Abstract
The effects of supplemental Zn within steroidal implant strategy on performance, carcass characteristics, trace mineral status, and muscle gene expression were tested in a 59-d study using 128 Angus-crossbred steers (492 ± 29 kg) in a 2 × 4 complete randomized design. Implant strategies included no implant (NoIMP) or Component TE-200 (TE200; Elanco, Greenfield, IN) administered on day 0. Zinc was supplemented at 0, 30, 100, or 150 mg Zn/kg dry matter (Zn0, Zn30, Zn100, Zn150, respectively) from ZnSO4. Steers were stratified by body weight (BW) to pens (n = 5 or 6 steers/pen) equipped with GrowSafe bunks (GrowSafe Systems Ltd., Airdrie, AB, Canada) and assigned treatments (n = 15, 16, or 17 steers/treatment). Cattle were weighed on days −1, 0, 18, and 59 with blood collected on days −1, 18, 40, and 59. Muscle samples were collected from the longissimus thoracis on day 11 and liver samples were collected on day 55 or 56. Data were analyzed using the Mixed Procedure of SAS via contrast statements testing the linear and quadratic response to Zn supplementation within implant treatment and NoIMP vs. TE200 for performance, carcass, blood, and liver parameters. Specific contrast statements were formed for the analysis of gene expression in muscle including: Zn0 vs. Zn150 within NoIMP and TE200, NoIMP vs. TE200 (Zn0 and Zn150 only), and the linear effect of supplementing Zn0, Zn100, and Zn150 within TE200. Steer was the experimental unit. Day 18 BW and days 0 to 18 average daily gain (ADG) were linearly increased due to Zn supplementation within TE200 (P ≤ 0.002) in conjunction with a linear increase from Zn in day 11 muscle epidermal growth factor receptor, matrix metalloproteinase 2, and phosphodiesterase 4B gene expression of TE200 steers (P ≤ 0.05). Plasma Zn on days 18 and 40 linearly increased with increasing Zn supplementation regardless of implant treatment (P ≤ 0.03) and was lesser for TE200 than NoIMP steers on day 18 (P = 0.001). Day 59 BW and hot carcass weight (HCW) were greater for TE200 vs. NoIMP (P ≤ 0.002) and HCW of implanted steers tended to linearly increase with increasing Zn supplementation (P = 0.09). No effects of Zn supplementation were observed in NoIMP for HCW, BW, or ADG (P ≥ 0.17). Yield grade and 12th rib fat tended to quadratically decrease within NoIMP (P ≤ 0.09), with Zn100 being the most lean. These data indicate increasing supplemental Zn influences steroidal implant signaling machinery while increasing the Zn status and implant-induced growth of feedlot cattle.
Keywords: cattle, steroidal implant, zinc
Supplemental zinc enhances the effects of growth implants in beef cattle, improving weight gain and carcass dressing percentage.
Introduction
Steroidal implants have been commonly used in US beef production since the 1950s (Preston, 1999), resulting in improvements in the average daily gain (ADG) of cattle by 16% to 20% (Bartle et al., 1992; Duckett and Pratt, 2014). This steroidal implant-induced growth response is elicited through genomic and nongenomic modes of action that regulate gene transcription (Yen, 2015) and stimulate growth processes through secondary messengers (Heinlein and Chang, 2002; Filardo and Thomas, 2012). Zinc may augment both genomic and nongenomic steroidal implant signaling. For example, Zn acts on the nongenomic G protein-coupled estrogen receptor (GPER1) to phosphorylate epidermal growth factor receptor (EGFR) and insulin-like growth factor-1 receptor (IGF1R) in a dose-dependent manner (Pisano et al., 2017), leading to the activation of growth processes. Zinc supports transcription factors, deoxyribonucleic acid, and protein synthesis (Oberleas and Prasad, 1969; Duncan and Dreosti, 1976; Cousins et al., 2006), all vital components of growth. Zinc’s role in DNA synthesis was specifically demonstrated when thymidine kinase activity, a key enzyme for the incorporation of thymidine into DNA, was decreased in the liver of Zn-deficient rats (Duncan and Dreosti, 1976). The steroidal implant, zeranol, increased Zn absorption and retention in lambs (Hufstedler and Greene, 1995) and the supplementation of 200 mg Zn/kg dry matter (DM) basis from ZnSO4 to steers resulted in a greater ADG response to a potent steroidal implant than steers receiving a control diet that exceeded NASEM (30 mg Zn/kg DM; 2016) Zn recommendations (Huerta et al., 2002). These data suggest administration of steroidal implants may increase the Zn requirements of the animal to accommodate increased growth rates and greater supplementation of Zn may promote steroidal implant-induced growth. Therefore, the objective of this study was to determine the effects of increasing dietary Zn supplementation within steroidal implanted and non-steroidal implanted steers on performance, carcass characteristics, liver and plasma Zn concentrations, and the expression of genes associated with growth and Zn metabolism. It was hypothesized that optimal Zn supplementation to improve growth and subsequent effects on relative gene expression measures would differ between steroidal implanted and non-steroidal implanted steers.
Materials and Methods
All procedures and protocols utilized in this study were approved by the Iowa State University Institutional Animal Care and Use Committee (log number: IACUC-20-053).
Animals and experimental design
One hundred and twenty-eight Angus-crossbred steers (492 ± 29 kg) were utilized in a complete randomized design arranged as a 2 × 4 factorial resulting in 8 treatments to test the effect of Zn supplementation within steroidal implant treatment. Steroidal implant treatments included no steroidal implant (NoIMP) or a Component TE-200 (TE200; 200 mg trenbolone acetate + 20 mg estradiol; donated by Elanco Animal Health, Greenfield, IN) administered on day 0. Cattle were fed 0, 30, 100, or 150 mg Zn/kg DM (Zn0, Zn30, Zn100, or Zn150, respectively) from ZnSO4 starting on day 0. Zinc treatments were added to the diet through dried distiller grains with solubles-based premix. Prior to the start of the experiment, all steers received a corn silage-based growing diet supplemented with 30 mg Zn/kg DM from ZnSO4. Steers were fed once daily (0800 h) and transitioned to a dry-rolled corn-based diet during the first 14 d of the experiment and remained on the finishing diet through the remainder of the 59-d study (Table 1). The Zn0 diet analyzed 48, 40, and 42 mg Zn/kg DM for transition 1, transition 2, and finishing, respectively. Cattle were stratified by body weight (BW) into pens (n = 5 or 6 steers per pen) to evenly disperse BW across treatments. Pens were equipped with a single GrowSafe (GrowSafe Systems Ltd., Airdrie, AB, Canada) bunk and cattle were provided ad libitum access to feed. Radio-frequency tags on each steer relayed individual steer feed disappearance data from the bunk to GrowSafe software. Cattle were randomly assigned to steroidal implant and supplemental Zn treatments within pen (n = 3 pens/treatment). All cattle within a pen received the same steroidal implant and supplemental Zn treatment, and individual intake and performance data were collected for each steer. Therefore, the steer was the experimental unit. Cattle were harvested on day 61 at a commercial abattoir (Greater Omaha Beef, Omaha, NE) via industry-accepted practices. Trained Greater Omaha Beef personnel collected hot carcass weight (HCW) while ribeye area (REA), 12th rib fat (BF), marbling score, and yield grade (YG) were obtained through a camera grading system following a 48-h chill.
Table 1.
Compositional analysis of non-zinc supplemented diet
| Ingredient, % of diet DM | Transition 1 days 0 to 6 |
Transition 2 days 7 to 13 |
Finisher days 14 to 59 |
|---|---|---|---|
| Dry rolled corn | 30 | 45 | 57 |
| Sweet Bran1 | 25 | 20 | 20 |
| DDGS2 | 18.06 | 13.06 | 13.06 |
| Bromegrass hay | 5 | 8 | 8 |
| Corn silage | 20 | 12 | - |
| Limestone | 1.5 | 1.5 | 1.5 |
| Salt | 0.31 | 0.31 | 0.31 |
| Vitamin & mineral premix3 | 0.1159 | 0.1159 | 0.1159 |
| Rumensin | 0.0135 | 0.0135 | 0.0135 |
| Analyzed composition, % | |||
| Crude protein4 | 16.6 | 13.1 | 13.9 |
| NDF4 | 28.0 | 31.2 | 20.9 |
| Ether extract4 | 5.5 | 5.0 | 4.4 |
| Zn5, mg/kg DM | 48 | 40 | 42 |
| Cu5, mg/kg DM | 17 | 13 | 12 |
| Calculated composition6, Mcal/kg | |||
| NEm | 1.97 | 1.98 | 2.05 |
| NEg | 1.32 | 1.33 | 1.39 |
1Branded wet corn gluten feed (Cargill Corn Milling, Blair, NE).
2Dried distillers grains with solubles.
3Premix provided 2,200 IU vitamin A and 25 IU vitamin E/kg diet. All diets included NASEM (2016) recommendations for Co, Cu, I, Mn, and Se. Diets were supplemented with Zn at 0, 30, 100, or 150 mg Zn/kg DM. All trace minerals were from inorganic sources.
