Abstract
The rare and endangered wild plant, Horsfieldia hainanensis, has been listed as a second-level key protected plant in China. Currently, its habitat is severely damaged, and the population has dramatically declined, necessitating urgent intervention for protection. In this study, the aim was to explore the correlations and differences from the perspectives of photosynthetic characteristics and leaf structure, providing scientific references for in-situ conservation and ex-situ cultivation. The results revealed the following: (1) The maximum net photosynthetic rate (Pmax) and light saturation point (LSP) of mature trees were significantly higher than those of seedlings, while the light compensation point (LCP), CO2 compensation point (CCP), and CO2 saturation point (CSP) were significantly lower in seedlings. (2) The average daily net photosynthetic rate of mature trees was significantly higher than that of seedlings. When both mature trees and seedlings exhibited a “midday depression” phenomenon, accompanied by an increase in intercellular CO2 concentration (Ci), it indicated that the “midday depression” was caused by non-stomatal limiting factors. (4) Both mature trees and seedlings showed peak values of water use efficiency (WUE) under low light conditions. Mature trees had smaller upper and lower epidermis thickness but larger leaf thickness, and their leaf structure, characterized by well-developed palisade and spongy tissues, conformed to the cellular structure adaptations for low light. Therefore, both were more adapted to low light conditions. (5) The stomatal density (SD) and individual stomatal area (SA) of seedlings were significantly higher than those of mature trees. (6) The total chlorophyll content of mature trees was significantly higher than that of seedlings, while the chlorophyll a/chlorophyll b ratio was significantly lower in mature trees and remained below three in both cases. In summary, the photosynthetic capacity and light adaptability of mature trees are stronger than those of seedlings, but both mature trees and seedlings exhibit shade-tolerant characteristics. For in-situ conservation, it is possible to promote the growth and development of seedlings by appropriately employing artificial “windowing” or shading methods based on the actual growth environment of the seedlings. In the case of ex-situ cultivation, seedlings should be provided with appropriate shading initially, while ensuring sufficient moisture and CO2 concentration. As the plants grow, the shading intensity can be gradually reduced. Once the plants reach maturity, they have a broader range of light adaptability and can be transplanted to environments with less shading.
Keywords: Horsfieldia hainanensis, Photosynthetic characteristics, Leaf structure, Photosynthetic pigments, Photosynthetic response, Population conservation
Introduction
Horsfieldia hainanensis, a tall tree of the Myristicaceae family, is primarily found in Hainan and southern Guangxi, China. This species typically inhabits shady, moist forests in valleys and hills at elevations between 400 and 450 m above sea level. It has been listed in both the “List of Extremely Small Population Wild Plants in Guangxi” and the “List of Key Protected Wild Plants in China.” Due to its limited geographical distribution, population decline, and rarity, H. hainanensis is considered part of an extremely small population. Such populations are critical reservoirs of biological and genetic diversity. Without timely conservation efforts, their unique biological traits and genetic resources may be lost. As a result, protecting plants of extremely small populations is crucial for preserving biodiversity in China.
Photosynthesis is the fundamental process by which green plants utilize light energy to produce and store organic compounds. It is also a vital physiological process closely associated with the growth and development of plants (Zong et al., 2022). The competitive advantage of plants in population competition and their distribution range are indirectly influenced by the strength of photosynthetic capacity. By studying plant photosynthesis and its influencing factors, it is possible to optimize environmental conditions during cultivation and breeding processes. This is an important approach in the protection of endangered plants, population restoration, and reintroduction efforts (John et al., 2007). For instance, the related research findings indicate significant differences in photosynthetic and photoprotective characteristics between Nerium oleander seedlings and mature trees, leading to distinct physiological traits in response to different seasons (Chondrogiannis et al., 2023). A comparative analysis of leaf characteristics of seedlings and mature trees of 17 species in tropical rainforests revealed significant differences in photosynthetic characteristics and leaf structures between seedlings and mature trees of all species, which are correlated with their environmental adaptability and growth status (Houter & Pons, 2012). Additionally, leaf microstructure and photosynthetic pigment content are crucial methods for observing plant diversity. Pan et al. (2024a) found significant differences in photosynthetic characteristics between seedlings and mature trees of Manglietia aromatica, primarily attributed to differences in spongy tissue thickness, leaf thickness, and chlorophyll content. From the aforementioned studies, it is evident that understanding the differences in photosynthetic characteristics and physiological structures of the same plant species at different stages is crucial for elucidating their endangerment mechanisms and implementing appropriate conservation measures.
