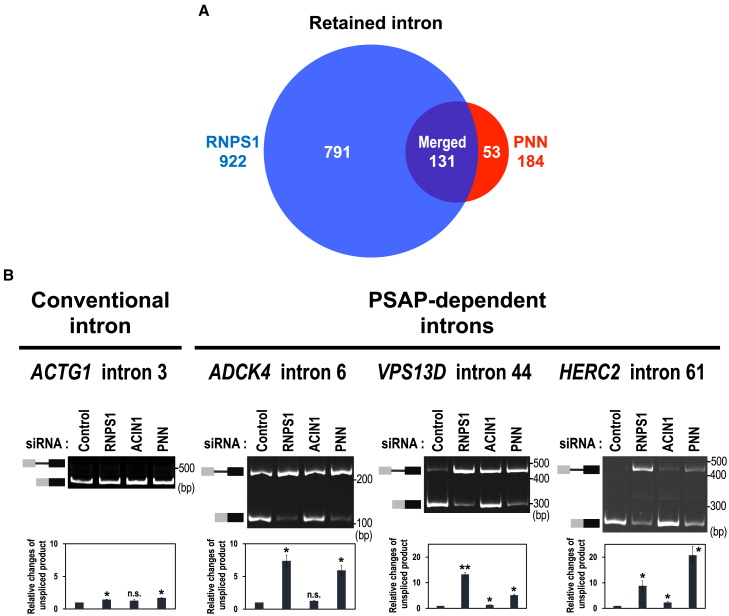
To test whether PSAP might have a general role in controlling splicing, we performed siRNA-mediated knockdown of RNPS1 and PSAP-specific component PNN in HEK293 cells followed by the whole-transcriptome sequencing (RNA-Seq). The sequencing reads were mapped to the human genome sequences and we analyzed splicing patterns, both negatively and positively changed, in five major classes (Figure S4; Table S2 “The list of splicing changes in RNPS1-deficient HEK293 cells”; Table S3 “The list of splicing changes in PNN-deficient HEK293 cells”).
The most frequent class of the overlapping events was intron retention type (131/289; Figure S4; Table S4 “The list of overlapping splicing changes in RNPS1- and PNN-deficient HEK293 cells”), and remarkably, these are all intron retention promoted, not repressed (Figure 2A), indicating that these 131 introns are PSAP-dependently spliced. The data also reveal that RNPS1 and PNN solely (not in PSAP complex) promote splicing in 791 and 53 introns, respectively (Figure 2A).
Figure 2.
RNPS1 and PNN are general splicing factors for individual subsets of introns
(A) Venn diagram of retained introns that are generated by RNPS1- and PNN-knockdown HEK293 cells. See also Figure S4 and Table S2. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1-deficient HEK293 cells (related to Figures 2A, S4), Table S3. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in PNN-deficient HEK293 cells (related to Figures 2A, S4), Table S4. The list of overlapping splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1- and PNN-deficient HEK293 cells (related to Figures 2A, S4).
(B) In cellulo splicing assays of the pre-mRNAs including a conventional intron and three representative PSAP-dependent introns. After the indicated siRNA-mediated knockdown in HEK293 cells, indicated endogenous splicing was analyzed by RT–PCR followed by PAGE (upper panel). The unspliced RNA products were quantitated by RT–qPCR and the relative values were standardized to that in the control siRNA (lower graph). Means ± SE are given for three independent experiments and Welch’s t-test values were calculated (∗p < 0.05, ∗∗p < 0.005, n.s. p > 0.05).
We validated the PSAP-dependent splicing defect by RT–PCR with three representative PSAP-dependent introns (arbitrarily chosen from 131 introns in Table S2). By depletion of PSAP components (RNPS1 and PNN), we observed unspliced pre-mRNAs with all the PSAP-dependent introns, whereas the control authentic ACTG1 intron 3 was fully spliced out (Figure 2B). These results implicate that PSAP promotes splicing in a subset of introns.
PSAP-controlled introns are periodically spliced over the cell cycle
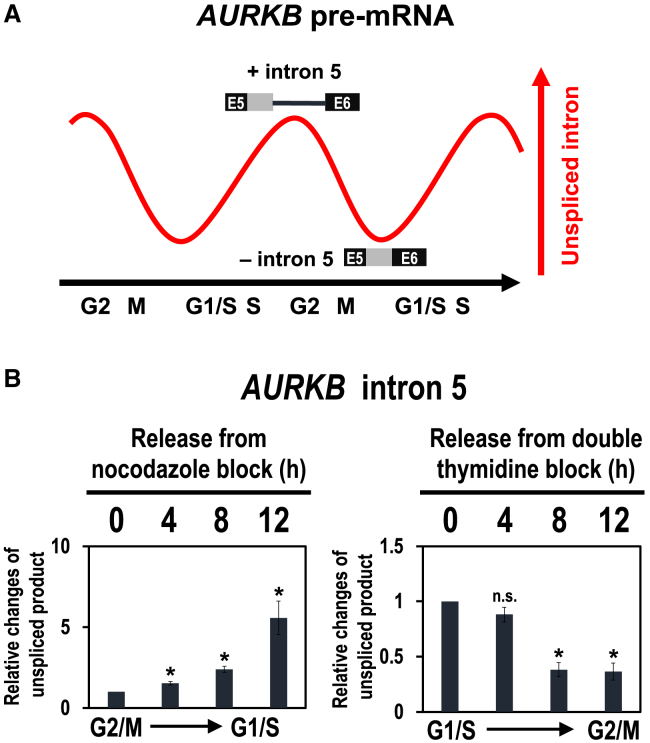
A previous RNA-Seq analysis at different stages of the cell cycle identified ∼1,300 genes with changes of cell cycle-dependent alternative splicing, and notably, the intron retention event of AURKB intron 5 was characterized as a representative model.19 It was reported that the excision of AURKB intron 5 is periodically modulated and this splicing activity reaches the maximum around M phase (Figure 3A). Using the same AURKB pre-mRNA including intron 5, we demonstrated that RNPS1 in PSAP complex binds upstream of the 5′ splice site and promotes its precise splicing (Figures 1C and 1D).18 We thus hypothesized that PSAP also plays a role in cell cycle-dependent splicing observed in AURKB intron 5.
