Abstract
Tissue regeneration in many skin defects is progressing with new treatments in recent years. Tissue engineering with the use of scaffolds offers more versatile and faster solutions in treatment. Extracellular matrix (ECM) and its three-dimensional (3D) network structure as a biological bond by imitating the tissue microstructure has been used for tissue repair, which can answer many existing challenges. Vitamin A, which comes in several forms such as retinols, retinals, and retinoic acids, is a necessary vitamin that is crucial for wound healing. In this research, sheep kidney capsule tissue decellularized with sodium dodecyl sulfate (SDS) containing different doses of vitamin A has been used as an ECM in skin tissue engineering. The above scaffold was evaluated in terms of properties such as biocompatibility, analysis of mechanical properties, attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), hydrophilicity, antibacterial, and cell adhesion. The findings reported suitable properties for wound dressing, especially at a dose of 15,000 U/ml vitamin A for this scaffold. Then, the above scaffold was evaluated on the full-thickness wound model in rat, which showed good wound contraction, and increased VEGF factor. It showed a decrease in IL-1β level. Therefore, the use of the above-mentioned decellularized scaffold in combination with medicinal agents effective in wound healing can be introduced for further pre-clinical studies.
Keywords: Kidney capsule, Vitamin A, Decellularization, Wound healing
1. Introduction
The skin is the largest organ in the human body that covers the body's whole surface [1]. This tissue is an intricate organ that uses a variety of defense mechanisms, including chemical, physical, and microbiological barriers, to shield the host from harm from the outside world [2]. The integumentary system is formed by the skin and its appendages that originate from the epidermis, such as hair follicles, sweat glands, sebaceous glands, nails, and mammary glands. Histologically, the skin is composed of two primary layers: the dermis and the epidermis [3]. Growth factors, cytokines, chemokines, and other cells coordinately interact during the dynamic and intricate multiple-phase process that is normal wound healing. If these steps are not followed, wounds could become chronic and develop atypical scars. Patients' quality of life is impacted by chronic wounds since they necessitate frequent care and high medical expenses. As a result, a lot of work has gone into creating cutting-edge therapeutic methods for treating wounds [4]. Several causes may cause the skin, which is the biggest organ in the body, to take longer to heal after an injury. Abrasions, burns, tumors, and chronic wounds are frequent skin conditions. Skin tissue engineering, lotions, solutions, dressings, autografts, and allografts are among the wound care options. The dressing should prevent infection, maintain moisture in the area, and encourage rapid healing [5,6]. The development of skin tissue engineering scaffolds to facilitate wound healing is one of the main goals of regenerative medicine. These scaffolds, with their structural emulation of bodily tissues, offer a novel perspective on the treatment of skin injuries. Natural scaffolds derived from decellularized tissues that preserve their essential components can serve as an appropriate substrate for cellular functions [7]. In recent years, tissue engineering has made significant strides toward creating artificial scaffolds as biological substitutes for organ restoration and enhancement. Three-dimensional (3D) scaffolds with suitable mechanical, biological, biophysical, and biochemical properties can be created by tissue engineering and used as support structures. These scaffolds mimic bodily tissues and offer the ideal environment for cell adhesion, development, proliferation, and migration. The above causes biological exchanges in the system [8,9]. Because it preserves active chemicals that promote tissue regeneration, the extracellular matrix (ECM) is an essential biomaterial in tissue engineering. In addition to its complex structural composition and natural components, the extracellular matrix (ECM) facilitates cell-cell interactions and provides mechanical integrity [10]. Thus, a suitable ECM design is always considered. Decellularized tissues provide cell-free matrices that are minimally immunogenic and retain bioactive extracellular matrix elements such as growth factors, hyaluronic acid, collagen, proteoglycans, elastin, and structural and specialized proteins [11,12]. It appears promising to decellularize natural tissues to provide an extracellular matrix for 3D scaffolds and tissue microenvironment research. Owing to the lack of a single accepted method, current research endeavors aim to create novel approaches for the decellularization of complete or partial tissues that promote cell differentiation and transplantation for tissue regeneration [13,14]. There are several different types of vitamin A, such as retinol, retinal, and retinoic acids. It is an essential micronutrient. Retinol from preformed retinoids or pro-vitamin A carotenoids that are transformed into retinol in the enterocyte are the two forms of dietary vitamin A that are absorbed. These are subsequently transported by chylomicrons to the liver, where they are stored until they are released into the bloodstream and bound to retinol-binding protein in the liver's physiologically active tissues. Numerous vital and varied biological processes, including growth, immunity, cellular differentiation, reproduction, embryological development, and eyesight, depend on vitamin A. Nuclear retinoic acid receptors, retinoid receptors, and peroxisome proliferator-activated receptors are the main mechanisms by which vitamin A acts. Insufficient levels of retinoid control the proliferation and differentiation of several cell types in the skin, resulting in aberrant epithelial keratinization. Vitamin A promotes epidermal turnover, speeds up re-epithelialization, and repairs epithelial structure in injured tissue. The special power of retinoids is their capacity to counteract the anti-inflammatory steroids' inhibiting effects on wound healing. Apart from its function in the inflammatory stage of wound healing, research has indicated that retinoic acid also promotes the synthesis of extracellular matrix elements like collagen type I and fibronectin, boosts the growth of keratinocytes and fibroblasts, and lowers the amounts of degrading matrix metalloproteinases [15]. Retinoids are generally referred to as vitamin A. A lack of vitamin A can cause a range of cutaneous symptoms. By modifying the activity of certain cell lines via retinoic acid receptors, it also has hormone-like properties. Regarding the physiologic effect of vitamin A on acute or chronic wounds via systemic or topical treatment, there are many research conducted on animals and few on humans. Supplementing with vitamin A is most commonly used to counteract the effects of steroids A vitamin A deficit slows down healing [16]. The aim of this study is to research the effect of decellularized scaffolds containing vitamin A in wound healing. The effects of the scaffold alone and with vitamin A are investigated.
2. Materials
Penicillin-streptomycin, trypsin- EDTA, and fetal bovine serum (FBS) were obtained from Bioidea, Iran. Xylene and paraffin were purchased from Asiapajohesh, Iran. Phosphate-buffered saline (PBS), sodium dodecyl sulfate (SDS), Dulbecco's Modified Eagle's medium (DMEM), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), glutaraldehyde, hematoxylin-eosin (H-E), Masson's trichrome (MT), Alcian blue (AB), and glutaraldehyde were obtained from Sigma Aldrich, Germany. Vitamin A 50,000U/1 mL ampoules were purchased from Osve, Iran. Adipose mesenchymal stem cells (AMSCs) were obtained from Pasteur Institute, in Iran. Xylazine and ketamine were purchased from Alfasan, Woerden, Netherlands. ELISA kits were obtained from Sunlong Medical, China.
