Abstract
Purpose
Systemic inflammation biomarkers, derived from routine blood tests, have been demonstrated to be associated with prognosis of patients undergoing peritoneal dialysis (PD). However, studies focusing on the comparisons of their role on predictive efficacy for prognosis of PD patient are limited and results are inconsistent. The purpose of this study was to evaluate the prognostic value of various systemic inflammation biomarkers and to identify the optimal one in PD patients.
Patients and Methods
This longitudinal study involved 3,225 patients undergoing PD across China. The prognostic accuracy of systemic inflammatory biomarkers was evaluated using C-statistics. Independent prognostic biomarkers of outcomes were determined using multivariate Cox proportional hazards regression analysis.
Results
During a 46-month follow-up, 829 (25.7%) patients died, with 458 (55.3%) deaths attributed to cardiovascular disease (CVD). The highest C-statistics were observed for the IBI, with 0.619 and 0.621 for all-cause and CVD mortality, respectively. The optimal threshold of the IBI for predicting prognosis in patients undergoing PD was 50.0. An elevated IBI was a significant independent predictor of all-cause mortality, with a 1-SD increase associated with higher risks of all-cause and CVD mortality. Participants in the upper two quartiles of IBI exhibited increased risks of all-cause mortality by 41.2% and 67.6%, respectively, compared to those in the lowest quartile. Similar results were observed for CVD mortality.
Conclusion
The IBI is a superior prognostic indicator of survival and could be broadly applied for prognosis of patients undergoing PD. Elevated IBI is an independent risk factor for all-cause and CVD mortality.
Keywords: biomarker, peritoneal dialysis, prognosis, systemic inflammation
Introduction
The incidence of end-stage renal disease (ESRD) has surged, becoming a widespread concern globally.1 Peritoneal dialysis (PD), as a crucial therapy option for patients with ESRD, is increasingly favored owing to its minimal staffing needs, modest infrastructure requirements, and patient-centric self-management.2 The Chinese National Renal Data System anticipates that the number of individuals requiring PD will exceed 140,000 by 2022. Nevertheless, patients undergoing PD face significant challenges with suboptimal long-term outcomes, including a 5-year survival rate of 48.4%,3 and a notable rate of cardiovascular disease (CVD) mortality.4 Therefore, a pressing need exists for reliable biomarkers that can predict the prognosis of patients undergoing PD to improve their survival prospects.
Hypertension, protein energy wasting, the peritoneal solute transport rate,5 and overhydration6 are well-recognized risk factors for PD. Systemic inflammation, a pivotal aspect of the microenvironment, plays a crucial role in disease progression and prognosis in patients undergoing PD.7,8 Exposure to glucose-based peritoneal dialysis fluid may elevate the risk of hyperglycemia, which is associated with oxidative stress and inflammation.8 Additionally, the accumulation of uremic toxins and increased vulnerability to infections may contribute to an intensified inflammatory burden.9 A chronic and continuous status of systemic inflammation have been associated with high peritoneal solute transport rate (PSTR) in the early stages of peritoneal dialysis10, and the relationship between systemic inflammation and survival has been consistently reported in published studies, including mortality and cardiovascular death risk.11,12
Serum inflammatory parameters estimated using routine blood test results (eg, neutrophil and lymphocyte counts, platelet counts, and platelet distribution width) can effectively reflect the systemic inflammatory state. Furthermore, several systemic inflammation biomarkers, including inflammatory indicators, metabolic factors, and nutritional values, are crucial for predicting the prognosis of various diseases.13–16 These biomarkers include the platelet-to-lymphocyte ratio (PLR), neutrophil-to-lymphocyte ratio (NLR), and systemic immune inflammation index (SII). Among these biomarkers, the most optimal predictive factor remains uncertain. It is reported that NLR was strongly linked to the new-onset cardiovascular (CV) event and CVD mortality in PD patients under the age of 60.17 While PLR was associated with superior predictive power than NLR in another study.18 Yang et al found that the highest Area Under the Curve (AUC) was obtained for SII in PD patients, followed by systemic inflammation response index (SIRI).19 Notably, the inflammatory burden index (IBI), which represents the homeostatic balance between the inflammatory and immune states, is an emerging biomarker whose prognostic potential for patients undergoing PD has yet to be fully explored.
Therefore, the primary objective of this study was to conduct a comprehensive and systematical comparison in the prognostic value of systemic inflammatory biomarkers, and to identify the optimal biomarker with superior prognostic value for patients undergoing PD. Notably, we further emphasis on the the relationship between IBI and mortality to assess its predictive efficacy for the prognosis of PD patients.
Materials and Methods
Study Participants
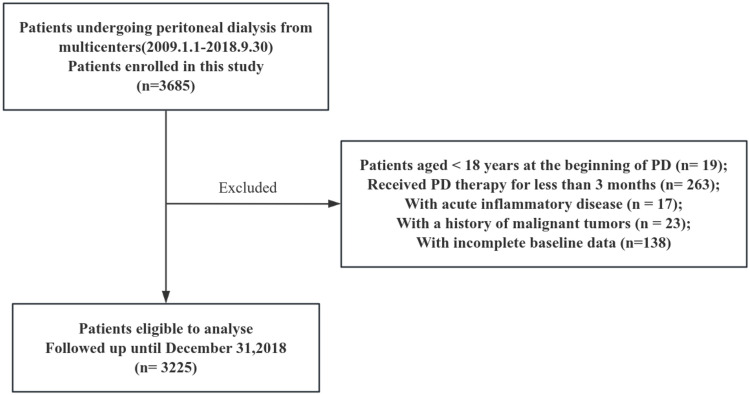
This multicenter, observational cohort study enrolled 3,685 patients undergoing continuous ambulatory peritoneal dialysis (CAPD) at different hospitals in China between January 1, 2009, and September 30, 2018. Patients younger than 18 years at the initiation of PD (n = 19), those who underwent PD for less than 3 months (n = 263), had acute inflammatory disease (as it may lead the instability of clinical laboratory indicators) during the baseline period (n = 17), had a history of malignant tumors (as their potentially limited life expectancy) (n = 23), or had incomplete baseline data (n = 138) were excluded. Ultimately, 3,225 patients were included in the study and followed up until December 31, 2018, or until one of the study endpoints (death, kidney transplantation, transfer to hemodialysis, transfer to other centers, or loss to follow-up) occurred (Figure 1). This study was conducted in accordance with the ethical standards of the Declaration of Helsinki and its later amendments, and it received approval from the Human Ethics Committee.
Figure 1.
Flow diagram of the study.
Data Collection
All baseline demographic and clinical data were collected within the first 3 months after the initiation of PD. Baseline demographic data included age, sex, comorbidities (history of CVD and diabetes mellitus), lifestyle habits (smoking and alcohol consumption), and medication use (eg, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, α-blockers, β-blockers, diuretics, and aspirin). Clinical and laboratory data included body mass index (BMI), systolic and diastolic blood pressure, white blood cell count, hemoglobin level, neutrophil count, monocyte count, lymphocyte count, platelet count, serum albumin level, uric acid level, fasting blood glucose level, total cholesterol level, triglyceride level, serum calcium level, serum phosphorus level, serum intact parathyroid hormone level, C-reactive protein (CRP) level, estimated glomerular filtration rate (eGFR), and total K dialyzer clearance of urea (Kt/V). Systemic inflammation biomarkers were assessed using a range of biochemical or hematological markers measured through routine blood tests, or by deriving their ratios from these measurements.20 In total, 19 systemic inflammation biomarkers were collected and analyzed for their established associations with increased risk of mortality. The IBI was calculated as the CRP level multiplied by the neutrophil level and divided by the lymphocyte level, and detailed measurement of other inflammation markers were provided in Table 1.