4Analysis of Zn0 TMR was conducted by Dairyland Laboratories (Arcadia, WI).
5Trace minerals were analyzed by inductively coupled plasma optical emission spectrometry (ICP Optima 7000 DV, Perkin Elmer, Waltham, MA). Analyses were of Zn0 TMR. Analysis of Zn30, Zn100, and Zn150 were 68, 122, and 148 mg Zn/kg DM, 77, 121, and 107 mg Zn/kg DM, and 48, 101, and 137 mg Zn/kg DM for transition 1, transition 2, and finishing diets, respectively.
6Net energy of maintenance (NEm) and gain (NEg) were calculated using ingredient nutrient values from NASEM (2016).
Sample collection and analysis
Total mixed ration (TMR) samples of each treatment were collected weekly to calculate DM by drying in a forced air oven for 48 h at 70 °C. Dried TMR samples were ground through a 2-mm screen (Retsch Zm100 grinder; Glen Mills Inc., Clifton, NJ) and composited by month within each dietary Zn treatment. The composited Zn0 TMR was sent to Dairyland Laboratories (Arcadia, WI) for nutrient analysis (methods denoted in Heiderscheit and Hansen, 2020). BWs were taken on days −1, 0, 18, 40, 55/56, and 59 (n = 15, 16, or 17 steers/treatment). Blood was collected on days −1, 18, and 40 (n = 8, 9, or 10 steers/treatment) via jugular venipuncture in vacuum-capped tubes (Becton Dickerson, Rutherford, NJ) containing trace mineral grade K2EDTA for plasma trace mineral analysis or sodium heparin for plasma urea nitrogen (PUN) analysis. Blood tubes were spun in a temperature-controlled centrifuge (4 °C) at 1,000 × g for 10 min for K2EDTA tubes and 20 min for sodium heparin tubes before storing at −20 °C until analysis was completed. A commercial kit (Teco Diagnostics, Anaheim, CA) was utilized to determine PUN concentrations with an intra-assay and inter-assay CV of 6.33% and 7.15%, respectively. PUN data are not discussed herein but are shown in Supplementary Table 1.
Muscle biopsies were conducted on 9 randomly selected steers per treatment (n = 3 steers/pen) on d 11 utilizing procedures adapted from Pampusch et al. (2008). At time points split across 2 d, half of the cattle were biopsied on each day. Biopsies were extracted from the longissimus thoracis between the 10th and 13th rib spaces. Muscle samples were flash-frozen in liquid nitrogen and stored at −80 °C prior to analysis.
Due to limitations in sample analysis, muscle samples from only 5 of the 8 treatments (NoIMP: Zn0 and Zn150 and TE200: Zn0, Zn100, and Zn150) were analyzed for quantitative gene expression using the 48.48 Dynamic Array Integrated Fluidic Circuit (Fluidigm, San Francisco, CA) as described by Suasnavas et al. (2015). Prior to quantitative gene expression analysis, RNA was isolated from muscle samples using Trizol Reagent (Life Technologies, Carlsbad, CA, USA) and cDNA was synthesized using random primers and Superscript III Reverse Transcriptase (Invitrogen, Waltham, MA, USA) in accordance with manufacturer’s instructions (McGill et al., 2016). Twenty-four genes specific to the steroidal implant growth mechanism and the biological functions of Zn were targeted for analysis (Supplementary Table 2) and primer sets for each gene were designed and validated by Fluidigm. In brief, a Specific Target Amplification (STA) was performed to enrich each sample for target-specific cDNA prior to quantitative gene expression analysis, in accordance with Fluidigm protocol. For STA thermal cycling, each reaction consisted of 1.25 µL of the primer mix, 2.5 µL of the TaqMan PreAmp Master Mix (Applied Biosystems, Foster City, CA), and 1.25 µL of cDNA diluted 1:20 in nuclease-free water. Enzyme activation took place at 95 °C for 10 min before amplification was conducted for 14 cycles (95 °C for 15 s then 60 °C for 4 min). The Fluidigm IFC chip was run on the Biomark thermocycler/detection module. The average expression of housekeeping genes eukaryotic translation elongation factor 1 alpha 2 (EEF1A2) and ribosomal protein S9 (RPS9) was utilized as a reference to determine the relative gene expression of each parameter using the 2−∆∆Ct method (Livak and Schmittgen, 2001). Relative gene expression was calculated relative to the NoIMP-Zn0 treatment.
Liver biopsies using procedures described by Engle and Spears (2000) were conducted on day 55/56 with half of the steers sampled on each day (n = 8, 9, or 10 steers/treatment). Liver samples were stored at −20 °C before analysis for common trace minerals involved in growth including Cu, Fe, Mn, and Zn. Liver and composited TMR samples were acid-digested following the procedures of Pogge and Hansen (2013) and Richter et al. (2012), respectively. Trace mineral concentrations of liver, TMR, and plasma were measured via inductively coupled plasma optical emission spectrometry (Optima 7000 DV, Perkin Elmer, Waltham, MA) as described by Pogge and Hansen (2013) and Richter et al. (2012). A standard was utilized on each run to verify instrument accuracy (Trace Elements Serum Control #66816; UTAK Laboratories Inc., Valencia, CA; Bovine Liver #1577c; National Institute of Standards and Technology, Gaithersburg, MD).
Statistical analysis
Data were analyzed via the Mixed Procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC) with the fixed effect of treatment. Contrast statements were formed using the IML Procedure of SAS 9.4 (SAS Inst. Inc.) to specifically test for linear and quadratic effects of Zn supplementation within NoIMP and TE200 treatments as well as to test NoIMP vs. TE200, regardless of Zn treatment. Steer served as the experimental unit for all analyses (n = 15, 16, or 17 steers/treatment for performance and carcass parameters or n = 8, 9, or 10 steers/treatment for blood and liver data). Initial BW was utilized as a covariate in all performance and carcass data analysis and initial plasma Zn concentrations served as covariates for subsequent timepoint analysis. Because not all treatments were included in the assessment of relative gene expression in day 11 muscle samples additional contrast statements were formed. These statements included testing the effect of Zn0 vs. Zn150 within NoIMP and TE200 treatments, NoIMP vs. TE200 (Zn0 and Zn150 only), and the linear effect of Zn0, Zn100, and Zn150 within TE200. Outliers were assessed using the Cook’s D statistical test with values above 0.20 removed from analysis. One steer was removed from performance and carcass parameters (NoIMP-Zn30) due to poor performance related to health concerns. Data are reported as the least squares mean with the standard error of the mean, and statistical significance was determined as P ≤ 0.05 with tendencies between 0.05 < P ≤ 0.10.
Results
Performance
No differences in BW were observed on day 0 (Table 2; P ≥ 0.67) between Zn treatments. However, day 18 BW, days 0 to 18 ADG, and days 0 to 18 feed efficiency (G:F) linearly increased as Zn supplementation increased within TE200 (P ≤ 0.02) and were greater for steroidal implanted steers than non-steroidal implanted steers (P ≤ 0.01). Steroidal implanted steers also tended to have greater days 0 to 18 dry matter intake (DMI) than NoIMP (P = 0.09). Zinc supplementation tended to quadratically decrease days 0 to 18 DMI (P = 0.09) and quadratically increase days 0 to 18 G:F (P = 0.05) of NoIMP.
Table 2.
Effects of zinc1 supplementation on performance parameters of non-steroidal implanted and steroidal implanted2 beef feedlot steers
| NoIMP | TE200 | Contrasts3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn0 | Zn30 | Zn100 | Zn150 | Zn0 | Zn30 | Zn100 | Zn150 | SEM | L-NoIMP | Q-NoIMP | L- TE200 |
Q- TE200 |
NoIMP vs. TE200 | |
| Steer (n)4 | 17 | 15 | 16 | 16 | 15 | 16 | 16 | 16 | ||||||
| BW, kg | ||||||||||||||
| day 0 | 490 | 495 | 491 | 492 | 495 | 492 | 492 | 489 | 7.6 | 0.99 | 0.91 | 0.67 | 0.95 | 0.98 |
| day 18 | 528 | 532 | 532 | 531 | 531 | 534 | 534 | 540 | 1.8 | 0.34 | 0.18 | 0.002 | 0.34 | 0.005 |
| day 59 | 595 | 596 | 603 | 596 | 605 | 607 | 608 | 603 | 3.8 | 0.50 | 0.17 | 0.81 | 0.26 | 0.002 |
| days 0 to 18 | ||||||||||||||
| ADG, kg | 2.01 | 2.24 | 2.22 | 2.18 | 2.17 | 2.31 | 2.32 | 2.65 | 0.101 | 0.34 | 0.18 | 0.002 | 0.34 | 0.005 |
| DMI, kg | 11.4 | 11.2 | 11.2 | 12.1 | 11.5 | 11.4 | 11.7 | 12.0 | 0.24 | 0.11 | 0.09 | 0.09 | 0.48 | 0.52 |
| G:F | 0.177 | 0.192 | 0.200 | 0.181 | 0.188 | 0.203 | 0.199 | 0.222 | 0.0094 | 0.71 | 0.05 | 0.02 | 0.61 | 0.01 |
| Carcass-adjusted overall5 | ||||||||||||||
| Final BW | 594 | 593 | 600 | 593 | 604 | 606 | 610 | 613 | 4.0 | 0.81 | 0.35 | 0.10 | 0.99 | <0.0001 |
| ADG | 1.70 | 1.69 | 1.79 | 1.68 | 1.87 | 1.89 | 1.96 | 2.01 | 0.066 | 0.81 | 0.35 | 0.10 | 0.99 | <0.0001 |
| G:F | 0.151 | 0.152 | 0.163 | 0.145 | 0.162 | 0.168 | 0.170 | 0.174 | 0.0053 | 0.74 | 0.04 | 0.12 | 0.86 | <0.0001 |
1Cattle were supplemented 0, 30, 100, or 150 mg Zn/kg DM (Zn0, Zn30, Zn100, or Zn150, respectively) from ZnSO4.