Figure 3.
Reported periodic AURKB pre-mRNA splicing is recapitulated in synchronized cells during the cell cycle
(A) Schematic representation of cyclical splicing in AURKB intron 5 (modified from Dominguez et al.19).
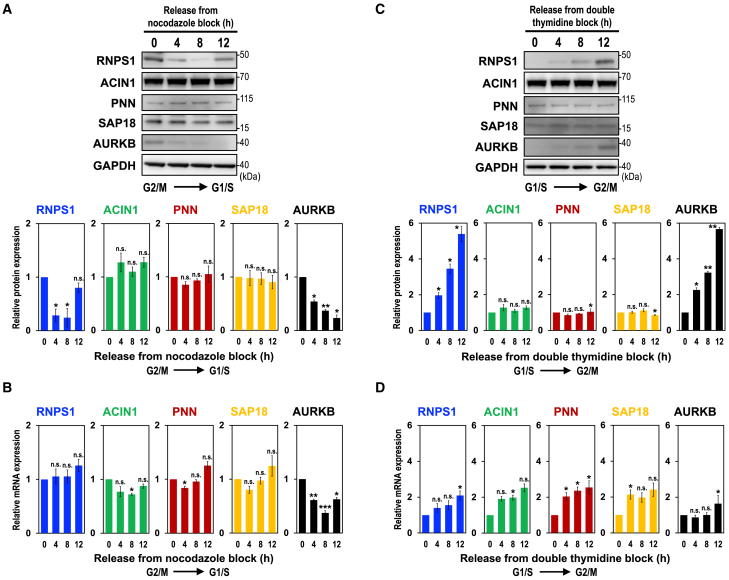
(B) HEK293 cells were synchronized in G2/M phase by treating with nocodazole and synchronized in G1/S phase by treating with double thymidine. Cells were washed, released, and harvested at the indicating time points. Endogenous splicing in the AURKB intron 5 was analyzed by RT–qPCR and the unspliced products were standardized to that at the start time, 0 h. Means ± SE are given for three independent experiments and Welch’s t-test values were calculated (∗p < 0.05, n.s. p > 0.05). See also Figures S5 and S6.
We first recapitulated the periodic splicing of AURKB intron 5 during cell cycle progression with HEK293 cells (Figure 3B). We treated the cells with the microtubule-destabilizing reagent, nocodazole, which arrests cell cycle at the start of the G2/M phase (nocodazole release 0 h). Using RT–PCR followed by PAGE analysis and quantitative RT–PCR (RT–qPCR), we observed increasing unspliced intron 5 from G2/M to G1/S phase (Figures S5 and 3B). Whereas from G1/S to G2/M phase, we found decreasing unspliced intron 5 in synchronized HEK293 cells after release from the double thymidine block at the G1/S boundary (Figure 3C).
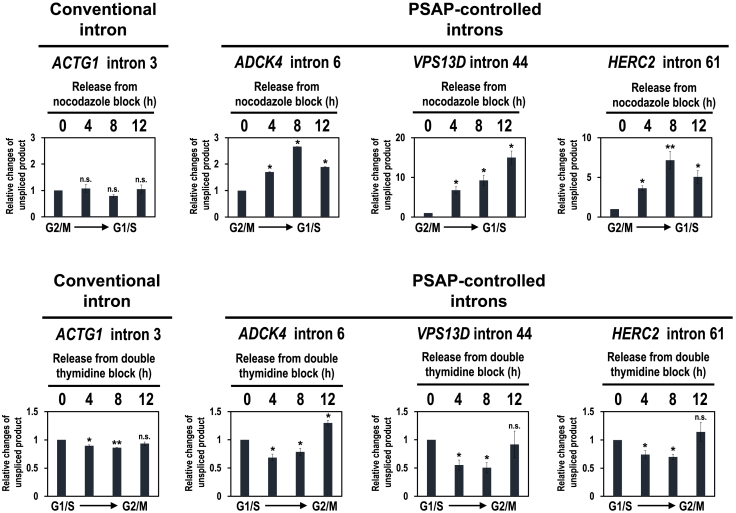
It is important to confirm whether the observed cyclical splicing modulation is a general event in the PSAP-controlled introns. Using three representative PSAP-dependently spliced introns (Figure 2B), we examined the splicing changes during the cell cycle using nocodazole and double thymidine to synchronize HEK293 cells (Figures 4 and S5). Remarkably in these PSAP-controlled introns, we also observed periodic changes of unspliced introns as observed in AURKB intron 5 pre-mRNA (Figure 4; cf. Figure 3B). These periodic patterns are evidently unique when compared with those observed in a conventional intron. Our results thus provided the mechanistic basis underlying the reported periodic pattern of splicing activity (Figure 3A). Here we propose that PSAP promotes periodic splicing linked to the cell cycle.
Figure 4.
Periodic modulation of unspliced introns across the cell cycle was observed in PSAP-controlled introns but not in conventional intron
HEK293 cells were synchronized in G2/M phase by nocodazole block (upper panels) and synchronized in G1/S phase by double thymidine block (lower panels). Endogenous splicing in the indicated conventional intron and PSAP-controlled introns was analyzed by RT–qPCR. Quantification and the statistical analysis were performed as described in Figure 3B (∗p < 0.05, ∗∗p < 0.005, n.s. p > 0.05). See also Figures S5 and S6.
