Abstract
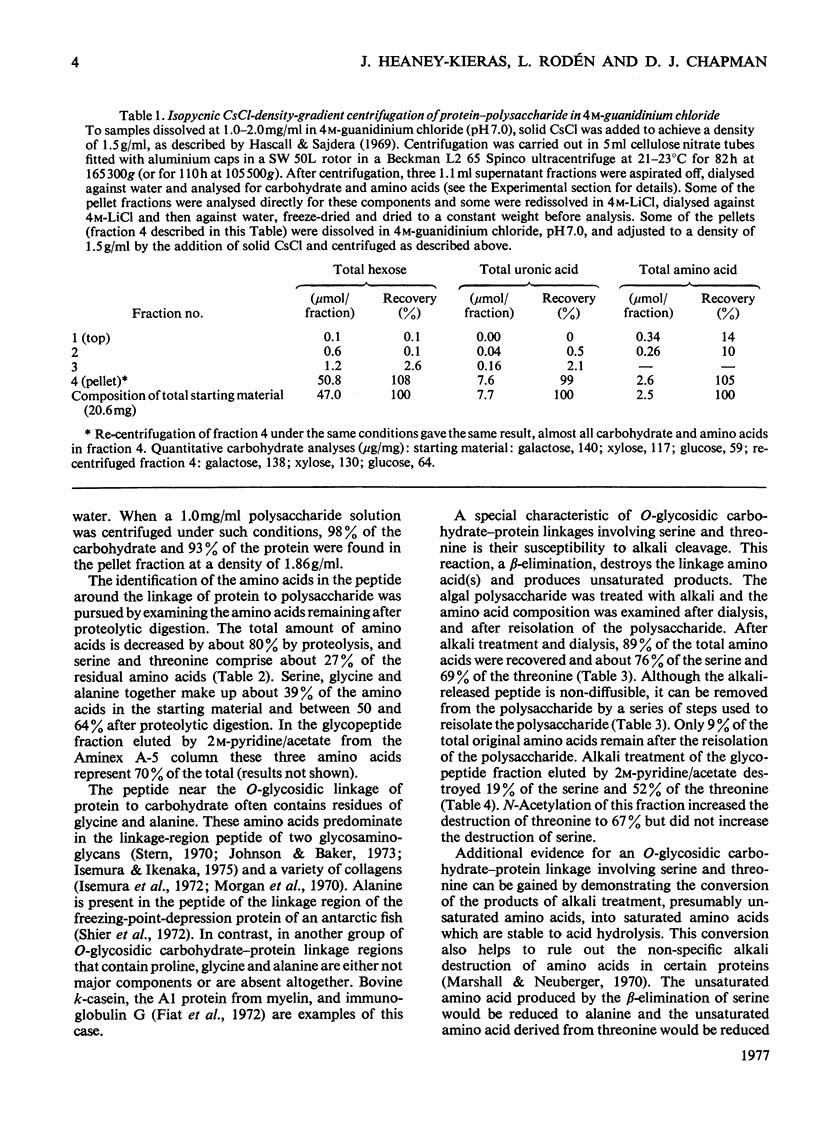
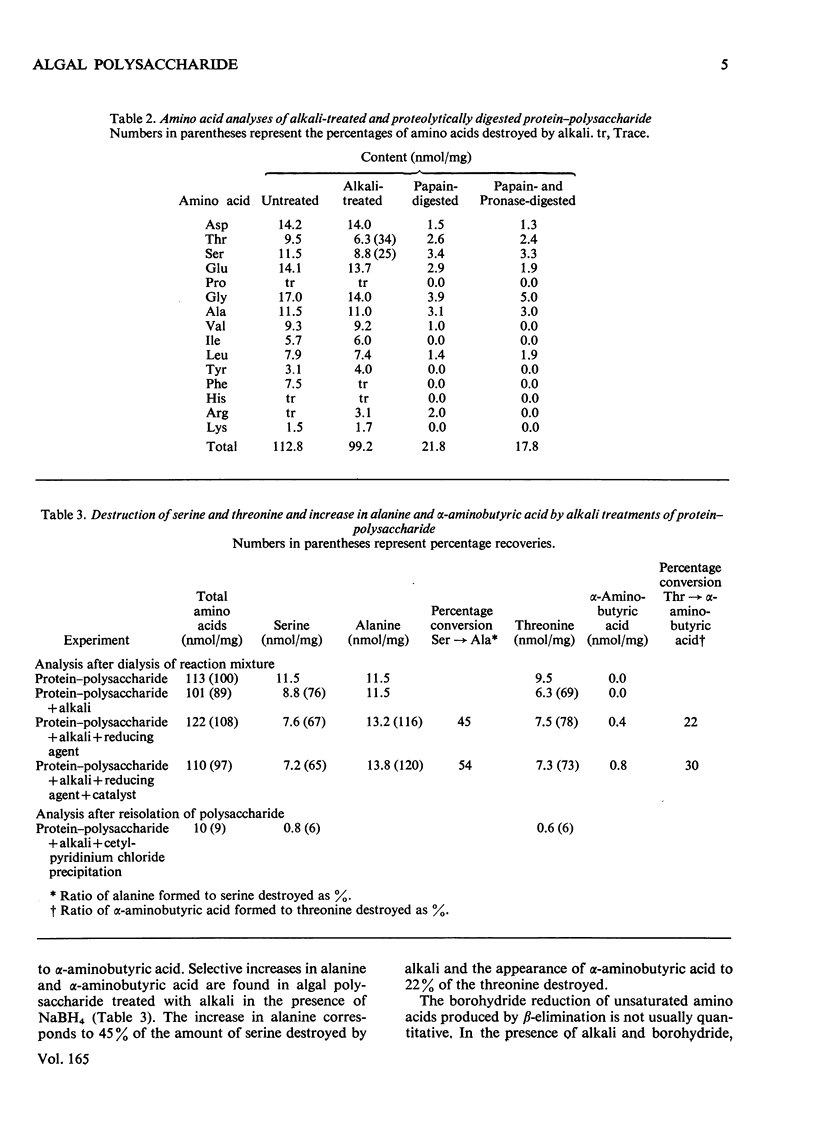
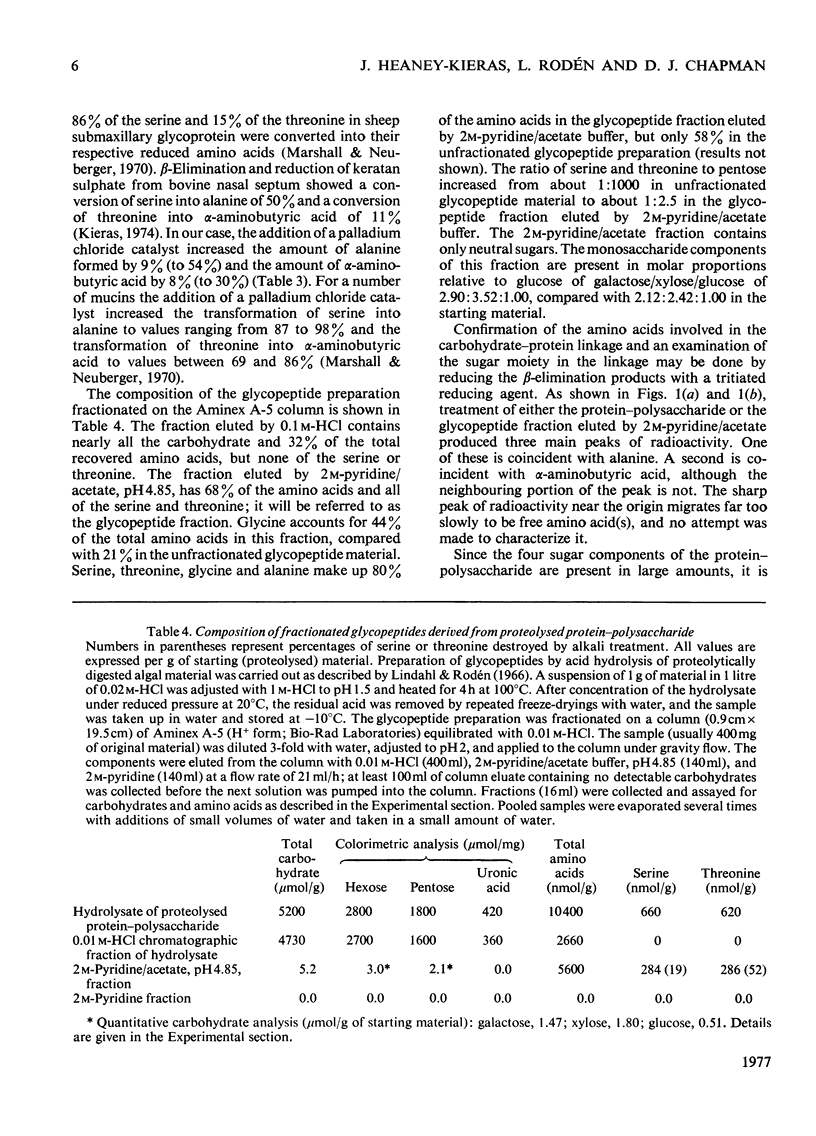
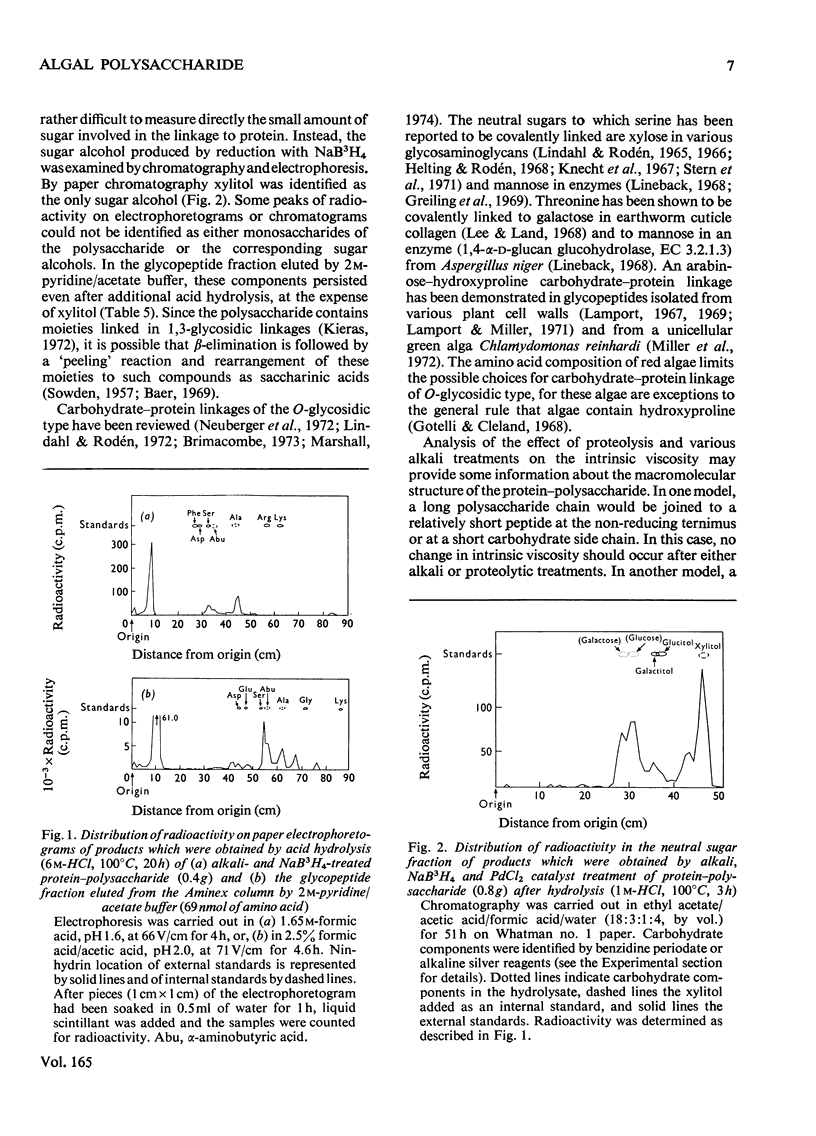
The extracellular anionic polysaccharide isolated from cultures of a unicellular red alga, Porphyridium cruentum, contains a small amount of protein after extensive purification. The polysaccharide and protein are recovered in the same fraction after isopycnic CsCl-density-gradient centrifugation in 4M-guanidinium chloride, under conditions designed to separate proteins from polysaccharide. The peptide portion of the protein-polysaccharide is released from the polysaccharide by alkali under conditions for beta-elimination. The released peptide is non-diffusible, but in can be separated from the polysaccharide by precipitation of the polysaccharide as the cetylpyridinium complex. Under conditions for beta-elimination of certain O-glycosidic carbohydrate-protein linkages, selective destruction of serine and threonine occurs. The addition of a reducing agent to the alkali mixture produces a selective increase in alanine and alpha-aminobutyric acid. Addition of a tritiated reducing agent to the alkali mixture produces radioactive alanine and alpha-aminobutyric