Abstract
We report herein a convenient one-pot synthesis for the shelf-stable molecular complex [Mn(NO3)3(OPPh3)2] (2) and describe the properties that make it a powerful and selective one-electron oxidation (deelectronation) reagent. 2 has a high reduction potential of 1.02 V versus ferrocene (MeCN) (1.65 vs normal hydrogen electrode), which is one the highest known among readily available redox agents used in chemical synthesis. 2 exhibits stability toward air in the solid state, can be handled with relative ease, and is soluble in most common laboratory solvents such as MeCN, dichloromethane, and fluorobenzene. 2 is substitutionally labile with respect to the coordinated (pseudo)halide ions enabling the synthesis of other new Mn(III) nitrato complexes also with high reduction potentials ranging from 0.6 to 1.0 V versus ferrocene.
Introduction.
Single-electron oxidation, sometimes referred to as deelectronation,1 is one of the most fundamental reactions in chemistry. Most very strong oxidants (>0.8 V; all potentials vs FeCp2 unless noted) such as main group fluorides (e.g., AsF6) or transition-metal hexafluoride compounds (WF6, PtF6, etc.) are impractical because they are difficult to synthesize and/or handle in standard laboratory settings.2 Nitrosonium and Ag+ salts of weakly coordinating anions (WCAs) are also established reagents for deelectronation,3 but, as with most strong oxidants, oxidation is often accompanied by side reactions in addition to electron removal. Some recent progress toward more selective single-electron oxidants such as [TCNQ·4B(C6F5)3] (TCNQ = tetracyanoquinodimethane), [phenazineF][Al(ORF)4] (phenazineF = perfluoro-5,10-bis(perfluorophenyl)-5,10-dihydrophenazine), and aminium-cation carborane-anion pairs, like T13,4 have been reported, but the multistep syntheses and air and moisture sensitivities limit their utility.5 Hence, the search for selective single-electron oxidants continues, driven by the need for ease of synthesis and handling while maintaining high reduction potentials.
We noted that a characteristic of the Mn(III) ion is its high reduction potential, a property that is critical to life,6 battery materials,7 and oxidation in organic transformations such as alkene difunctionalization,8 alkene epoxidation,9 and free-radical additions and cyclizations.10 Within this framework of reactivity, we recently observed that the mononuclear Mn(III) complex [MnIIICl3(OPPh3)2] (1) is a potent Cl-atom transfer reagent11 and mediates C–H chlorination in specific cases,12 properties that appear to be intrinsic to the highly reactive MnCl3 entity. We reasoned that the coordination of pnictogen-oxide ylide triphenylphosphine oxide (Ph3PO) enabled the room-temperature stabilization of highly reactive MnCl3, which otherwise decomposes above −40 °C.13 As an extension of this rationale, we hypothesized that analogous Mn(III) complexes with weakly coordinating pseudohalide ligands could likewise exhibit desirable bench-stability characteristics while maintaining some of the intrinsic chemical properties, such as the high reduction potential.
Exploring this hypothesis with nitrate as the anion, reported herein, resulted in successful gram-scale synthesis of [MnIII(NO3)3(OPPh3)2] (2) and demonstration of desirable bench-stability and redox properties for oxidation. 2 has a strikingly high reduction potential of 1.02 V in MeCN (1.65 vs NHE) (Figure 1), which is one of the highest among available stoichiometric oxidants.14 While other reactants with higher potentials are known,5 2 can be synthesized in a single step on the bench from commercially available reactants, is capable of open-to-air manipulations, and is soluble in common laboratory solvents. The byproducts of oxidation with 2 are [MnII(NO3)2(OPPh3)2] (9) and non-nucleophilic nitrateion.15 Except for use in exotic cases, where extremely electrophilic products are generated, 2 is an important addition to the growing class of powerful oxidants. 2 also is substitutionally labile with respect to halide/pseudohalide ion metathesis enabling the synthesis of new Mn(III) nitrato16 complexes, effectively allowing us to tune the reduction potential in a series of strong to very strong oxidants spanning 0.6 to 1.0 V.
Figure 1.
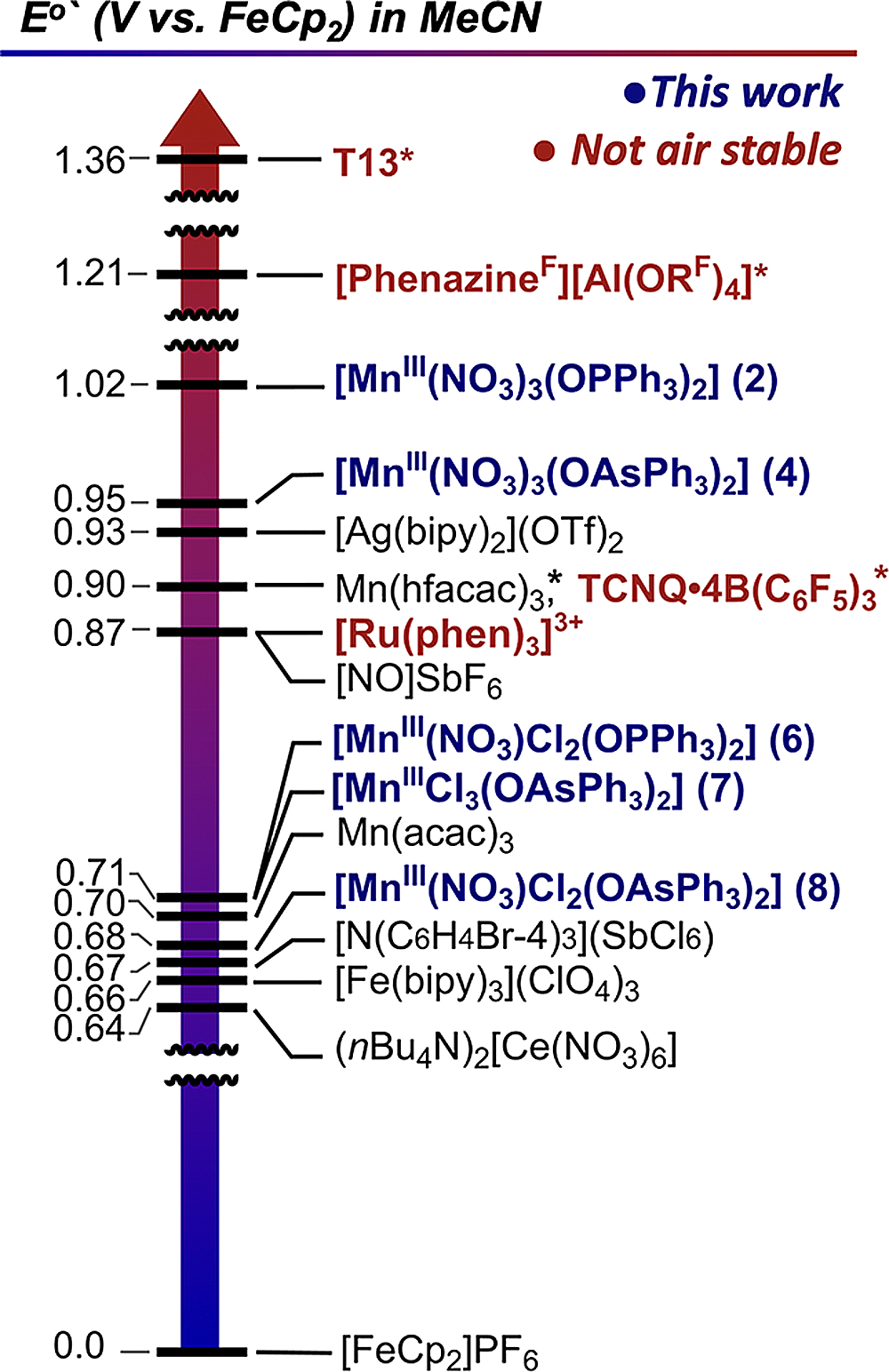
Reduction potentials of common air-stable oxidants in MeCN (see the text for citations). (*) Potentials in DCM.