Table 1.
Systemic Inflammation Biomarkers Evaluated in This Study
| Biomarkers | Biomarker Formulas |
|---|---|
| C-reactive protein-albumin-lymphocyte index (CALLY) | Albumin (g/dL) ×Lymphocyte(/uL) /CRP (mg/dL) |
| C-reactive protein-to-albumin ratio (CAR) | C-reactive protein (mg/dL)/albumin (g/dL) |
| Glucose to lymphocyte Ratio (GLR) | Glucose (mmol/L)/Lymphocyte(/uL) |
| Inflammatory burden index (IBI) | C-reactive protein (mg/L) ×neutrophil (/uL)/lymphocyte (/uL) |
| Lymphocyte–albumin score (LA) | Lymphocyte (/uL) ×albumin (g/dL) |
| Lymphocyte-to-C-reactive protein ratio (LCR) | Lymphocyte (/uL) / C-reactive protein (mg/L) |
| Monocyte-to-lymphocyte Ratio (MLR) | Monocyte (/uL) / Lymphocyte (/uL) |
| Neutrophil-to-albumin ratio (NAR) | Neutrophil (/uL)/albumin (g/dL) |
| Neutrophil-C-reactive protein score (NC) | Neutrophil (/uL) ×C-reactive protein (mg/L) |
| Neutrophil-to-lymphocyte ratio (NLR) | Neutrophil (/uL) / lymphocyte (/uL) |
| Neutrophil–platelet score (NP) | Neutrophil (/uL) ×platelet (/uL) |
| Platelet-to-albumin ratio (PAR) | Platelet (/uL)/albumin (g/dL) |
| Platelet-C-reactive protein score (PC) | Platelet (/uL) ×C-reactive protein (mg/L) |
| Platelet distribution width to lymphocyte ratio (PDWLR) | Platelet distribution width (%) / lymphocyte (/uL) |
| Platelet distribution width to platelet count ratio (PDWPCR) | Platelet distribution width (%) / Platelet (/uL) |
| Platelet-to-lymphocyte ratio (PLR) | Platelet (/uL) / lymphocyte (/uL) |
| Platelet-to-Neutrophil Ratio (PNR) | Platelet (/uL) / Neutrophil (/uL) |
| Systemic-immune-inflammation index (SII) | Platelet (/uL) ×neutrophil (/uL)/lymphocyte (/uL) |
| Systemic inflammation response index (SIRI) | Neutrophil (/uL) ×Monocyte (/uL) / lymphocyte (/uL) |
Follow-Up
The primary outcomes were all-cause and cardiovascular mortality. CVD mortality was defined according to the following diagnostic codes of the International Classification of Diseases, Tenth Revision: acute myocardial ischemia or infarction (I20 and I21); cardiomyopathy (I42); arrhythmia (I44, I45, I47, I48, and I49); cardiac arrest (I46); congestive heart failure (I50); cerebrovascular accident (I60, I61, I62, I63, and I64); and peripheral vascular disease (I70 and I71).21 All patients were followed up until death, transfer to hemodialysis, kidney transplantation, transfer to other centers, loss to follow-up, or until the study’s end date (December 31, 2018).
Statistical Analysis
Continuous variables are expressed as the mean ± standard deviation (SD) for normally distributed data, or as the median (25–75% interquartile range [IQR]) for non-normally distributed data. Continuous variables were compared using analysis of variance or the Kruskal–Wallis test, while categorical variables were compared using the χ2 test or Fisher’s exact test. The predictive accuracy of systemic inflammatory biomarkers for determining the prognosis of patients undergoing PD was assessed using C-statistics. The optimal threshold for IBI was determined using maximally selected log-rank statistics in R version 4.3.3.22 Participants were categorized into four groups based on IBI quartiles. Spearman correlation was used to assess the relationship between IBIs and other clinical characteristics. Kaplan–Meier curves were generated to compare survival among the four groups. Univariate and multivariate Cox proportional hazards regression models were used to examine associations and identify independent prognostic factors influencing outcomes. A restricted cubic spline curve was created to determine the relationship between IBI and survival in patients undergoing PD. The groups were stratified by age, sex, BMI, smoking, history of diabetes mellitus, and history of CVD after adjusting for multiple variables, and subgroup analyses were performed. The interaction P-values correspond to the interaction between IBI and the subgroup variables of interest. Internal cohort validation was used to further validate the association between IBI and survival in patients undergoing PD. Results are reported as hazard ratios (HRs) with 95% confidence intervals (CIs). Statistical analyses were conducted using the Statistical Package for Social Science version 26.0 (IBM, Armonk, NY, USA) and Empower® (www.empowerstats.com; X&Y Solutions, Inc., Boston, MA, USA). P-values < 0.05 were considered significant.
Results
Participant Characteristics
A total of 3,225 patients were enrolled in this study, including 1,435 (44.5%) women and 1,790 (55.5%) men, with a mean age of 52.6 ± 14.8 years. Among them, 34.2% had diabetes and 29.3% had a history of CVD. During a mean follow-up of 46.00 ± 27.07months, 829 (25.7%) patients died, with 458 (55.3%) deaths attributed to CVD.
Comparison of Serum Systemic Inflammation Biomarkers
Systemic inflammatory biomarkers were independently associated with all-cause and CVD mortality in this cohort (Table 2, Figure S1). Furthermore, the highest C-statistics were observed for the IBI, with 0.619 (0.597–0.642) for all-cause mortality and 0.621 (0.594–0.648) for CVD mortality. Pairwise comparisons of the C-statistics indicated that the IBI had superior performance over other systemic inflammatory biomarkers, suggesting its potential as a reliable prognostic indicator of systemic inflammation. Therefore, the potential value of the IBI as a prognostic biomarker was further assessed.
Table 2.