The natural regeneration of H. hainanensis is challenging, with low survival rates of seedlings impeding their growth into mature trees, thereby severely affecting population propagation and expansion. In order to investigate the reasons behind these challenges and implement rational conservation measures for the H. hainanensis, researchers have conducted studies on aspects such as reproductive cultivation, genetic diversity, ecological characteristics, and population structure (He, 2013; Chai et al., 2021; Jiang et al., 2016; Zhong et al., 2018). However, there is limited information regarding its photosynthetic characteristics and leaf microstructure. This study aims to address the following questions through the measurement and analysis of photosynthetic characteristics, leaf structure features, and photosynthetic pigment content of mature trees and seedlings of the H. hainanensis: (1) Are there differences in photosynthetic characteristics between mature trees and seedlings of the H. hainanensis? (2) If differences in photosynthetic capacity exist, are these differences related to leaf structure and photosynthetic pigment content? The research results aim to explore the photosynthetic characteristics and differences at different growth stages of the H. hainanensis, with the intention of providing a theoretical basis for translocation conservation and population restoration of the species and investigating protective planting methods for the seedlings of the H. hainanensis.
Materials And methods
Overview of the experimental site
The experimental site is located within the Guangxi Institute of Botany in Guilin, Guangxi. It is situated at approximately 25°01′N latitude and 110°17′E longitude, with an elevation of 180 m. The site falls within the subtropical monsoon climate zone of Central Asia. The region benefit from favorable climatic conditions with abundant sunlight and ample rainfall, it is an ecological habitat suitable for the growth of H. hainanensis. The average annual temperature is 19.4 °C, with the hottest month averaging around 28.5 °C and the coldest month averaging only about 8.3 °C. The average annual accumulative precipitation is approximately 1,974 mm, and the average annual relative humidity ranges from 73% to 79%. The average annual sunshine duration is around 1,670 h (Wu et al., 2000).
Materials
The test materials consisted of mature trees and seedlings of H. hainanensis. The 12-year-old mature trees were planted in the endangered plant germplasm nursery of the Guangxi Institute of Botany. The experimental seedlings are 1-year-old seedlings cultivated from seeds collected from mature trees in the study. They were planted in plastic pots with an inner diameter of 21 cm and depth of 18 cm, with one seedling per pot. Both mature trees and seedlings are grown in red soil as the soil substrate. Three healthy and vigorous mature trees and seedlings without diseases or pests were selected for the experiments.
Methods
Measurement of photosynthesis-light response curves
Under clear weather conditions, from 8:00 to 13:00 in the morning, the photosynthetic parameters of H. hainanensis were measured using a portable Li-6400 photosynthesis system (Li-6400; Li-Cor, Lincoln, NE, USA). Prior to measurement, the leaves were pre-conditioned for 30 min under a light intensity of 600 µmol·m−2·s−1 (using the built-in red-blue light source of the instrument) to fully activate the photosynthetic system. The measurement was conducted with an open gas exchange system, with an airflow rate of 0.5 L·min−1, leaf temperature set at 28 °C, and CO2 concentration set at 400 μmol·mol−1. The light intensity gradient was set at 1,500, 1,200, 1,000, 800, 600, 400, 200, 150, 100, 50, 20, and 0 µmol·m−2·s−1. Three individuals each of mature trees and seedlings were measured. The photosynthetic-light response curve was plotted with photosynthetic photon flux density (PPFD) as the x-axis and net photosynthesis rate (Pn) as the y-axis. The photosynthetic parameters were fitted to the Pn-PPFD curve using the following equation (Ye, Yu & Kang, 2012):
| (1) |
In the equation, Pn represents the net photosynthesis rate, AQY is the apparent quantum yield, β and γ are coefficients, PPFD denotes the photosynthetic photon flux density, and Rd stands for dark respiration rate. After conducting a goodness-of-fit test and obtaining satisfactory results, the following formulas were used to calculate the light saturation point (LSP), maximum net photosynthesis rate (Pmax), and light compensation point (LCP):
| (2) |
| (3) |
| (4) |
During the extraction of the light response curves, stomatal conductance (Gs) and transpiration rate (Tr) were analyzed at different light intensities. Water use efficiency (WUE = Pn/Tr) was calculated (Wen, 1997).
Measurement of photosynthesis-CO2 response curves
Three healthy plants at each growth stage, including both mature trees and seedlings, previously used for photosynthesis-light response curve measurements were selected for analysis, with each plant replicated three times. Measurements were conducted on the test leaves in the morning at 8:00 after induction. The measurement of photosynthesis-CO2 response curves was conducted using the Li-6400 portable photosynthesis system. The airflow rate was set at 0.5 L·min−1, and the leaf temperature was set at 28 °C. Based on the results of the light response curve measurement, the fixed light intensity for mature trees was set at 1,000 μmol·m−2·s−1, and for seedlings, it was set at 500 μmol·m−2·s−1. The CO2 concentration gradient was set at 400, 300, 200, 150, 100, 50, 400, 400, 600, 800, 1,000, 1,200, 1,500, and 2,000 μmol·mol−1 (controlled using CO2 cylinders). During the measurement, a balance of 150–180 s was maintained at each CO2 concentration, and the net photosynthesis rate (Pn) at different CO2 concentrations was automatically recorded by the system. The Pn-Ci curve was fitted and plotted using a rectangular hyperbolic correction model. Net photosynthesis rate (Pn), CO2 compensation point (CCP), CO2 saturation point (CSP), and potential maximum net photosynthesis rate (Amax) were calculated using the following formulas.
| (5) |
| (6) |
| (7) |
| (8) |
In the equation, Ci represents the intercellular CO2 concentration, α is the initial carboxylation efficiency of the CO2 response curve, β and γ are coefficients, and Rp is the rate of photorespiration.