Synthesis and Characterization of [MnIII(NO3)3(OPPh3)2].
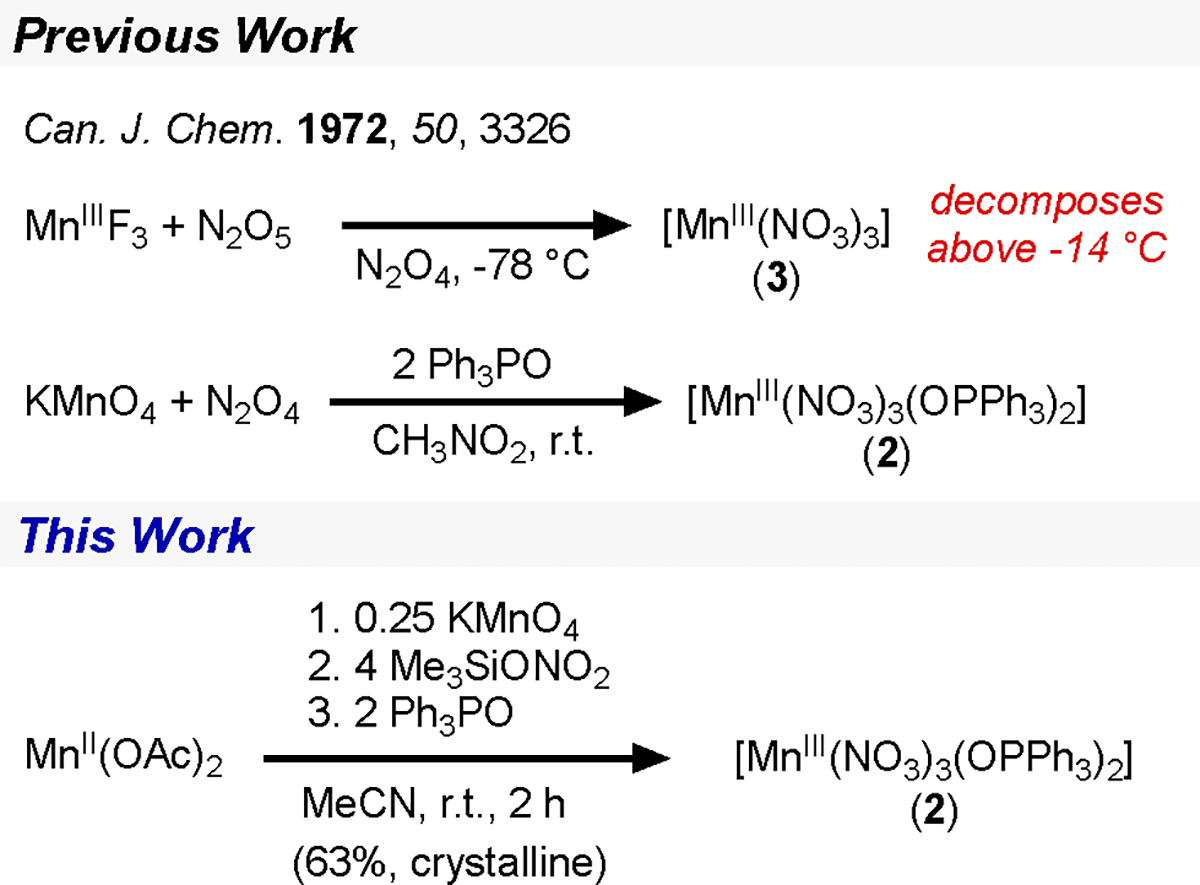
A procedure to prepare what is nominally [MnIII(NO3)3] (3) has been known since 1972,17 but it requires the use of highly toxic and inconvenient reagents like N2O5 and N2O4 and requires storage below −14 °C (Figure 2). 2 was synthesized from [MnIII(NO3)3] via this cumbersome route and, aside from a solid-state magnetic measurement and select vibrational data, virtually nothing was known about it prior to this work.17 Motivated by the premise that 2 would exhibit a kind of tempered stability like 1, we aimed to develop convenient ways of preparing 2 and new pnictogen-oxide stabilized complexes of Mn(III); pyridine-N-oxide (Opy), Ph3PO, and triphenylarsine oxide (Ph3AsO) were the chosen ligands for this study.
Figure 2.