3. Methods
3.1. Preparation, decellularization, and load vitamin a in kidney capsule
The present work aims to choose relevant data and implant a better scaffold in mice after the chemical-physical evaluation of the scaffold and determination of vitamin A dose was completed in a laboratory model. Based on pilot testing, scaffolds of 1.5 × 1.5 cm2 were cut, and various dosages of vitamin A were added to the scaffolds. The kidney capsule was broken up into tiny pieces, each measuring 1.5 by 1.5 cm2. A combined physico-chemical method was used for decellularization. Using the immersion-agitation approach, decellularization by 0.5 % sodium dodecyl sulfate (SDS) (chemical detergent) was carried out. During the decellularization process, tissues were shaken at 80 revolutions per minute (rpm) for 24 h while immersed in detergent. The detergent was changed every 8 h. Then, to remove the detergent from the tissue, they were washed with distilled water under a shaker for 24 h (70 rpm). The distilled water was changed every 8 h. In our previous study, we used several different methods for decellularization, and the best results were obtained from the use of 0.5 % SDS. Therefore, we used this method to continue our research work [17]. Decellularized kidney capsules (DKC) were dried with a freeze-dryer (Alpha 2–4 LD, Christ) and divided into three groups to load amounts of 5000, 10,000, and 15,000 U/ml of vitamin A. Scaffolds were incubated with different amounts of vitamin A for 24 h.
3.2. Biocompatibility evaluation (MTT assay)
An indirect evaluation of the biocompatibility of DKCs was carried out using the ISO 10993-5 standard. Sterilized DKCs were incubated in a Dulbecco's Modified Eagle's medium (DMEM) culture medium for 24 h. The medium condition was taken and added to produced adipose mesenchymal stem cells (AMSCs) from the Pasteur Institute in Iran (1 × 104 in each well of 96 plates) to examine biocompatibility at 48 and 72 h using the MTT assay [18].
3.3. Attenuated total reflection fourier transform infrared spectroscopy (ATR-FTIR) analysis
FTIR spectroscopy was utilized to assess the scaffolds' molecular structure, functional group identification, and bond formation. DKCs loaded with different amounts of vitamin A were subjected to infrared spectra recording using a Nicolet Is10 (Irprestige-21, Shimadzu) equipped with an ATR mode. The spectra were acquired across a range of 500–4000 cm-1, with an average resolution of 4 cm-1 over 64 scans [19].
3.4. Mechanical test
The mechanical properties of scaffolds were studied using the Santam st-1 device (Iran). The tensile strength of the device was evaluated by inserting its clamp between DKCs loaded with different amounts of vitamin A that had been cut into 10 mm × 40 mm specifications. Young's modulus, strain, and stress were calculated using the following formulas. The test was stopped after the samples were broken down, and the load cell's output was 1000 N (N) (3). F is for force, Aˆ is for cross-section, ΔL is for length change, Lˆ is for initial length, and ΔS is for stress change (stress). F = force, A˚ = cross-section, ΔL = change in length, L˚ = initial length, ΔS = change in stress
| (Stress) S F / A˚, (Strain) E = ΔL / L˚, (Young's modulus) E = ΔS / Δe |
3.5. Cell attachment (SEM)
An SEM (scanning electron microscope) (Philips Company, the Netherlands) was used to examine the ultrastructure of cell attachment DKCs loaded with different amounts of vitamin A. 1 × 104 AMSCs were grown on DKC to investigate cell adhesion on the scaffold. After that, the cells were incubated for 72 h. Samples were treated using a 2.5 % glutaraldehyde solution. The specimens were coated with gold palladium to facilitate microscopic inspection [20].
3.6. Contact angle
Using a syringe and a 27 G needle, deionized water was poured onto the scaffold surfaces (Jikan CAG-20, Iran). The drop was photographed on the scaffold, and Image J software was used to determine the contact angles based on the drop shape [21].
3.7. Water retention capacity (WRC)
After weighing the dry tissues and soaking them in PBS for a while to test the scaffolds' capacity to hold water, they were incubated at 37 °C for a full day. The scaffolds were put in a centrifuge tube with filter paper underneath and spun for 3 min at 500 rpm. After weighing the scaffolds once more, the following formula was applied [17]. Ws: the weight of the swollen scaffold, Wi: weight of the dry scaffolds WR (%) = (Ws−Wi)/Wi 100.
3.8. Antibacterial test
The sensitive (ATCC25923) and methicillin-resistant (M30) strains of Staphylococcus aureus, the sensitive (ATCC7853) and imipenem-resistant (IMI10) strains of Pseudomonas aeruginosa, and the meropenem-sensitive (MEM10) Escherichia coli strain (ATCC25922) were used. The bacteria were incubated in the BHI enrichment medium at 37 °C and 170 rpm overnight. Then they were cultured on blood agar medium for 18-24 h at 37 °C. The previously prepared cultures were taken out of the refrigerator and kept at room temperature for 15 min to reach room temperature. Scaffolds were placed in 3 groups. Scaffold group 1 contained 5000 units of vitamin A, group 2 contained 10,000 units of vitamin A, and group 3 contained 15,000 units of vitamin A. The scaffolds were exposed to UV light for 20 min. Normal saline (0.9 % w/v) was used to prepare a microbial suspension with a concentration of 1.5 × 108 CFU mL−1. To perform the antibiogram test, sterile swabs were used to disperse the prepared suspension onto an agar plate. After adding 100 μL of broth medium to the scaffolds, they were placed on blank disks and the disks were placed on Mueller Hinton agar medium. To evaluate the antibacterial activity, the blank disks containing the scaffolds were placed against the antibiotic disks of imipenem, meropenem, and methicillin. 15 min after performing the antibiogram, the plates were incubated at 37 °C for 18-24 h. After the mentioned period, the diameter of the inhibition zone around the paper disks was measured.
3.9. In vivo wound model
The effectiveness of the DKCs loaded with vitamin A in wound healing was assessed using a full-thickness wound model. At the age of eight weeks, 30 healthy adult male rats weighing between 220 and 240 gr were procured from the Animal Breeding and Maintenance Center, Kermanshah University of Medical Sciences, Iran. The ethics committee of Kermanshah University of Medical Sciences gave its clearance, and the experiments were carried out by university policies (ethical code: code IR.KUMS.AEC.1402.046). Rats were housed in environments that were temperature-controlled between 20 and 23 °C, had a 12-h light and dark cycle, and had humidity levels between 40 and 60 %. Normal food and unrestricted access to water were provided to the mice. The animals were then split into three groups: 1. control group (no treatment group), 2. DKC group (decellularized kidney capsules, treatment group), and 3. DKC-15000 vA group (decellularized kidney capsules + 15,000 U/ml vitamin A, treatment group). Intraperitoneal injections of xylazine (10 mg/kg) and ketamine (100 mg/kg) were used to induce general anesthesia. Following that, the rat's back developed a 1.5 × 1.5 cm2) full-thickness wound. Each group (n = 5) received the treatments during two periods of 7 and 14 days. A digital camera was used to take pictures of the wound at 7- and 14-days following surgery to track the healing process. Utilizing Image J software (1.52v), the size of the wound was measured. Lastly, the wound closure was computed using the following formula.
| (Open wound area / Primary wound area) x 100 = % wound closure |
3.10. Scaffold implantation into the rat wound
Before grafting, the scaffolds were UV-exposed for 15 min on each side after being soaked for 30 min in sterile PBS containing 2 % antibiotics. The DKC-15000 vA group was chosen and implanted in the wound model due to its superior performance in tests of toxicity, mechanical characteristics, contact angle, antibacterial test, and cell adherence. The scaffolds were positioned on the wound so that the scaffold's edge was under the skin, negating the need for sutures and allowing researchers to assess the therapeutic effects of each scaffold in full-thickness wound healing. Following dressing, Tegaderm elastic tapes (TegadermTM, 3 M Health Care, Germany) were used to bind the wounds. Additionally, the same coating was applied to the control group's wounds, which were not received untreated.