Measurement of diurnal variation in photosynthesis
Three healthy plants at each growth stage, including both mature trees and seedlings, previously used for photosynthesis-light response curve measurements were selected for analysis, with each plant replicated three times. The parameters of photosynthetic diurnal changes were measured using the Li-6400 portable photosynthesis system. Measurements were taken at intervals of 1.5 h between 8:30 and 17:30 Beijing time. The following photosynthetic parameters and environmental factors were measured: net photosynthesis rate (Pn, μmol·m−2·s−1), transpiration rate (Tr, mmol·m−2·s−1), stomatal conductance (Gs, mol·m−2·s−1), intercellular CO2 concentration (Ci, μmol·mol−1), stomatal limitation (Ls = 1 - Ci/Ca), water use efficiency (WUE = Pn/Tr, μmol·mmol−1). Additionally, environmental factors such as photosynthetically active radiation (PAR, μmol·m−2·s−1), air temperature (Ta, °C), and relative humidity (RH, %) were recorded.
Measurement of leaf anatomy parameters
Three healthy and disease-free leaves, located 2–3 pairs down from the top, were separately collected from three mature trees and three seedlings, resulting in a total of three samples per treatment. Small sections measuring 1 cm × 1 cm were excised and fixed in 70% Formalin-Acetic Acid-Alcohol (FAA) fixative solution for 24 h. Subsequently, conventional paraffin sectioning technique (Li et al., 2019) was employed to prepare slides, which were then observed and photographed using a Nikon Eclipse E100 optical microscope. Six slides were obtained for each treatment, with three fields of view captured per slide. Additionally, the leaf anatomy parameters were measured using caseviewer software, including leaf thickness, upper epidermis thickness, lower epidermis thickness, palisade parenchyma thickness, and spongy parenchyma thickness. Furthermore, the ratios of palisade to spongy tissue, relative palisade parenchyma thickness, and relative spongy parenchyma thickness were calculated as follows: (1) Palisade-to-spongy tissue ratio = palisade parenchyma thickness/spongy parenchyma thickness; (2) Relative palisade parenchyma thickness = palisade parenchyma thickness/leaf thickness; (3) Relative spongy parenchyma thickness = spongy parenchyma thickness/leaf thickness.
Measurement of leaf stomatal structure
By comparing the leaf stomatal structure parameters of mature trees and seedlings, we further analyzed the differences in stomatal structure between the upper and lower epidermises of leaves of mature trees and seedlings. We adopted the same sampling method as that for determining leaf anatomical structure. A total of 18 leaves from mature trees and seedlings were picked. For each leaf, three 1 cm × 1 cm samples were cut from both the upper and lower epidermises. For each sample, 2.5% glutaraldehyde solution was used for fixation, followed by rinsing with phosphate buffer. Dehydration, critical point drying and gold plating were carried out in sequence. The VEGA3 TESCAN vacuum electron scanning electron microscope was used for photographing and observation. Ten fields of view were randomly observed for each sample. Finally, Axiovision was used to measure stomatal length, stomatal width, stomatal density and single stomatal area.
Measurement of photosynthetic pigment content
The leaf samples used for the photosynthetic measurements were individually weighed to 0.5 g, excluding the veins. They were then cut into small pieces and transferred to 25 mL volumetric flasks. Next, 95% ethanol was added to each flask to reach the volume mark. The flasks were placed in dark conditions for 24 h to allow for pigment extraction. Afterward, the absorbance of the extraction solution was measured at wavelengths of 665 nm, 649 nm, and 470 nm. This process was repeated three times for each sample. Using the following formulas, the content of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl a+b), and carotenoids (Car) was calculated, along with the ratio of Chl a to Chl b (Chl a/b) and the ratio of carotenoids to total chlorophyll content (Car/Chl a+b) (Li et al., 2020b):
| (9) |
| (10) |
| (11) |
| (12) |
Data analysis
The mean and standard deviation data of photosynthetic characteristic parameters, leaf structure parameters, and photosynthetic pigment content were calculated using Excel 2016. A t-test was performed using SPSS 26.0 (IBM, Armonk, NY, USA), graphs were plotted using Origin 2022 software, and the photosynthetic parameters of the photosynthetic response curve were fitted and calculated using the rectangular hyperbolic correction model from the Photosynthesis Calculation 4.1.1 software (Ye, 2010).