2Steroidal implant strategies included no steroidal implant (NoIMP) or a Component TE-200 (TE200; 200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0.
3Contrast statements were formed to test for linear (L) and quadratic (Q) effects of Zn supplementation within non-steroidal implanted (L-NoIMP and Q-NoIMP) or steroidal implanted (L-TE200 or Q-TE200) steers. A separate contrast statement was formed to test for differences between non-steroidal implanted steers and all steroidal implanted steers (NoIMP vs. TE200).
4Initial body weight (BW) was utilized as a covariate in all performance analyses including body weight (BW), average daily gain (ADG), dry matter intake (DMI), and gain:feed (G:F).
5Carcass adjusted performance was calculated using the average dressing percentage for all treatments: 62.25%.
By the end of the trial, TE200 were 8.25 and 13.25 kg heavier than NoIMP for day 59 BW and carcass-adjusted final BW, respectively (P ≤ 0.002). Furthermore, TE200 steers had greater days 0 to 59 and carcass-adjusted ADG and G:F (P ≤ 0.02). However, days 0 to 59 DMI was not influenced by Zn supplementation or steroidal implant treatment (P ≥ 0.15). A tendency for a linear increase due to Zn supplementation in carcass-adjusted final BW and ADG was observed in steroidal implanted steers (P ≤ 0.10), while carcass-adjusted G:F quadratically increased within NoIMP (P = 0.04) with Zn100 having the greatest G:F.
Carcass characteristics
HCW tended to linearly increase (Table 3; P = 0.09) while dressing percentage linearly increased (P = 0.01) with increasing Zn supplementation within TE200. Steroidal implanted steers had greater HCW, dressing percentage, and REA (P ≤ 0.02) than NoIMP. Both BF and YG tended to quadratically decrease with increasing Zn supplementation within NoIMP (P ≤ 0.09) with Zn100 having lesser BF and YG than other Zn treatments. However, marbling was not influenced by Zn supplementation or steroidal implant treatment (P ≥ 0.17).
Table 3.
Effects of zinc1 supplementation within non-steroidal implanted and steroidal implanted2 beef steers on carcass characteristics
| NoIMP | TE200 | Contrasts3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn0 | Zn30 | Zn100 | Zn150 | Zn0 | Zn30 | Zn100 | Zn150 | SEM | L-NoIMP | Q-NoIMP | L- TE200 |
Q- TE200 |
NoIMP vs. TE200 | |
| Steer (n)4 | 17 | 15 | 16 | 16 | 15 | 16 | 16 | 16 | ||||||
| Hot carcass weight, kg | 370 | 369 | 373 | 369 | 376 | 377 | 380 | 381 | 2.5 | 0.80 | 0.39 | 0.09 | 0.98 | <0.0001 |
| Dress, % | 62.2 | 62.0 | 61.9 | 62.0 | 62.3 | 62.1 | 62.4 | 63.2 | 0.30 | 0.62 | 0.65 | 0.01 | 0.13 | 0.02 |
| Ribeye area, cm2 | 84.4 | 81.7 | 86.5 | 83.1 | 89.4 | 88.8 | 87.9 | 87.6 | 1.96 | 0.76 | 0.56 | 0.48 | 0.91 | 0.001 |
| 12thrib fat, cm | 1.61 | 1.67 | 1.43 | 1.69 | 1.50 | 1.44 | 1.52 | 1.56 | 0.086 | 0.87 | 0.09 | 0.43 | 0.67 | 0.12 |
| Marbling5 | 491 | 470 | 483 | 495 | 472 | 460 | 452 | 472 | 22.0 | 0.70 | 0.52 | 0.96 | 0.43 | 0.17 |
| Yield grade6 | 3.6 | 3.6 | 3.4 | 3.7 | 3.5 | 3.4 | 3.5 | 3.5 | 0.08 | 0.87 | 0.09 | 0.43 | 0.67 | 0.12 |
1Zinc was supplemented to cattle at 0, 30, 100, or 150 mg Zn/kg DM from ZnSO4 (Zn0, Zn30, Zn100, or Zn150, respectively).
2Steroidal implant strategies included no steroidal implant (NoIMP) or a Component TE-200 (TE-200; 200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0.
3Contrast statements were formed to test for linear (L) and quadratic (Q) effects of Zn supplementation within non-steroidal implanted (L-NoIMP and Q-NoIMP) or steroidal implanted (L-TE200 or Q-TE200) steers. A separate contrast statement was formed to test for differences between non-steroidal implanted steers and all steroidal implanted steers (NoIMP vs. TE200).
4Intial body weight was utilized as a covariate for carcass characteristics.
5Marbling scores: slight = 300, small = 400, modest = 500, moderate = 600, slightly abundant = 700, moderately abundant = 800.
6Yield grade was assigned by personnel of the commercial abattoir.
Trace mineral and PUN concentrations
As expected, day −1 plasma Zn concentrations were not influenced by Zn or steroidal implant treatments (Table 4; P ≥ 0.16). Day 18 and 40 plasma Zn concentrations linearly increased with increasing Zn supplementation within both NoIMP and TE200 (P ≤ 0.03). Furthermore, plasma Zn was lesser for steroidal implanted steers on day 18 than NoIMP (P = 0.001), but no effect of steroidal implant was observed for day 40 plasma Zn concentrations (P = 0.49).
Table 4.
Effects of zinc1 supplementation within non-steroidal implanted and steroidal implanted2 beef steers on trace mineral and plasma urea nitrogen concentrations
| NoIMP | TE200 | Contrasts3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn0 | Zn30 | Zn100 | Zn150 | Zn0 | Zn30 | Zn100 | Zn150 | SEM | L- NoIMP |
Q-NoIMP | L- TE200 |
Q- TE200 |
NoIMP vs. TE200 | |
| Steer (n) | 10 | 9 | 9 | 9 | 8 | 9 | 9 | 9 | ||||||
| Plasma Zn4, mg/L | ||||||||||||||
| day −1 | 1.22 | 1.31 | 1.32 | 1.32 | 1.28 | 1.28 | 1.37 | 1.35 | 0.053 | 0.17 | 0.41 | 0.16 | 0.67 | 0.42 |
| day 18 | 1.31 | 1.34 | 1.43 | 1.44 | 1.25 | 1.21 | 1.33 | 1.39 | 0.037 | 0.003 | 0.53 | 0.001 | 0.36 | 0.001 |
| day 40 | 1.33 | 1.25 | 1.36 | 1.42 | 1.29 | 1.27 | 1.35 | 1.38 | 0.038 | 0.01 | 0.12 | 0.03 | 0.82 | 0.49 |
| day 55 liver5, mg/kg DM | ||||||||||||||
| Zn | 115 | 113 | 122 | 115 | 118 | 118 | 125 | 127 | 6.1 | 0.69 | 0.59 | 0.20 | 0.97 | 0.20 |
| Mn | 8.7 | 7.9 | 8.6 | 8.6 | 8.2 | 7.9 | 7.6 | 8.4 | 0.46 | 0.65 | 0.55 | 0.82 | 0.15 | 0.10 |
| Cu | 310 | 274 | 277 | 252 | 278 | 268 | 268 | 293 | 29.9 | 0.15 | 0.86 | 0.67 | 0.49 | 0.94 |
| Fe | 181 | 181 | 173 | 184 | 178 | 170 | 171 | 184 | 11.2 | 0.99 | 0.47 | 0.59 | 0.28 | 0.59 |
1Zinc was supplemented to cattle at 0, 30, 100, or 150 mg Zn/kg DM from ZnSO4 (Zn0, Zn30, Zn100, or Zn150, respectively).
2Steroidal implant strategies included no steroidal implant (NoIMP) or a Component TE-200 (TE-200; 200 mg trenbolone acetate + 20 mg estradiol; Elanco Animal Health, Greenfield, IN) on day 0.
3Contrast statements were formed to test for linear (L) and quadratic (Q) effects of Zn supplementation within non-steroidal implanted (L-NoIMP and Q-NoIMP) or steroidal implanted (L-TE200 or Q-TE200) steers. A separate contrast statement was formed to test for differences between non-steroidal implanted steers and all steroidal implanted steers (NoIMP vs. TE200).