acid, and xylitol as the only sugar alcohol. Similar results are obtained from glycopeptides isolated from partial acid hydrolysates. A macromolecular structure of the protein-polysaccharide is suggested by a comparison of the intrinsic viscosity of material before and after treatment with alkali and proteolytic enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fiat A. M., Alais C., Jollès P. The amino-acid and carbohydrate sequences of a short glycopeptide isolated from bovine kappa-casein. Eur J Biochem. 1972 Jun 9;27(3):408–412. doi: 10.1111/j.1432-1033.1972.tb01852.x. [DOI] [PubMed] [Google Scholar]
- Gotelli I. B., Cleland R. Differences in the occurrence and distribution of hydroxyproline-proteins among the algae. Am J Bot. 1968 Sep;55(8):907–914. [PubMed] [Google Scholar]
- Greiling H., Vögele P., Kisters R., Ohlenbusch H. D. Uber eine Kohlenhydrat-Protein-Bindung in der beta-Fructofuranosidase. Hoppe Seylers Z Physiol Chem. 1969 Apr;350(4):517–518. [PubMed] [Google Scholar]
- Hascall V. C., Sajdera S. W. Proteinpolysaccharide complex from bovine nasal cartilage. The function of glycoprotein in the formation of aggregates. J Biol Chem. 1969 May 10;244(9):2384–2396. [PubMed] [Google Scholar]