3.11. Histopathology study
The animals of each group were killed using sodium thiopental on the 7th and 14th days. Skin tissues were removed and fixed in 10 % formalin for 72 h. After that, the paraffin-embedded tissues were cut to a thickness of 5 μm, and the tissues were stained with hematoxylin and eosin (H&E) and Masson trichrome (MT). Finally, the development of tissue granulation, collagen deposition, and epithelialization in different groups were investigated using light microscopy [22].
3.12. Angiogenesis study
On the seventh and fourteenth days, mouse vascular endothelial growth factor (VEGF) levels were found in the wound area, per the high-sensitivity enzyme-linked immunosorbent test (ELISA) methodology. Briefly, the tissues were centrifuged for 15 min at 12,000 rpm and 4 °C to extract the supernatant. A 96-well plate coated with an antibody specific to the mouse VEGF marker was layered with diluted biotin-conjugated antibody and left to be in contact with the supernatant (100 μl) for 2 h at room temperature. After that, streptavidin-HRP and biotinylated VEGF antibody were mixed and incubated for 30 min at 37 °C. After incubation, unbound streptavidin-HRP washed away [23]. The process was stopped by adding sulfuric acid, and the absorbance at 450 nm was measured (Elisa Reader, Bio Tek, USA).
3.13. Inflammatory study
The ELISA kit methodology was followed to quantify the amount of interleukin-1 beta (IL-1β). In brief, skin supernatant (100 μL) was plated on a 96-well plate that had been precoated with an antibody specific to the mouse IL-1β marker for 2 h at room temperature on days 7 and 14. After that, the sample's IL-1β was coupled to a biotinylated mouse IL-1β antibody, which was then stored for an hour at 37 °C. After that, it was mixed with streptavidin-HRP and allowed to bind to biotinylated IL-1β antibody for half an hour at 37 °C [24]. At 450 nm, absorbance was measured using sulfuric acid to halt the addition process (Elisa Reader, Bio Tek, USA).
3.14. Statistical analysis
All data were statistically evaluated, and diagrams were produced using GraphPad Prism (version 8). When examining the data using the two-way ANOVA, P < 0.05 was considered significant.
4. Results
4.1. Biocompatibility
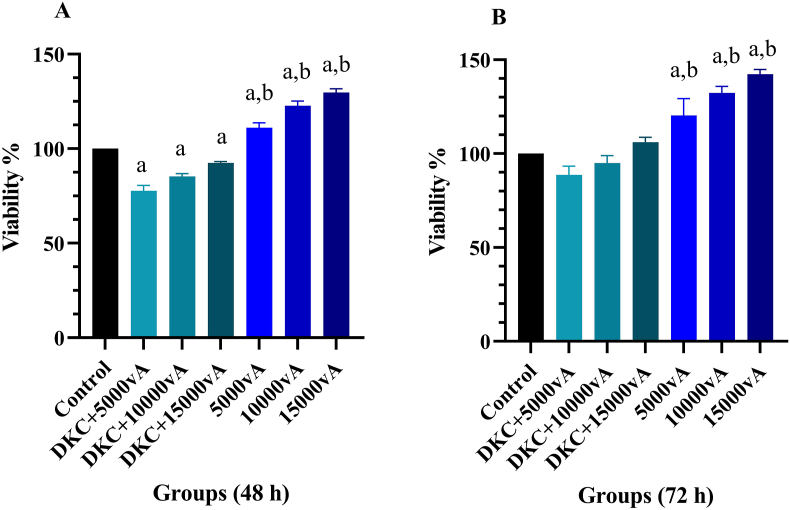
MTT test was performed to determine the biocompatibility of the scaffolds during 48 and 72 h. More than 80 % cell viability was reported in all scaffolds containing vitamin A. Cell survival was observed at 72 h more than at 48 h. The effect of vitamin A alone on cell viability was more than its loaded amounts in the scaffold. Therefore, scaffolds containing different concentrations of vitamin A were biocompatible and did not cause cytotoxicity (Fig. 1 A and B).
Fig. 1.
A) MTT assay in 48 h and B) MTT assay in 72 h, Cytotoxicity after 48 and 72 h reported good cell survival in all experimental groups, survival at 72 h was reported higher than 48 h, %, a: Significant compared to the control group, b: Significant compared to DKCs containing vitamin A, the data presented are mean ± SD, n = 3, DKC: decellularized kidney capsules, 5000vA: 5000 U/ml vitamin A, 10000vA: 10,000 U/ml vitamin A, 15000vA: 15,000 U/ml vitamin A.
4.2. ATR-FTIR
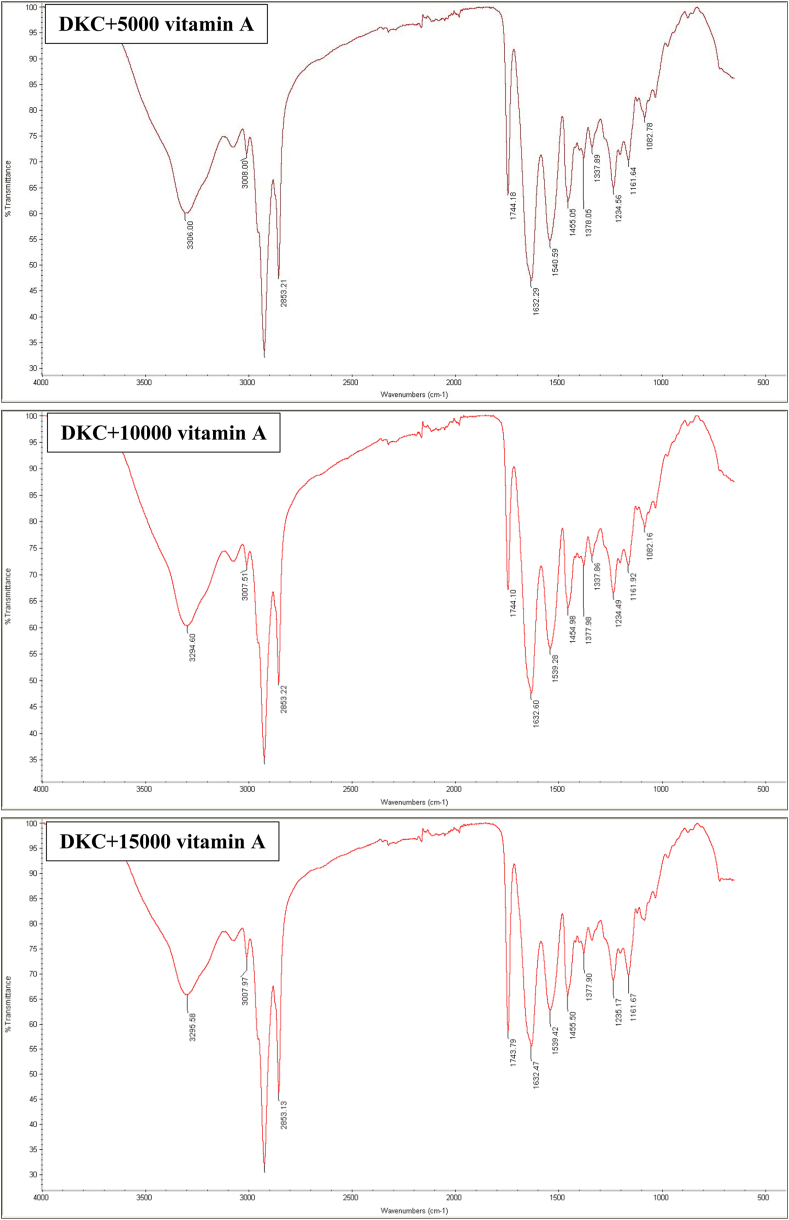
In a previous study, the scaffold without vitamin A was examined in terms of chemical agents [17]. The ATR-FTIR spectrum of all three graphs obtained from the decellularized tissue containing different amounts of vitamin A can be seen in Fig. 2. The peak of the 1632 cm−1 region is related to the C=C bond and the peak of the 3008 cm−1 region is related to the C=H bond. Also, the peak area of 3306 cm−1 shows OH groups. These peaks can confirm the load of vitamin A in the DKCs.
