Abstract
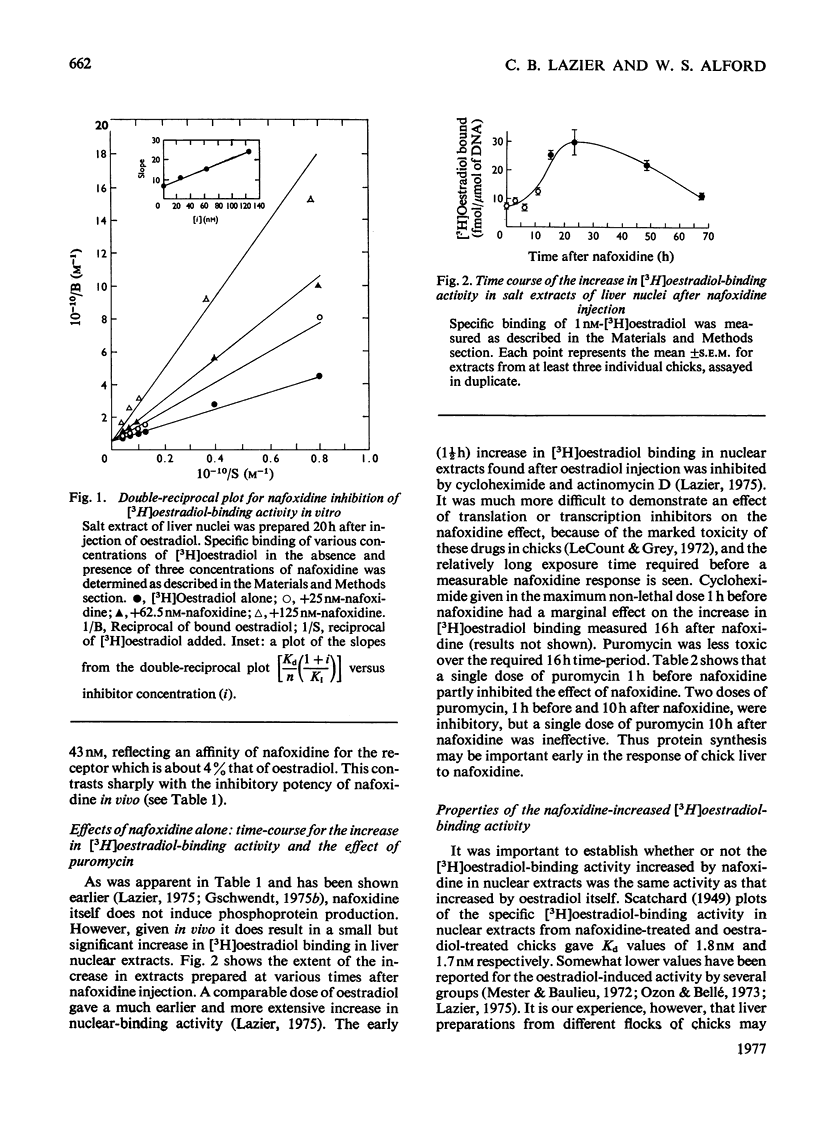
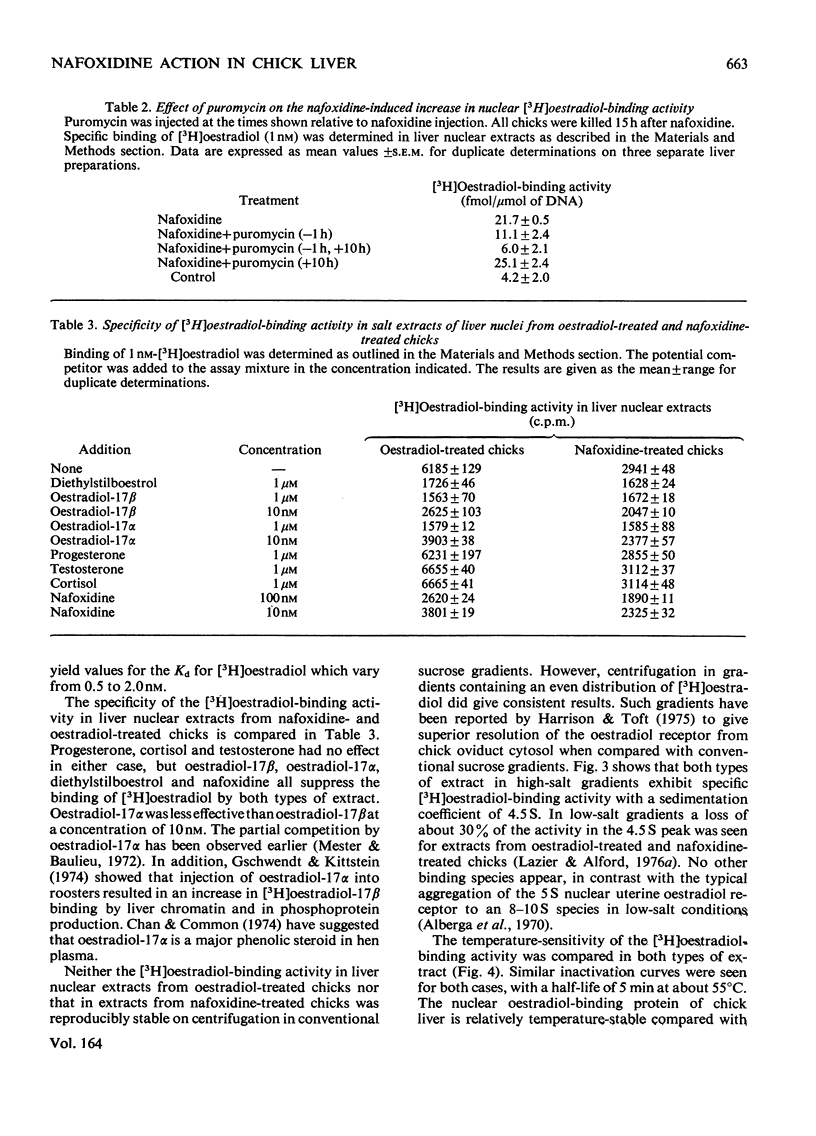
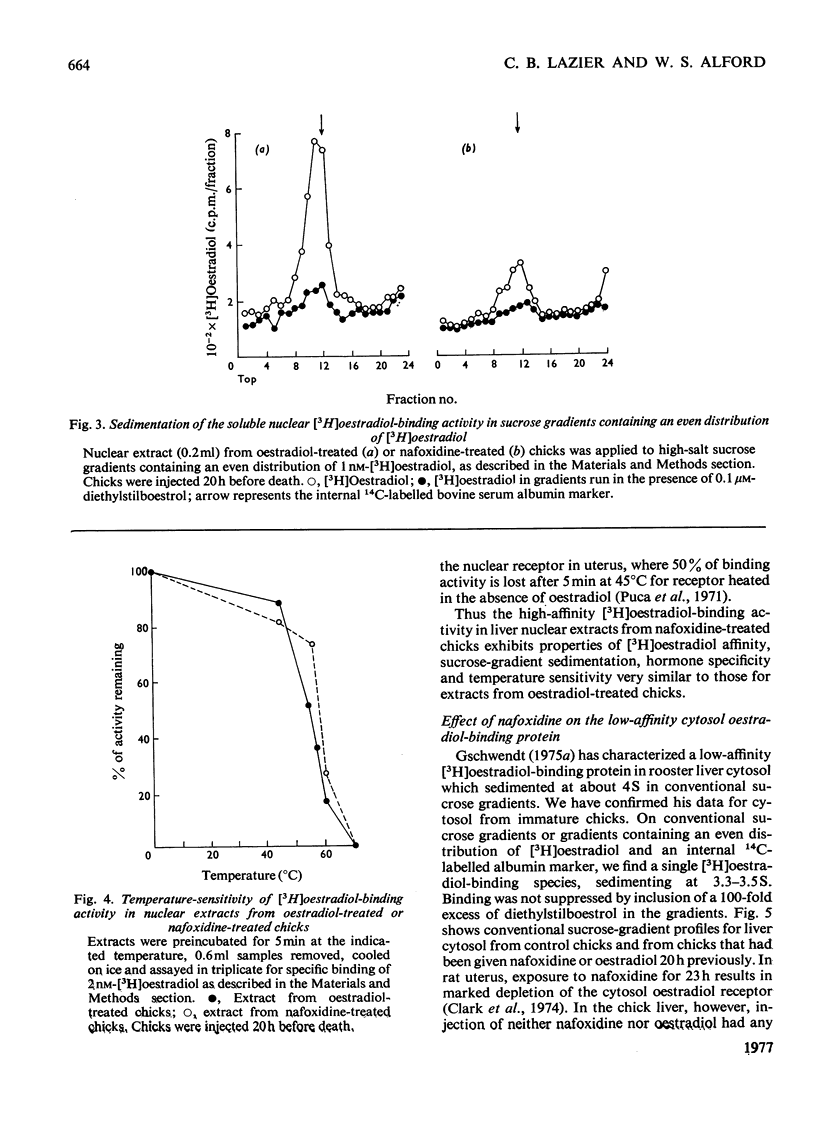
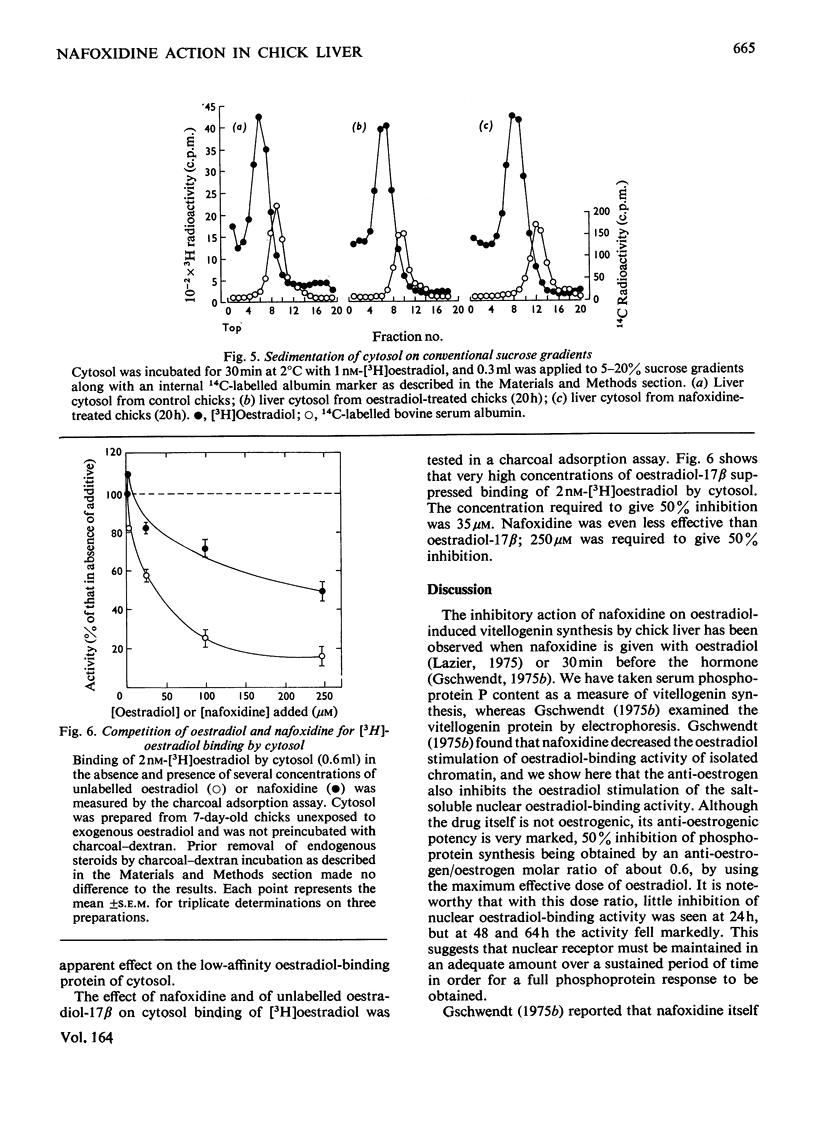
Nafoxidine hydrochloride (Upjohn, 11100A)injected with oestradiol into immature chicks inhibits the hormone-induced increase in [3H]oestradiol-binding activity in salt extracts of liver nuclei as well as the subsequent production by liver of egg-yolk phosphoprotein. Substantial inhibition of both oestradiol-induced responses is seen when nafoxidine is given in a dose approximately equimolar with that of oestradiol. In vitro nafoxidine competitively inhibits binding of [3H]oestradiol in nuclear extracts. The Ki for the inhibition is 43 nM, which indicates an affinity of nafoxidine for the binding protein about 4% of that of oestradiol. The inhibitory action of nafoxidine in vivo thus is more potent than the relative binding affinity determined in vitro might indicate. One possible explanation is that the primary site of nafoxidine action is at a point proximal to nuclear receptor interaction. Nafoxidine injected alone into the chick does not induce phosphoprotein synthesis, but it does increase [3H]oestradiol-binding activity in extracts of liver nuclei to a limited extent. No differences in the properties of the oestradiol-binding activity in extracts from nafoxidine-treated chicks or from oestradiol-treated chicks were detected. Chick liver cytosol does not contain detectable high-affinity oestradiol-binding activity. A low-affinity oestradiol-binding component with a sedimentation coefficient of 3.5S was