Results
Diurnal variation of photosynthetic parameters
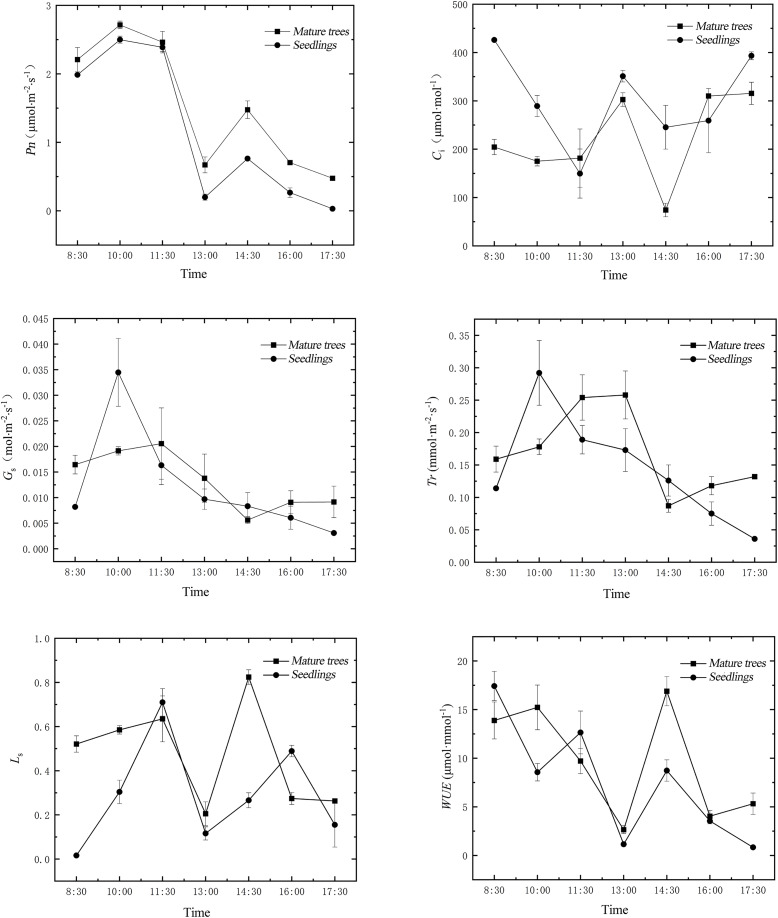
From Fig. 1, it can be observed that the Pn of both mature trees and seedlings of H. hainanensis showed a low value at 13:00 Beijing time, exhibiting a typical “midday depression” phenomenon, with the highest Pn value at 10:00. The diurnal variations of Ls and WUE were similar to Pn, showing low values at 13:00 and relatively high values at 11:30 and 14:30, then dropping to low values at 13:00, with the difference that WUE remained at relatively high values before 11:30. The Ci showed high values at 8:30, 13:00, and 17:30, with a low value at 11:30. The diurnal variation trends of Gs and Tr were similar. Gs of mature trees showed a peak at 11:30 and a decrease at 14:30, while seedlings exhibited a unimodal trend with the highest value at 10:00 and the lowest value at 17:30. Table 1 indicates that the daily average net photosynthetic rate and daily average stomatal limitation of mature trees were greater than those of seedlings, showing extremely significant differences (P < 0.01). On the other hand, the daily average intercellular CO2 concentration and daily average water use efficiency of seedlings were higher than those of mature trees, showing significant differences (P < 0.05). There were no significant differences (P > 0.05) in the daily average stomatal conductance and daily average transpiration rate between mature trees and seedlings.
Figure 1. Diurnal variation of photosynthetic parameters in leaves of mature trees and seedlings of H. hainanensis.
Table 1. Daily average values of photosynthetic parameters in leaves of mature trees and seedlings of H. hainanensis.
| Type | Pn (µmol·m−2·s−1) | Gs (mol·m−2·s−1) | Ci (μmol·mol−1) | Tr (mmol·m−2·s −1) | L s | WUE (μmol·mmol−1) |
|---|---|---|---|---|---|---|
| Mature trees | 1.530 ± 0.099 | 0.013 ± 0.003 | 223.328 ± 19.767 | 0.169 ± 0.037 | 0.472 ± 0.040 | 7.553 ± 0.804 |
| Seedlings | 1.161 ± 0.041 | 0.012 ± 0.002 | 301.949 ± 29.546 | 0.144 ± 0.028 | 0.294 ± 0.044 | 9.673 ± 1.083 |
| P value | <0.001** | 0.656 | 0.019* | 0.394 | 0.007** | 0.046* |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
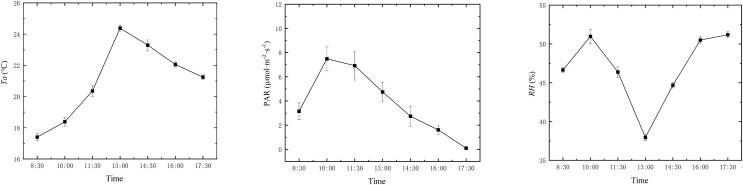
Diurnal variation of environmental factors
The diurnal variation of environmental factors, as shown in Fig. 2, revealed that temperature (Ta) and photosynthetically active radiation (PAR) exhibited a unimodal trend with an initial increase followed by a decrease. Ta reached its peak at 13:00 (Beijing time), with a value of 24.384 °C. PAR reached its peak at 10:00, with a value of 7.492 µmol·m−2·s−1. RH reached its lowest point at 13:00, with a value of 37.955%, which may be related to the ambient temperature at that time. It then reached its highest value at 17:30, measuring 51.183%.