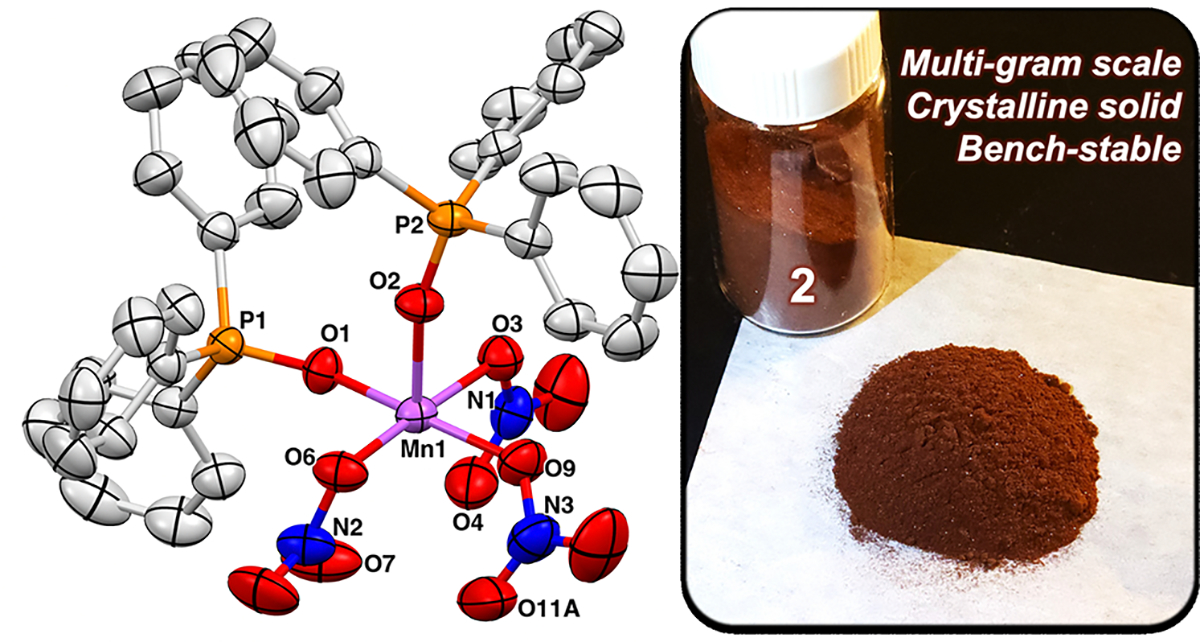
X-ray crystal structure of 2 (ellipsoids shown at 50% probability; H atoms omitted and only one component of disordered atoms for clarity) and material isolated from multigram scale synthesis.
Two convenient routes to 2 were devised, both using an acetonitrile solution of trimethylsilylnitrate (Me3SiONO2) generated in situ from AgNO3 and Me3SiCl.18 In the first route, 36 equiv of Me3SiONO2 was added to [Mn12Ol2(OAc)16(H2O)4]·2HOAc·4H2O (Mn12)19 and then treated with 24 equiv of Ph3PO to give 2 in low yields (26% crystalline). The more efficient second route is analogous to the one-pot synthesis that we developed for 1 (Scheme 1),11 employing Me3SiONO2 instead of Me3SiCl. Adding Me3SiONO2 to a mixture of Mn(OAc)2 and KMnO4 in a 16:4:1 ratio formed a brown solution, presumably containing 3, and upon addition of Ph3PO 2 was obtained in good yields. The reaction is scalable; we routinely perform syntheses that furnish 6 g of crude product (73%, Figure 2) which after recrystallization in a glovebox furnishes 5 g (63%) of crystalline, analytically pure material. In the solid state, 2 is stable to air and can be stored for months without noticeable change on the bench (Figure S5). Performing an entire synthesis (8 g scale) on a Schlenk line under Ar followed by open-to-air filtration does not diminish the yield of the crude product.
X-ray diffraction (XRD) characterization of red-brown crystals of 2 revealed a pseudo-square-pyramidal geometry with the Ph3PO ligands in the cis position (Figure 2), similar to 1.11 The solid-state ATR-FTIR spectrum of 2 contains a characteristic P–O stretch at 1436 cm−1 as well as nitrato stretches at 1528, 1260, and 951 cm−1. The solution-state magnetic moment of 2 in DCM is consistent with a high-spin Mn(III) center (μeff = 5.46 μB), and the X-band EPR spectrum (77 K) is silent.
Synthesis of Other Mn(III) Nitrato Complexes.
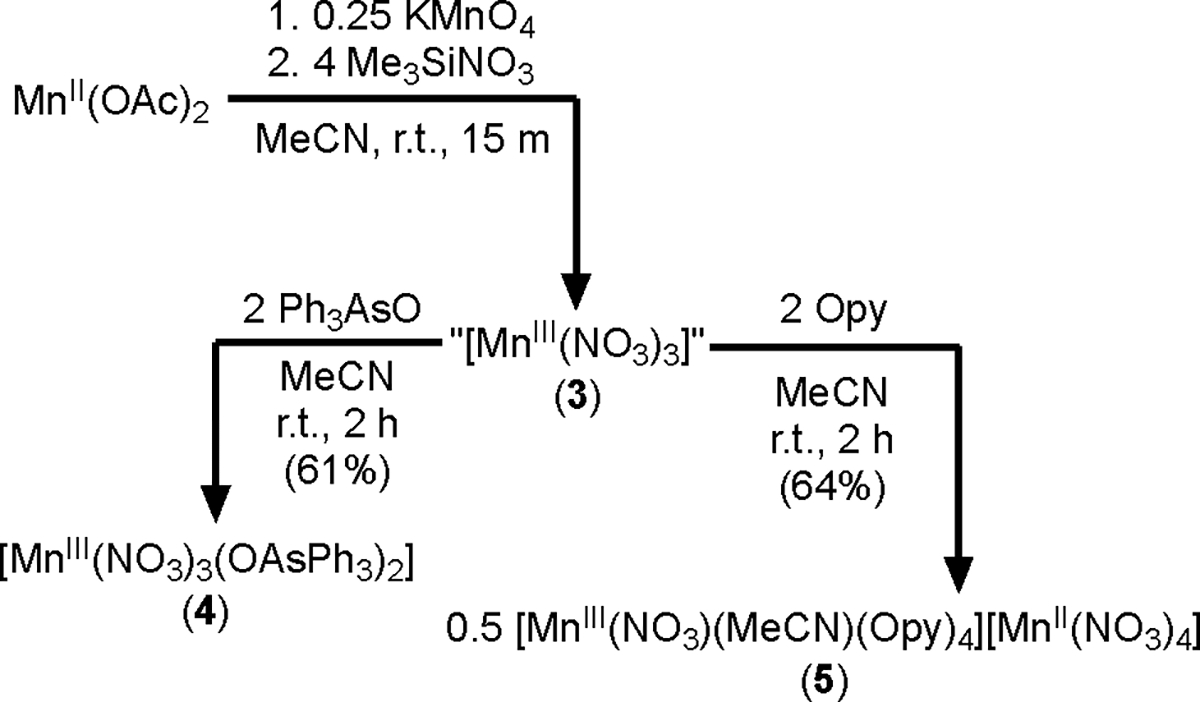
Mn(III) nitrato complexes with other pnictogen-oxide ligands were synthesized via 3 that was prepared directly from Mn(OAc)2 and KMnO4 (Scheme 2). Addition of 2 equiv of Ph3AsO to 3 produced [MnIII(NO3)3(OAsPh3)2] (4) in 61% crystalline yield. XRD analysis of deep purple crystals of 4 revealed a pseudo-trigonal-bipyramidal geometry with the Ph3AsO ligands in the trans position (Figure 3). Addition of 2 equiv of Opy to 3 did not produce the expected [MnIII(NO3)3(Opy)2], and instead the mononitrato Mn(III) complex salt [MnIII(NO3)(MeCN)(Opy)4][MnII(NO3)4] (5) was obtained in 64% crystalline yield (Figure 3). The six-coordinate manganese center of 5 is in the +3 oxidation state. The bond lengths and angles of the other manganese center are consistent with the known anion [MnII(NO3)4]2−.20 While 2 and 4 are bench stable in the solid state, 5 readily undergoes hydration when exposed to air. Furthermore, 2 and 4 are soluble in polar organic solvents like DCM, MeCN, and THF while 5 is only soluble in MeCN, consistent with its salt-like state in contrast to neutral 2. In fact, 2 is soluble in fluorobenzene (2.8 mg/mL) and is sparingly soluble in toluene and benzene (<1 mg/mL).