- Heaney-Kieras J., Chapman D. J. Structural studies on the extracellular polysaccharide of the red alga, Porhyridium. Carbohydr Res. 1976 Dec;52:169–177. doi: 10.1016/s0008-6215(00)85957-1. [DOI] [PubMed] [Google Scholar]
- Helting T., Rodén L. The carbohydrate-protein linkage region of chondroitin 6-sulfate. Biochim Biophys Acta. 1968 Dec 23;170(2):301–308. doi: 10.1016/0304-4165(68)90010-x. [DOI] [PubMed] [Google Scholar]
- Isemura M., Ikenaka T. Beta-Elimination and sulfite addition reaction of chondroitin sulfate peptidoglycan and the peptide structure of the linkage region. Biochim Biophys Acta. 1975 Nov 10;411(1):11–21. doi: 10.1016/0304-4165(75)90280-9. [DOI] [PubMed] [Google Scholar]
- Isemura M., Ikenaka T., Matsushima Y. Evidence for occurrence of a characteristic amino acid sequence of glycopeptides in the linkage region between peptide and carbohydrate. Biochem Biophys Res Commun. 1972 Jan 31;46(2):457–462. doi: 10.1016/s0006-291x(72)80160-8. [DOI] [PubMed] [Google Scholar]
- KIMMEL J. R., SMITH E. L. Crystalline papain. I. Preparation, specificity, and activation. J Biol Chem. 1954 Apr;207(2):515–531. [PubMed] [Google Scholar]
- Kieras F. J. The linkage region of cartilage keratan sulfate to protein. J Biol Chem. 1974 Dec 10;249(23):7506–7513. [PubMed] [Google Scholar]