RNPS1 in PSAP controls periodic splicing over the cell cycle
We assumed that PSAP-controlled periodic splicing was modulated by the expression level changes in any of the PSAP component. We thus examined the protein levels of PSAP components during the cell cycle using nocodazole synchronized HEK293 cell (Figure 5A). Intriguingly, RNPS1 protein level was at a maximum in G2/M phase (nocodazole release 0 h) and decreased through cell cycle progression (nocodazole release 4–8 h). The decrease in RNPS1 protein was coordinated with the level of AURKB protein, which was at a high level in G2/M and declined to a low level in G1/S (Figure 5A).20 In contrast, protein levels of other PSAP components PNN and SAP18, as well as an ASAP component ACIN1, were not significantly changed. Notably, we did not observe such a change in RNPS1 mRNA expression level during cell cycle (Figure 5B) as previously reported in human RNPS1 (E5.1).21 These data together indicate that the reduction of RNPS1 protein level is regulated at the protein level, but not at the mRNA level.
Figure 5.
The level of RNPS1 protein, but not other ASAP/PSAP proteins, is coordinated with AURKB protein level during the cell cycle
(A) HEK293 cells were synchronized in G2/M phase by nocodazole block and released cells were harvested at indicated time points. ASAP/PSAP proteins were analyzed by Western blotting with indicated antibodies (upper panel). Individual bands on the Western blots were quantified and the relative values were standardized to that at the start time, 0 h (lower graph). Means ± SE are given for three independent experiments and Welch’s t-test values were calculated (∗p < 0.05, ∗∗p < 0.005, ∗∗∗p < 0.0005, n.s. p > 0.05). See also Figure S6.
(B) mRNA levels in (A) were analyzed by RT–qPCR and the relative values were standardized to that at the start time, 0 h. See (A) for the statistical analysis.
(C) HEK293 cells were synchronized at G1/S phase by double thymidine block and ASAP/PSAP proteins were analyzed as described in (A). See (A) for the statistical analysis. See also Figure S6.
(D) mRNA levels in (C) were analyzed by RT–qPCR and the relative values were standardized to that at the start time, 0 h. See (A) for the statistical analysis.
We also examined the levels of PSAP components for the latter part of the cell cycle using the double thymidine block synchronized HEK293 cells (Figure 5C). We found that RNPS1 protein was minimal at G1/S phase (double thymidine release 0 h) and greatly increased as the cell cycle progressed to G2/M phase (double thymidine block 4–12 h), which was well coordinated again with the level of AURKB protein. The RNPS1 protein increase could be due to the mRNA level in which RNPS1 was increased, together with AURKB, from G1/S into G2/M phase (Figure 5D).
Since RNPS1 and AURKB protein expressions were synchronized during the cell cycle (Figures 5A and 5C), we assumed that the splicing activator RNPS1 was responsible for the observed cell cycle-dependent splicing modulation (Figures 3 and 4). To test this assumption, we performed nocodazole treatment in RNPS1-knockdown HEK293 cells, and analyzed splicing changes in the two PSAP-controlled introns (Figure S6A). Even at the RNPS1-upregulated G2/M phase, we observed that RNPS1-knockdown generated the unspliced product. Nocodazole treatment in PNN-knockdown HEK 293 cells also increased the unspliced product (Figure S6B) confirmed that RNPS1, as a component of the PSAP, promotes the periodic pre-mRNA splicing linked to cell cycle progression.
Periodic decrease of RNPS1 protein is mediated by the ubiquitin-proteasome pathway
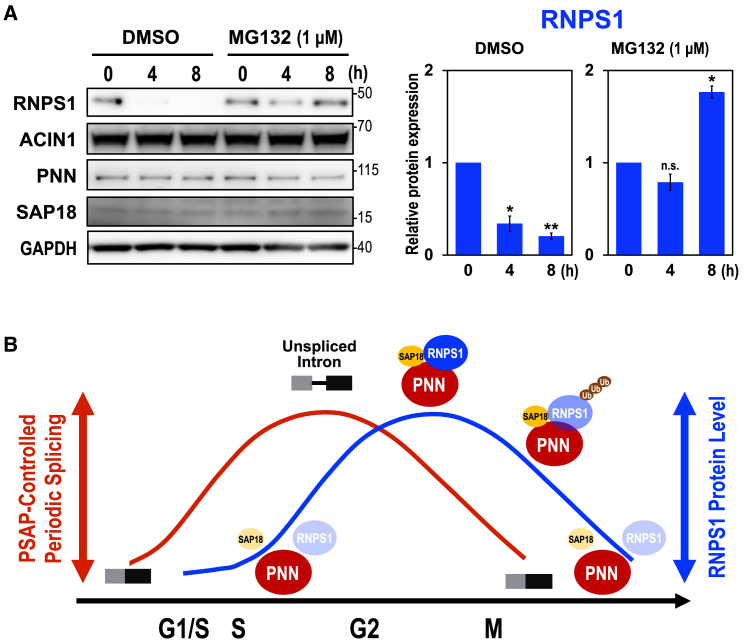
Ubiquitin-mediated proteolysis is one of the keys to regulating cell cycle progression.22 RNPS1 is indeed polyubiquitinated by K48-linkage and degraded by the proteasome.23 We assumed that periodic degradation of RNPS1 protein is controlled by ubiquitin-proteasome pathway.
We treated HEK293 cells with proteasome inhibitor MG132 and we examined the protein level of PSAP components. As we expected, RNPS1 protein was stabilized in the presence of MG132 whereas the level of other ASAP/PSAP proteins, ACIN1, PNN and SAP18, were not changed at all (Figure 6A). Since the degradation of RNPS1 causes co-destabilization of SAP18 (Figure 1A), the functional PSAP complex appears to be dissociated, that is the cause of generated unspliced intron. Taken together, we conclude that ubiquitin-proteasome pathway plays a key role in the periodic fluctuation of RNPS1 protein level, leading to observed periodic splicing in accordance with the cell cycle (Figure 6B).
Figure 6.
PSAP-mediated cyclical splicing is controlled by RNPS1 protein level through the ubiquitin-proteasome pathway
(A) HEK293 cells were cultured for 4 h and 8 h with proteasome inhibitor MG132 and the protein levels were analyzed by Western blotting using the antibody against each ASAP/PSAP protein. A common solvent, dimethyl sulfoxide (DMSO), was added to the medium (final concentration at 0.1%). The RNPS1 band on the Western blots was quantified and the relative values were standardized to that at the start time, 0 h (right graph). Means ± SE are given for three independent experiments and Welch’s t-test values were calculated (∗p < 0.05, ∗∗p < 0.005, n.s. p > 0.05).