Figure 3.
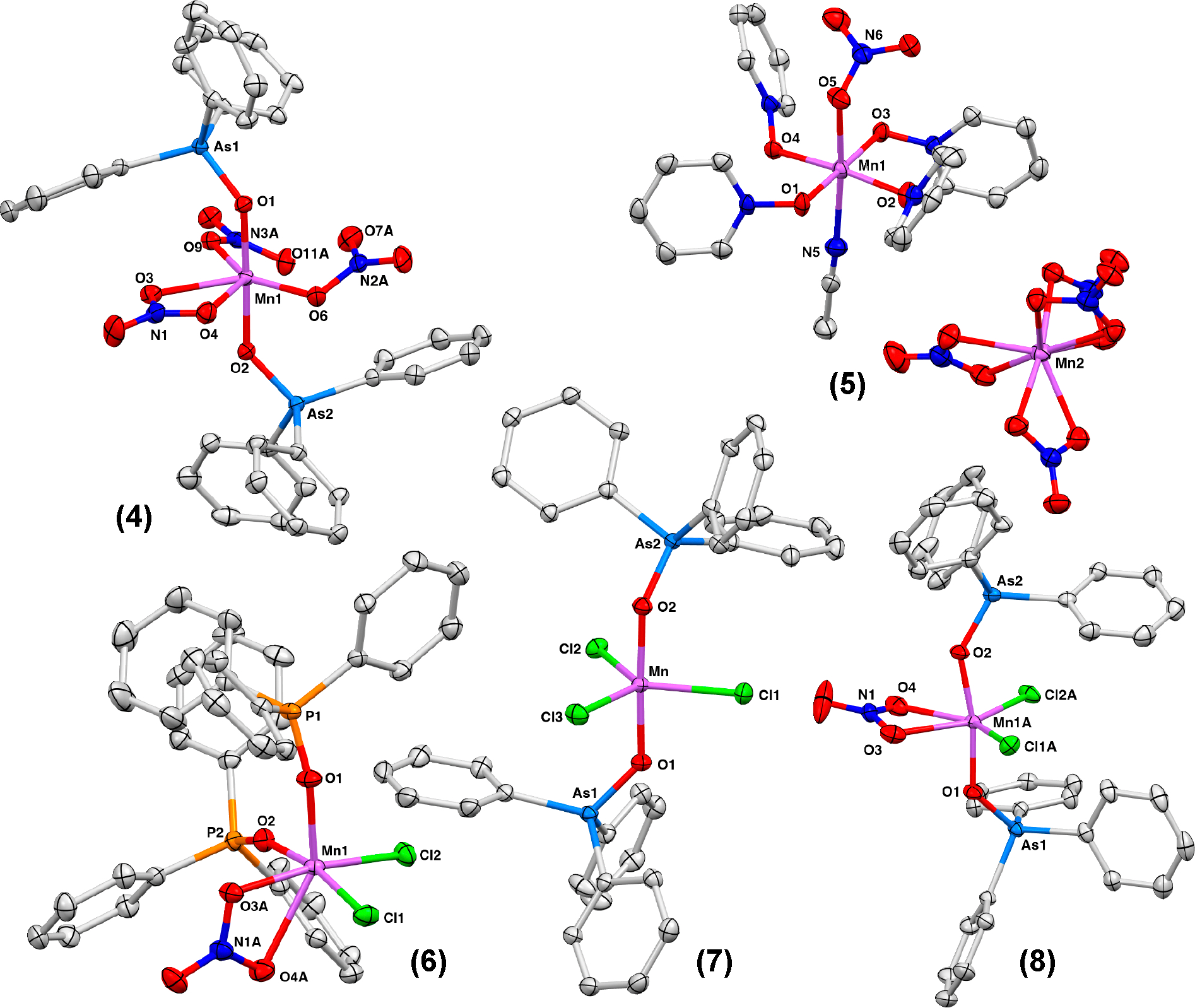
Molecular structures determined with XRD for 4–8 (ellipsoids shown at the 50% probability). H atoms are omitted, and only one component of disordered atoms is shown for clarity.