4Plasma Zn from day −1 was utilized as a covariate in days 18 and 40 plasma Zn analysis.
5Liver samples were collected on days 55/56 with half of the samples collected on each d.
Liver Zn, Cu, and Fe concentrations measured on days 55 or 56 were not affected by increasing Zn supplementation (P ≥ 0.15) or steroidal implant treatment (P ≥ 0.20). Liver Mn tended to be lesser for TE200 steers than NoIMP steers (P = 0.10) and was not affected by Zn supplementation (P ≥ 0.15).
Gene expression
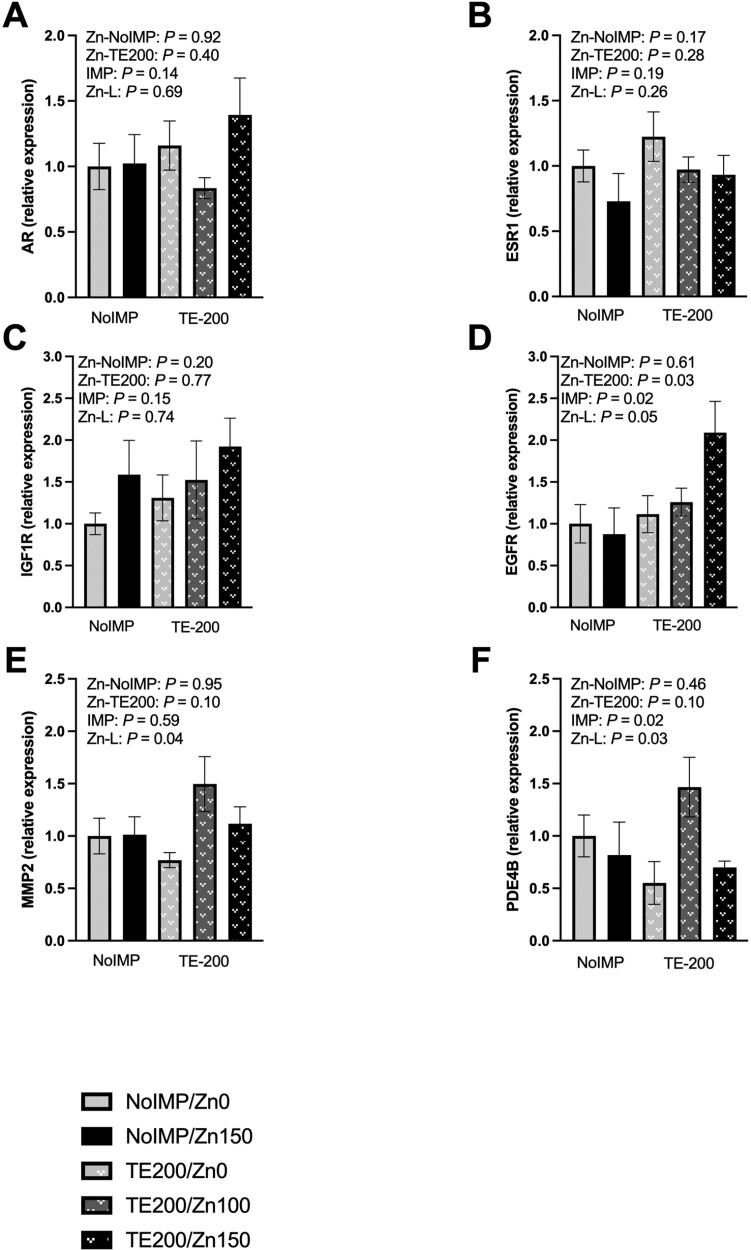
The relative gene expression of steroidal implant hormone receptors and signaling machinery are displayed in Figure 1. Neither androgen receptor (Figure 1A), estrogen receptor 1 (ESR1; Figure 1B), or IGF1R (Figure 1C) were influenced by Zn0 or Zn150 supplementation within NoIMP or TE200 treatments (P ≥ 0.17), steroidal implant treatment (P ≥ 0.14), or a linear response to 0, 100, or 150 mg Zn/kg DM supplementation within TE200 steers (P ≥ 0.26). However, EGFR relative gene expression was greater for Zn150 than Zn0 within TE200 (Figure 1D; P = 0.03). Furthermore, EGFR gene expression was greatest for steroidal implanted cattle (P = 0.02) and linearly increased with supplementation of 0, 100, and 150 mg Zn/kg DM within TE200 (P = 0.05).
Figure 1.
Relative gene expression of steroidal implant receptors and growth signaling machinery from the muscle of steers receiving no steroidal implant (NoIMP) or a steroidal implant (TE200) and fed 0, 100, or 150 mg Zn/kg DM (Zn0, Zn100, Zn150, respectively). Samples were collected from the longissimus thoracis on day 11 and relative gene expression was calculated relative to NoIMP-Zn0 treatment. Contrast statements were formed to test the effect of increasing Zn from Zn0 to Zn150 within NoIMP (Zn-NoIMP) and TE200 (Zn-TE200), in addition to the main effect of steroidal implant (IMP; Zn0 and Zn150) and the linear effect of increasing Zn as Zn0, Zn100, and Zn150 within TE200 (Zn-L). A) Androgen receptor (AR), B) estrogen receptor (ESR1), and C) insulin-like growth factor-1 receptor (IGF1R) gene expression were not influenced by Zn or steroidal implant contrast statements (P ≥ 0.14). D) Epidermal growth factor receptor (EGFR) gene expression was greater for Zn150 than Zn0 steers administered TE200 (P = 0.03) and linearly increased with 0, 100, and 150 mg/kg DM of supplemental Zn within TE200 steers (P = 0.05). Steroidal implanted cattle (Zn0 and Zn150) had greater EGFR gene expression than NoIMP (P = 0.02). E) Matrix metalloproteinase 2 (MMP2) gene expression tended to be greater for Zn150 than Zn0 within TE200 (P = 0.10) and linearly increased with 0, 100, and 150 mg/kg DM of supplemental Zn for TE200 steers (P = 0.04). F) Phosphodiesterase 4B (PDE4B) gene expression tended to be greater for Zn150 than Zn0 within TE200 (P = 0.10) and linearly increased in TE200 steers with 0, 100, and 150 mg/kg DM of supplemental Zn (P = 0.03). Steroidal implanted cattle had lesser muscle PDE4B gene expression than NoIMP for Zn0 and Zn150 cattle (P = 0.02).
Matrix metalloproteinase 2 (MMP2) tended to be greater for Zn150 than Zn0 within TE200 (Figure 1E; P = 0.10) and linearly increased (P = 0.04) with increasing supplemental Zn at 0, 100, and 150 mg Zn/kg DM within TE200. Similarly, phosphodiesterase 4B (PDE4B) relative gene expression tended to be greater for Zn150 than Zn0 within TE200 steers (Figure 1F; P = 0.10) but was lesser for steroidal implanted steers than NoIMP supplemented Zn0 or Zn150 (P = 0.02). However, PDE4B gene expression linearly increased with the supplementation of 0, 100, and 150 mg Zn/kg DM within TE200 (P = 0.03). The linear responses observed in both MMP2 and PDE4B gene expression were largely driven by high relative gene expression of Zn100-TE200 steers.