Comparative Analysis of the Discrimination of Each Systemic Inflammation-Related Biomarkers for All-Cause and CVD Mortality in PD Patients
| Discrimination ability | All-Cause Mortality | CVD Mortality | ||||
|---|---|---|---|---|---|---|
| C-Statistic | Difference | p | C-Statistic | Difference | p | |
| IBI | 0.619 (0.597,0.642) | Ref | 0.621 (0.594,0.648) | Ref | ||
| CALLY | 0.614 (0.592,0.637) | −0.003 (−0.011,-0.004) | <0.001 | 0.612 (0.585,0.640) | −0.007 (−0.016,0.003) | 0.155 |
| LCR | 0.606 (0.584,0.629) | −0.011 (−0.018,-0.004) | 0.002 | 0.608 (0.580,0.635) | −0.011 (−0.020,-0.003) | 0.011 |
| NC | 0.603 (0.581,0.625) | −0.017 (−0.025,-0.008) | <0.001 | 0.596 (0.569,0.623) | −0.025 (−0.036,-0.014) | <0.001 |
| CAR | 0.601 (0.579,0.624) | −0.018 (−0.029,-0.007) | 0.001 | 0.590 (0.562,0.617) | −0.031 (−0.045,-0.017) | <0.001 |
| PC | 0.599 (0.577,0.621) | −0.021 (−0.033,-0.009) | 0.001 | 0.590 (0.563,0.617) | −0.031 (−0.047,-0.015) | <0.001 |
| SIRI | 0.593(0.570,0.616) | −0.026 (−0.052,-0.001) | 0.041 | 0.590 (0.562,0.619) | −0.031 (−0.061,0.000) | 0.051 |
| SII | 0.590 (0.567,0.614) | −0.029 (−0.055,-0.004) | 0.026 | 0.603 (0.574,0.631) | −0.018 (−0.048,0.12) | 0.245 |
| NAR | 0.588 (0.565,0.611) | −0.031 (−0.057,-0.006) | 0.016 | 0.574 (0.546,0.602) | −0.047 (−0.078,-0.016) | 0.003 |
| NLR | 0.579 (0.556,0.602) | −0.040 (−0.064,-0.017) | 0.001 | 0.603 (0.574,0.632) | −0.018 (−0.047,0.011) | 0.225 |
| LA | 0.578 (0.555,0.601) | −0.042 (−0.068,-0.015) | 0.002 | 0.595 (0.567,0.623) | −0.026 (−0.058,0.007) | 0.118 |
| MLR | 0.577 (0.553,0.600) | −0.043 (−0.071,-0.015) | 0.002 | 0.573 (0.544,0.602) | −0.047 (−0.081,-0.013) | 0.006 |
| PAR | 0.574 (0.551,0.598) | −0.045 (−0.077,-0.013) | 0.005 | 0.556 (0.526,0.586) | −0.065 (−0.104,-0.026) | 0.001 |
| PLR | 0.571 (0.548,0.594) | −0.049 (−0.076,-0.021) | 0.001 | 0.594 (0.566,0.622) | −0.027 (−0.060,0.007) | 0.119 |
| GLR | 0.570 (0.547,0.593) | −0.049 (−0.079,-0.020) | 0.001 | 0.591 (0.563,0.619) | −0.030 (−0.065,0.006) | 0.101 |
| NP | 0.568 (0.545,0.591) | −0.051 (−0.080,-0.023) | <0.001 | 0.560 (0.531,0.589) | −0.061 (−0.095,-0.026) | 0.001 |
| PDWLR | 0.556 (0.528,0.585) | −0.075 (−0.108,-0.042) | <0.001 | 0.580 (0.545,0.615) | −0.045 (−0.087,-0.004) | 0.034 |
| PNR | 0.526 (0.503,0.549) | −0.094 (−.012,-0.067) | <0.001 | 0.526 (0.497,0.555) | −0.095 (−0.129,-0.061) | <0.001 |
| PDWPCR | 0.518 (0.489,0.546) | −0.113 (−0.154,-0.073) | <0.001 | 0.503 (0.467,0.540) | −0.121 (−0.172,-0.071) | <0.001 |
Abbreviations: IBI, inflammatory burden index (C-reactive protein–neutrophil–lymphocyte ratio); CALLY, C-reactive protein-albumin-lymphocyte index; LCR, Lymphocyte-to-C-reactive protein ratio; NC, Lymphocyte C-reactive protein score; CAR, C-reactive protein-to-albumin ratio; PC, Platelet-C-reactive protein score; SIRI, Systemic inflammation response index; SII, Systemic-immune-inflammation index; NAR, Neutrophil-to-albumin ratio; NLR, Neutrophil-to-lymphocyte ratio; LA, Lymphocyte–albumin score; MLR, Monocyte-to-lymphocyte Ratio; PAR, Platelet-to-albumin ratio; PLR, Platelet-to-lymphocyte ratio; GLR, Glucose to lymphocyte Ratio; NP, Neutrophil–platelet score; PDWLR, Platelet distribution width to lymphocyte ratio; PNR, Platelet-to-Neutrophil Ratio; PDWPCR, Platelet distribution width to platelet count ratio.
Distribution of Baseline IBI Levels and Factors Associated with the IBI
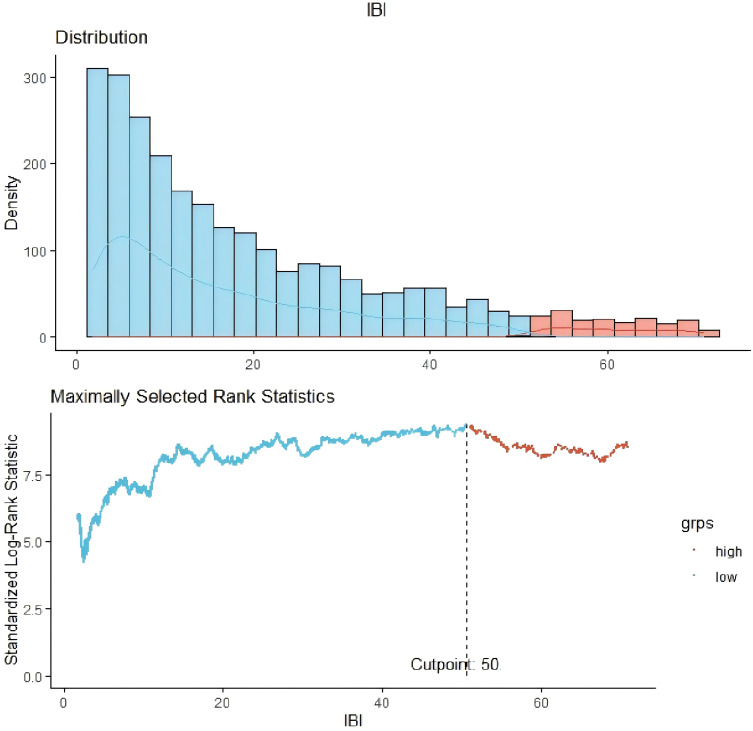
We observed an abnormal distribution of baseline IBIs across the study population (Table 3), with a median value of 13.95. According to the C-statistics, the optimal threshold of the IBI for predicting prognosis in patients undergoing PD was 50.0 (Figure 2). It revealed positive associations between IBI levels and age, BMI, systolic blood pressure, and triglyceride levels, while negative correlations were observed between IBI and hemoglobin, albumin, and serum calcium levels (all P < 0.05; Table 4).
Table 3.