Figure 2. Diurnal variation of environmental factors.
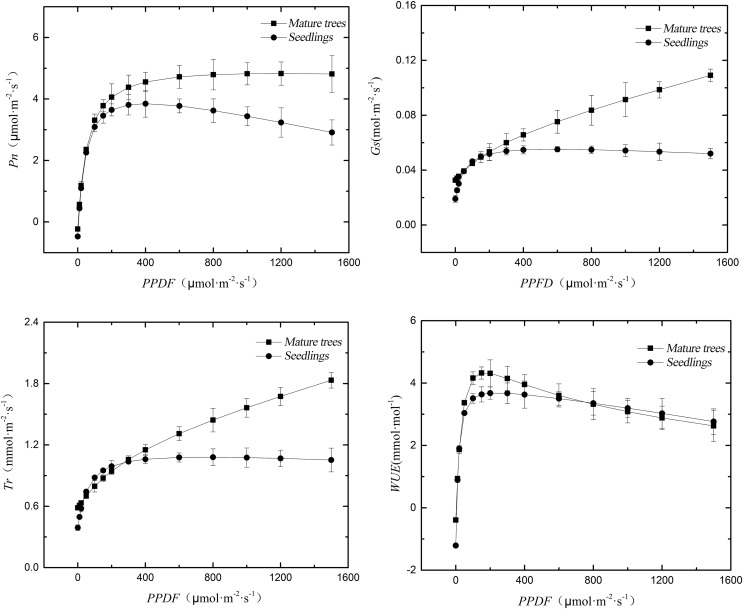
Response of photosynthetic physiological indices to light intensity
The determination coefficients (R2) for the fitting of the photosynthesis-light response curves in mature trees and seedlings of H. hainanensis were both above 0.95, indicating a good fitting effect (Fig. 3). As the photosynthetic photon flux density (PPFD) increased, the Pn of mature trees gradually increased and then stabilized, while the Pn of seedlings initially increased and then showed a declining trend. The Pn of the two groups started to diverge after a PPFD of 200 µmol·m−2·s−1. The stomatal conductance (Gs) of mature trees increased with increasing light intensity, while the Gs of seedlings showed an initial increase followed by a flattening trend. Differences in Gs between the two groups gradually emerged after a PPFD of 400 µmol·m−2·s−1, indicating that mature trees possess a stronger potential for gas exchange, thereby enabling them to have a higher photosynthetic rate. The trend of Tr was similar to that of Gs, indicating that transpiration is stronger under strong light conditions, thereby promoting the transport of internal water and inorganic salts. The WUE of mature trees and seedlings initially increased and then decreased with increasing light intensity. The WUE of mature trees reached its peak at 150 µmol·m−2·s−1 and rapidly decreased thereafter. The WUE of seedlings reached its peak at 200 µmol·m−2·s−1 and slowly declined afterward. Mature trees exhibit higher WUE under low light intensity, possibly related to their adaptation to harsh karst habitats. However, under high light intensity, WUE shows a decreasing trend, indicating a weaker adaptation to high light intensity environments.
Figure 3. Response of photosynthetic parameters in leaves of mature trees and seedlings of H. hainanensis to light intensity.
Photosynthesis-light response parameters
Mature trees exhibited higher overall Pn compared to seedlings, with significant differences observed in the maximum net photosynthetic rate (Pmax) between mature trees (4.83 µmol·m−2·s−1) and seedlings (3.85 µmol·m−2·s−1) (P < 0.05), indicating that mature trees have stronger photosynthetic capabilities than seedlings and a greater ability to accumulate energy. Seedlings had significantly higher light compensation point (LCP) and dark respiration rate (Rd) (P < 0.05), measuring 4.70 µmol·m−2·s−1 and 0.50 µmol·m−2·s−1, respectively. The light saturation point (LSP) of mature trees was significantly higher (P < 0.05) at 1,185.31 µmol·m−2·s−1. However, there were no significant differences (P > 0.05) in apparent quantum yield (AQY) between mature trees and seedlings (Table 2).
Table 2. Photosynthesis-light response parameters of leaves in mature trees and seedlings of H. hainanensis.