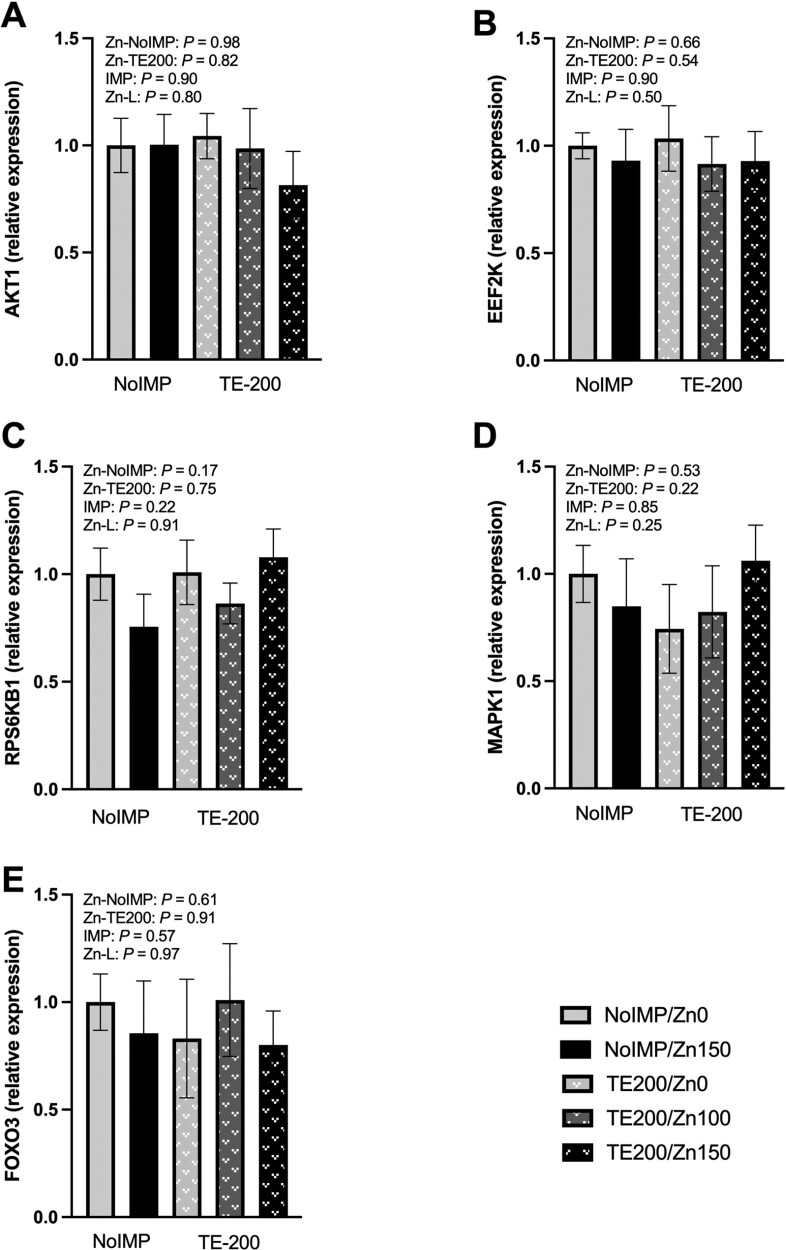
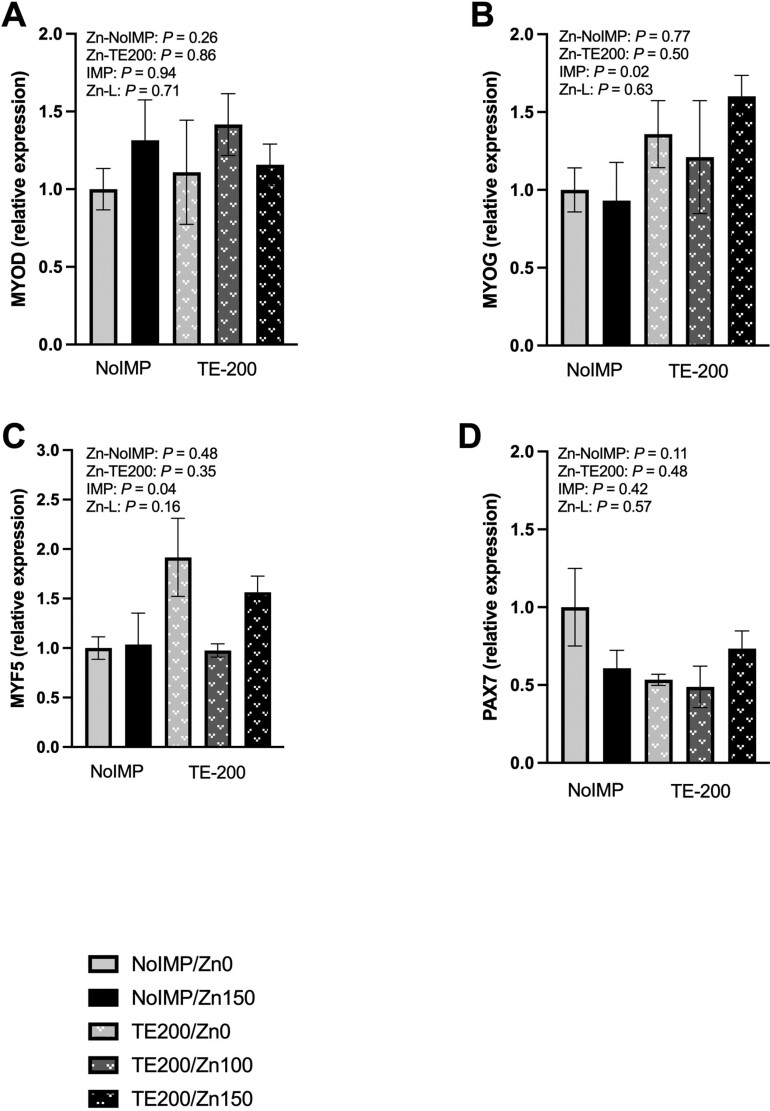
The relative gene expression of select genes involved in growth signaling are found in Figure 2. No effects of Zn or IMP were observed through contrast statements formed for gene expression for protein kinase B (AKT1; Figure 2A), eukaryotic elongation factor 2 kinase (EEF2K; Figure 2B), ribosomal protein S6 kinase B1 (RPS6KB1; Figure 2C), mitogen-activated protein kinase 1 (MAPK1; Figure 2D), or forkhead box O-3 (FOXO3; Fig 2E; P ≥ 0.17). However, some markers of satellite cell development were influenced by steroidal implant strategy (Figure 3). Both myogenin (MYOG; Figure 3B) and myogenic factor 5 (MYF5; Figure 3C) were greater for Zn0 and Zn150 steroidal implanted steers than NoIMP counterparts (P ≤ 0.04). No further differences in relative gene expression were observed for markers of satellite cell development (P ≥ 0.11).
Figure 2.
Relative gene expression of growth signaling proteins from the muscle of steers not steroidal implanted (NoIMP) or steroidal implanted (TE200) and supplemented 0, 100, or 150 mg Zn/kg DM (Zn0, Zn100, Zn150, respectively). Samples were collected on day 11 from the longissimus thoracis. Relative gene expression was calculated relative to NoIMP-Zn0 treatment. Contrast statements were formed to test the effect of increasing Zn from Zn0 to Zn150 within NoIMP (Zn-NoIMP) and TE200 (Zn-TE200), in addition to the main effect of steroidal implant (IMP; Zn0 and Zn150) and the linear effect of increasing Zn as Zn0, Zn100, and Zn150 within TE200 (Zn-L). A) Protein kinase B (AKT1), B) eukaryotic elongation factor 2 kinase (EEF2K), C) ribosomal protein S6 kinase B1 (RPS6KB1), D) mitogen-activated protein kinase 1 (MAPK1), and E) forkhead box O-3 (FOXO3) gene expression were not affected by Zn or steroidal implant contrast statements (P ≥ 0.17).
Figure 3.
The relative gene expression of markers of satellite cell development was analyzed in non-steroidal implanted (NoIMP) and steroidal implanted (TE200) steers that were supplemented 0, 100, or 150 mg Zn/kg DM (Zn0, Zn100, Zn150, respectively). Samples from the longissimus thoracis were collected on day 11 and relative gene expression was calculated relative to NoIMP-Zn0 treatment. Contrast statements were formed to test the effect of increasing Zn from Zn0 to Zn150 within NoIMP (Zn-NoIMP) and TE200 (Zn-TE200), in addition to the main effect of steroidal implant (IMP; Zn0 and Zn150) and the linear effect of increasing Zn as Zn0, Zn100, and Zn150 within TE200 (Zn-L). A) Myogenic differentiation 1 (MYOD) gene expression was not influenced by the tested contrast statements (P ≥ 0.26). B) Myogenin (MYOG) and C) myogenic factor 5 (MYF5) gene expression were greater for TE200 than NoIMP for Zn0 and Zn150 treatments (P ≤ 0.04). D) Paired box protein 7 (PAX7) gene expression was not influenced by the tested contrast statements (P ≥ 0.11).
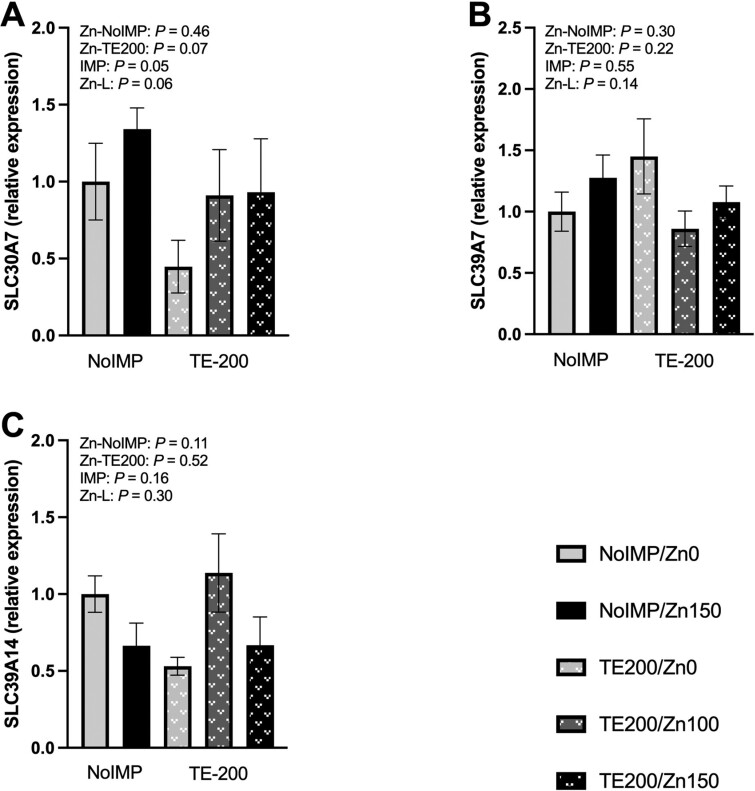
Within TE200, Zn150 tended to have greater relative gene expression of the Zn transporter solute carrier family 30 member 7 (SLC30A7) than Zn0 (Figure 4A; P = 0.07), and SLC30A7 gene expression tended to linearly increase with supplementation of 0, 100, and 150 mg Zn/kg DM within TE200 steers (P = 0.06). Furthermore, TE200 steers had lesser SLC30A7 relative gene expression than NoIMP (P = 0.05). Neither solute carrier family 39 member 7 (SLC39A7; Figure 4B) or solute carrier family 39 member 14 (SLC39A14; Figure 4C) relative gene expression were influenced by the tested contrast statements (P ≥ 0.11).