Baseline Characteristics of the Study Participants Stratified by IBI Quartile
| Variables | Total (n = 3225) | Q1 | Q2 | Q3 | Q4 | p value |
|---|---|---|---|---|---|---|
| ≤4.99 | 4.99–13.95 | 13.95–34.12 | ≥34.12 | |||
| (n = 806) | (n = 806) | (n = 807) | (n = 806) | |||
| Sex, female (%) | 1435 (44.5) | 390 (48.4) | 380 (47.1) | 339 (42) | 326 (40.4) | 0.002* |
| Age (years) | 52.6 ± 14.8 | 49.5 ± 14.5 | 52.1 ± 14.1 | 52.5 ± 14.9 | 56.1 ± 14.9 | < 0.001* |
| BMI (kg/m2) | 22.3 ± 3.4 | 21.8 ± 3.2 | 22.4 ± 3.5 | 22.5 ± 3.5 | 22.5 ± 3.4 | < 0.001* |
| Smoking, n (%) | 276 (8.6) | 55 (6.8) | 51 (6.3) | 76 (9.4) | 94 (11.7) | <0.001* |
| Alcohol consumption, n (%) | 75 (2.3) | 10 (1.2) | 16 (2) | 24 (3) | 25 (3.1) | 0.042* |
| Diabetes, n (%) | 1103 (34.2) | 263 (32.6) | 287 (35.6) | 277 (34.3) | 276 (34.2) | 0.660 |
| CVD, n (%) | 944 (29.3) | 205 (25.4) | 226 (28) | 246 (30.5) | 267 (33.1) | 0.005* |
| Systolic pressure (mmHg) | 146.1 ± 23.7 | 143.9 ± 21.6 | 146.2 ± 24.0 | 146.7 ± 24.0 | 147.7 ± 25.0 | 0.012* |
| Diastolic pressure (mmHg) | 85.2 ± 15.3 | 85.0 ± 14.7 | 85.0 ± 15.0 | 85.6 ± 15.3 | 85.0 ± 16.1 | 0.782 |
| Medication | ||||||
| CCB, n (%) | 2027 (62.9) | 501 (62.2) | 511 (63.4) | 511 (63.3) | 504 (62.5) | 0.944 |
| ACEI, n (%) | 781 (24.2) | 205 (25.4) | 180 (22.3) | 204 (25.3) | 192 (23.8) | 0.428 |
| ARB, n (%) | 936 (29.0) | 246 (30.5) | 229 (28.4) | 242 (30) | 219 (27.2) | 0.435 |
| β-blocker, n (%) | 1112 (34.5) | 285 (35.4) | 275 (34.1) | 295 (36.6) | 257 (31.9) | 0.235 |
| ɑ-blocker, n(%) | 670 (20.8) | 187 (23.2) | 163 (20.2) | 171 (21.2) | 149 (18.5) | 0.128 |
| Diuretic, n(%) | 175 (5.4) | 42 (5.2) | 40 (5) | 51 (6.3) | 42 (5.2) | 0.628 |
| Aspirin, n (%) | 236 (7.3) | 61 (7.6) | 53 (6.6) | 60 (7.4) | 62 (7.7) | 0.823 |
| Laboratory variables | ||||||
| White blood cell (10^9/L) | 6.79 ± 2.34 | 6.20 ± 1.89 | 6.52 ± 2.18 | 6.71 ± 2.03 | 7.70 ± 2.87 | < 0.001* |
| Platelet (10^9/L) | 203.8 ± 70.1 | 204.1 ± 65.9 | 200.9 ± 66.5 | 201.8 ± 70.5 | 208.4 ± 76.7 | 0.143 |
| Hemoglobin (g/L) | 96.4 ± 19.8 | 98.6 ± 21.0 | 95.7 ± 18.6 | 96.4 ± 20.0 | 94.9 ± 19.3 | 0.001* |
| Neutrophils (10^9/L) | 4.62 ± 2.02 | 3.89 ± 1.54 | 4.35 ± 1.79 | 4.56 ± 1.70 | 5.67 ± 2.50 | < 0.001* |
| Monocytes (10^9/L) | 0.50 ± 0.26 | 0.49 ± 0.29 | 0.48 ± 0.25 | 0.50 ± 0.25 | 0.53 ± 0.25 | 0.006* |
| Lymphocytes (10^9/L) | 1.31 ± 0.53 | 1.45 ± 0.50 | 1.36 ± 0.54 | 1.31 ± 0.53 | 1.14 ± 0.52 | < 0.001* |
| Glucose (mmol/L) | 5.43 ± 2.14 | 5.23 ± 1.81 | 5.36 ± 2.25 | 5.45 ± 2.16 | 5.67 ± 2.29 | < 0.001* |
| Albumin (g/L) | 34.8 ± 5.6 | 36.0 ± 5.6 | 34.9 ± 5.5 | 34.6 ± 5.5 | 33.7 ± 5.5 | < 0.001* |
| Uric acid (mmol/L) | 416.7 ± 113.8 | 412.8 ± 110.6 | 421.3 ± 117.2 | 412.5 ± 112.3 | 420.0 ± 115.0 | 0.255 |
| Cholesterol (mmol/L) | 4.65 ± 1.34 | 4.70 ± 1.34 | 4.63 ± 1.32 | 4.65 ± 1.38 | 4.60 ± 1.30 | 0.504 |
| Triglyceride (mmol/L) | 1.70 ± 1.37 | 1.64 ± 1.31 | 1.66 ± 1.29 | 1.79 ± 1.54 | 1.71 ± 1.31 | 0.151 |
| Calcium (mmol/L) | 2.11 ± 0.28 | 2.14 ± 0.28 | 2.10 ± 0.26 | 2.11 ± 0.28 | 2.08 ± 0.30 | < 0.001* |
| Phosphorus (mmol/L) | 1.65 ± 0.56 | 1.67 ± 0.55 | 1.66 ± 0.53 | 1.65 ± 0.54 | 1.63 ± 0.60 | 0.619 |
| Intact Parathyroid hormone (pg/mL) | 326.3 ± 259.2 | 343.5 ± 278.9 | 333.6 ± 268.6 | 315.8 ± 243.2 | 312.4 ± 243.6 | 0.049* |
| CRP (mg/L) | 6.66 ± 7.23 | 0.95 ± 0.71 | 3.06 ± 1.74 | 7.07 ± 3.77 | 15.53 ± 8.18 | < 0.001* |
| eGFR (mL/min/1.73m2) | 6.82 ± 3.08 | 6.71 ± 3.05 | 6.81 ± 3.15 | 6.84 ± 3.07 | 6.93 ± 3.05 | 0.554 |
| Kt/V | 2.13 ± 0.59 | 2.17 ± 0.55 | 2.15 ± 0.60 | 2.09 ± 0.61 | 2.11 ± 0.59 | 0.034* |
Note: Continuous variables are shown as the mean ± SD or median (IQR). Bold formatting and *: p < 0.05.
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; CCB, calcium channel blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRP, C-reactive protein; eGFR, estimated glomerular; Kt/V, K dialyzer clearance of urea.
Figure 2.
Cut-off of the inflammatory burden index in patients undergoing peritoneal dialysis.
Table 4.