- Kieras J. H., Kieras F. J., Bowen D. V. 2-O-methyl-D-glucuronic acid, a new hexuronic acid of biological origin. Biochem J. 1976 Apr 1;155(1):181–185. doi: 10.1042/bj1550181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht J., Cifonelli J. A., Dorfman A. Structural studies on heparitin sulfate of normal and Hurler tissues. J Biol Chem. 1967 Oct 25;242(20):4652–4661. [PubMed] [Google Scholar]
- LINDAHL U., RODEN L. THE ROLE OF GALACTOSE AND XYLOSE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2821–2826. [PubMed] [Google Scholar]
- Lamport D. T., Miller D. H. Hydroxyproline arabinosides in the plant kingdom. Plant Physiol. 1971 Oct;48(4):454–456. doi: 10.1104/pp.48.4.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamport D. T. The isolation and partial characterization of hydroxyproline-rich glycopeptides obtained by enzymic degradation of primary cell walls. Biochemistry. 1969 Mar;8(3):1155–1163. doi: 10.1021/bi00831a049. [DOI] [PubMed] [Google Scholar]
- Lee Y. C., Lang D. D-galactose di- and trisaccharides from the earthworm cuticle collagen. J Biol Chem. 1968 Feb 10;243(3):677–680. [PubMed] [Google Scholar]
- Lindahl U., Rodén L. The chondroitin 4-sulfate-protein linkage. J Biol Chem. 1966 May 10;241(9):2113–2119. [PubMed] [Google Scholar]