| Type | Pmax (µmol·m−2·s−1) | LCP (µmol·m−2·s−1) | LSP (µmol·m−2·s−1) | AQY (µmol·µmol−1) | Rd (µmol·m−2·s−1) |
|---|---|---|---|---|---|
| Mature trees | 4.83 ± 0.16 | 2.69 ± 0.28 | 1,185.31 ± 23.65 | 0.025 ± 0.002 | 0.25 ± 0.07 |
| Seedlings | 3.85 ± 0.29 | 4.70 ± 0.44 | 403.45 ± 34.47 | 0.025 ± 0.007 | 0.50 ± 0.09 |
| P value | 0.015* | 0.005** | <0.001** | 0.448 | 0.038* |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
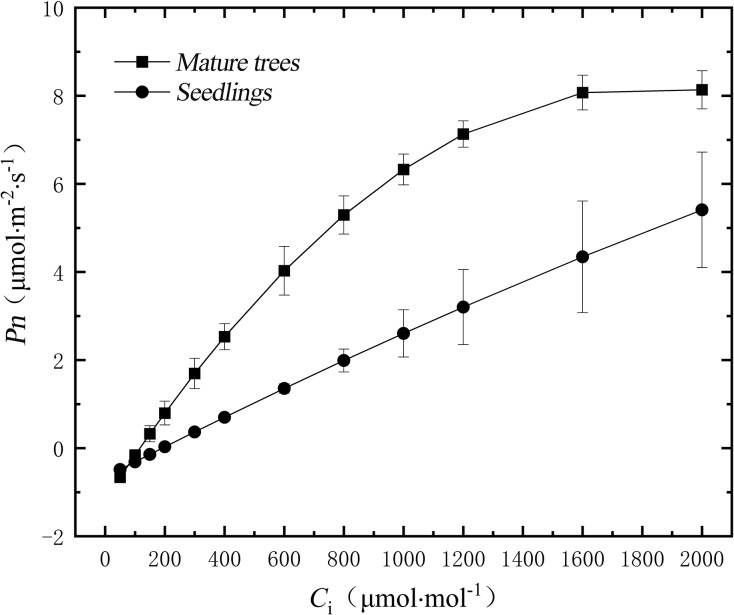
Photosynthesis-CO2 response parameters
The determination coefficients (R2) for the fitting of the photosynthesis-CO2 response curves in mature trees and seedlings of H. hainanensis were both above 0.95, indicating a good fitting effect (Fig. 4). With the increase of Ci, the Pn of mature trees showed an initial increase followed by a flattening trend, while the Pn of seedlings exhibited a continuous increase. After Ci reached 100 µmol·mol−1, the Pn of mature trees surpassed that of seedlings, and the final performance of Pmax showed mature trees > seedlings. According to Table 3, the initial carboxylation efficiency (α), potential maximum net photosynthetic rate (Amax), and light respiration rate (Rp) of mature trees were all higher than those of seedlings, with Rp showing significant differences (P < 0.05) and α, Amax showing extremely significant differences (P < 0.01). The CO2 compensation point (CCP) and CO2 saturation point (CSP) of seedlings were both higher than those of mature trees, and both showed extremely significant differences (P < 0.01).
Figure 4. Photosynthesis-CO2 response curves of leaves in mature trees and seedlings of H. hainanensis.
Table 3. Photosynthesis-CO2 response parameters of leaves in mature trees and seedlings of H. hainanensis.
| Type | α (µmol·m−2·s−1) | Amax (µmol·m−2·s−1) | CCP (µmol·m−2·s−1) | CSP (µmol·m−2·s−1) | Rp (µmol·m−2·s−1) |
|---|---|---|---|---|---|
| Mature trees | 0.010 ± 0.001 | 8.214 ± 0.382 | 117.756 ± 21.054 | 1,829.814 ± 34.317 | 1.179 ± 0.133 |
| Seedlings | 0.004 ± 0.001 | 5.822 ± 0.259 | 253.453 ± 23.528 | 2,438.832 ± 76.247 | 0.852 ± 0.069 |
| P value | 0.002** | <0.001** | 0.002** | <0.001** | 0.019* |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
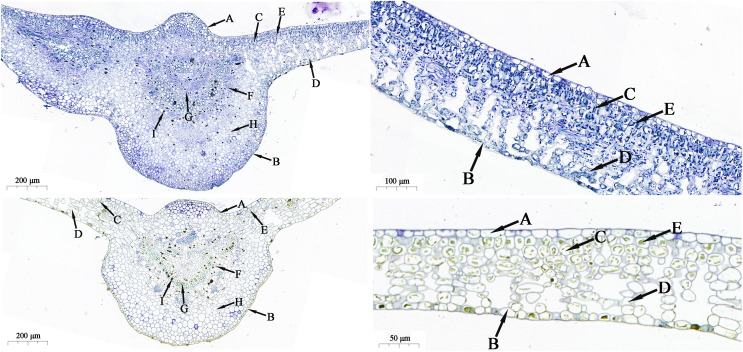
Foliar morphological characteristics
The cross-sectional structural morphological characteristics of leaf blades in mature trees and seedlings of H. hainanensis are shown in Fig. 5. The leaf blades are composed of upper epidermis, lower epidermis, palisade tissue, spongy tissue, and other differentiated cells. According to Table 4, the leaf thickness (LT), palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT), palisade-to-spongy ratio (PPT/SPT), and relative palisade parenchyma thickness (RPPT) of mature trees were significantly higher than those of seedlings (P < 0.05), with LT, PPT, SPT, and RPPT showing extremely significant differences (P < 0.01). The upper epidermis thickness (UET), lower epidermis thickness (LET), and relative spongy parenchyma thickness (RSPT) of seedlings were higher than those of mature trees, with significant differences observed in UET (P < 0.05) and extremely significant differences in LET (P < 0.01). However, there were no significant differences in RSPT (P > 0.05), indicating that the leaves of mature trees are better developed and have a stronger ability to photosynthesize.