Spearman Correlation Analysis Between IBI and Clinical Parameters
| r | p value | |
|---|---|---|
| Sex, female (%) | −0.075 | <0.001 |
| Age(years) | 0.161 | <0.001 |
| BMI (kg/m2) | 0.077 | <0.001 |
| Smoking | 0.073 | <0.001 |
| Alcohol consumption | 0.037 | 0.037 |
| Diabetes | 0.013 | 0.446 |
| CVD | 0.063 | <0.001 |
| Systolic pressure (mmHg) | 0.07 | <0.001 |
| Diastolic pressure (mmHg) | 0.006 | 0.719 |
| Hemoglobin (g/L) | −0.06 | 0.001 |
| Albumin (g/L) | −0.107 | <0.001 |
| Calcium (mmol/L) | −0.08 | <0.001 |
| Phosphorus (mmol/L) | −0.029 | 0.103 |
| Intact Parathyroid hormone (pg/mL) | −0.033 | 0.063 |
| Cholesterol (mmol/L) | −0.028 | 0.109 |
| Triglyceride (mmol/L) | 0.035 | 0.047 |
| eGFR (mL/min/1.73m2) | 0.023 | 0.19 |
| Kt/V | −0.024 | 0.197 |
| CCB, n (%) | 0.006 | 0.731 |
| ACEI, n (%) | −0.004 | 0.838 |
| ARB, n (%) | −0.021 | 0.235 |
| β-blocker, n (%) | −0.015 | 0.386 |
| ɑ-blocker, n(%) | −0.026 | 0.142 |
| Diuretic, n(%) | 0.003 | 0.85 |
| Aspirin, n (%) | 0.002 | 0.912 |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; eGFR, estimated glomerular; Kt/V, K dialyzer clearance of urea; CCB, calcium channel blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Association Between Baseline IBI and All-Cause and CVD Mortality
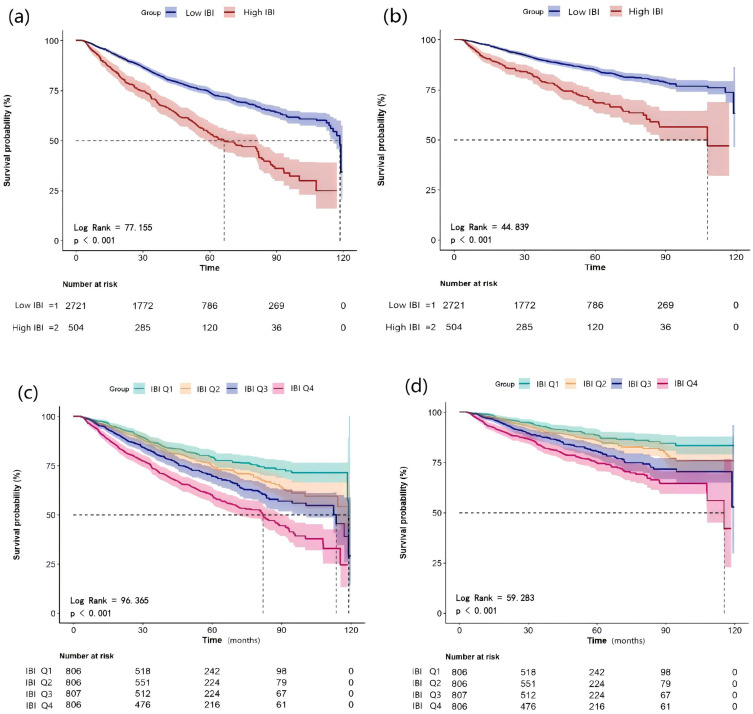
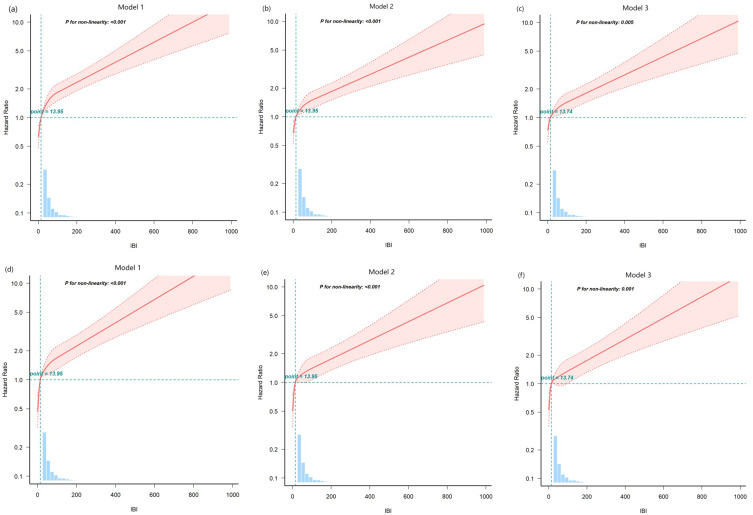
Our study indicated that the cumulative rates of overall survival (Figure 3a and c) and CVD mortality-free survival (Figure 3b and d) decreased with increasing IBI levels (all log-rank P < 0.001). Both univariate and multivariate restricted cubic spline curves demonstrated a declining survival rate with increasing IBI levels in patients undergoing PD, including both all-cause and CVD mortality (all P < 0.05; Figure 4).
Figure 3.
Kaplan–Meier curves of all-cause and CVD mortality stratified by IBI. Cumulative mortality curves for all-cause mortality (a) and cardiovascular mortality (b) according to the IBI cut-off value. Cumulative mortality curves for all-cause mortality (c) and cardiovascular mortality (d) according to quartiles of IBI.
Abbreviations: CVD, cardiovascular disease; IBI, inflammatory burden index.
Figure 4.
Association between inflammatory burden index and overall survival in patients. A restricted cubic spline curve for all-cause mortality (a–c) and cardiovascular mortality (d–f). Model 1: Unadjusted. Model 2: Adjusted for age, sex, BMI, systolic pressure, diastolic pressure, DM, and CVD history. Model 3: Model 2 plus smoking, alcohol consumption, medication (CCB, ACEI, ARB, β-blocker, ɑ-blocker, diuretic, aspirin), PLT, HB, UA, ALB, TC, TG, calcium, phosphorus, iPTH, eGFR, and Kt/V.
Abbreviations: BMI, body mass index; DM, diabetes mellitus, CVD, cardiovascular disease.
In Cox proportional hazard regression models, the unadjusted model (model 1) showed a significant association between the baseline IBI level (as a continuous variable) and an increased risk of poor survival (Table 5), which was further verified using age-, sex-, and covariate-adjusted models. Each 1-SD increase in the IBI was independently associated with a 15.5% (95% CI: 1.113–1.198) increased risk of all-cause mortality and a 17.5% (95% CI: 1.122–1.230) increased risk of CVD mortality. When assessed categorically (≥ cutoff vs < cutoff), the IBI remained an independent risk factor for adverse outcomes, including all-cause mortality (HR: 1.724; 95% CI: 1.455–2.042) and CVD mortality (HR: 1.887; 95% CI: 1.512–2.357). Using the lowest quartile group (Q1) as a reference, the risk of adverse outcomes increased progressively in the Q3 and Q4 groups, with HRs of 1.412 (95% CI: 1.129–1.767) and 1.676 (95% CI: 1.350–2.081), respectively, for all-cause mortality. Similar trends were observed for CVD mortality. The prognostic value of other systemic inflammation-related biomarkers in the prognosis of patients undergoing PD is shown in Table S1.
Table 5.