(B) A schematic model of RNPS1-mediated periodic splicing during cell cycle. RNPS1 protein, but neither PNN nor SAP18 protein, in PSAP plays an essential role to control periodic splicing over the cell cycle progression. The ubiquitin-mediated proteolysis of RNPS1 also destabilizes SAP18 that induces dissociation of PSAP complex, leading to the splicing defect.
Discussion
The PSAP complex is critical to maintain splicing fidelity
We previously reported that RNPS1 interacts with a specific cis-element and ensures precise splicing in the AURKB pre-mRNA, which is essential for chromosome segregation and cytokinesis.18 Here, we demonstrate that RNPS1, as a component of PSAP complex with PNN and SAP18, binds to the same cis-element in the upstream exon, leading to the precise splicing on AURKB intron 5 (Graphical abstract, upper panel).
The PSAP components (PNN, SAP18, and RNPS1) are all associated with the EJC core as peripheral factors (reviewed in Le Hir et al.6 and Schlautmann and Gehring7). Therefore, it is conceivable that the EJC recruits the PSAP complex on the target pre-mRNA and PSAP itself functions to promote precise splicing. Indeed, we previously found that EJC core on pre-mRNA recruits RNPS1 as a key factor to promote splicing involved in mitotic cell cycle.17
Regarding the mechanism of PSAP-mediated control to ensure precise splicing in AURKB pre-mRNA, our data implicate that it occurs either through repression of the upstream pseudo 5′ splice site (without direct masking) or through activation of the downstream authentic 5′ splice site, likely through the interaction with U1 snRNP (Figure S3A; see Limitations of the study). Since the downstream authentic 5′ splice site is much weaker than the upstream pseudo 5′ splice site,18 the intrinsic RNPS1 activity as a general splicing activator,3 in a component of PSAP, must be a prerequisite for the use of the weak authentic 5′ splice site. On the other hand, repression of the pseudo 5′ splice site cannot be ruled out because RNPS1-knockdown produces five aberrant mRNAs using one common upstream pseudo 5′ splice site and the downstream five 3′ splice sites in endogenous AURKB pre-mRNA.18 This “repression” scenario is consistent with two recent observations that EJC-recruited PSAP represses distant pseudo and recursive 5′ splice site24,25, however, the underlying mechanism to repress such distant 5′ splice site without a steric hindrance remains unknown.
RNPS1 functions in periodic splicing during the cell cycle
Cyclical removal of AURKB intron 5 contributes to periodic AURKB protein expression during the mitotic cell cycle19; however, the involved mechanism remains to be elucidated. Our finding could provide a breakthrough in addressing this important open question. Here we propose that splicing activator RNPS1 in PSAP complex not only functions to ensure precise splicing, but also plays an essential role in controlling periodic splicing linked to cell cycle (Graphical abstract, lower panel).
Our RNA-Seq analysis in RNPS1- and PNN-knockdown cells showed that most affected splicing events were intron retentions, and we could validate that these intron retention events were all induced from G2/M phase to G1/S phase as observed previously in AURKB intron 519. We eventually discovered that the level of RNPS1 protein is downregulated in G1/S phase by the ubiquitin proteasome pathway and it is gradually upregulated to its maximum level at M phase. This characteristic temporal fluctuation of the RNPS1 protein level is well accounted for by the periodic splicing in accordance with the cell cycle (Figure 6B). Since the protein levels of other PSAP components, PNN and SAP18, remain constant during the cell cycle, it is likely that the functional PSAP complex is dissociated by the lack of RNPS1 component.
Previously, it was reported that knockdown of EJC-core component, RBM8A (Y14), induces abnormal nuclear structure, multinucleated cells, G2/M cell-cycle arrest, and genome instability (Fukumura et al.17, Ishigaki et al.26; and references therein), which is a similar phenotype to that caused by the knockdown of PSAP component, RNPS1.18 In mouse models, it was reported that haploinsufficiency of MAGOH, the other EJC core component, causes the defect of mitosis of neural stem cells, ending up in microcephaly.27 Together, it is very likely that the EJC recruits the functional PSAP complex on a subset of pre-mRNAs to control periodic splicing during mitotic cell cycle, as well as to ensure efficient and precise splicing at least in AURKB pre-mRNA.
We demonstrate that PSAP, but not ASAP, controls the faithful splicing and periodic splicing of a transcript involved in the mitotic cell cycle. Therefore, the function of these two complexes is determined by only one distinct component of PSAP and ASAP, i.e., PNN and ACIN1 (Figure 1A, top schemes). Consistently, a recent report showed that PSAP and ASAP form functionally distinct complexes with the EJC core to confer distinct alternative splicing activities.14 On the other hand, since RNPS1 is a common component of PSAP and ASAP, periodic changes of RNPS1 protein would also be expected to modulate the function of ASAP during the cell cycle. Indeed, a possible function of ASAP in the cell cycle is suggested by the fact that depletion of the component of ASAP, ACIN1, cause defects in cell cycle progression.28 Moreover, the genome-wide identification of RNA targets suggests the role of ACIN1 in cell cycle.12 Taken together, we speculate that PSAP and ASAP complexes may target distinct subsets of pre-mRNAs and control periodic changes of splicing during the cell cycle. Although it is out of scope for this article, there is considerable interest in identifying these distinct subsets of pre-mRNAs.
Limitations of the study
We previously found that RNPS1 can bind to a component of U1 snRNP, LUC7L3 (one of the human paralogs of yeast Luc7A).4 It was also shown that LUC7L3 overexpression changes the selection of the alternative 5′ splice site in cellulo.29 Moreover, recent study showed that three human paralogs, LUC7L, LUC7L2, and LUC7L3, as components of the U1 snRNP, regulate unique alternative splicing profiles.30
We thus tested our hypothesis that the weak authentic 5′ splice site is activated through RNPS1 binding to LUC7L3 in the U1 snRNP. However, depletion of LUC7L3 has no effects on the PSAP-dependent AURKB and MDM2 pre-mRNA splicing (Figure S1B), suggesting that RNPS1–LUC7L3 interaction is not involved in these two PSAP-dependent splicing events. Of course, we cannot rule out the possibility of RNPS1 interaction with other U1 snRNP components.