Figure 5. Leaf structure of H. hainanensis in mature trees and seedlings (top: mature tree leaf; bottom: seedling leaf).
UE, Upper epidermal; LE, Lower epidermal; PT, Palisade tissue; ST, Spongy tissue; Ch, Chloroplasts; VB, Vascular bundles; X, Xylem; AT, Aqueous tissue; AC, Abnormal cells.
Table 4. Anatomical parameters of leaf structure in mature trees and seedlings of H. hainanensis.
| Index | Mature trees | Seedlings | P value |
|---|---|---|---|
| Leaf thickness (LT, μm) | 319.567 ± 0.939 | 253.300 ± 2.642 | <0.001** |
| Upper epidermis thickness (UET, μm) | 21.633 ± 0.946 | 24.533 ± 0.736 | 0.027* |
| Lower epidermis thickness (LET, μm) | 14.700 ± 0.245 | 24.367 ± 0.822 | <0.001** |
| Palisade parenchyma thickness (PPT, μm) | 139.300 ± 0.909 | 100.767 ± 1.852 | <0.001** |
| Spongy parenchyma thickness (SPT, μm) | 138.567 ± 1.731 | 110.567 ± 1.725 | <0.001** |
| PPT/SPT | 1.005 ± 0.009 | 0.911 ± 0.031 | 0.014* |
| Relative palisade parenchyma thickness (RPPT) | 0.436 ± 0.002 | 0.398 ± 0.003 | <0.001** |
| Relative spongy parenchyma thickness (RSPT) | 0.434 ± 0.004 | 0.437 ± 0.011 | 0.741 |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
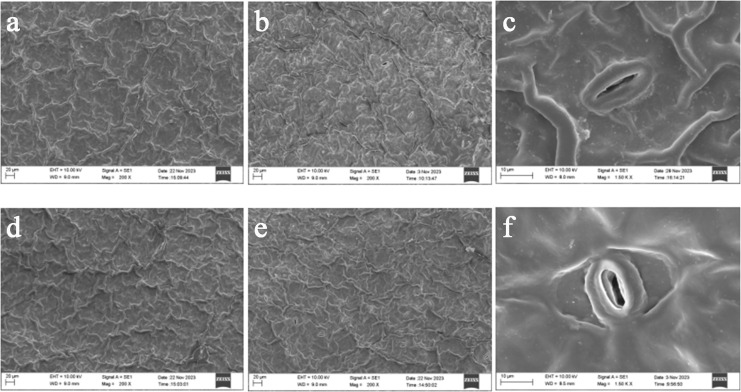
Leaf stomatal characteristics
Based on the leaf stomatal characteristics of mature trees (Figs. 6A–6C) and seedling (Figs. 6D–6F) leaves of H. hainanensis, it can be observed that, from left to right, they respectively represent the upper and lower epidermis and stomatal structures of the leaves. The leaf stomata are mainly distributed on the lower epidermis, and both the stomata and their guard cells appear elongated, primarily to avoid direct sunlight and reduce water loss. As indicated in Table 5, the average values of stomatal density, stomatal width, and individual stomatal area for seedlings are all greater than those of mature trees, the average values of stomatal width and individual stomatal area showing extremely significant differences (P < 0.01), the average values of stomatal density showing significant differences (P < 0.05), while the average values of stomatal length exhibits no significant difference (P > 0.05).
Figure 6. The stomatal characteristics of mature trees (top) and seedling leaves (bottom) of H. hainanensis.
Table 5. The stomatal parameters of mature trees and seedling leaves of H. hainanensis.
| Type | Stomatal density (SD) (counts/mm2) | Stomatal length (SL) (µm) | Stomatal width (SW) (µm) | Stomatal area (SA) (µm2) |
|---|---|---|---|---|
| Mature trees | 95.26 ± 5.35 | 14.57 ± 1.30 | 3.24 ± 0.91 | 37.34 ± 12.15 |
| Seedlings | 106.23 ± 3.66 | 14.04 ± 0.35 | 6.93 ± 0.37 | 76.45 ± 5.95 |
| P value | 0.031* | 0.533 | 0.003** | 0.007** |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
Leaf photosynthetic pigment content and ratio
According to Table 6, it can be observed that the Chl a, Chl b, Chl (a+b), and Car content of mature trees of H. hainanensis are higher than those of seedlings. However, the Chl a/b ratio and Car/Chl (a+b) ratio of seedlings are higher than those of mature trees. Among them, Chl a, Chl b, Chl (a+b), Car, and Car/Chl (a+b) show extremely significant differences (P < 0.01), while Chl a/b ratio shows a significant difference (P < 0.05).