Fig. 2.
ATR-FTIR spectrum of DKCs loaded with different amounts of vitamin A, DKC: decellularized kidney capsules, 5000vA: 5000 U/ml vitamin A, 10000vA: 10,000 U/ml vitamin A, 15000vA: 15,000 U/ml vitamin A.
4.3. Mechanical test
The test of mechanical properties shows that Young's modulus has increased with the rise in the concentration of vitamin A in the scaffold (312.5 MPa in the DKC loaded with 15,000 U/ml vitamin A compared to 183.8 and 182.1 MPa in the DKCs containing 10,000 and 5000 U/ml vitamin A, respectively). Also, the breaking point and ultimate tensile strength (UTS) of the 15,000 U/ml vitamin A sample is higher than the other two samples. which indicates the increase of force tolerance in this scaffold compared to the other two samples (Fig. 3).
Fig. 3.
Mechanical test of DKCs loaded with different amounts of vitamin A, DKC: decellularized kidney capsules, 5000vA: 5000 U/ml vitamin A, 10000vA: 10,000 U/ml vitamin A, 15000vA: 15,000 U/ml vitamin A.
4.4. SEM
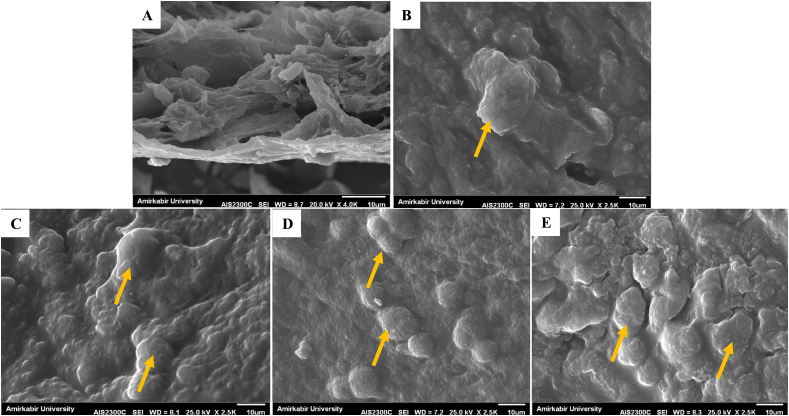
SEM images showed in the 0.5 % SDS decellularization method (DKC), fibers are broken to a certain extent and cell adhesion is seen (Fig. 4 A and B). Cell adhesion has also occurred in the scaffolds loaded with different amounts of vitamin A, and it seems that adhesion has occurred in a better way with an increase in the concentration of vitamin A (Fig. 4C: 5000vA: 5000 U/ml vitamin A, Fig. 4 D: 10000vA: 10,000 U/ml vitamin A, Fig. 4 E: 15000vA: 15,000 U/ml vitamin A).
Fig. 4.
SEM images, A) X-4.0K cross-section of DKC, B) cell adhesion on DKC surface without vitamin A, C) cell adhesion on DKC loaded with 5000 U/ml vitamin A, D) cell adhesion on DKC loaded with 10,000 U/ml vitamin A, E) cell adhesion on DKC loaded with 15,000 U/ml vitamin A, The magnification in the images is related to adhesion X-2.5K, cells are indicated by orange arrows.
4.5. Hydrophilicity tests
A contact angle test was performed to measure the hydrophilicity of the scaffold surface (Fig. 5 A). The degree of hydrophilicity of DKC with different concentrations of vitamin A has shown different results. The contact angle in the DKC loaded with 15,000 U/ml vitamin A (37.20 ± 0.30) was reported to be higher than 10,000 U/ml vitamin A (29.40 ± 0.30) and 5000 U/ml vitamin A (17.40 ± 0.40). All three scaffolds showed degrees of hydrophilicity. WRC plays an important role in the physiological absorption of liquids. The amount of water retention decreased with increasing concentration of vitamin A in the DKC (108.66 ± 1.02, 57.00 ± 0.20, and 42.00 ± 1.03, in 5000, 1000, and 15,000 U/ml vitamin A respectively) (Fig. 5 B).
Fig. 5.
To measure the hydrophilicity of the surface of the DKCs containing vitamin A, the contact angle test was performed in Fig. 6 A, and WRC in Fig. 6 B, DKC: decellularized kidney capsules, 5000vA: 5000 U/ml vitamin A, 10000vA: 10,000 U/ml vitamin A, 15000vA: 15,000 U/ml vitamin A.