Further studies are needed to elucidate the PSAP-mediate molecular mechanism to ensure splicing fidelity observed in AURKB and MDM2 pre-mRNAs.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Akila Mayeda (mayeda@fujita-hu.ac.jp).
Materials availability
All plasmids and cell lines generated in this study are available from the lead contact with a completed materials transfer agreement.
Data and code availability
-
•
The raw data derived from RNA-Seq analysis (see Figures 2A and S4; Table S2. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1-deficient HEK293 cells (related to Figures 2A, S4), Table S3. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in PNN-deficient HEK293 cells (related to Figures 2A, S4), Table S4. The list of overlapping splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1- and PNN-deficient HEK293 cells (related to Figures 2A, S4)) have been deposited in DDBJ database and are publicly available as of the date of publication. Accession number is listed in the key resources table.
-
•
This article does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.
Acknowledgments
We thank H. Shirasaki and T. Kanehisa for technical support, and members of our labs for constructive discussions. K.F. was partly supported by Grants-in-Aid for Scientific Research (C) and Challenging Research (Exploratory) (grant numbers: JP18K07304, JP21K07202, and JP24K21953) from the Japan Society for the Promotion of Science (JSPS), a research grant from the Hori Sciences and Arts Foundation, a research grant from Nitto Foundation, a research grant from Takeda Science Foundation, a research grant from Aichi Cancer Research Foundation, and a research grant from the Mochida Memorial Foundation. A. Masuda was partly supported by Grants-in-Aid for Scientific Research (B) and Challenging Research (Exploratory) (grant numbers: JP21H02476, JP21H02838, and JP22K19269) from JSPS, and a grant from the Japan Agency for Medical Research and Development (grant number: JP23ek0109497). K.O. was partly supported by Grants-in-Aid for Scientific Research (B) (grant number: JP23H02794) from JSPS and Health and Labour Science Research Grant (grant number: JP23FC1014) from the Ministry of Health Labour and Welfare. A. Mayeda was partly supported by Grants-in-Aid for Scientific Research (B) and Scientific Research (C) (grant number: JP16H04705 and JP21K06024) from JSPS. A. Mayeda was awarded a Professor Emeritus of the Fujita Health University in April 2024.
Author contributions
K.F. and A. Mayeda conceived and designed the experiments; K.F. performed most of the experiments and analyses, organized the data, and drafted the manuscript; J.-i.T. and A. Masuda performed bioinformatics analyses of the sequencing data; K.F., K.O., and A. Mayeda revised and edited the manuscript; O.N. and H.S. provided facility and financial support; A. Mayeda coordinated and supervised the whole project. All authors read, corrected, and approved the final manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-RNPS1 | Mayeda et al.3 | |
| Rabbit anti-ACIN | Cell Signaling Technology | #4934 |
| Rabbit anti-PNN | Sigma-Aldrich | #HPA001378 |
| Rabbit anti-SAP18 | Proteintech | #13841-1-AP |
| Rabbit anti-AURKB | Cell Signaling Technology | #HPA052943 |
| Mouse anti-U2AF | Sigma-Aldrich | #U4758 |
| Mouse anti-GAPDH | MBL Life Science | #M171-3 |
| Mouse anti-Flag | MBL Life Science | #M185-3L |
| Mouse IgG | SANTA CRUZ | #sc-2025 |
| Bacterial and virus strains | ||
| E. coli strain DH5α | TOYOBO | #DNA-913F |
| Chemicals, peptides, and recombinant proteins | ||
| Lipofectamine 2000 | Thermo Fisher Scientific | #11668019 |
| Lipofectamine RNAiMAX Transfection Reagent | Thermo Fisher Scientific | #13778150 |
| Nocodazole | Sigma-Aldrich | #M1404 |
| Thymidine | Sigma-Aldrich | #T9250 |
| MG132 | Sigma-Aldrich | #7449 |
| Deposited data | ||
| RNA-Seq raw data | This paper | DDBJ database under accession number DRA016802 |
| Experimental models: Cell lines | ||
| HeLa cells | ATCC | |
| HEK293 cells | ATCC | |
| Oligonucleotides | ||
| See Table S1 | This paper | |
| Recombinant DNA | ||
| pcDNA3-Flag-RNPS1 | Fukumura et al.18 | N/A |
| pcDNA3-AURKB/E5-WT | Fukumura et al.18 | N/A |
| pcDNA3-AURKB/E5-Δ#3 | Fukumura et al.18 | N/A |
| pcDNA3-AURKB/E5/inR-E6 | This paper | N/A |
| pcDNA3-AURKB/E5/inL-E6 | This paper | N/A |
| Software and algorithms | ||
| Trimmomatic v0.36 | Bolger et al.31 | https://bioweb.pasteur.fr/packages/pack@Trimmomatic@0.36 |
| Toolkit v0.0.13 | http://hannonlab.cshl.edu/fastx_toolkit/index.html | |
| TopHat v2.1.1 | Kim et al.32 | https://bioweb.pasteur.fr/packages/pack@tophat@2.1.1 |
| Cufflinks v2.2.1 | Trapnell et al.33 | https://bioweb.pasteur.fr/packages/pack@cufflinks@2.2.1 |
| MISO v0.5.2 | Katz et al.34 | http://hollywood.mit.edu/burgelab/miso/ |
Experimental model and participant details
Human cell line
HeLa and HEK293 cells (originally purchased from ATCC) were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wako) supplemented with 10% fetal bovine serum (Sigma-Aldrich) and 1% penicillin-streptomycin (Nakarai Tesque). Cells were grown at 37°C in 5% CO2.