Figure 4.
Relative gene expression of Zn transporters in the muscle of non-steroidal implanted (NoIMP) and steroidal implanted (TE200) steers supplemented 0, 100, or 150 mg Zn/kg DM (Zn0, Zn100, Zn150, respectively). Samples were collected on day 11 from the longissimus thoracis and relative gene expression was determined relative to NoIMP-Zn0 treatment. Contrast statements were formed to test the effect of increasing Zn from Zn0 to Zn150 within NoIMP (Zn-NoIMP) and TE200 (Zn-TE200), in addition to the main effect of steroidal implant (IMP; Zn0 and Zn150) and the linear effect of increasing Zn as Zn0, Zn100, and Zn150 within TE200 (Zn-L). A) Solute carrier family 30 member 7 (SLC30A7) gene expression tended to be greater for Zn150 than Zn0 within TE200 steers (P = 0.07) and tended to linearly increase with increasing Zn supplementation (Zn0, Zn100, and Zn150) within TE200 (P = 0.06). Gene expression of SLC30A7 was lesser for TE200 than NoIMP (P = 0.05). B) Solute carrier family 39 member 7 (SLC39A7) and C) solute carrier family 39 member 14 (SLC39A14) were not affected by the tested contrast statements (P ≥ 0.11).
Discussion
Steroidal implants improve cattle ADG (Bartle et al., 1992; Duckett and Pratt, 2014) with the greatest potential for gain occurring in the first 40 d post-steroidal implant during peak hormonal payout (Johnson et al., 1996). It was hypothesized increasing Zn concentrations up to 5 times NASEM (2016) recommendations (30 mg Zn/kg DM) may be needed to optimize steroidal implant-induced growth of cattle. Zinc is involved in DNA and protein synthesis through a multitude of biological pathways including phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), mitogen-activated protein kinase (MAPK), and mammalian target of rapamycin (MTOR; Jung et al., 2009). Indeed, increasing supplemental Zn linearly increased ADG of steroidal implanted cattle within 18 d of steroidal implant administration, though no effects of Zn were observed for non-steroidal implanted steers at this time. Similarly, Huerta et al. (2002) found steers supplemented with 200 mg Zn/kg DM from ZnSO4 had a 9.8% greater growth response to a high potency steroidal implant than steers fed a control diet containing 84 mg Zn/kg DM. Niedermayer et al. (2018) observed improved gain and HCW in steers supplemented with trace minerals (Cu, Co, Fe, Mn, Se, and Zn) at 2 to 3 times NASEM (2016) recommendations, regardless of receiving a steroidal implant or not. In contrast to the current study, Niedermayer et al. (2018) supplemented cattle with a high concentration of trace minerals for twice as long (124 d). The short feeding period (59 d) of the current study may have limited potential Zn responses within non-steroidal implanted steers. However, the proximity of cattle to a mature BW may have also contributed to the lack of response to Zn supplementation within non-steroidal implanted cattle. Spears and Kegley, (2002) found Zn supplemented steers (33 mg Zn/kg DM) had improved performance during the growing period followed by a diminished Zn growth response during the finishing period. The current study was conducted during the Spring of 2020 during the COVID-19 pandemic explaining the heavy initial BW (492 kg) of cattle enrolled in the study. Perhaps, cattle maturity hindered the potential growth benefits of supplemental Zn in non-steroidal implanted steers. As cattle mature on the growth curve, lean tissue accretion lessens (Owens et al., 1995).
Biopsies of the longissimus thoracis collected on day 11 post-steroidal implant administration allow for a closer examination of growth processes affected by Zn. Considering no effects of steroidal implant or Zn supplementation were observed for androgen and E2 receptor relative gene expression, the current study was unable to assess the direct effects of Zn on the genomic steroidal implant mode of action. However, upon estrogen stimulation from a steroidal implant, GPER initiates non-genomic signaling through the cleaving action of MMP2 or 9 on heparin-binding epidermal growth factor-like growth factor and the subsequent activation of EGFR to trigger growth processes including cell proliferation (Thornton et al., 2015). Pisano et al. (2017) found Zn acts through GPER to activate signaling through IGF1R and EGFR as Zn depletion hindered IGF1R and EGFR signaling. The linear response of EGFR relative gene expression to increasing Zn supplementation within steroidal implanted steers aligns with the findings of Pisano et al. (2017) and the linear increase in days 0 to 18 ADG of the current study. As a component of the non-genomic steroidal implant signaling mechanism, EGFR leads to the activation of downstream proteins such as extracellular-signal-regulated-kinase 1 and 2 (ERK-1/-2) that induce cell proliferation and migration (Wells, 1999; Filardo et al., 2000). Both ERK and AKT activation in human breast cancer cells have been observed with increasing Zn exposure (Pisano et al., 2017), indicating the effects of Zn supplementation on growth pathways are observed downstream of cell surface receptors. However, MAPK1 (representative of ERK-2) gene expression and AKT1 gene expression were not influenced by steroidal implant or Zn supplementation within steroidal implanted steers of the current study. Measurements at the protein level may be more reflective of Zn’s effects on steroidal implant-induced signaling as Zn is associated with increased protein phosphorylation (Wu et al., 1999; Samet et al., 2003; Pisano et al., 2017). Although the small sample size in the present study may have limited relative gene expression results, maintaining an α level of P ≤ 0.05 for statistical significance and 0.05 < P ≤ 0.10 for tendencies protected relative gene expression data from both Type I and Type II if the α level was too high or not high enough, respectively.
As components of non-genomic steroidal implant-induced signaling (Shakur et al., 2001; Thornton et al., 2015), MMP2 and PDE4B showed interesting responses to Zn supplementation. The linear response to Zn for MMP2 and EGF4 gene expression corresponds with increased days 0 to 18 growth of steroidal implanted steers. As a regulator of G protein-coupled receptor signaling, PDE4B degrades intracellular cyclic adenosine monophosphate (cAMP) to inhibit downstream non-genomic signaling (Shakur et al., 2001). Gene expression of PDE4B is inhibited by Zn in cell culture (von Bulow et al., 2005). Therefore, it was hypothesized increasing Zn supplementation would decrease the relative gene expression of PDE4B within steroidal implanted steers in a dose-dependent manner, leading to sustained cAMP signaling and increased growth rates. Indeed, steroidal implanted steers had lesser PDE4B gene expression than non-steroidal implanted steers. However, a linear increase in PDE4B relative gene expression with increasing Zn supplementation of steroidal implanted steers was observed, largely attributed to high Zn100 expression. It is uncertain why PDE4B gene expression would increase with Zn supplementation, though the small sampling size may have exaggerated this response.
Interestingly, non-steroidal implanted steers supplemented with 100 mg Zn/kg DM seemed to perform comparably to steroidal implanted steers throughout the trial and had numerically greater HCW than NoIMP counterparts. Therefore, supplementing 100 mg Zn/kg DM to non-steroidal implanted cattle may make up for the lost growth potential of not utilizing a steroidal implant. Although Zn supplementation did not affect day 59 BW, the early effects of Zn on steroidal implant-induced growth performance were apparent in HCW, and Zn linearly increased the dressing percentage of steroidal implanted steers. It is worth considering that as carcass transfer increases late in the feeding period (Macdonald et al., 2007), Zn may be particularly important to help transfer live gain to carcass gain (Genther-Schroeder et al., 2016).
Within the limited literature focused on Zn supplementation to cattle utilizing growth-promoting technologies, the growth response to supplemental Zn is inconsistently transferred to HCW. For example, the 7 kg BW advantage Messersmith et al. (2021) observed in steroidal implanted heifers supplemented 100 vs. 30 mg Zn/kg DM 48 d before harvest was not observed in HCW. Similarly, Huerta et al. (2002) found no differences in HCW due to Zn supplementation of 0 or 200 mg Zn/kg DM in steers and heifers, despite ADG differences throughout the trial. More statistical power appears necessary to pick up these subtle, yet impactful effects of Zn on HCW. Indeed, a post hoc power analysis revealed a n of 64 to detect an HCW response between Zn0 and Zn150 of steroidal implanted steers. However, power was sufficient to detect differences in days 0 to 8 ADG (n = 13).