4.6. Antibacterial results
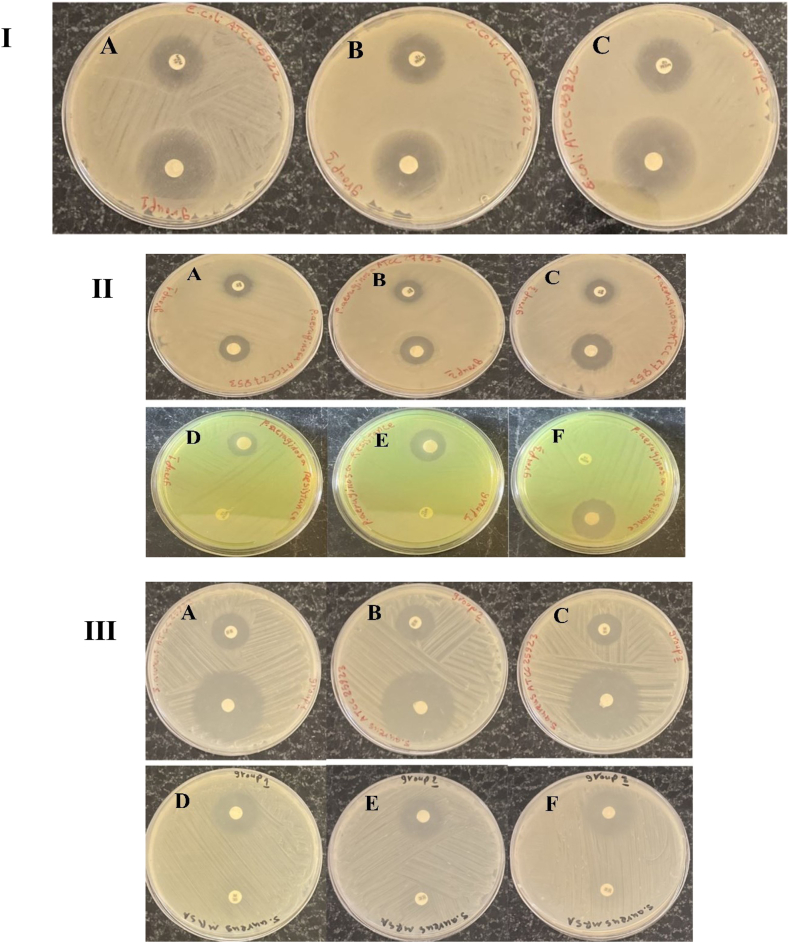
The antibacterial effect of the scaffold containing different doses of vitamin A was investigated on these bacteria. The measurement of the inhibition zone and the antibacterial effect of the scaffolds showed that the diameter of the inhibition zone of the scaffolds is much greater than the inhibition zone of the antibiotics. The diameter of the inhibition zone for sensitive and resistant strains is shown in Table 1 and Fig. 6. As can be seen, with the increase in the dose of vitamin A, the diameter of the inhibition zone and the antibacterial effect of the scaffolds have increased. Compared to scaffolds, antibiotics showed little antibacterial effect. The excellent effectiveness of scaffolds against antibiotic-resistant strains is very important. These observations confirm the strong antibacterial effect of scaffolds containing vitamin A against common Gram-positive and negative bacteria that cause wound infection.
Table 1.
The diameter of the inhibition zone of the scaffolds containing vitamin A.
| Bacteria | diameter of the inhibition zone group 1 |
diameter of the inhibition zone group 2 |
diameter of the inhibition zone group 3 |
|||
|---|---|---|---|---|---|---|
| Scaffolds + 5000 U/ml vitamin A |
Antibiotics | Scaffolds + 10,000 U/ml vitamin A |
Antibiotics | Scaffolds + 15,000 U/ml vitamin A |
Antibiotics | |
| E. coli ATCC | 35 | 29 | 40 | 28 | 42 | 25 |
| P. areuginosa ATCC | 21 | 19 | 24 | 19 | 33 | 21 |
| P. areuginosa resistance | 19 | 0 | 20 | 0 | 25 | 0 |
| S. aureus ATCC | 35 | 19 | 38 | 20 | 40 | 19 |
| S. aureus resistance | 30 | 0 | 32 | 0 | 35 | 0 |
E. coli: Escherichia coli, P. areuginosa: Pseudomonas aeruginisa, S. aureus: Staphylococcus aureus
Fig. 6.
Diameter of inhibition zone of around scaffolds containing vitamin A in three group, and antibiotic disks, I) antibacterial assay of scaffolds against E. coli ATCC25922. The scaffolds containing Vitamin A shown significant antibacterial activity against E. coli ATCC25922. Bactericidal efficiency of scaffold in all three groups was better than meropenem antibiotic. Antibacterial activity of the scaffold in group 1 (Plate. A), group 2 (Plate. B), and group 3 (Plate. C) was 35 mm, 40 mm, and 42 mm, respectively, II) significant antibacterial activity of scaffolds against P. aeruginosa ATCC27853. Diameter of the growth inhibition zone of the scaffold in all three groups was higher than that of the imipenem antibiotic. Antibacterial activity of the scaffold in group 1 (Plate. A), group 2 (Plate. B), and group 3 (Plate. C) was 21 mm, 24 mm, and 23 mm, respectively. Antibacterial activity against P. aeruginosa resistant to meropenem. The highest antibacterial effect was related to the in group 3 (Plate. F), which created an inhibition zone with a diameter of 25 mm, but group 1(Plate. D), and group 2 (Plate. E) created an inhibition zone with a diameter of 19 mm, and 20 mm, III) the scaffolds show significant antibacterial activity against S. aureus ATCC27853. Diameter of the growth inhibition zone of the scaffold in all groups was higher than that of the methicillin antibiotic. Antibacterial activity in group 1 (Plate. A), group 2 (Plate. B), and group 3 (Plate. C) was 35 mm, 38 mm, and 40 mm, respectively. The highest antibacterial effect against S. aureus resistant to methicillin in group 3 (Plate. F) was significantly higher than that of the group 1 (Plate. D), and group 2 (Plate. E).
4.7. In vivo wound healing
The macroscopic appearance of the wound sites was investigated in the study (Fig. 7 A). At the site of the wound, there were no indications of infection or inflammation. The macroscopic view showed that the size of wounds in the groups treated with scaffolds was smaller compared to the control group. To quantify the wound healing process, the speed of wound closure was measured (Fig. 7 B). Wound closure in the control groups were 35.53 ± 0.55 % and 80.64 ± 1.46 % 7 and 14 days after wound formation, respectively. Wound healing in the DKC groups were 45.85 ± 0.45 % and 92.46 ± 0.83 % 7 and 14 days after wound formation, respectively. Wound closure in the DKC + vitamin A groups was 68.00 ± 1.00 % on day 7 and 96.84 ± 0.45 % on day 14.
Fig. 7.
Studies on in vivo wound healing, A) 7 and 14 days following the damage, the macroscopic state of the wounds was evaluated, B) A comparison of the wound closure on days 7 and 14 following wounding is shown in the diagram. The values represent the mean ± standard deviation (n = 5). a, a’: significant compared to the control group (days 7 and 14), b, b’: significant compared to the DKC group (day 7 and 14), c’: significant compared to day 7 of the same group.
4.8. Histological results
The wound site in the control groups (days 7 and 14) showed the highest number of scabs. The process of epithelization is better observed by increasing the healing time and using the scaffold in the wound site. In the groups where the scaffold with and without vitamin A was used as a wound dressing, the process of collagen tissue formation can be seen, although it is immature. Collagen bundles were formed in groups of the DKC + vitamin A on the 14th day. The formation of hair follicles can also be seen in this group. Regeneration on the 14th day is described to be better than on the 7th day (Fig. 8 A and B).
Fig. 8.