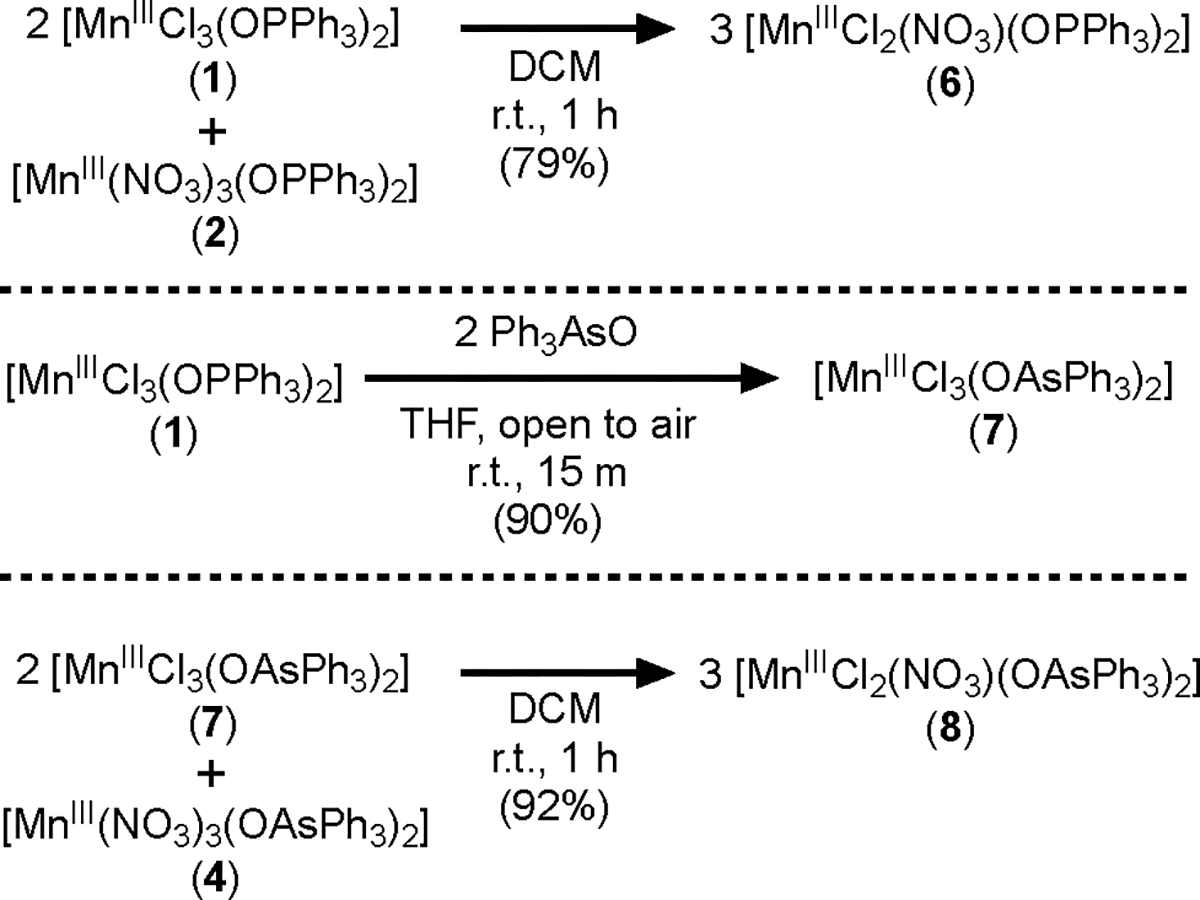
We also prepared mixed nitrato dichlorido Mn(III) complexes via halide/pseudohalide metathesis (Scheme 3). Reaction of 1 and 2 in a 2:1 ratio cleanly produces 6 (Figure 3). Similarly, complex [MnIIICl2(NO3)(OAsPh3)2] (8) was synthesized from 4 and [MnIIICl3(OAsPh3)2] (7) (Figure 3).21
Electrochemical Investigation of the Mn(III) Complexes.
The complexes prepared here were analyzed with cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in DCM, MeCN (Figure 4), and THF (Figure S47). The reduction potentials are remarkably high and span the range of 0.6 to 1.0 V (Table 1).
Figure 4.
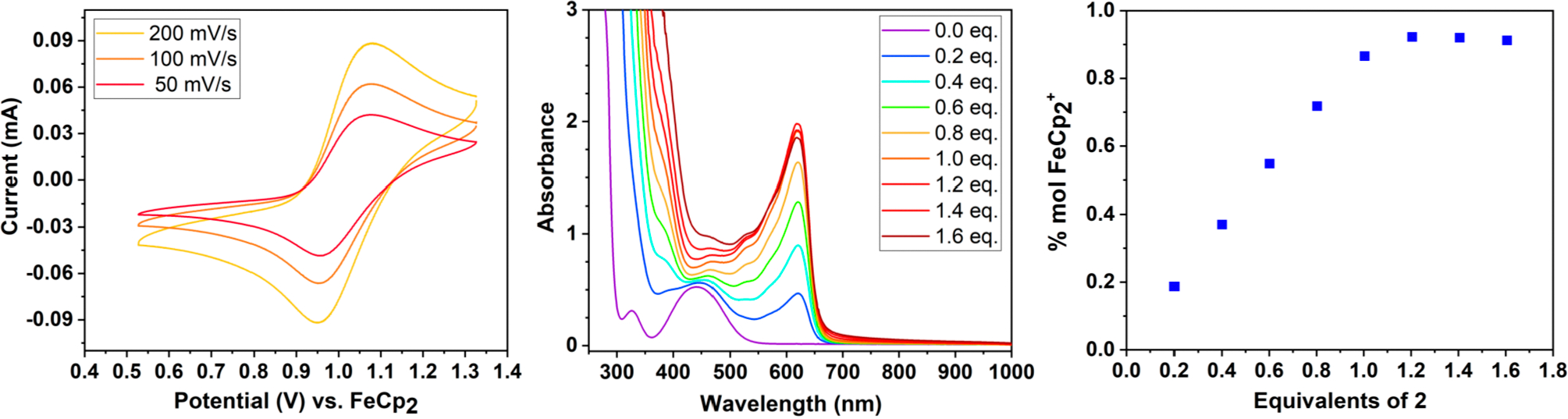
(Left) Cyclic voltammograms of 0.006 M 2 in MeCN with 0.500 M [nBu4N][PF6] (see Supporting Information for details). (Middle & right) Titration of 2 with ferrocene in DCM showing one-to-one stoichiometry.
Table 1.
Reduction Potentials for Mn(III) Complexes
|
E1/2 (V vs FeCp2) |
||
|---|---|---|
| Complex | MeCN | DCM |
|
| ||
| [MnIII(NO3)3(OPPh3)2] (2)a | 1.02 | 0.96 |
| [MnIII(NO3)3(OAsPh3)2] (4) | 0.95 | 0.91 |
| [MnIII(NO3)(MeCN)(Opy)4][MnII(NO3)4] (5) | 1.02 | |
| [MnIII(NO3)(Cl)2(OPPh3)2] (6) | 0.72 | 0.68 |
| [MnIII(Cl)3(OAsPh3)2] (7) | 0.72 | 0.78 |
| [MnIII(NO3)(Cl)2(OAsPh3)2] (8) | 0.68 | 0.68 |
Potentials of 2 in MeCN measured in triplicate were within 1 mV. Potentials in DCM measured in triplicate varied by ±10 mV. Electrolyte: 0.5 M [nBu4N][PF6]. Analyte conc.: 6 mM.
To confirm that 2 acts as a one-electron oxidant, we titrated a solution of ferrocene with 2 and found that one equivalent was required to cleanly generate FeCp2+ in DCM (Figure 4). The same titration was conducted for [FeII(bipy)3](PF6)2 (0.66 V in MeCN) and N(4-bromophenyl)3 (0.70 V in DCM), and likewise a clean one-electron transformation occurred to [FeIII(bipy)3]3+ 22 and N(4-bromophenyl)3+•, respectively.