Plasma Zn concentrations linearly increased with increasing Zn supplementation regardless of steroidal implant treatment on days 18 and 40. Interestingly, Zn100 and Zn150 plasma Zn concentrations were relatively similar and did not exceed 1.44 mg Zn/L, supporting the tightness of plasma Zn regulation when fed these physiological (not pharmacological) Zn diets. Considering the adequacy range of 0.8 to 1.4 mg Zn/L for plasma Zn concentrations proposed by Kincaid (2000), cattle Zn status was adequate to high.
Plasma Zn concentrations were lesser on day 18 in steroidal implanted steers. These data agree with the steroidal implant-induced decrease in plasma Zn concentrations observed by Messersmith (2018) 13 d post-steroidal implant administration. However, the depression in plasma Zn concentrations observed by Messersmith (2018) persisted through day 73, unlike the present study where day 40 plasma Zn was not affected by steroidal implant administration. Perhaps the depression of plasma Zn concentrations did not persist through day 40 due to the lesser growth response after the first 18 d post steroidal implant administration. The lesser plasma Zn concentrations of steroidal implanted vs. non-steroidal implanted steers may be due to increased Zn demand in the muscle for growth processes (Oberleas and Prasad, 1969; Duncan and Dreosti, 1976) corresponding with the linear increase in days 0 to 18 performance and gene expression of steroidal implant signaling machinery such as EGFR and MMP2 of TE200 steers due to Zn supplementation. Furthermore, this steroidal implant-induced growth response appears to influence the Zn transporter SLC30A7 (ZnT7) that is important in the uptake of cytosolic Zn by both the Golgi apparatus and sarcoplasmic reticulum (Kirschke and Huang, 2002; Tuncay et al., 2017).
No effects of Zn supplementation or steroidal implant administration were observed on d 55/56 liver Cu, Fe, or Zn concentrations. Interestingly, Reichhardt et al. (2021) observed a decrease in liver Zn concentrations 2 d post-steroidal implant administration, but not on day 10 suggesting mobilization of liver Zn occurs during a limited window following steroidal implant administration. Furthermore, changes in liver Cu and Fe due to steroidal implants have been inconsistent when measured (Niedermayer et al., 2018; Reichhardt et al., 2021). The inconsistent response to steroidal implant in liver Cu and Fe concentrations may be due to the high concentrations of these minerals stored in the liver offsetting the mobilization of these minerals into the bloodstream. In contrast, on days 55/56 liver Mn concentrations were decreased due to steroidal implant administration, consistent with Smerchek et al. (2024) who reported on the relationship between liver Mn concentrations and decreasing PUN in steroidal implanted cattle, especially early in the steroidal implant period.
In summary, this study demonstrates that Zn supplementation can enhance the growth response to steroidal implants in beef cattle, particularly during the initial weeks following steroidal implant administration. The observed effects on performance parameters, coupled with changes in gene expression related to growth factor signaling and Zn homeostasis, suggest Zn plays a crucial role in supporting steroidal implant-induced growth. The benefits of Zn supplementation were most pronounced in steroidal implanted cattle, with minimal effects observed in non-steroidal implanted animals. These findings have important implications for beef cattle management, indicating that current Zn recommendations may be insufficient for maximizing the growth potential of steroidal implanted cattle. However, the complex interactions between Zn status, steroidal implant response, and time underscore the need for further research to refine Zn supplementation strategies in beef cattle production.
Supplementary Material
Glossary
Abbreviations
- ADG
Average daily gain
- AKT1
protein kinase B
- BF
12th rib fat thickness
- BW
Body weight
- DM
Dry matter
- DMI
Dry matter intake
- EEF1A2
eukaryotic translation elongation factor 1 alpha 2
- EEF2K
eukaryotic elongation factor 2 kinase
- EGFR
epidermal growth factor receptor
- ESR1
estrogen receptor 1
- FOXO3
forkhead box O-3
- G:F
Gain to feed ratio
- GPER1
G protein-coupled estrogen receptor 1
- HCW
Hot carcass weight
- IGF1R
insulin-like growth factor 1 receptor
- MAPK1
mitogen-activated protein kinase 1
- MMP2
matrix metalloproteinase 2
- MMP9
matrix metalloproteinase 9
- MTOR
mammal target of rapamycin kinase
- MYF5
myogenic factor 5
- MYOD
myogenic differentiation 1
- MYOG
myogenin
- NoIMP
No Implant treatment
- PAX7
paired box protein 7
- PDE4B
phosphodiesterase 4B
- PUN
Plasma urea nitrogen
- REA
Ribeye area
- RPS6KB1
ribosomal protein S6 kinase B1
- RPS9
ribosomal protein S9
- SLC30A10
solute carrier family 30 member 10
- SLC30A7
solute carrier family 30 member 7
- SLC39A14
solute carrier family 39 member 14
- SLC39A7
solute carrier family 39 member 7
- STA
Specific target amplification
- TE200
Component TE-200 implant treatment
- YG
Yield grade
- Zn0
Zinc at 0 mg/kg DM
- Zn30
Zinc at 30 mg/kg DM
- Zn100
Zinc at 100 mg/kg DM
- Zn150
Zinc at 150 mg/kg DM
Contributor Information
Elizabeth M Messersmith, Department of Animal Science, College of Agriculture and Life Sciences, Iowa State University, Ames, IA 50011, USA.
Stephanie L Hansen, Department of Animal Science, College of Agriculture and Life Sciences, Iowa State University, Ames, IA 50011, USA.
Conflict of interest statement
The authors declare no conflict of interest.
Author Contributions
Elizabeth Messersmith (Conceptualization, Data curation, Project administration, Writing—original draft, Writing—review & editing), and Stephanie Hansen (Conceptualization, Data curation, Funding acquisition, Project administration, Writing—original draft, Writing—review & editing)
Literature Cited
- Bartle, S. J., Preston R. L., Brown R. E., and Grant R. J... 1992. Trenbolone acetate/estradiol combinations in feedlot steers: dose-response and implant carrier effects. J. Anim. Sci. 70:1326–1332. doi: 10.2527/1992.7051326x [DOI] [PubMed] [Google Scholar]
- Cousins, R. J., Liuzzi J. P., and Lichten L. A... 2006. Mammalian zinc transport, trafficking, and signals. J. Biol. Chem. 281:24085–24089. doi: 10.1074/jbc.R600011200 [DOI] [PubMed] [Google Scholar]
- Duckett, S. K., and Pratt S. L... 2014. Meat Science and Muscle Biology Symposium—Anabolic implants and meat quality. J. Anim. Sci. 92:3–9. doi: 10.2527/jas.2013-7088 [DOI] [PubMed] [Google Scholar]
- Duncan, J. R., and Dreosti I. E... 1976. A proposed site of action for zinc in DNA synthesis. J. Comp. Pathol. 86:81–85. doi: 10.1016/0021-9975(76)90031-1 [DOI] [PubMed] [Google Scholar]
- Engle, T. E., and Spears J. W... 2000. Effects of dietary copper concentration and source on performance and copper status of growing and finishing steers 1. J. Anim. Sci. 78:2446–2451. doi: 10.2527/2000.7892446x [DOI] [PubMed] [Google Scholar]
- Filardo, E. J., and Thomas P... 2012. Minireview: G protein-coupled estrogen receptor-1, GPER-1: Its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology 153:2953–2962. doi: 10.1210/en.2012-1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo, E. J., Quinn J. A., Bland K. I., and Frackelton A. R... 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans -activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol. 14:1649–1660. doi: 10.1210/mend.14.10.0532 [DOI] [PubMed] [Google Scholar]
- Genther-Schroeder, O. N., Branine M. E., and Hansen S. L... 2016. The influence of supplemental Zn-amino acid complex and ractopamine hydrochloride feeding duration on growth performance and carcass characteristics of finishing beef cattle. J. Anim. Sci. 94:4338–4345. doi: 10.2527/jas.2015-0159 [DOI] [PubMed] [Google Scholar]
- Heiderscheit, K. J., and Hansen S. L... 2020. Effect of rumen-protected lysine on growth performance, carcass characteristics, and plasma amino acid profile in feedlot steers. Transl Anim Sci 4:txaa128. doi: 10.1093/tas/txaa128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinlein, C. A., and Chang C... 2002. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 16:2181–2187. doi: 10.1210/me.2002-0070 [DOI] [PubMed] [Google Scholar]
- Huerta, M., Kincaid R. L., Cronrath J. D., Busboom J., Johnson A. B., and Swenson C. K... 2002. Interaction of dietary zinc and growth implants on weight gain, carcass traits and zinc in tissues of growing beef steers and heifers. Anim. Feed Sci. Technol. 95:15–32. doi: 10.1016/s0377-8401(01)00334-0 [DOI] [Google Scholar]
- Hufstedler, G. D., and Greene L. W... 1995. Mineral and nitrogen balance in lambs implanted with zeranol. J. Anim. Sci. 73:3785–3788. doi: 10.2527/1995.73123785x [DOI] [PubMed] [Google Scholar]
- Johnson, B. J., Anderson P. T., Meiske J. C., and Dayton W. R... 1996. Effect of a combined trenbolone acetate and estradiol implant on feedlot performance, carcass characteristics, and carcass composition of feedlot steers. J. Anim. Sci. 74:363–371. doi: 10.2527/1996.742363x [DOI] [PubMed] [Google Scholar]
- Jung, M. R., Min Y. L., Seung P. Y., and Ho J. H... 2009. Zinc chloride stimulates DNA synthesis of mouse embryonic stem cells: Involvement of PI3K/Akt, MAPKs, and mTOR. J. Cell. Physiol. 218:558–567. doi: 10.1002/jcp.21628 [DOI] [PubMed] [Google Scholar]
- Kincaid, R. L. 2000. Assessment of trace mineral status of ruminants: A review. J. Anim. Sci. 77:1–10. doi: 10.2527/jas2000.77e-suppl1x [DOI] [Google Scholar]
- Kirschke, C. P., and Huang L... 2002. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the golgi apparatus*. J. Biol. Chem. 278:4096–4102. doi: 10.1074/jbc.M207644200 [DOI] [PubMed] [Google Scholar]
- Livak, K. J., and Schmittgen T. D... 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Macdonald, J. C., Klopfenstein T. J., Erickson G. E., and Vander Pol K. J... 2007. Changes in gain through the feeding period. Nebr. Beef Cattle Rep. 55–57.