Evaluations of wound healing in histopathology, A) day 7, B) day 14, The yellow lines show the boundary between the healthy tissue and the tissue being repaired, white arrows: scab skin, red arrows: immature collagens, pink arrows: collagen in Masson trichrome staining and orange arrow: hair follicle.
4.9. Angiogenesis and inflammatory results
ELISA results show that VEGF levels in the tissue graft site are higher in the treated groups than in the control group (Fig. 8 A). The increase in VEGF level in all groups shows a time-dependent increase. On day 14, the level of VEGF in the DKC + vitamin A group was significantly higher than in other groups. After that, the DKC group reported a high level of VEGF on day 14. The level of IL-1β, dependent on time, showed a decreasing trend in the treated samples compared to the control group. The DKC + vitamin A group showed a significant reduction in inflammation, especially on the fourteenth day, compared to other groups, but this difference was not significant compared to other treatment groups (Fig. 9 B).
Fig. 9.
A) The effect of DKC and DKC + vitamin A treatments on VEGF levels and B) IL-1β level in skin wounds after 7 and 14 days, the values represent the mean ± standard deviation (n = 5). a, a’: significant compared to the control group (days 7 and 14), b’: significant compared to the DKC group (day 7), c’: significant compared to day 7 of the same group.
5. Discussion
In our previous study, for the first time, we started the research on kidney capsule decellularization [17]. This tissue was decellularized by different methods and the best scaffold in terms of mechanical properties, and biocompatibility was obtained in decellularization with 0.5 % SDS. In the present study, vitamin A was loaded in the above scaffold and after checking its various properties, it was grafted on the full-thickness wound model. In the development of scaffold materials, the focus is on providing a suitable platform for establishing cell interactions with each other and with the ECM to create a suitable environment for the treatment of skin injuries [25]. The emergence of decellularized extracellular matrix (dECM) is a promising new technique to provide a biological environment mimicking the body. dECM is widely used as a bioactive biomaterial with low immunogenicity and is readily available for the regeneration and repair of transformed skin [26]. Vitamin A is an essential nutrient in skin health, and vitamin A deficiency causes failure in the keratinization of epithelial cells. Although the exact mechanism of its action is not fully known, retinoids are widely used to treat skin disorders and have shown good results in healing and accelerating wound healing, mainly in animals and humans [15]. It showed the effect of vitamin A on three cell lines (L929, HUVEC, and RAW264.7) by evaluating the MTT method [27]. Decellularized amniotic membrane tissues containing exosome nanoparticles derived from adipose tissue stem cells also showed good cell proliferation [28]. In the present study, the survival of mesenchymal cells in the treatment with different concentrations of vitamin A, and in the scaffolds loaded with vitamin A was reported, which increased with the increase in the dose. Therefore, ECMs obtained from decellularized tissues loaded with compounds effective in tissue repair can maintain cell survival and be a good source for scaffold construction ideas.
In a study, the FTIR spectrum of retinol (one of the forms of vitamin A) showed peaks related to asymmetric and symmetric C–H stretching and C C stretching [29], which were reported in the same direction as our study. Young's modulus is often somewhat reduced after decellularization, as reported in fish skin decellularization [30], but often the drug load in decellularized tissues affected Young's modulus compared to the untreated group. It shows some increase. For example, the resveratrol load in the decellularized tissue of the pericardium showed a higher Young's modulus than the group without resveratrol [31]. In scaffolds prepared by methods other than decellularization, increasing the concentration of compounds such as rosuvastatin in the scaffold has increased Young's modulus and thus improved tissue strength [21]. The results of our study also showed that Young's modulus increased with increasing vitamin A concentration. Decellularized tissues will show cell adhesion if the above process has been done well and the detergents have been completely removed from the tissue, especially the surface of the tissue [32]. In the decellularized tissues containing compounds such as curcumin, cell adhesion was also observed in the groups that did not have curcumin [33]. In our study, it was also seen that cell adhesion occurred both in the groups containing vitamin A and in the scaffold without this compound. Therefore, it can be concluded that in the case of optimal decellularization of the scaffold and if the compounds loaded in the scaffold do not have toxic effects, cell adhesion is done well. The design of safe and effective formulations to improve and treat wound infection due to bacterial agents is a challenging issue. Antibacterial properties and power of wound dressing biomaterials play an important role in healing and repairing wounds [34]. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli are the most common bacteria associated with wound infection through biofilm formation in wound tissue [35]. Vitamin A has a significant effect on wound healing through several mechanisms, including stimulation of epidermal efficiency, increasing the rate of re-epithelialization, and regeneration of epithelial structure [36]. It has also been shown that vitamin A plays an important role in increasing the inflammatory response, angiogenesis, and reparative collagen synthesis in wounds [37]. Since antibiotic resistance of common bacteria causing wound infection is one of the main obstacles in the treatment of infected wounds [38], the use of scaffolds with an acceptable dose of vitamin A (15,000 units) Provides a more suitable treatment condition for healing and repairing infected wounds. Scaffolds containing different doses of vitamin A provided acceptable results, but the antibacterial activity of the scaffold containing a higher dose (15,000 units) of vitamin A provided stronger efficacy to overcome bacterial resistance. Vitamin A plays an important role in maintaining the health and function of mucous membranes and skin, which are the body's first line of defense against pathogens such as bacteria. This vitamin strengthens the epithelial cells that are present in the skin, respiratory system, and digestive system. Vitamin A increases the production of antibodies by stimulating B cells in the immune system. Antibodies can identify and destroy bacteria. It is now known that in vitamin A deficiency, epithelial cells shrink and squamous keratinization may occur in the skin, and at the same time, the resistance of keratinized epithelial tissues to foreign pathogens decreases As a result, it reduces the function of innate immunity [[39], [40], [41]]. Therefore, it seems that vitamin A can have antibacterial effects indirectly.
Reaching the ideal contact angle for skin scaffolds is essential to promoting proper cell adhesion and proliferation. Empirical evidence indicates that a contact angle within the 40–70-degree range is advantageous for fostering these characteristics. Compared to very hydrophobic or overly hydrophilic surfaces, this range denotes a moderately hydrophilic surface, which promotes better cell adhesion and proliferation [42,43]. Biomaterials exhibit an increase in water absorption at very low contact angles, resulting in a decrease in protein absorption—a necessary component for cell attachment and recognition. Cellular receptors, if they exist at all, are unable to supply the mechanical force necessary to encourage cell division. Consequently, the right contact angle is required to maximize the activity of growth factors, while improper contact angles lead to protein denaturation [44].