found, but it was unaffected by treatment of chicks with earlier nafoxidine or oestradiol. The results suggest a difference in the mechanism of oestradiol action in the chick liver and in the widely studied rat uterus, on which the usual model for oestradiol action is largely based.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias F., Warren J. C. An estrophilic macromolecule in chicken liver cytosol. Biochim Biophys Acta. 1971;230(3):550–559. doi: 10.1016/0304-4165(71)90188-7. [DOI] [PubMed] [Google Scholar]
- Bergink E. W., Kloosterboer H. J., Gruber M., Ab G. Estrogen-induced phosphoprotein synthesis in roosters. Kinetics of induction. Biochim Biophys Acta. 1973 Feb 4;294(1):497–506. doi: 10.1016/0005-2787(73)90105-6. [DOI] [PubMed] [Google Scholar]
- Best-Belpomme M., Mester J., Weintraub H., Baulieu E. E. Oestrogen receptors in chick oviduct. Characterization and subcellular distribution. Eur J Biochem. 1975 Sep 15;57(2):537–547. doi: 10.1111/j.1432-1033.1975.tb02329.x. [DOI] [PubMed] [Google Scholar]
- Beuving G., Gruber M. Induction of phosvitin synthesis in roosters by estradiol injection. Biochim Biophys Acta. 1971 Mar 25;232(3):529–536. doi: 10.1016/0005-2787(71)90607-1. [DOI] [PubMed] [Google Scholar]
- Capony F., Rochefort H. In vivo effect of anti-estrogens on the localisation and replenishment of estrogen receptor. Mol Cell Endocrinol. 1975 Sep;3(3):233–251. doi: 10.1016/0303-7207(75)90047-7. [DOI] [PubMed] [Google Scholar]