Associations of IBI and IBI Categories with All-Cause and CVD Mortality. (Cox Regression Analysis)
| Model 1 | p value | Model 2 | p value | Model 3 | p value | |
|---|---|---|---|---|---|---|
| All-cause mortality | ||||||
| Continuous (per SD) | 1.203(1.169,1.239) | <0.001 | 1.158(1.121,1.196) | <0.001 | 1.155(1.113,1.198) | <0.001 |
| Cut-off value | ||||||
| C1(<50) | Ref | Ref | Ref | |||
| C2(≥50) | 2.132(1.1.825,2.492) | <0.001 | 1.775(1.514,2.079) | <0.001 | 1.724(1.455,2.042) | <0.001 |
| Quartiles | ||||||
| Q1(<4.99) | Ref | Ref | Ref | |||
| Q2(4.99–13.95) | 1.277(1.022,1.595) | 0.315 | 1.184 (0.947,1.480) | 0.139 | 1.133 (0.897,1.431) | 0.296 |
| Q3(13.95–34.12) | 1.624(1.312,2.010) | 0.001 | 1.466 (1.183,1.817) | <0.001 | 1.412 (1.129,1.767) | 0.003 |
| Q4(>34.12) | 2.438(1.995,2.980) | <0.001 | 1.869 (1.524,2.293) | <0.001 | 1.676 (1.350,2.081) | <0.001 |
| CVD mortality | ||||||
| Continuous (per SD) | 1.216(1.173,1.261) | <0.001 | 1.164(1.119,1.211) | <0.001 | 1.175(1.122,1.230) | <0.001 |
| Cut-off value | ||||||
| C1(<50) | Ref | Ref | Ref | |||
| C2(≥50) | 2.301(1.876,2.823) | <0.001 | 1.915(1.555,2.358) | <0.001 | 1.887(1.512,2.357) | <0.001 |
| Quartiles | ||||||
| Q1(<4.99) | Ref | Ref | Ref | |||
| Q2(4.99–13.95) | 1.334(0.981,1.013) | 0.066 | 1.235 (0.907,1.680) | 0.18 | 1.228 (0.890,1.694) | 0.211 |
| Q3(13.95–34.12) | 1.888(1.414,2.520) | <0.001 | 1.714 (1.283,2.291) | <0.001 | 1.670 (1.232,2.265) | 0.001 |
| Q4(>34.12) | 2.605(1.976,3.434) | <0.001 | 2.036 (1.538,2.696) | <0.001 | 1.884 (1.398,2.538) | <0.001 |
Notes: Model 1: unadjusted. Model 2: adjusted for age, sex, BMI, systolic pressure, diastolic pressure, DM, and CVD history. Model 3: Model 2 plus smoking, alcohol consumption, medication (CCB, ACEI, ARB, β-blocker, ɑ-blocker, Diuretic, Aspirin), PLT, HB, UA, ALB, TC, TG, calcium, phosphorus, iPTH, eGFR and Kt/V.
Abbreviations: IBI, inflammatory burden index; BMI, body mass index; DM,diabetes; CVD, cardiovascular disease; CCB, calcium channel blocker; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; PLT, platelet; HB, hemoglobin; UA, Uric acid; ALB, albumin; TC, cholesterol; TG, triglyceride; iPTH, intact Parathyroid hormone; eGFR, estimated glomerular; Kt/V, K dialyzer clearance of urea.
Subgroup and Interaction Analyses
Multivariable subgroup analysis showed that higher IBI was an independent risk factor for all-cause and CVD mortality across most subgroups, indicating a stable correlation between IBI and outcomes. Statistically significant interactions were observed between IBI, age, and CVD history for both all-cause and CVD mortality (P < 0.05; Figure S2).
Internal Cohort Validation
To further verify the association between IBI and the prognosis of patients undergoing PD, the study population was randomly divided into two validation cohorts at a 7:3 ratio (cohort A: n = 2,257 and cohort B: n = 968). A comparison of clinical characteristics confirmed a good fit between the two cohorts (Table S2). Multivariable analysis revealed that IBI was an independent factor influencing the prognosis of patients undergoing PD in validation cohort A (HR: 1.145; 95% CI: 1.098–1.193; P < 0.001, Table S3) and validation cohort B (HR: 1.278; 95% CI: 1.132–1.442; P < 0.001, Table S4).
Discussion
In this large-scale multicenter study involving 3,225 patients undergoing PD, we investigated the association between systemic inflammatory biomarkers and prognosis. Our findings indicated a positive relationship between the IBI and adverse prognosis, even after accounting for key prognostic factors. The IBI emerged as a potential target for therapeutic interventions aimed at systemic inflammation. Notably, among the 19 systemic inflammatory biomarkers, the IBI exhibited the highest predictive accuracy for the prognosis of patients undergoing PD.
Despite rapid technological advancements in PD, mortality rates remain high, with CVD mortality being particularly concerning. We observed mortality and CVD mortality rates of 25.71% and 55.25%, respectively, which are consistent with previous research findings. Systemic inflammation plays a crucial role in the poor prognosis of patients undergoing PD.23 The pathogenesis of chronic systemic inflammation is intricate and multifaceted, involving both disease- and dialysis-related factors, such as high levels of uremic toxins, inadequate dialysis, catheter-associated chronic irritation, contamination of the dialysis solution, release of plastic materials, and exposure to glucose-based dialysis solutions.24
Emerging evidence underscores the significant role of systemic inflammation in the progression of ESRD and its association with poor prognosis in patients undergoing PD. Zeng et al identified a link between the NLR and cardiovascular outcomes among 1,652 patients undergoing PD, suggesting that individuals in the highest NLR tertile were at increased risk of cardiovascular events.17 Wen et al performed a multicenter retrospective study involving 1,753 patients and reported that a higher monocyte-to-lymphocyte ratio (MLR) was independently associated with CVD mortality.25 Chen et al found that an increased PLR was an independent risk factor for CVD among patients undergoing PD, outperforming the predictive power of the NLR.18 Moreover, Li et al and Tang et al demonstrated that both the systemic inflammation response index (SIRI) and the SII were promising indicators of prognosis in patients undergoing PD, with predictive power comparable to that of the NLR and MLR.26,27 Our study confirmed that most systemic inflammatory biomarkers were independently associated with all-cause and CVD mortality. Among these biomarkers, the IBI exhibited superior prognostic value, with the highest C-statistics (0.619 for all-cause mortality and 0.621 for CVD mortality).
The IBI, a novel biomarker that comprehensively reflects the inflammatory status, has been proposed as a prognostic indicator in various populations. Song et al systemically evaluated the prognostic ability of inflammatory indicators in patients with primary hepatocellular carcinoma and identified the IBI as the optimal predictor (area under the curve: 0.698), with superior prognostic value compared to other traditional inflammatory markers such as the NLR, PLR, and SII.28 Xie et al investigated the relationship between the IBI and survival in patients with non-small-cell lung cancer and reported that the IBI was superior among systemic inflammation-related biomarkers.22 Additionally, a study from the National Health and Nutrition Examination Survey (NHANES) involving 8,827 participants indicated that higher IBIs were associated with a higher risk of all-cause mortality among individuals aged 45 years or older in the general population.29 Our results revealed that an IBI threshold of 50.0 was optimal for predicting outcomes in patients undergoing PD. Patients with high IBIs were at significantly higher risk of poor outcomes, as shown by a declining survival rate with increasing IBI levels. Internal validation cohorts further confirmed the reproducibility and efficacy of the IBI for assessing the prognosis of patients undergoing PD.