Numerous significant and varied biological processes, such as reproduction, embryonic development, cell differentiation, growth, immunity, and eyesight, depend on vitamin A. Nuclear retinoic acid receptors, retinoid X receptors, and peroxisome proliferator-activated receptors are the primary mechanisms by which vitamin A operates. The proliferation and differentiation of many skin cell types are regulated by retinoids and aberrant epithelial keratinization results from its shortage. Vitamin A promotes re-epithelialization, boosts epidermal circulation, and repairs epithelial structure in injured tissue. The special power of retinoids is their capacity to counteract the anti-inflammatory steroids' inhibiting effects on wound healing. Apart from its function in the inflammatory stage of wound healing, research has demonstrated that retinoic acid also stimulates the synthesis of extracellular matrix elements like collagen type I and fibronectin, boosts the growth of keratinocytes and fibroblasts, and lowers the amounts of degrading matrix metalloproteinases [15,16]. In a study, the angiogenic and anti-inflammatory effects of vitamin A were obtained in vitro models [27]. Decellularized tissue with compounds such as monophosphoryl lipid A (MPLA) increases the speed of cell migration, and more collagen and angiogenesis were observed in the transplant on the skin wound model [45,46]. As in the present study, the results showed that the groups that had decellularized tissue containing vitamin A showed a better process of wound healing, angiogenesis, and anti-inflammatory properties. The properties of compounds such as vitamin A when they are loaded in scaffolds, probably due to the creation of a suitable biological substrate, show more effective properties in the process of skin regeneration.
6. Conclusion
Decellularized kidney capsules with 0.5 % SDS were loaded with different doses of vitamin A and presented good properties as skin scaffolds. These features include appropriate mechanical behavior, biocompatibility, cell attachment, hydrophilicity, and antibacterial properties. A scaffold loaded with 15,000 U/ml vitamin A showed full-thickness wound healing within 14 days in rats with increased angiogenesis and VEGF levels. On the other hand, it caused the suppression of pro-inflammatory mediators such as IL-1β. These findings can introduce new evidence of a decellularized kidney capsule scaffold containing vitamin A for the treatment of skin wounds.
Funding
This paper was funded research deputy of Kermanshah University of Medical Sciences, Kermanshah, Iran.
Declaration of competing interest
The authors declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgment
The results described in this paper were part of a student thesis. This paper originated from an MD dissertation (research code: 4020878 and 4020973) and with the ethics code IR.KUMS.MED.REC.1402.262 and IR.KUMS.AEC.1402.046.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Khavkin J., Ellis D.A. Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin. 2011;19(2):229–234. doi: 10.1016/j.fsc.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen A.V., Soulika A.M. The dynamics of the skin's immune system. Int J Mol Sci. 2019;20(8):1811. doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arda O., Göksügür N., Tüzün Y. Basic histological structure and functions of facial skin. Clin Dermatol. 2014;32(1):3–13. doi: 10.1016/j.clindermatol.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Nourian Dehkordi A., Mirahmadi Babaheydari F., Chehelgerdi M., Raeisi Dehkordi S. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019;10:1–20. doi: 10.1186/s13287-019-1212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asghari F., Faradonbeh D.R., Malekshahi Z.V., Nekounam H., Ghaemi B., Yousefpoor Y., et al. Hybrid PCL/chitosan-PEO nanofibrous scaffolds incorporated with A. euchroma extract for skin tissue engineering application. Carbohydr Polym. 2022;278:118926. doi: 10.1016/j.carbpol.2021.118926. [DOI] [PubMed] [Google Scholar]
- 6.Javanmardi S., Safari I., Aghaz F., Khazaei M. Wound healing activities of Gundelia tournefortii L extract and milk-cream ointment on second-degree burns of rat skin. Int J Low Extrem Wounds. 2021;20(3):272–281. doi: 10.1177/1534734620921589. [DOI] [PubMed] [Google Scholar]
- 7.Khazaei M., Rahmati S., Khazaei M.R., Rezakhani L. Accelerated wound healing with resveratrol-loaded decellularized pericardium in mice model. Cell Tissue Bank. 2023:1–9. doi: 10.1007/s10561-023-10117-w. [DOI] [PubMed] [Google Scholar]
- 8.Rahmani Del Bakhshayesh A., Annabi N., Khalilov R., Akbarzadeh A., Samiei M., Alizadeh E., et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cell Nanomed Biotechnol. 2018;46(4):691–705. doi: 10.1080/21691401.2017.1349778. [DOI] [PubMed] [Google Scholar]
- 9.Khazaei F., Rezakhani L., Alizadeh M., Mahdavian E., Khazaei M. Exosomes and exosome-loaded scaffolds: characterization and application in modern regenerative medicine. Tissue Cell. 2023;80:102007. doi: 10.1016/j.tice.2022.102007. [DOI] [PubMed] [Google Scholar]
- 10.Khoshnood N., Zamanian A. Decellularized extracellular matrix bioinks and their application in skin tissue engineering. Bioprinting. 2020;20 [Google Scholar]
- 11.Solarte David V.A., Güiza-Argüello V.R., Arango-Rodríguez M.L., Sossa C.L., Becerra-Bayona S.M. Decellularized tissues for wound healing: towards closing the gap between scaffold design and effective extracellular matrix remodeling. Front Bioeng Biotechnol. 2022;10:821852. doi: 10.3389/fbioe.2022.821852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alizadeh M., Rezakhani L., Khodaei M., Soleimannejad M., Alizadeh A. Evaluating the effects of vacuum on the microstructure and biocompatibility of bovine decellularized pericardium. J Tissue Engin Rege Med. 2021;15(2):116–128. doi: 10.1002/term.3150. [DOI] [PubMed] [Google Scholar]
- 13.Alizadeh A., Rezakhani L., Anjom Shoa M., Ghasemi S. Frequency of CD44 positive cells in MKN45 cell line after treatment with docetaxel in two and three-dimensional cell cultures. Tissue Cell. 2020;63:101324. doi: 10.1016/j.tice.2019.101324. [DOI] [PubMed] [Google Scholar]
- 14.Alizadeh M., Rezakhani L., Alizadeh A. Characterization of the decellularized ovine pericardium for skin tissue engineering. J Shahrekord Univ Med Sci. 2020;22(4):173–180. [Google Scholar]
- 15.Polcz M.E., Barbul A. The role of vitamin A in wound healing. Nutr Clin Pract. 2019;34(5):695–700. doi: 10.1002/ncp.10376. [DOI] [PubMed] [Google Scholar]
- 16.Zinder R., Cooley R., Vlad L.G., Molnar J.A. Vitamin A and wound healing. Nutr Clin Pract. 2019;34(6):839–849. doi: 10.1002/ncp.10420. [DOI] [PubMed] [Google Scholar]