- Chan A. H., Common R. H. Identification of radioactive oestradiol-17alpha and oestradiol-17beta in the plasma of the laying hen after injection of oestrone-4-14C. Comp Biochem Physiol B. 1974 Sep 15;49(1B):105–111. doi: 10.1016/0305-0491(74)90229-6. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J., Jr, Anderson J. N. Oestrogen receptors and antagonism of steroid hormone action. Nature. 1974 Oct 4;251(5474):446–448. doi: 10.1038/251446a0. [DOI] [PubMed] [Google Scholar]
- Clemens M. J. The regulation of egg yolk protein synthesis by steroid hormones. Prog Biophys Mol Biol. 1974;28:69–108. doi: 10.1016/0079-6107(74)90017-0. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., Eldridge J. D., Goldberger R. F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J Biol Chem. 1975 Dec 10;250(23):9060–9066. [PubMed] [Google Scholar]
- Gschwendt M. A cytoplasmic oestrogen-binding component in chicken liver. Hoppe Seylers Z Physiol Chem. 1975 Feb;356(2):157–165. doi: 10.1515/bchm2.1975.356.1.157. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Gschwendt M. The effect of antiestrogens on egg yolk protein synthesis and estrogen-binding to chromatin in the rooster liver. Biochim Biophys Acta. 1975 Aug 13;399(2):395–402. [PubMed] [Google Scholar]
- Harrison R. W., Toft D. O. Estrogen receptors in the chick oviduct. Endocrinology. 1975 Jan;96(1):199–205. doi: 10.1210/endo-96-1-199. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., DeSombre E. R. Estrogen-receptor interaction. Science. 1973 Oct 12;182(4108):126–134. doi: 10.1126/science.182.4108.126. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Jacobson H. I., Smith S., Jungblut P. W., De Sombre E. R. The use of estrogen antagonists in hormone receptor studies. Gynecol Invest. 1972;3(1):108–123. doi: 10.1159/000301747. [DOI] [PubMed] [Google Scholar]
