Introduction
Nail bed cancers are rare, with an estimated incidence of 1.2 out of every 100,000 primary care consultations and 28 out of every 100,000 dermatology consultations.1 However, the incidence may be underestimated as most nail bed tumors go undiagnosed or misdiagnosed.2 Squamous cell carcinoma (SCC) typically progresses slowly and painlessly.3 The thumb and index finger are the most commonly affected areas.4 SCC is most often present in adult men, with a peak incidence between 50 and 69 years old.5 Predisposing factors for SCC development include trauma, radiation exposure, smoking, infection with human papillomavirus types 16 and 18, and chemical insults.6–8
Approximately 71% of SCC cases involving the nail unit are localized to the nail bed, 27% of cases are in lateral nail folds, and 1.7% of cases involve both the nail bed and nail folds.2 SCC is often referred to as "the great mimicker nail tumor" as it mimics chronic paronychia, onychomycosis, pyogenic granulomas, subungual glomus tumors, and enchondromas.7
Case Presentation
A 46-year-old male, RG, visited an occupational medicine clinic with a chronic paronychia/subungual ulcer on his left ring finger. He had experienced trauma to his finger 3 times and had injured his nail bed 3 years ago when it got caught between a meter reader lid and a concrete wall. During the physical examination, RG’s left ring finger appeared typical, but the nail plate had grown and elevated from the nail bed with a thick layer of brown scab-like tissue formation (Figure 1). It was slightly red, warm, and swollen on the tip, and purulent fluid extruded from the center of this material. There was tenderness to palpation at the tip, the finger was warm and well-perfused, and the range of motion was full. The sensation was intact to light touch to the radial and ulnar side of the tip. The remainder of the focused physical examination was otherwise unremarkable.
Figure 1:
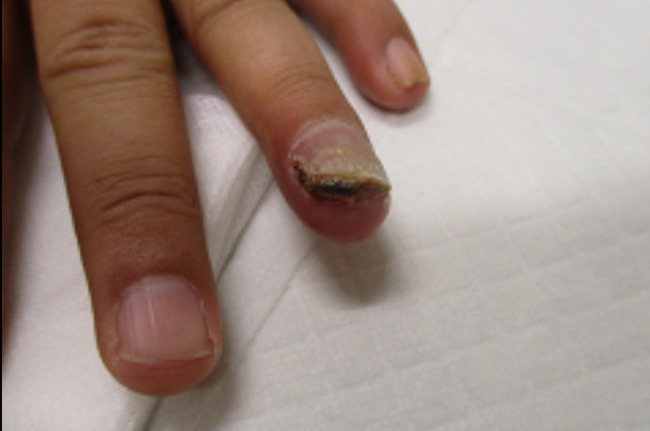
Dystrophic nail on the left ring finger.
A plastic surgeon removed the damaged nail, but the new nail grew deformed, not connecting with the radial edge of the nail fold. Nine months later, RG visited an occupational medicine physician for dystrophic nail/chronic paronychia and was treated for paronychia. However, a discharge culture showed Pseudomonas aeruginosa and Candida parapsilosis complex. After multiple concurrent courses of antibiotics, a nail biopsy and avulsion were necessary as the symptoms were persistent and refractory to conservative management. Pathology revealed SCC with well-to-moderately differentiated section margins involved. RG underwent Mohs micrographic surgery (MMS) for the SCC, and the wound healed by secondary intention (Figure 2). Physical therapy helped improve the patient’s range of motion, and he fully recovered. No surveillance was required as it was not a high-risk lesion.
Figure 2:
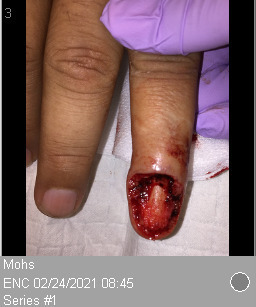
Left ring finger post-Mohs micrographic surgery.
The patient has given consent for the publication of this case report.
Clinical Reasoning and Diagnosis
Bacteriological and fungal cultures of purulent fluid from the left ring finger were obtained and P aeruginosa grew. A hand x-ray was done to rule out a fracture, and a chest x-ray was obtained as SCC can rarely metastasize from the lung. Both x-rays were reported as negative.
The histological examination revealed well-to-moderately differentiated SCC with atypical squamous proliferation that assumed an infiltrative pattern.