- McGill, J. L., Rusk R. A., Guerra-Maupome M., Briggs R. E., and Sacco R. E... 2016. Bovine gamma delta T cells contribute to exacerbated IL-17 production in response to co-infection with bovine RSV and Mannheimia haemolytica. S. M. Varga, editor. PLoS One 11:e0151083. doi: 10.1371/journal.pone.0151083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messersmith, E. M. 2018. The effect of copper supplementation on performance and carcass characteristics of cattle utilizing growth promoting technologies. Graduate Theses and Dissertations. 16857. Ames (Iowa): Iowa State University. https://lib.dr.iastate.edu/etd/16857 [Google Scholar]
- Messersmith, E. M., Niedermayer E. K., Thornton K. J., Crawford G. I., and Hansen S. L... 2021. Zinc supplementation strategies in feedlot heifers receiving an extended-release implant or an aggressive re-implant program. J. Anim. Sci. 99:1–8. doi: 10.1093/JAS/SKAB212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NASEM. 2016. Nutrient Requirements of Beef Cattle. 8th ed.Washington, DC: National Academy Press. [Google Scholar]
- Niedermayer, E. K., Genther-Schroeder O. N., Loy D. D., and Hansen S. L... 2018. Effect of varying trace mineral supplementation of steers with or without hormone implants on growth and carcass characteristics. J. Anim. Sci. 96:1159–1170. doi: 10.1093/jas/skx063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleas, D., and Prasad A. S... 1969. Growth as affected by zinc protein nutrition. Am. J. Clin. Nutr. 22:1304–1314. doi: 10.1093/ajcn/22.10.1304 [DOI] [PubMed] [Google Scholar]
- Pampusch, M. S., White M. E., Hathaway M. R., Baxa T. J., Chung K. Y., Parr S. L., Johnson B. J., Weber W. J., and Dayton W. R... 2008. Effects of implants of trenbolone acetate, estradiol, or both, on muscle insulin-like growth factor-I, insulin-like growth factor-I receptor, estrogen receptor-α, and androgen receptor messenger ribonucleic acid levels in feedlot steers. J. Anim. Sci. 86:3418–3423. doi: 10.2527/jas.2008-1085 [DOI] [PubMed] [Google Scholar]
- Pisano, A., Santolla M. F., De Francesco E. M., De Marco P., Rigiracciolo D. C., Perri M. G., Vivacqua A., Abonante S., Cappello A. R., Dolce V.,. et al. 2017. GPER, IGF-IR, and EGFR transduction signaling are involved in stimulatory effects of zinc in breast cancer cells and cancer-associated fibroblasts. Mol. Carcinog. 56:580–593. doi: 10.1002/mc.22518 [DOI] [PubMed] [Google Scholar]
- Pogge, D. J., and Hansen S. L... 2013. Supplemental vitamin C improves marbling in feedlot cattle consuming high sulfur diets. J. Anim. Sci. 91:4303–4314. doi: 10.2527/jas.2012-5638 [DOI] [PubMed] [Google Scholar]
- Preston, R. L. 1999. Hormone containing growth promoting implants in farmed livestock. Adv. Drug Deliv. Rev. 38:123–138. doi: 10.1016/s0169-409x(99)00012-5 [DOI] [PubMed] [Google Scholar]
- Reichhardt, C. C., Messersmith E. M., Brady T. J., Motsinger L. A., Briggs R. K., Bowman B. R., Hansen S. L., and Thornton K. J... 2021. Anabolic implants varying in hormone type and concentration influence performance, feeding behavior, carcass characteristics, plasma trace mineral concentrations, and liver trace mineral concentrations of angus sired steers. Animals. 11:1964. doi: 10.3390/ani11071964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter, E. L., Drewnoski M. E., and Hansen S. L... 2012. Effects of increased dietary sulfur on beef steer mineral status, performance, and meat fatty acid composition. J. Anim. Sci. 90:3945–3953. doi: 10.2527/jas.2011-4512 [DOI] [PubMed] [Google Scholar]
- Samet, J. M., Dewar B. J., Wu W., and Graves L. M... 2003. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol. Appl. Pharmacol. 191:86–93. doi: 10.1016/s0041-008x(03)00219-9 [DOI] [PubMed] [Google Scholar]
- Shakur, Y., Stenson Holst L., Rahn Landstrom T., Movsesian M., Degerman E., and Manganiello V... 2001. Regulation and function of the cyclic nucleotide phosphodiesterase (PDE3) gene family. Prog. Nucleic Acid Res. Mol. Biol. 66:241–277. doi: 10.1016/s0079-6603(00)66031-2 [DOI] [PubMed] [Google Scholar]
- Smerchek, D. T., Rients E. L., McLaughlin A. M., Henderson J. A., Ortner B. M., Thornton K. J., and Hansen S. L... 2024. The influence of steroidal implants and manganese sulfate supplementation on growth performance, trace mineral status, hepatic gene expression, hepatic enzyme activity, and circulating metabolites in feedlot steers. J. Anim. Sci. 102:skae062. doi: 10.1093/jas/skae062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spears, J. W., and Kegley E. B... 2002. Effect of zinc source (zinc oxide vs zinc proteinate) and level on performance, carcass characteristics, and immune response of growing and finishing steers. J. Anim. Sci. 80:2747–2752. doi: 10.2527/2002.80102747x [DOI] [PubMed] [Google Scholar]
- Suasnavas, E. A., Heywood S., Ward A., Cox L., O’Grady M., Zhao Y., Gilbert L., and Isom S. C... 2015. Isolation and characterization of trophoblast-derived stem-like cells from peri-implantation porcine embryos. Anim. Reprod. Sci. 154:128–141. doi: 10.1016/j.anireprosci.2015.01.012 [DOI] [PubMed] [Google Scholar]
- Thornton, K. J., Kamange-Sollo E., White M. E., and Dayton W. R... 2015. Role of G protein–coupled receptors (GPCR), matrix metalloproteinases 2 and 9 (MMP2 and MMP9), heparin-binding epidermal growth factor–like growth factor (hbEGF), epidermal growth factor receptor (EGFR), erbB2, and insulin-like growth factor 1 receptor (I. J. Anim. Sci. 93:4291–4301. doi: 10.2527/jas.2015-9191 [DOI] [PubMed] [Google Scholar]
- Tuncay, E., Bitirim V. C., Durak A., Carrat G. R. J., Taylor K. M., Rutter G. A., and Turan B... 2017. Hyperglycemia-induced changes in ZIP7 and ZnT7 expression cause Zn2+ release from the sarco(endo)plasmic reticulum and mediate ER stress in the heart. Diabetes 66:1346–1358. doi: 10.2337/db16-1099 [DOI] [PubMed] [Google Scholar]
- von Bulow, V., Rink L., and Haase H... 2005. Zinc-mediated inhibition of cyclic nucleotide phosphodiesterase activity and expression suppresses TNF- and IL-1 production in monocytes by elevation of guanosine 3’,5’-cyclic monophosphate. J. Immunol. 175:4697–4705. doi: 10.4049/jimmunol.175.7.4697 [DOI] [PubMed] [Google Scholar]
- Wells, A. 1999. EGF receptor. Int. J. Biochem. Cell Biol. 31:637–643. doi: 10.1016/s1357-2725(99)00015-1 [DOI] [PubMed] [Google Scholar]
- Wu, W., Graves L. M., Jaspers I., Devlin R. B., Reed W., and Samet J. M... 1999. Activation of the EGF receptor signaling pathway in human airway epithelial cells exposed to metals. Am. J. Physiol. Lung Cell. Mol. Physiol. 277:L924–L931. doi: 10.1152/ajplung.1999.277.5.L924 [DOI] [PubMed] [Google Scholar]
- Yen, P. M. 2015. Classical nuclear hormone receptor activity as a mediator of complex biological responses: a look at health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 29:517–528. doi: 10.1016/j.beem.2015.07.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.