- Miller D. H., Lamport D. T., Miller M. Hydroxyproline heterooligosaccharides in Chlamydomonas. Science. 1972 May 26;176(4037):918–920. doi: 10.1126/science.176.4037.918. [DOI] [PubMed] [Google Scholar]
- Morgan P. H., Jacobs H. G., Segrest J. P., Cunningham L. W. A comparative study of glycopeptides derived from selected vertebrate collagens. A possible role of the carbohydrate in fibril formation. J Biol Chem. 1970 Oct 10;245(19):5042–5048. [PubMed] [Google Scholar]
- SOWDEN J. C. The saccharinic acids. Adv Carbohydr Chem. 1957;12:35–79. doi: 10.1016/s0096-5332(08)60204-0. [DOI] [PubMed] [Google Scholar]
- Shier W. T., Lin Y., De Vries A. L. Structure and mode of action of glycoproteins from an antarctic fish. Biochim Biophys Acta. 1972 Apr 15;263(2):406–413. doi: 10.1016/0005-2795(72)90092-x. [DOI] [PubMed] [Google Scholar]
- Stern E. L., Lindahl B., Rodén L. The linkage of dermatan sulfate to protein. II. Monosaccharide sequence of the linkage region. J Biol Chem. 1971 Sep 25;246(18):5707–5715. [PubMed] [Google Scholar]
- Stoolmiller A. C., Dorfman A. The biosynthesis of hyaluronic acid by Streptococcus. J Biol Chem. 1969 Jan 25;244(2):236–246. [PubMed] [Google Scholar]