RG underwent nail removal. The pathology report revealed that RG had a well-to-moderately differentiated SCC. Following the diagnosis, RG underwent MMS to remove the SCC on the left ring finger. After the successful surgery, RG underwent physical therapy and fully recovered.3
Treatment Pathway
Surgical treatment
The main goal of treating SCC is to remove the tumor altogether. The available treatment options depend on the tumor’s invasion and bone involvement. For primary tumors without bone involvement, conventional surgery with safety margins or MMS can be performed. Conventional surgery with safety margins is a feasible option and is used for tumors that do not involve the bone and are invasive.9 If the tumor size is < 2 cm, then wide local excision can be performed, where 4 mm of surrounding unaffected tissue is removed, followed by a full-thickness skin graft. According to studies, this method has a recurrence rate of 4% after 5 years of follow-up, with high cosmetic and functional outcomes, and a cure rate of 95% to 97%.10,11 The European Dermatology Forum and the European Association of Dermato-Oncology recommend minimal 5 mm surrounding margins for low-risk tumors.12
MMS is commonly used when treating noninvasive SCC without bone involvement. MMS is a good option because it preserves tissue, provides clear margins, and helps to distinguish between inflammation or compression and periosteal invasion. MMS aims to achieve a complete margin-negative resection where no tumor remains, ie, R0 resection above 90%. According to a recent prospective study conducted in Australia, the recurrence rate of primary SCC after undergoing MMS was found to be 2.6% as compared to conventional surgery with safety margins over 5 years.13–15
A meta-analysis found that MMS has a 97% success rate.16 After 5 years, the recurrence rate with MMS is 3.5%.4
There is no agreement on how long patients should be monitored for tumor recurrence, but some experts recommend follow-up after 5 years and advise patients to report any clinical changes.2
Radiotherapy
Patients who are not suitable for surgical removal may be able to undergo radiotherapy as an alternative, although this treatment has the potential to cause SCC.17 After follow-up at 5 years, the local recurrence rate is estimated to be around 6.4%.18 In cases where there is a high chance of recurrence, such as with positive margins or invasive tumors with perineural invasion, postoperative radiotherapy is recommended.19 When the disease is progressive and challenging to treat, photon irradiation using a water bath may be used.20
Immunotherapy
For individuals with metastatic, positive margins, and locally advanced SCC that cannot be treated solely through surgery, immunotherapy with monoclonal antibodies and PD-1 inhibitors (such as cemiplimab) is recommended.21 In a recent phase 2 study, cemiplimab was found to positively impact tissue reaction. As for second-line treatments, platin-based chemotherapy and epidermal growth factor receptor inhibitors are viable options.22
Conclusions
In conclusion, nail bed cancers are rare, but the most prevalent type of malignant tumor affecting the nail unit is SCC. Health care practitioners should be aware of potential nail bed issues such as infections (bacterial or viral) and nail bed tumors. This is especially important when a patient presents with persistent inflammation with nail bed deformity.
It is important to conduct additional research to determine if there are ways to improve current treatment practices. Despite the different treatment methods available, the rate of recurrence after conventional surgery that involves tumor removal is still high. More studies and awareness are needed to establish the actual incidence of SCC in the nail bed.
By sharing experiences and educating others, SCC of the nail bed can be better identified, and appropriate treatment can be promptly implemented to improve patient outcomes.
Relevancy Statement.
This case report demonstrates a team-based approach to patient care, with different disease-specific clinicians providing treatment and reaching a consensus diagnosis of a rare disease.
Footnotes
Author Contributions: Dr Shiu, Dr Newton, and Ms Varela treated the patient, provided them with appropriate antibiotics, diagnosed them correctly, and provided the figures. Dr Chaudhry wrote a large portion of the manuscript, edited it, and submitted it for publication. Ms Varela wrote a small portion of the manuscript. Dr Durant completed the final critical assessment of the manuscript.