Method details
Construction of expression plasmids
The pcDNA3-Flag-RNPS1 expression plasmid was described previously.18 To construct the expression plasmids, pcDNA3-AURKB/E5-WT and the pcDNA3-AURKB/E5-Δ#3, the fragments containing the 3′ end of intron 4, exon 5 and the 5′ end of intron 5 were amplified by PCR from pcDNA3-AURKB/E5-E6 and pcDNA3-AURKB/E5-E6-Δ#3 plasmids,18 respectively, and these were subcloned into pcDNA3 vector (Invitrogen–Thermo Fisher Scientific). The pcDNA3-AURKB/E5/inR-E6 and pcDNA3-AURKB/E5/inL-E6 plasmids were constructed using overlap extension PCR as previously described.35 All PCRs were performed with PrimeSTAR Max DNA Polymerase (Takara Bio) using the described primer sets (Table S1).
siRNA-mediated knockdown and splicing assay
The transfection of HeLa and HEK293 cells with siRNAs (100 pmol each) was performed using Lipofectamine RNAi max (Invitrogen-Thermo Fisher Scientific) according to manufacturer’s protocol. The siRNAs targeting RNPS1, ACIN1, PNN, SAP18, GPATCH8 and LUC7L3 (Table S1) were purchased (Nippon Gene).
At 72 h post-transfection, total RNAs were isolated from HeLa and HEK293 cells using the NucleoSpin RNA kit (Macherey-Nagel). To examine endogenous mRNA expression levels and the splicing products, total RNAs were reverse transcribed using PrimeScript II reverse transcriptase (Takara Bio) with oligo-dT and random primers, and the obtained cDNAs were analyzed by PCR and quantitative PCR (qPCR) using specific primer sets (Table S1).
All primers oligonucleotides were purchased (Fasmac) and all PCRs were performed with Blend Taq polymerase (Toyobo). The PCR products were analyzed by 6% polyacrylamide gel electrophoresis (PAGE). PCR products of aberrantly spliced and unspliced pre-mRNAs were quantified using NIH ImageJ software. Eco Real-Time PCR system (Illumina) was used for qPCR analysis with Thunderbird SYBR qPCR Mix (Toyobo). Relative changes of splicing efficiency is calculated as described previously.36 All the experiments were independently repeated three times. Mean values, standard error of the mean (SEM), and Welch’s t-test were calculated using Excel (Microsoft).
Western blotting analysis
At 72 h post-transfection, total protein was isolated from siRNA-treated HeLa and HEK293 cells to examine the endogenous protein expression levels. Cells were suspended in buffer D [20 mM HEPES-KOH (pH 7.9), 50 mM KCl, 0.2 mM EDTA, 20% glycerol], sonicated for 20 s, centrifuged to remove debris, and the lysates were subjected to Western blotting. The procedure, detection and analysis were described previously.35
The following antibodies were used to detect target proteins: anti-RNPS1 (1:750 dilution),3 anti-ACIN1 (1:1500 dilution; Cells Signaling Technology), anti-PNN (1:1500 dilution; Sigma-Aldrich), anti-SAP18 (1:1500 dilution; Proteintech), anti-AURKB (1:1500 dilution; Cells Signaling Technology) and anti-GAPDH (1:1500 dilution; MBL Life Science) antibodies.
RNA immunoprecipitation assay
HeLa cells (in 60-mm dishes) were co-transfected with pcDNA3-Flag-RNPS1 and pcDNA3-AURKB/E5-WT or the pcDNA3-AURKB/E5-Δ#3 using Lipofectamine 2000 (Invitrogen–Thermo Fisher Scientific). After culture for 48 h, the transfected cells were suspended in 100 μL of buffer D (see above) and sonicated for 20 s (UR-20P; Tomy Seiko). The lysate was spun down to remove debris and 5 μL of supernatant was saved as 5% of input. The remaining supernatant was rocked at 4°C for 3 h with each antibody conjugated with Dynabeads Protein A (Invitrogen–Thermo Fisher Scientific) in 0.5 mL of NET2 buffer [50 mM Tris-HCl (pH7.5), 150 mM NaCl, 0.05% Nonidet P-40]. The beads were washed six times with 0.8 mL of NET2 buffer and bound RNA, together with the supernatant (5% of input), were eluted separately by TRI Reagent (Molecular Research Center). These RNAs were reverse transcribed by PrimeScript II reverse transcriptase (Takara Bio) with SP6 primer, and qPCRs were performed using specific primer sets (Table S1).
The values of % input (Figure 1D) were calculated from obtained RT–qPCR-based threshold cycle (Ct) values of immunoprecipitated RNA (Ct1) and 5% input RNA (Ct2) with the formula: 2(Ct2–Ct1) 20.
Whole-transcriptome sequencing (RNA-Seq) analysis
The RNA libraries were prepared from three RNA sources; HEK293 cells transfected with siRNAs targeting RNPS1 and PNN (with control siRNA). mRNA isolation, cDNA library construction and whole-transcriptome sequencing was performed by Macrogen Japan Corporation.
The obtained reads were mapped to the human genome as follows. FASTQ files were filtered by Trimmomatic v0.3633 with the parameters “PE-phred33 ILLUMINACLIP:Trimmomatic-0.36/adapters/TruSeq3-PE.fa:2:30:10 LEADING:3 TRAILING:3 SLIDINGWINDOW:4:15 MINLEN:150” and trimmed by fastx_trimmer included in the FASTX-Toolkit v0.0.13 (http://hannonlab.cshl.edu/fastx_toolkit/index.html) with the parameter “-l 150”. The qualified FASTQ files were mapped to human genome hg19 by TopHat v2.1.134 and the transcripts were assembled by Cufflinks v2.2.135 with default parameters. PSI (percent-spliced-in) values were estimated by MISO v0.5.236 using the assembled transcripts as previously described.37 RNA-Seq raw data have been deposited in DDBJ database (http://www.ddbj.nig.ac.jp/index-e.html) under accession No. DRA16802.