The byproducts of oxidation with 2 are a weakly coordinating nitrate anion and 9, which are easy to separate from reaction mixtures and will not likely interfere with subsequent chemistries if they are still present. The potentials of oxidants like KMnO4 or ceric ammonium nitrate (CAN) in aqueous HClO4 that are more powerful than 2 are only available in aqueous conditions, and such comparisons with aqueous oxidants are misleading. For instance, the E1/2 for tetrabutylammonium ceric nitrate (CTAN)23 is 0.6 V (Figure 1) in MeCN and DCM and requires addition of strong acids like HOTf to achieve higher oxidizing strength in organic solvent.24
C–H Nitrooxylation and Stability Limits of 2.
Single-electron oxidation is not the only reaction that 2 mediates. We found that 2 and 4 react with certain benzylic C–H bonds to form nitrate esters (nitrooxylation) (Scheme 4, Table 2).25
Table 2.
Nitrooxylation of HMB with Mn(NO3)3 Complexesa
| Entry | Complex | % yield |
|---|---|---|
|
| ||
| 1 | 2 | 99 |
| 2 | 4 b | 58 |
| 3 | 2 (1 equiv) | 50 |
| 4 | 2 (crude) | 82 |
Conditions shown in Scheme 4.
24 h. Average yields for duplicated runs determined by 1H NMR.
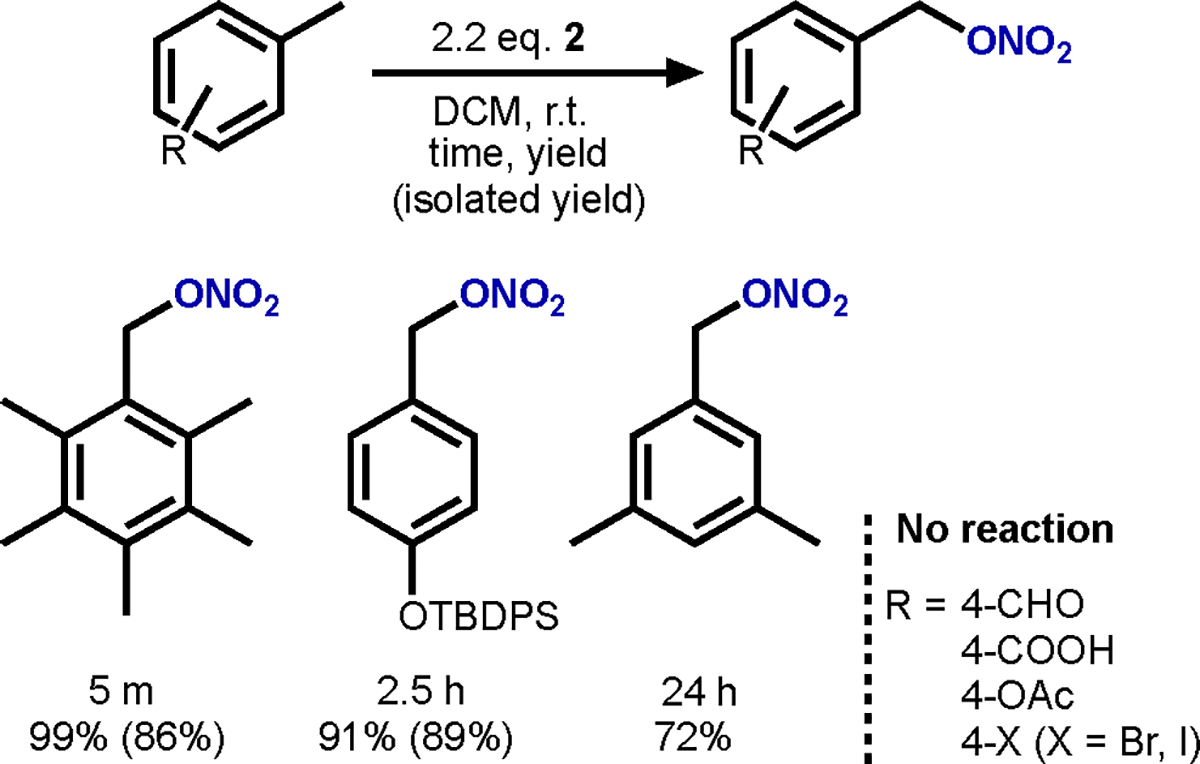
Hexamethylbenzene (HMB) was chosen as model substrate to investigate this reactivity since it has a weak benzylic C–H bond dissociation free energy (BDFE) in MeCN of 81.0 kcal/mol26 and low ionization potential (IP) of 7.85 eV.27 Reaction of HMB with two equiv of 2 in DCM afforded quantitative conversion to 2,3,4,5,6-pentamethylbenzyl nitrate in 5 min (entry 1). The same reaction but with 4 gave only 58% conversion in 24 h (entry 2). Reaction of 1 equiv of 2 with HMB resulted in 50% conversion (entry 3) consistent with the proposed mechanism (vide infra). Due to the efficiency and short reaction time of 2 mediated nitrooxylation of HMB, it can serve as an effective batch control for the purity of 2 (entry 4; see Supporting Information for details).
The reactivity of 2 with benzylic C–H bonds is limited, boding well for use of 2 as a selective single-electron oxidant. For example, 2 did not react with a range of benzylic substrates (e.g, aromatic aldehydes, ketones, or esters; Scheme 4), even under thermal (refluxing DCM) or photolytic (100 W Xe-arc lamp) conditions. Other substrates affording clean nitrooxylation were tert-butyldiphenylsilane (TBDPS) protected p-cresol and mesitylene (Scheme 4). One possible mechanism of the reaction with HMB is a C–H cleavage akin to what has been proposed for Ni(IV) nitrato-mediated C–H cleavage by Jackson and Wang,28 chlorination of HMB using 1 by us,12 and CF3 functionalization of arenes using Ag2+ photooxidants by Nocera.29 The first equivalent of 2 cleaves a C–H bond forming nitric acid and a benzylic radical, the latter of which couples with a second molecule of 2 explaining the need for two equivalents of 2 and observation of 9 as the byproduct in HMB nitrooxylation. Given that 2 has an E1/2,MeCN of 1.02 V and HNO3 has a pKa,MeCN of 9.4,30 the H atom abstracting ability (i.e., effective BDFE) is estimated to be 89 kcal/mol.26 This bond energy is high enough for C–H nitrooxylation of HMB, but it is not so high as to interfere with selective single-electron oxidation under a wide range of conditions (most C–H strengths are above 89 kcal/mol). An alternative mechanism to C–H activation could initiate with single-electron oxidation of HMB to form the HMB+• given its low IP.27 2 also reacts, albeit slowly, with mesitylene (IP = 8.39 eV) but is stable in dry fluorobenzene (IP = 9.19 eV), DCM (IP = 11.35 eV), and MeCN (IP = 12.20 eV),31,32 which puts an upper limit of single-electron oxidation of <9.2 eV.33 This high IP and low BDFEeff reflects the high stability of 2 toward air and common organic solvents while maintaining its potent oxidizing ability. In addition to this and the other properties of 2 that we have described herein, it is plain that 2 and other Mn(III) nitrato complexes are ideal powerful oxidants for organometallic chemists.