Conflicts of Interest: None declared
Funding: None declared
References
- 1.High WA, Tyring SK, Taylor RS. Rapidly enlarging growth of the proximal nail fold. Dermatol Surg. 2003;29(9):984–986. 10.1046/j.1524-4725.2003.29266.x [DOI] [PubMed] [Google Scholar]
- 2.Dijksterhuis A, Friedeman E, van der Heijden B. Squamous cell carcinoma of the nail unit: Review of the literature. J Hand Surg Am. 2018;43(4):374–379. 10.1016/j.jhsa.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 3.Lecerf P, Richert B, Theunis A, André J. A retrospective study of squamous cell carcinoma of the nail unit diagnosed in a Belgian general hospital over a 15-year period. J Am Acad Dermatol. 2013;69(2):253–261. 10.1016/j.jaad.2013.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Dika E, Fanti PA, Patrizi A, Misciali C, Vaccari S, Piraccini BM. Mohs surgery for squamous cell carcinoma of the nail unit: 10 years of experience. Dermatol Surg. 2015;41(9):1015–1019. 10.1097/DSS.0000000000000452 [DOI] [PubMed] [Google Scholar]
- 5.Tang N, Maloney ME, Clark AH, Jellinek NJ. A retrospective study of nail squamous cell carcinoma at 2 institutions. Dermatol Surg. 2016;42 Suppl 1:S8–S17. 10.1097/DSS.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 6.Shimizu A, Kuriyama Y, Hasegawa M, Tamura A, Ishikawa O. Nail squamous cell carcinoma: A hidden high-risk human papillomavirus reservoir for sexually transmitted infections. J Am Acad Dermatol. 2019;81(6):1358–1370. 10.1016/j.jaad.2019.03.070 [DOI] [PubMed] [Google Scholar]
- 7.Lobato-Berezo A, Fernandez-Valencia-Kettunen CK, Burgos-Lazaro F, Martinez-Perez M, Aguilar-Martinez A, Gallego-Valdes MA. Ungual squamous cell carcinoma mimicking a chronic paronychia: clinical, pathological and radiological correlation. Dermatol Online J. 2015;21(11). [PubMed] [Google Scholar]
- 8.Potter JA, Griffin PA. Polydactylous subungual squamous cell carcinoma caused by chemical contact. Plast Reconstr Surg Glob Open. 2013;1(4):e28. 10.1097/GOX.0b013e31829c48d6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Starace M, Alessandrini A, Piraccini BM. Nail Disorders in Children. Skin Appendage Disord. 2018;4(4):217–229. 10.1159/000486020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Topin-Ruiz S, Surinach C, Dalle S, Duru G, Balme B, Thomas L. Surgical treatment of subungual squamous cell carcinoma by wide excision of the nail unit and skin graft reconstruction: An evaluation of treatment efficiency and outcomes. JAMA Dermatol. 2017;153(5):442–448. 10.1001/jamadermatol.2017.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodland DG, Zitelli JA. Surgical margins for excision of primary cutaneous squamous cell carcinoma. Journal of the American Academy of Dermatology. 1992;27(2):241–248. 10.1016/0190-9622(92)70178-I [DOI] [PubMed] [Google Scholar]
- 12.Stratigos AJ, Garbe C, Dessinioti C, et al. European interdisciplinary guideline on invasive squamous cell carcinoma of the skin: Part 2. Treatment. Eur J Cancer. 2020;128:83–102. 10.1016/j.ejca.2020.01.008 [DOI] [PubMed] [Google Scholar]
- 13.Leibovitch I, Huilgol SC, Selva D, Hill D, Richards S, Paver R. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia I. Experience over 10 years. J Am Acad Dermatol. 2005;53(2):253–260. 10.1016/j.jaad.2005.02.059 [DOI] [PubMed] [Google Scholar]
- 14.Brantsch KD, Meisner C, Schönfisch B, et al. Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: A prospective study. Lancet Oncol. 2008;9(8):713–720. 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 15.Chren M-M, Linos E, Torres JS, Stuart SE, Parvataneni R, Boscardin WJ. Tumor recurrence 5 years after treatment of cutaneous basal cell carcinoma and squamous cell carcinoma. J Invest Dermatol. 2013;133(5):1188–1196. 10.1038/jid.2012.403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26(6):976–990. 10.1016/0190-9622(92)70144-5 [DOI] [PubMed] [Google Scholar]
- 17.Cuperus E, Leguit R, Albregts M, Toonstra J. Post radiation skin tumors: basal cell carcinomas, squamous cell carcinomas and angiosarcomas. A review of this late effect of radiotherapy. Eur J Dermatol. 2013;23(6):749–757. 10.1684/ejd.2013.2106 [DOI] [PubMed] [Google Scholar]
- 18.Babington S, Veness MJ, Cakir B, Gebski VJ, Morgan GJ. Squamous cell carcinoma of the lip: is there a role for adjuvant radiotherapy in improving local control following incomplete or inadequate excision? ANZ J Surg. 2003;73(8):621–625. 10.1046/j.1445-2197.2003.t01-1-02710.x [DOI] [PubMed] [Google Scholar]
- 19.Likhacheva A, Awan M, Barker CA, et al. Definitive and postoperative radiation therapy for basal and squamous cell cancers of the skin: Executive summary of an American Society for Radiation Oncology clinical practice guideline. Pract Radiat Oncol. 2020;10(1):8–20. 10.1016/j.prro.2019.10.014 [DOI] [PubMed] [Google Scholar]
- 20.Goodman CR, DeNittis A. Photon irradiation using a water bath technique for treatment of confluent carcinoma in situ of the hand, digits, and nail bed: A case report. J Med Case Reports. 2017;11(1):86. 10.1186/s13256-017-1233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maubec E. Update of the management of cutaneous squamous-cell carcinoma. Acta Derm Venereol. 2020;100(11):adv00143. 10.2340/00015555-3498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: Management of advanced and high-stage tumors. J Am Acad Dermatol. 2018;78:249–261. [DOI] [PubMed] [Google Scholar]