Compared to the general population, the level of IBI tended to be much higher in PD patients.30 The advantage of the IBI over other prognostic biomarkers may stem from its ability to measure the balance between immune regulation and persistent inflammatory responses by integrating peripheral hematological biomarkers with CRP, neutrophils, and lymphocytes. CRP, synthesized by the liver, is an acute-phase protein produced in response to inflammatory stimuli, tissue damage, or malignant neoplasia, and is also known for precipitating the somatic C-polysaccharide of Streptococcus pneumoniae.31 Increased CRP levels observed in PD patients are probably due to the inflammatory reaction being exaggerated by both peritoneal irritation and less removal of cytokines due to uremic status.32 Besides, exposure to non-biocompatible or glucose-based dialysis solutions, have been proved to induce microenvironmental hypoxia and activate chronic inflammation.33 CRP is associated with cardiovascular risk factors such as stroke, myocardial infarction, sudden death, and peripheral vascular disease by inducing endothelial cell dysfunction and activating monocytes.34 Neutrophils are the first cell type to respond to inflammatory stimuli and may activate the vascular endothelium and migrate toward inflammatory foci. During this multistep process, activated neutrophils release various mediators, most of which contribute to atherosclerosis by stimulating platelet adhesion, thereby boosting monocyte recruitment and promoting the activation of macrophages and endothelial cells.14,35 However, an imbalance in lymphocyte metabolism, delayed hypersensitivity response to various antigens, and diminished lymphocyte proliferation are widespread issues among patients undergoing PD.36 Therefore, the elevated level of neutrophils to lymphocytes ratio (NLR), may associated with the progression of atherosclerosis and adverse cardiovascular outcomes in PD patients.37 Combined with CRP and NLR, IBI shown a more significant advantage in predicting prognosis in PD patients.
Based on the adverse impact of systemic inflammation, several potential therapeutic strategies may be actualized to reduce inflammatory burden in PD patients: (1) Avoid nephrotoxic drugs and use biocompatible PD fluids to preserve kidney function and reduce inflammation. (2) Use prebiotics, probiotics, and symbiotics to maintain gut health and decrease systemic inflammation. (3) Optimize fluid status and avoid overhydration. (4)Prevent infections related to catheters and peritonitis.24 In addition, both ACEI and ARBs are known for their anti-inflammatory effects beyond their blood pressure-lowering actions.38 An overall pooled analysis suggested that both of them could significantly reduce markers of inflammation such as CRP and nuclear factor-kappaB (NF-κB), which are associated with cardiovascular risk. They also limit endothelial dysfunction and vascular inflammation, which are key factors in the development of atherosclerosis and other cardiovascular diseases. However, no correlations were observed between IBI level and the use of ACEI and ARBs in our study. Notwithstanding, It is noteworthy that only baseline use of medications were collected in our study, which restricts the analysis of casual effect of certain medications on systemic inflammation.
To our knowledge, this is the first large-scale study to systematically and comprehensively evaluate the prognostic value of hematological systemic inflammation biomarkers in patients undergoing PD. The multicenter design is a crucial aspect of this study, as it enriches the diversity and generalizability of our cohort, culminating in findings that are both robust and broadly applicable. Our findings highlight that the IBI is the most effective systemic inflammation biomarker for patients undergoing PD. Furthermore, we established an optimal IBI threshold of 50.0 for prognostic assessment. These results deepen our understanding of the relationship between systemic inflammation and survival in patients undergoing PD and offer valuable insights for using systemic inflammation biomarkers in prognostic assessments, treatment efficacy predictions, and follow-up of such patients.
Limitations
However. this study has several limitations that warrant consideration. First, due to its observational design, causal interpretations cannot be drawn. Some unmeasured confounders, such as the data of use of prebiotics, probiotics, or symbiotics, and the infections related to catheters and peritonitis during the follow-ups were unavailable, which may limite the causal inference. Also, the collection of baseline medication use may restrict the analysis of casual effect of certain medications on systemic inflammation. Future studies aimed at exploring this casual effect were needed. In addition, dynamic monitoring of IBI changes during follow-up remains necessary and warrants further investigation. Third, the selection bias of a single-center study was not a concern, potential biases caused by variations in treatment protocols among the centers could not be entirely ruled out. Although internal cohort validation was performed to avoid overoptimistic estimates of the model’s predictive accuracy, the generalizability of our findings is constrained, due to the study’s selection limited in China’s tertiary hospitals, including the unique healthcare system, economic factors, population characteristics and medical practice. The complex interaction among these suggests that our results may not be readily applicable to other countries and regions. Therefore, an external validation about the predictive value of IBI in other regions will be necessary to enhance the study’s generalization.
Conclusion
The results of this study demonstrate that the IBI is an inexpensive and easily accessible marker, which may be a superior biomarker of systemic inflammation in patients undergoing PD. A higher IBI was identified as an independent predictor of all-cause and CVD mortality, suggesting that the IBI represents a novel and effective prognostic tool for widespread application in patients undergoing PD. Although the results of this study were internally validated, further external validation is needed, particularly among other ethnic groups. Future studies should employ stricter inclusion criteria, larger sample sizes, and prospective designs.
Acknowledgments
All authors are responsible for the reliability and unbiased presentation of the data. We extend our gratitude to the staff directly involved in this cohort study for their meticulous work in patient care and data collection.
Funding Statement
This work was supported by the Project of Jiangmen Science and Technology Bureau, China. (2024YL01002, 2024YL01004) and the Key Project of Jiangmen Basic and Applied Basic Research (No.2320002000884).
Ethics Approval and Consent to Participate
This study was conducted in accordance with the ethical standards of the Helsinki Declaration and its later amendments. The study protocol was approved by the Human Ethics Committee. Considering the retrospective nature of the study, the Human Ethics Committee of Jiangmen Central Hospital [2022, NO.101] waived the need for informed consent. All clinical data were obtained from pre-existing datasets at the hospital. The confidentiality and security of subjects’ personal information are guaranteed.
Disclosure
The author(s) report no conflicts of interest in this work.