- 17.Khazaei M.R., et al. Decellularized kidney capsule as a three-dimensional scaffold for tissue regeneration. Cell Tissue Bank. 2024:1–14. doi: 10.1007/s10561-024-10136-1. [DOI] [PubMed] [Google Scholar]
- 18.Khazaei M., et al. Antioxidant properties of Brown algae in 3D model for colorectal cancer. Cell Tissue Biol. 2024;18(2):163–172. [Google Scholar]
- 19.Gharibshahian M., et al. Magnesium-oxide-enhanced bone regeneration: 3D-printing of gelatin-coated composite scaffolds with sustained Rosuvastatin release. Int J Biol Macromol. 2024;266 doi: 10.1016/j.ijbiomac.2024.130995. [DOI] [PubMed] [Google Scholar]
- 20.Khazaei M., et al. Decellularized prostate for cancer studies. Cell Tissue Biol. 2024;18(3):280–288. [Google Scholar]
- 21.Gharibshahian M., et al. Fabrication of rosuvastatin-incorporated polycaprolactone-gelatin scaffold for bone repair: a preliminary in vitro study. Cell J (Yakhteh) 2024;26(1):70. doi: 10.22074/CELLJ.2023.2009047.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alizadeh M., et al. The effect of Scrophularia striata on cell attachment and biocompatibility of decellularized bovine pericardia. Cell Tissue Bank. 2022;23(2):261–269. doi: 10.1007/s10561-021-09939-3. [DOI] [PubMed] [Google Scholar]
- 23.Kilic Bektas C., et al. A bilayer scaffold prepared from collagen and carboxymethyl cellulose for skin tissue engineering applications. J Biomater Sci Polym Ed. 2018;29(14):1764–1784. doi: 10.1080/09205063.2018.1498718. [DOI] [PubMed] [Google Scholar]
- 24.Khazaei M., et al. Accelerated wound healing with resveratrol-loaded decellularized pericardium in mice model. Cell Tissue Bank. 2024;25(1):245–253. doi: 10.1007/s10561-023-10117-w. [DOI] [PubMed] [Google Scholar]
- 25.Hama R., et al. Recent tissue engineering approaches to mimicking the extracellular matrix structure for skin regeneration. Biomimetics. 2023;8(1):130. doi: 10.3390/biomimetics8010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang S., Zhuang Y., Cai M., Wang X., Lin K. Decellularized extracellular matrix: a promising strategy for skin repair and regeneration. Eng Reg. 2023;4:357–374. [Google Scholar]
- 27.Neghab H.K., Soheilifar M.H., Djavid G.E. AG. Hogrefe; 2021. (An in vitro model for investigation of vitamin A effects on wound healing). [DOI] [PubMed] [Google Scholar]
- 28.Xiao S., Xiao C., Miao Y., Wang J., Chen R., Fan Z., Hu Z. Human acellular amniotic membrane incorporating exosomes from adipose-derived mesenchymal stem cells promotes diabetic wound healing. Stem Cell Res Ther. 2021;12(1):255. doi: 10.1186/s13287-021-02333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa J.R., et al. Silica microparticles from sugarcane by-products as an encapsulation system for retinoids aimed at topical sustained release. Int J Mol Sci. 2024;25(6):3215. doi: 10.3390/ijms25063215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lau C.S., Hassanbhai A., Wen F., Wang D., Chanchareonsook N., Goh B.T., et al. Evaluation of decellularized tilapia skin as a tissue engineering scaffold. J Tissue Eng Reg Med. 2019;13(10):1779–1791. doi: 10.1002/term.2928. [DOI] [PubMed] [Google Scholar]
- 31.Khazaei M., Alizadeh M., Rezakhani L. Resveratrol-loaded decellularized ovine pericardium: ECM introduced for tissue engineering. Biotechnol Appl Biochem. 2024;71(2):387–401. doi: 10.1002/bab.2547. [DOI] [PubMed] [Google Scholar]
- 32.Rahmati S., Jalili A., Dehkordi M.B., Przedborski M. An effective method for decellularization of human foreskin: implications for skin regeneration in small wounds. Cell Journal (Yakhteh) 2022;24(9):506. doi: 10.22074/cellj.2022.8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh H., Li H., Wang K., Chou K. Curcumin in decellularized goat small intestine submucosa for wound healing and skin tissue engineering. J Biomed Mater Res B Appl Biomater. 2022;110(1):210–219. doi: 10.1002/jbm.b.34903. [DOI] [PubMed] [Google Scholar]
- 34.Fan Z., Liu B., Wang J., Zhang S., Lin Q., Gong P., et al. A novel wound dressing based on Ag/graphene polymer hydrogel: effectively kill bacteria and accelerate wound healing. Adv Funct Mater. 2014;24(25):3933–3943. [Google Scholar]
- 35.Guo J., Sun W., Kim J.P., Lu X., Li Q., Lin M., et al. Development of tannin-inspired antimicrobial bioadhesives. Acta Biomater. 2018;72:35–44. doi: 10.1016/j.actbio.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polcz M.E., Barbul A. The role of vitamin A in wound healing. Nutr Clin Pract. 2019;34(5):695–700. doi: 10.1002/ncp.10376. [DOI] [PubMed] [Google Scholar]
- 37.Barbul A., Thysen B., Rettura G., Levenson S.M., Seifter E. White cell involvement in the inflammatory, wound healing, and immune actions of vitamin A. JPEN - J Parenter Enter Nutr. 1978;2(2):129–138. doi: 10.1177/014860717800200208. [DOI] [PubMed] [Google Scholar]
- 38.Filius P.M., Gyssens I.C. Impact of increasing antimicrobial resistance on wound management. Am J Clin Dermatol. 2002;3(1):1–7. doi: 10.2165/00128071-200203010-00001. [DOI] [PubMed] [Google Scholar]
- 39.Qi Y., Niu Q.L., Zhu X.-L., Zhao X.-Z., Wang W.-W., Wang X.-J. Relationship between deficiencies in vitamin A and E and occurrence of infectious diseases among children. Eur Rev Med Pharmacol Sci. 2016;20(23):5009–5012. [PubMed] [Google Scholar]
- 40.Stephensen C.B. Vitamin A, infection, and immune function. Annu Rev Nutr. 2001;21(1):167–192. doi: 10.1146/annurev.nutr.21.1.167. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z., Liu Y., Qi G., Brand D., Zheng S.G. Role of vitamin A in the immune system. J Clin Med. 2018;7(9):258. doi: 10.3390/jcm7090258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nosar M.N., Salehi M., Ghorbani S., Beiranvand S.P., Goodarzi A., Azami M. Characterization of wet-electrospun cellulose acetate based 3-dimensional scaffolds for skin tissue engineering applications: influence of cellulose acetate concentration. Cellulose. 2016;23:3239–3248. [Google Scholar]
- 43.Chang H.-I., Wang Y. Cell responses to surface and architecture of tissue engineering scaffolds, in Regenerative medicine and tissue engineering-cells and biomaterials. InTechOpen. 2011 [Google Scholar]
- 44.Abedalwafa M., Wang F., Wang L., Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications: a review. Rev Adv Mater Sci. 2013;34(2):123–140. [Google Scholar]
- 45.Baghaee R., Rahmati S., Banitalebi M., Afzali L. Comparison of human acellular amniotic membranes with acellular amniotic membranes pretreated with MPLA for repair of fascia in rats. Cell Tissue Bank. 2023;24(2):495–501. doi: 10.1007/s10561-022-10049-x. [DOI] [PubMed] [Google Scholar]
- 46.Jalili A., Shojaei-Ghahrizjani F., Tabatabaiefar M.A., Rahmati S. Decellularized skin pretreatment by monophosphoryl lipid A and lactobacillus casei supernatant accelerate skin recellularization. Mol Biol Rep. 2024;51(1):1–14. doi: 10.1007/s11033-024-09599-y. [DOI] [PubMed] [Google Scholar]