Supplementary Material
Scheme 1.
Synthesis of 2
Scheme 2.
Synthesis of 3–5
Scheme 3.
Synthesis of 6–8
Scheme 4.
C–H Nitrooxylation Mediated by 2
ACKNOWLEDGMENTS
NSF grant CHE-1847933, NIH-R21-GM141685-01, and the University at Buffalo (UB) provided support. X-ray diffraction (XRD) System Rigaku XtaLAB Synergy-S was purchased with NSF award CHE-2216151. A Q-exactive GC-Orbitrap tandem MS was purchased using NSF CHE-1919594 by the UB Chemistry Instrument Center (CIC). The Bruker Ascend-500 NMR spectrometer in the UB Magnetic Resonance Center was purchased with NSF CHE-2018160.
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/jacs.4c03411.
Additional figures, experimental details, and NMR, FTIR, UV–vis spectra, and reaction data (PDF)
Accession Codes
CCDC 2338924–2338930 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
Complete contact information is available at: https://pubs.acs.org/10.1021/jacs.4c03411
Contributor Information
Ananya Saju, Department of Chemistry, University at Buffalo, State University of New York, Buffalo, New York 14260, United States.
Matthew R. Crawley, Department of Chemistry, University at Buffalo, State University of New York, Buffalo, New York 14260, United States
Samantha N. MacMillan, Department of Chemistry and Chemical Biology, Cornell University, Ithaca, New York 14853, United States
David C. Lacy, Department of Chemistry, University at Buffalo, State University of New York, Buffalo, New York 14260, United States
REFERENCES
- (1).(a) Himmel D; Radtke V; Butschke B; Krossing I Basic Remarks on Acidity. Angew. Chem., Int. Ed. 2018, 57, 4386. [DOI] [PubMed] [Google Scholar]; (b) Radtke V; Himmel D; Pütz K; Goll SK; Krossing I The Protoelectric Potential Map (PPM): An absolute two-dimensional chemical potential scale for a global understanding of chemistry. Chem.-Eur. J. 2014, 20, 4194. [DOI] [PubMed] [Google Scholar]
- (2).(a) Malischewski M; Adelhardt M; Sutter J; Meyer K; Seppelt K Isolation and structural and electronic characterization of salts of the decamethylferrocene dication. Science 2016, 353, 678. [DOI] [PubMed] [Google Scholar]; (b) Boere RT; Kacprzak S; Keßler M; Knapp C; Riebau R; Riedel S; Roemmele TL; Ruhle M; Scherer H; Weber S Oxidation of closo-[B12Cl12]2− to the radical anion [B12Cl12]•− and to neutral B12Cl12. Angew. Chem., Int. Ed. 2011, 50, 549. [DOI] [PubMed] [Google Scholar]; (c) Seppelt K Molecular hexafluorides. Chem. Rev. 2015, 115, 1296–1306. [DOI] [PubMed] [Google Scholar]
- (3).(a) Malinowski PJ; Krossing I Ag[Fe(CO)5]2+: A Bare Silver Complex with Fe(CO)5 as a Ligand. Angew. Chem., Int. Ed. 2014, 53, 13460. [DOI] [PubMed] [Google Scholar]; (b) Schmitt M; Mayländer M; Goost J; Richert S; Krossing I Chasing the Mond cation: synthesis and characterization of the homoleptic nickel tetracarbonyl cation and its tricarbonylnitrosyl analogue. Angew. Chem., Int. Ed. 2021, 60, 14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).T13 is the aminium-cation carborane-anion pair derived from 2,4-dibromo-N-(2-bromo-4-(trifluoromethyl)phenyl)-N-(2,4-dibromophenyl)aniline. See ref 5c.
- (5).(a) Albrecht PA; Rupf SM; Sellin M; Schlögl J; Riedel S; Malischewski M Increasing the oxidation power of TCNQ by coordination of B(C6F5)3. Chem. Commun. 2022, 58, 4958. [DOI] [PubMed] [Google Scholar]; (b) Schorpp M; Heizmann T; Schmucker M; Rein S; Weber S; Krossing I Synthesis and application of a perfluorinated ammoniumyl radical cation as a very strong deelectronator. Angew. Chem., Int. Ed. 2020, 59, 9453. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Davidson JJ; Gunther SO; Leong DW; Ozerov OV Synthesis of fluorinated aminium cations coupled with carborane anions for use as strong one-electron oxidants. Dalton Trans. 2023, 52, 16027. [DOI] [PubMed] [Google Scholar]; (d) Sellin M; Friedmann C; Mayländer M; Richert S; Krossing I Towards clustered carbonyl cations [M3(CO)14]2+ (M = Ru, Os): the need for innocent deelectronation. Chem. Sci. 2022, 13, 9147–9158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Lingappa UF; Monteverde DR; Magyar JS; Valentine JS; Fischer WW How manganese empowered life with dioxygen (and vice versa). Free Radic. Biol. Med. 2019, 140, 113. [DOI] [PubMed] [Google Scholar]; (b) Zhu W; Richards NGJ Biological functions controlled by manganese redox changes in mononuclear Mn-dependent enzymes. Essays Biochem. 2017, 61, 259. [DOI] [PubMed] [Google Scholar]; (c) Mandal M; Kawashima K; Saito K; Ishikita H Redox potential of the oxygen-evolving complex in the electron transfer cascade of photosystem II. J. Phys. Chem. Lett. 2020, 11, 249. [DOI] [PubMed] [Google Scholar]
- (7).Liu C; Neale ZG; Cao G Understanding electrochemical potentials of cathode materials in rechargeable batteries. Mater. Today 2016, 19, 109. [Google Scholar]
- (8).(a) Fu N; Sauer GS; Saha A; Loo A; Lin S Metal-catalyzed electrochemical diazidation of alkenes. Science 2017, 357, 575. [DOI] [PubMed] [Google Scholar]; (b) Sauer GS; Lin S An Electrocatalytic approach to the radical difunctionalization of alkenes. ACS Catal. 2018, 8, 5175. [Google Scholar]; (c) Dong X; Roeckl JL; Waldvogel SR; Morandi B Merging shuttle reactions and paired electrolysis for reversible vicinal dihalogenations. Science 2021, 371, 507. [DOI] [PubMed] [Google Scholar]
- (9).Philip RM; Radhika S; Abdulla CMA; Anilkumar G Recent trends and prospects in homogeneous manganese-catalyzed epoxidation. Adv. Synth. Catal. 2021, 363, 1272. [Google Scholar]
- (10).Demir AS; Emrullahoglu M Manganese(III) acetate: a versatile reagent in organic chemistry. Current Organic Synthesis 2007, 4, 321. [Google Scholar]
- (11).Saju A; Griffiths JR; MacMillan SN; Lacy DC Synthesis of a bench-stable manganese(III) chloride compound: coordination chemistry and alkene dichlorination. J. Am. Chem. Soc. 2022, 144, 16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Saju A; Gunasekera PS; Morgante P; MacMillan SN; Autschbach J; Lacy DC Experimental and computational determination of a M–Cl homolytic bond dissociation free energy: Mn(III)Cl-mediated C–H cleavage and chlorination. J. Am. Chem. Soc. 2023, 145, 13384. [DOI] [PubMed] [Google Scholar]
- (13).Nachtigall O; Pataki A; Molski M; Lentz D; Spandl J Solvates of manganese trichloride revisited - synthesis, isolation, and crystal structure of MnCl3(THF)3. Z. Anorg. Allg. Chem. 2015, 641, 1164. [Google Scholar]
- (14).Connelly NG; Geiger WE Chemical redox agents for organometallic chemistry. Chem. Rev. 1996, 96, 877. [DOI] [PubMed] [Google Scholar]
- (15).Pearson RG; Sobel HR; Songstad J Nucleophilic reactivity constants toward methyl iodide and trans-dichlorodi(pyridine)-platinum(II). J. Am. Chem. Soc. 1968, 90, 319. [Google Scholar]
- (16).Henceforth, when “nitrate” can be explicitly linked to a coordinated ligand it will be written as “nitrato” to differentiate it from nitrate salts, counterion, organo-nitrate, etc.