Cell cycle synchronization experiments
To achieve cell synchronization at G2/M phase, HEK293 cells were plated (in 35-mm dishes) at 40% confluency. Cells were subsequently treated with 2 mM thymidine (Sigma-Aldrich) for 24 h, washed with PBS, and supplemented with fresh DMEM. After culture for 3 h, cells were followed by 12 h culture in the presence of microtubule-disrupting agent nocodazole at 100 μM concentration (Sigma-Aldrich). To remove nocodazole, cells were washed with phosphate-buffered saline (PBS) and cultured in fresh DMEM. Cells were harvested every 4 h for 12 h after the removal of nocodazole.
To achieve cell synchronization at G1/S phase, HEK293 cells were plated (in 35-mm dishes) at 20% confluency. Cells were treated with 2 mM thymidine for 18 h, washed with PBS, and supplemented with DMEM. After culture for 9 h, cells were treated with 2 mM thymidine for another 15 h. Cells were released from thymidine block by washing with PBS followed by culture in fresh DMEM. Cells were harvested every 4 h for 12 h after the removal of thymidine.
To inhibit proteasome, HEK293 cells were treated with MG132 (Sigma-Aldrich) at a final concentration of 1 μM. After 4 h and 8 h of culture, cells were harvested and total protein was isolated (see above).
Quantification and statistical analysis
Three independent experiments were performed. Mean values, SE values, and Welch’s t-test were calculated using Excel (Microsoft).
Published: November 15, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111400.
Contributor Information
Kazuhiro Fukumura, Email: fukumura@fujita-hu.ac.jp.
Akila Mayeda, Email: mayeda@fujita-hu.ac.jp.
Supplemental information
References
- 1.Will C.L., Lührmann R. Spliceosome structure and function. Cold Spring Harbor Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a003707. [DOI] [Google Scholar]
- 2.Kastner B., Will C.L., Stark H., Lührmann R. Structural insights into nuclear pre-mRNA splicing in higher eukaryotes. Cold Spring Harbor Perspect. Biol. 2019;11 doi: 10.1101/cshperspect.a032417. [DOI] [Google Scholar]
- 3.Mayeda A., Badolato J., Kobayashi R., Zhang M.Q., Gardiner E.M., Krainer A.R. Purification and characterization of human RNPS1: a general activator of pre-mRNA splicing. EMBO J. 1999;18:4560–4570. doi: 10.1093/emboj/18.16.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakashita E., Tatsumi S., Werner D., Endo H., Mayeda A. Human RNPS1 and its associated factors: a versatile alternative pre-mRNA splicing regulator in vivo. Mol. Cell Biol. 2004;24:1174–1187. doi: 10.1128/MCB.24.3.1174-1187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trembley J.H., Tatsumi S., Sakashita E., Loyer P., Slaughter C.A., Suzuki H., Endo H., Kidd V.J., Mayeda A. Activation of pre-mRNA splicing by human RNPS1 is regulated by CK2 phosphorylation. Mol. Cell Biol. 2005;25:1446–1457. doi: 10.1128/MCB.25.4.1446-1457.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Hir H., Saulière J., Wang Z. The exon junction complex as a node of post-transcriptional networks. Nat. Rev. Mol. Cell Biol. 2016;17:41–54. doi: 10.1038/nrm.2015.7. [DOI] [PubMed] [Google Scholar]
- 7.Schlautmann L.P., Gehring N.H. A day in the life of the exon junction complex. Biomolecules. 2020;10:866. doi: 10.3390/biom10060866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwerk C., Prasad J., Degenhardt K., Erdjument-Bromage H., White E., Tempst P., Kidd V.J., Manley J.L., Lahti J.M., Reinberg D. ASAP, a novel protein complex involved in RNA processing and apoptosis. Mol. Cell Biol. 2003;23:2981–2990. doi: 10.1128/MCB.23.8.2981-2990.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murachelli A.G., Ebert J., Basquin C., Le Hir H., Conti E. The structure of the ASAP core complex reveals the existence of a Pinin-containing PSAP complex. Nat. Struct. Mol. Biol. 2012;19:378–386. doi: 10.1038/nsmb.2242. [DOI] [PubMed] [Google Scholar]
- 10.Joselin A.P., Schulze-Osthoff K., Schwerk C. Loss of Acinus inhibits oligonucleosomal DNA fragmentation but not chromatin condensation during apoptosis. J. Biol. Chem. 2006;281:12475–12484. doi: 10.1074/jbc.M509859200. [DOI] [PubMed] [Google Scholar]
- 11.Barrett S.P., Wang P.L., Salzman J. Circular RNA biogenesis can proceed through an exon-containing lariat precursor. Elife. 2015;4 doi: 10.7554/eLife.07540. [DOI] [Google Scholar]
- 12.Rodor J., Pan Q., Blencowe B.J., Eyras E., Cáceres J.F. The RNA-binding profile of Acinus, a peripheral component of the exon junction complex, reveals its role in splicing regulation. RNA. 2016;22:1411–1426. doi: 10.1261/rna.057158.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P., Lou P.J., Leu S., Ouyang P. Modulation of alternative pre-mRNA splicing in vivo by pinin. Biochem. Biophys. Res. Commun. 2002;294:448–455. doi: 10.1016/S0006-291X(02)00495-3. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z., Ballut L., Barbosa I., Le Hir H. Exon Junction Complexes can have distinct functional flavours to regulate specific splicing events. Sci. Rep. 2018;8:9509. doi: 10.1038/s41598-018-27826-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Otani Y., Fujita K.I., Kameyama T., Mayeda A. The exon junction complex core represses cancer-specific mature mRNA Re-splicing: A potential key role in terminating splicing. Int. J. Mol. Sci. 2021;22:6519. doi: 10.3390/ijms22126519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenn V., Musacchio A. The Aurora B kinase in chromosome bi-orientation and spindle checkpoint signaling. Front. Oncol. 2015;5:225. doi: 10.3389/fonc.2015.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukumura K., Wakabayashi S., Kataoka N., Sakamoto H., Suzuki Y., Nakai K., Mayeda A., Inoue K. The exon junction complex controls the efficient and faithful splicing of a subset of transcripts involved in mitotic cell-cycle progression. Int. J. Mol. Sci. 2016;17:1153. doi: 10.3390/ijms17081153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukumura K., Inoue K., Mayeda A. Splicing activator RNPS1 suppresses errors in pre-mRNA splicing: A key factor for mRNA quality control. Biochem. Biophys. Res. Commun. 2018;496:921–926. doi: 10.1016/j.bbrc.2018.01.120. [DOI] [PubMed] [Google Scholar]