References
- 1.Vogt B, Painter DF, Saad Berreta R, et al. Hospitalization in maintenance peritoneal dialysis: a review. Hosp Pract. 2023;51(1):18–28. doi: 10.1080/21548331.2023.2170613 [DOI] [PubMed] [Google Scholar]
- 2.Briggs V, Davies S, Wilkie M. International variations in peritoneal dialysis utilization and implications for practice. Am J Kidney Dis. 2019;74(1):101–110. doi: 10.1053/j.ajkd.2018.12.033 [DOI] [PubMed] [Google Scholar]
- 3.Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol. 2017;13(2):90–103. doi: 10.1038/nrneph.2016.181 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Zhang X, Xue S. Left heart failure caused by capacity overload in peritoneal dialysis patients. BioMed Res Int. 2022;2022:5422333. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Janda K, Krzanowski M, Dumnicka P, et al. Peritoneal solute transport rate as an independent risk factor for total and cardiovascular mortality in a population of peritoneal dialysis patients. In: Advances in peritoneal dialysis Conference on Peritoneal Dialysis; 2014. 30:15–20. [PubMed] [Google Scholar]
- 6.Krediet RT, Balafa O. Cardiovascular risk in the peritoneal dialysis patient. Nat Rev Nephrol. 2010;6(8):451–460. doi: 10.1038/nrneph.2010.68 [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, He J, Zhang F, et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol. 2013;26(2):243–253. doi: 10.5301/jn.5000169 [DOI] [PubMed] [Google Scholar]
- 8.Cho Y, Hawley CM, Johnson DW. Clinical causes of inflammation in peritoneal dialysis patients. Int J Nephrol. 2014;2014:909373. doi: 10.1155/2014/909373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai L, Golembiewska E, Lindholm B, et al. End-stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. doi: 10.1159/000479254 [DOI] [PubMed] [Google Scholar]
- 10.Pecoits-Filho R, de Moraes TP. Dialysis: systemic IL-6 levels predict survival after peritoneal dialysis. Nature Reviews Nephrology. 2013;9(12):708–710. doi: 10.1038/nrneph.2013.231 [DOI] [PubMed] [Google Scholar]
- 11.Lambie M, Chess J, Donovan KL, et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol. 2013;24(12):2071–2080. doi: 10.1681/ASN.2013030314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang AY, Wang M, Woo J, et al. Inflammation, residual kidney function, and cardiac hypertrophy are interrelated and combine adversely to enhance mortality and cardiovascular death risk of peritoneal dialysis patients. J Am Soc Nephrol. 2004;15(8):2186–2194. doi: 10.1097/01.ASN.0000135053.98172.D6 [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Pan Y, Sheng Y, et al. Relationship between PLR and clinicopathological characteristics of patients with advanced NSCLC and its predictive value for the efficacy of chemotherapy and prognosis. Emerg Med Int. 2022;2022:5811219. doi: 10.1155/2022/5811219 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Chen C, Cong BL, Wang M, et al. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integrat Med Res. 2018;7(2):192–199. doi: 10.1016/j.imr.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian X, He J, Zhang R, et al. The combined effect of systemic immune-inflammation index and type 2 Diabetes Mellitus on the prognosis of patients undergoing percutaneous coronary intervention: a large-scale cohort study. J Inflamm Res. 2023;16:6415–6429. doi: 10.2147/JIR.S445479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu Y, Zhong Z, Yang W, et al. Neutrophil percentage-to-albumin ratio and risk of mortality in patients on peritoneal dialysis. J Inflamm Res. 2023;16:6271–6281. doi: 10.2147/JIR.S437256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeng Y, Chen Z, Chen Q, et al. Neutrophil to lymphocyte ratio predicts adverse cardiovascular outcome in peritoneal dialysis patients younger than 60 years old. Mediators Inflamm. 2020;2020:4634736. doi: 10.1155/2020/4634736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen T, Yang M. Platelet-to-lymphocyte ratio is associated with cardiovascular disease in continuous ambulatory peritoneal dialysis patients. Int Immunopharmacol. 2020;78:106063. doi: 10.1016/j.intimp.2019.106063 [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Xu Y, Liu S, et al. The systemic inflammation indexes predict all-cause mortality in peritoneal dialysis patients. Renal Fail. 2023;45(1):2160348. doi: 10.1080/0886022X.2022.2160348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nøst TH, Alcala K, Urbarova I, et al. Systemic inflammation markers and cancer incidence in the UK Biobank. Eur J Epidemiol. 2021;36(8):841–848. doi: 10.1007/s10654-021-00752-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang AY, Wang M, Woo J, et al. Cardiac valve calcification as an important predictor for all-cause mortality and cardiovascular mortality in long-term peritoneal dialysis patients: a prospective study. J Am Soc Nephrol. 2003;14(1):159–168. doi: 10.1097/01.ASN.0000038685.95946.83 [DOI] [PubMed] [Google Scholar]
- 22.Xie H, Ruan G, Wei L, et al. The inflammatory burden index is a superior systemic inflammation biomarker for the prognosis of non-small cell lung cancer. J Cachexia, Sarcopenia and Muscle. 2023;14(2):869–878. doi: 10.1002/jcsm.13199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bello AK, Okpechi IG, Osman MA, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. 2022;18(12):779–793. doi: 10.1038/s41581-022-00623-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li PK, Ng JK, McIntyre CW. Inflammation and Peritoneal Dialysis. Semin Nephrol. 2017;37(1):54–65. doi: 10.1016/j.semnephrol.2016.10.007 [DOI] [PubMed] [Google Scholar]
- 25.Wen Y, Zhan X, Wang N, et al. Monocyte/lymphocyte ratio and cardiovascular disease mortality in peritoneal dialysis patients. Mediators Inflamm. 2020;2020(9852507):1–9. doi: 10.1155/2020/9852507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Li Y, Zou Y, et al. Use of the systemic inflammation response index (SIRI) as a novel prognostic marker for patients on peritoneal dialysis. Renal Fail. 2022;44(1):1227–1235. doi: 10.1080/0886022X.2022.2100262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tang R, Chen J, Zhou Q, et al. Association between systemic immune inflammation index and all-cause mortality in incident peritoneal dialysis-treated CKD patients: a multi-center retrospective cohort study. BMC Nephrology. 2024;25(1):8. doi: 10.1186/s12882-023-03451-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song R, Ni H, Huang J, et al. Prognostic value of inflammation-immunity-nutrition score and inflammatory burden index for hepatocellular carcinoma patients after hepatectomy. J Inflamm Res. 2022;15:6463–6479. doi: 10.2147/JIR.S386407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He C, Wu D, Wei X, et al. Association between inflammatory burden index and all-cause mortality in the general population aged over 45 years: data from NHANES 2005–2017. Nutr Metab Cardiovasc Dis. 2024;34(1):64–74. doi: 10.1016/j.numecd.2023.10.006 [DOI] [PubMed] [Google Scholar]
- 30.Yu F, Peng J. Association between inflammatory burden index and cardiovascular disease in adult Americans: evidence from NHANES 2005–2010. Heliyon. 2024;10(18):e38273. doi: 10.1016/j.heliyon.2024.e38273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borazan A, Ustün H, Yilmaz A. The effects of haemodialysis and peritoneal dialysis on serum lipoprotein(a) and C-reactive protein levels. J Int Med Res. 2003;31(5):378–383. doi: 10.1177/147323000303100504 [DOI] [PubMed] [Google Scholar]
- 33.Li W, Liu H, Qian W, et al. Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer. Comput Struct Biotech J. 2018;16:479–487. doi: 10.1016/j.csbj.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devaraj S, Valleggi S, Siegel D, et al. Role of C-reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep. 2010;12(2):110–118. doi: 10.1007/s11883-010-0098-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baetta R, Corsini A. Role of polymorphonuclear neutrophils in atherosclerosis: current state and future perspectives. Atherosclerosis. 2010;210(1):1–13. doi: 10.1016/j.atherosclerosis.2009.10.028 [DOI] [PubMed] [Google Scholar]
- 36.Grzegorzewska AE, Leander M Lymphocyte subsets in the course of continuous ambulatory peritoneal dialysis. Advances in peritoneal dialysis Conference on Peritoneal Dialysis; 2001. 17:10–14. [PubMed] [Google Scholar]
- 37.Lu X, Wang S, Zhang G, et al. High neutrophil-to-lymphocyte ratio is a significant predictor of cardiovascular and all-cause mortality in patients undergoing peritoneal dialysis. Kidney Blood Pressure Res. 2018;43(2):490–499. doi: 10.1159/000488696 [DOI] [PubMed] [Google Scholar]
- 38.Awad K, Zaki MM, Mohammed M, et al. Effect of the renin-angiotensin system inhibitors on inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Mayo Clinic Proceed. 2022;97(10):1808–1823. doi: 10.1016/j.mayocp.2022.06.036 [DOI] [PubMed] [Google Scholar]