- (17).Johnson DW; Sutton D The Preparation, properties, and some complexes of anhydrous manganese(III) nitrate. Can. J. Chem. 1972, 50, 3326. [Google Scholar]
- (18).Vankar PS; Reddy MVR; Vankar YD Applications of trimethylsilyl halides-oxidants combinations in organic synthesis. a review. Org. Prep. Proced. Int. 1998, 30, 373. [Google Scholar]
- (19).Eppley HJ; Christou G Synthesis of dodecaoxohexadecacarboxylatotetraaquo-dodecamanganese [Mn12O12(O2CR)16(H2O)4] (R = Me,Et,Ph,Cr) complexes. Inorg. Syn. 2002, 33, 61. [Google Scholar]
- (20).Drummond J; Wood JS Crystal and molecular structure of tetraphenylarsonium tetranitratomanganate(II) and X-ray study of other M(NO3)42− ions (M = Ni, Cu, or Zn). J. Chem. Soc. A 1970, 226, 226. [Google Scholar]
- (21).(a)(b) Uson R; Riera V; Ciriano MA; Valderrama M Pentacoordinate neutral manganese(III) complexes. Transition Met. Chem. 1976, 1, 122. [Google Scholar]
- (22).Wong CL; Kochi JK Electron transfer with organometals. Steric effects as probes for outer-sphere and inner-sphere oxidations of homoleptic alkylmetals with iron(III) and iridate(IV) complexes. J. Am. Chem. Soc. 1979, 101, 5593. [Google Scholar]
- (23).Zhang Y; Raines AJ; Flowers RA II Solvent-dependent chemoselectivities in Ce(IV)-mediated oxidative coupling reactions. Org. Lett. 2003, 5, 2363. [DOI] [PubMed] [Google Scholar]
- (24).Jeong D; Lee Y; Lee Y; Kim K; Cho J Synthesis, characterization, and reactivity of a highly oxidative mononuclear manganese(IV)-bis(fluoro) Complex. J. Am. Chem. Soc. 2024, 146, 4172. [DOI] [PubMed] [Google Scholar]
- (25).Examples of CH nitrooxylation: (a) Li B; Han Y-Q; Yang X; Shi B-F Palladium-catalyzed C(sp3)-H nitrooxylation with tert-butyl nitrate and molecular oxygen. Org. Lett. 2020, 22, 9719–9723. [DOI] [PubMed] [Google Scholar]; (b) Calvo R; Le Tellier A; Nauser T; Rombach D; Nater D; Katayev D Synthesis, characterization, and reactivity of a hypervalent-iodine-based nitrooxylating reagent. Angew. Chem., Int. Ed. 2020, 59, 17162–17168. [DOI] [PubMed] [Google Scholar]
- (26).Agarwal RG; Coste SC; Groff BD; Heuer AM; Noh H; Parada GA; Wise CF; Nichols EM; Warren JJ; Mayer JM Free energies of proton-coupled electron transfer reagents and their applications. Chem. Rev. 2022, 122, 1–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Schorpp M; Rein S; Weber S; Scherer H; Krossing I Guilty and charged: a stable solution of the hexamethylbenzene radical cation as a ligand forming oxidising agent. Chem. Commun. 2018, 54, 10036. [DOI] [PubMed] [Google Scholar]
- (28).Kwon YM; Lee Y; Schmautz AK; Jackson TA; Wang D C-H bond activation by a mononuclear nickel(IV)-nitrate complex. J. Am. Chem. Soc. 2022, 144, 12072. [DOI] [PubMed] [Google Scholar]
- (29).Campbell BM; Gordon JB; Raguram ER; Gonzalez MI; Reynolds KG; Nava M; Nocera DG Electrophotocatalytic perfluoroalkylation by LMCT excitation of Ag(II) perfluoroalkyl carboxylates. Science 2024, 383, 279. [DOI] [PubMed] [Google Scholar]
- (30).Kütt A; Rodima T; Saame J; Raamat E; Mäemets V; Kaljurand I; Koppel IA; Garlyauskayte RY; Yagupolskii YL; Yagupolskii LM; Bernhardt E; Willner H; Leito I Equilibrium acidities of superacids. J. Org. Chem. 2011, 76, 391. [DOI] [PubMed] [Google Scholar]
- (31).Watanabe K Ionization potentials of some molecules. J. Chem. Phys. 1957, 26, 542. [Google Scholar]
- (32).de Petris G; Fornarini S; Crestoni ME; Troiani A; Mayer PM What ion is generated when ionizing acetonitrile? J. Phys. Chem. A 2005, 109, 4425. [DOI] [PubMed] [Google Scholar]
- (33).Malinowski PJ; Himmel D; Krossing I Coordination chemistry of diiodine and implications for the oxidation capacity of the synergistic Ag+/X2(X = Cl, Br, I) system. Angew. Chem., Int. Ed. 2016, 55, 9262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