- Jensen E. V., Mohla S., Gorell T. A., De Sombre E. R. The role of estrophilin in estrogen action. Vitam Horm. 1974;32:89–127. doi: 10.1016/s0083-6729(08)60007-2. [DOI] [PubMed] [Google Scholar]
- Joss U., Bassand C., Dierks-Ventling C. Rapid appearance of estrogen receptor in chick liver nuclei: partial inhibition by cycloheximide. FEBS Lett. 1976 Jul 15;66(2):293–298. doi: 10.1016/0014-5793(76)80525-x. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Ferguson E. R. Antiestrogen action in the uterus: biological ineffectiveness of nuclear bound estradiol after antiestrogen. Endocrinology. 1975 Jul;97(1):1–12. doi: 10.1210/endo-97-1-1. [DOI] [PubMed] [Google Scholar]
- Lazier C. (3H)-estradiol binding by chick liver nuclear extracts: mechanism of increase in binding following estradiol injection. Steroids. 1975 Sep;26(3):281–298. doi: 10.1016/0039-128x(75)90075-6. [DOI] [PubMed] [Google Scholar]
- Lebeau M. C., Massol N., Baulieu E. E. Extraction, partial purification and characterization of 'the insoluble estrogen receptor' from chick liver nuclei. FEBS Lett. 1974 Jul 1;43(1):107–111. doi: 10.1016/0014-5793(74)81117-8. [DOI] [PubMed] [Google Scholar]
- Lecount T. S., Grey R. D. Transient shortening of microvilli induced by cycloheximide in the duodenal epithelium of the chicken. J Cell Biol. 1972 May;53(2):601–605. doi: 10.1083/jcb.53.2.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mester J., Baulieu E. E. Nuclear estrogen receptor of chick liver. Biochim Biophys Acta. 1972 Jan 28;261(1):236–244. doi: 10.1016/0304-4165(72)90334-0. [DOI] [PubMed] [Google Scholar]