- 19.Dominguez D., Tsai Y.H., Weatheritt R., Wang Y., Blencowe B.J., Wang Z. An extensive program of periodic alternative splicing linked to cell cycle progression. Elife. 2016;5 doi: 10.7554/eLife.10288. [DOI] [Google Scholar]
- 20.Honda R., Körner R., Nigg E.A. Exploring the functional interactions between Aurora B, INCENP, and survivin in mitosis. Mol. Biol. Cell. 2003;14:3325–3341. doi: 10.1091/mbc.E02-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Badolato J., Gardiner E., Morrison N., Eisman J. Identification and characterisation of a novel human RNA-binding protein. Gene. 1995;166:323–327. doi: 10.1016/0378-1119(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 22.Gilberto S., Peter M. Dynamic ubiquitin signaling in cell cycle regulation. J. Cell Biol. 2017;216:2259–2271. doi: 10.1083/jcb.201703170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon S.K., Kim E.H., Baek K.H. RNPS1 is modulated by ubiquitin-specific protease 4. FEBS Lett. 2017;591:369–381. doi: 10.1002/1873-3468.12531. [DOI] [PubMed] [Google Scholar]
- 24.Boehm V., Britto-Borges T., Steckelberg A.L., Singh K.K., Gerbracht J.V., Gueney E., Blazquez L., Altmüller J., Dieterich C., Gehring N.H. Exon junction complexes suppress spurious splice sites to safeguard transcriptome integrity. Mol. Cell. 2018;72:482–495. doi: 10.1016/j.molcel.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Blazquez L., Emmett W., Faraway R., Pineda J.M.B., Bajew S., Gohr A., Haberman N., Sibley C.R., Bradley R.K., Irimia M., Ule J. Exon junction complex shapes the transcriptome by repressing recursive splicing. Mol. Cell. 2018;72:496–509. doi: 10.1016/j.molcel.2018.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishigaki Y., Nakamura Y., Tatsuno T., Hashimoto M., Shimasaki T., Iwabuchi K., Tomosugi N. Depletion of RNA-binding protein RBM8A (Y14) causes cell cycle deficiency and apoptosis in human cells. Exp. Biol. Med. 2013;238:889–897. doi: 10.1177/1535370213494646. [DOI] [Google Scholar]
- 27.Silver D.L., Watkins-Chow D.E., Schreck K.C., Pierfelice T.J., Larson D.M., Burnetti A.J., Liaw H.J., Myung K., Walsh C.A., Gaiano N., Pavan W.J. The exon junction complex component Magoh controls brain size by regulating neural stem cell division. Nat. Neurosci. 2010;13:551–558. doi: 10.1038/nn.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jang S.W., Yang S.J., Ehlén A., Dong S., Khoury H., Chen J., Persson J.L., Ye K. Serine/arginine protein-specific kinase 2 promotes leukemia cell proliferation by phosphorylating acinus and regulating cyclin A1. Cancer Res. 2008;68:4559–4570. doi: 10.1158/0008-5472.CAN-08-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Puig O., Bragado-Nilsson E., Koski T., Séraphin B. The U1 snRNP-associated factor Luc7p affects 5' splice site selection in yeast and human. Nucleic Acids Res. 2007;35:5874–5885. doi: 10.1093/nar/gkm505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniels N.J., Hershberger C.E., Gu X., Schueger C., DiPasquale W.M., Brick J., Saunthararajah Y., Maciejewski J.P., Padgett R.A. Functional analyses of human LUC7-like proteins involved in splicing regulation and myeloid neoplasms. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.108989. [DOI] [Google Scholar]
- 31.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapnell C., Hendrickson D.G., Sauvageau M., Goff L., Rinn J.L., Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013;31:46–53. doi: 10.1038/nbt.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Katz Y., Wang E.T., Airoldi E.M., Burge C.B. Analysis and design of RNA sequencing experiments for identifying isoform regulation. Nat. Methods. 2010;7:1009–1015. doi: 10.1038/nmeth.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukumura K., Yoshimoto R., Sperotto L., Kang H.S., Hirose T., Inoue K., Sattler M., Mayeda A. SPF45/RBM17-dependent, but not U2AF-dependent, splicing in a distinct subset of human short introns. Nat. Commun. 2021;12:4910. doi: 10.1038/s41467-021-24879-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harvey S.E., Cheng C. Methods for characterization of alternative RNA splicing. Methods Mol. Biol. 2016;1402:229–241. doi: 10.1007/978-1-4939-3378-5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masuda A., Takeda J.i., Okuno T., Okamoto T., Ohkawara B., Ito M., Ishigaki S., Sobue G., Ohno K. Position–specific binding of FUS to nascent RNA regulates mRNA length. Genes Dev. 2015;29:1045–1057. doi: 10.1101/gad.255737.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
The raw data derived from RNA-Seq analysis (see Figures 2A and S4; Table S2. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1-deficient HEK293 cells (related to Figures 2A, S4), Table S3. The list of splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in PNN-deficient HEK293 cells (related to Figures 2A, S4), Table S4. The list of overlapping splicing changes (alternative 3′ splice site, alternative 5′ splice site, mutually exclusive exons, intron retention and cassette exon) in RNPS1- and PNN-deficient HEK293 cells (related to Figures 2A, S4)) have been deposited in DDBJ database and are publicly available as of the date of publication. Accession number is listed in the key resources table.
-
•
This article does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon reasonable request.