- O'Malley B. W., Means A. R. Female steroid hormones and target cell nuclei. Science. 1974 Feb 15;183(4125):610–620. doi: 10.1126/science.183.4125.610. [DOI] [PubMed] [Google Scholar]
- Ozon R., Bellé R. Récepteurs de l'oestradiol-17 dans le foie de poule et de l'amphibien Discoglossus pictus. Biochim Biophys Acta. 1973 Jan 24;297(1):155–163. [PubMed] [Google Scholar]
- Puca G. A., Nola E., Sica V., Bresciani F. Estrogen-binding proteins of calf uterus. Partial purification and preliminary characterization of two cytoplasmic proteins. Biochemistry. 1971 Sep 28;10(20):3769–3780. doi: 10.1021/bi00796a020. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rochefort H., Lignon F., Capony F. Effect of antiestrogens on uterine estradiol receptors. Gynecol Invest. 1972;3(1):43–62. doi: 10.1159/000301744. [DOI] [PubMed] [Google Scholar]
- Rochefort H., Lignon F., Capony F. Formation of estrogen nuclear receptor in uterus: effect of androgens, estrone and nafoxidine. Biochem Biophys Res Commun. 1972 May 26;47(4):662–670. doi: 10.1016/0006-291x(72)90543-8. [DOI] [PubMed] [Google Scholar]
- Ruh T. S., Ruh M. F. The effect of antiestrogens on the nuclear binding of the estrogen receptor. Steroids. 1974 Aug;24(2):209–224. doi: 10.1016/0039-128x(74)90104-4. [DOI] [PubMed] [Google Scholar]
- Sheridan P. J. Is there an alternative to the cytoplasmic receptor model for the mechanism of action of steroids? Life Sci. 1975 Aug 15;17(4):497–502. doi: 10.1016/0024-3205(75)90082-x. [DOI] [PubMed] [Google Scholar]