Table 6. Leaf photosynthetic pigment content and ratio in mature trees and seedlings of H. hainanensis.
| Type | Chl a (mg·g−1) | Chl b (mg·g−1) | Chl (a+b) (mg·g−1) | Car (mg·g−1) | Chl a/b | Car/Chl (a+b) |
|---|---|---|---|---|---|---|
| Mature trees | 15.284 ± 1.243 | 11.314 ± 0.837 | 26.598 ± 2.314 | 3.250 ± 0.266 | 1.351 ± 0.213 | 0.122 ± 0.008 |
| Seedlings | 7.444 ± 0.773 | 4.206 ± 0.355 | 11.650 ± 1.147 | 2.323 ± 0.159 | 1.770 ± 0.140 | 0.199 ± 0.021 |
| P value | <0.001** | <0.001** | <0.001** | 0.005** | 0.045* | 0.004** |
Notes:
The values in the table are the mean and standard deviation.
Indicates significant difference.
Indicates extremely significant difference.
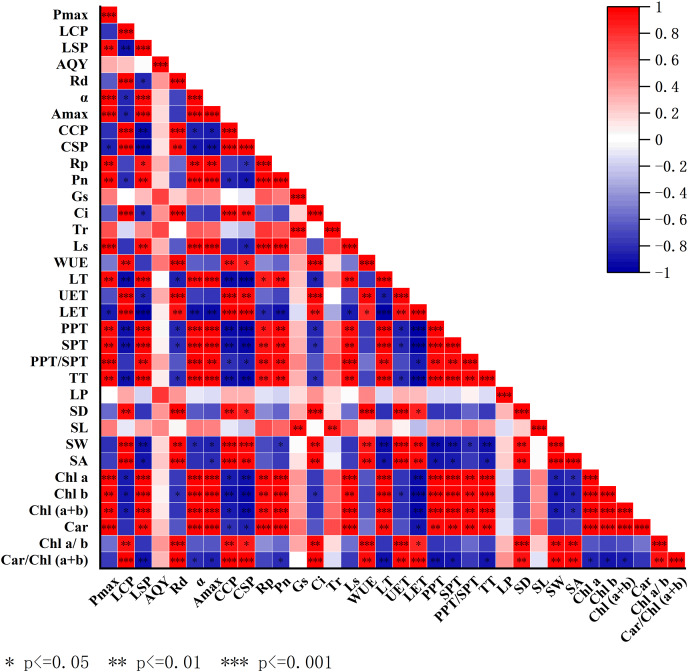
Correlation analysis of photosynthetic physiological parameters, leaf structural characteristics, and photosynthetic pigment content
Figure 7 shows the heatmap of the correlation analysis among photosynthetic physiological parameters, leaf structural characteristics, and photosynthetic pigment content in mature trees and seedlings of H. hainanensis. From Fig. 7, it can be observed that there is a strong correlation among photosynthetic physiological parameters, leaf structural characteristics, and photosynthetic pigment content in both mature trees and seedlings. Specifically, the photosynthetic pigments Chl a, Chl b, Chl (a+b), and Car show significant positive correlations with Pmax, LSP, α, Amax, Rp, Pn, Ls, LT, PPT, SPT, PPT/SPT, and TT. They also show significant negative correlations with CCP, CSP, and LET. Stomatal structural parameters SD, SW, and SA exhibit significant positive correlations with LCP, Rd, CCP, CSP, Ci, WUE, UET, and LET. Leaf structural parameters UET and LET show significant positive correlations with LCP, Rd, CCP, CSP, Ci, and WUE, while they exhibit significant negative correlations with PPT, SPT, and TT. Moreover, PPT, SPT, PPT/SPT, and TT show significant positive correlations with Pmax, LSP, α, Amax, Rp, Pn, Ls, and LT. Photosynthetic physiological parameters Rp, Pn, and Ls show significant positive correlations with Pmax, LSP, α, and Amax. α and Amax show significant positive correlations with Pmax and LSP. CCP and CSP exhibit significant positive correlations with LCP and Rd, while showing significant negative correlations with LSP, α, and Amax.
Figure 7. Heatmap of correlation analysis among photosynthetic physiological parameters, leaf structural characteristics, and photosynthetic pigments in mature trees and seedlings of H. hainanensis.
Discussion
Photosynthetic characteristics
By calculating the light and parameters of plants, further exploration of their suitable growth environments and their abilities to adapt to the environment can be conducted (Yokoya et al., 2007). A higher maximum net photosynthetic rate (Pmax) indicates a stronger carbon fixation ability and a more abundant accumulation of organic matter, making it easier to meet the organic material requirements for plant growth (Mahmud et al., 2018; Vona et al., 2018). The Pmax of mature trees of H. hainanensis (4.83 µmol·m−2·s−1) is significantly higher than that of seedlings (3.85 µmol·m−2·s−1) (Table 2), indicating that the photosynthetic capacity of mature trees is significantly higher than that of seedlings, and mature trees have a higher ability to accumulate organic matter compared to seedlings. One possible reason is that the organic matter required to sustain the normal growth of mature trees is greater than that of seedlings, so mature trees need a higher light utilization capacity to maintain normal physiological activities. To some extent, the palisade parenchyma thickness and chlorophyll content of mature tree leaves are significantly greater than those of seedlings.
The LCP and LSP reflect a plant’s adaptation and utilization abilities towards light, serving as important indicators for evaluating plant growth characteristics. A larger difference between LCP and LSP indicates a wider range of light adaptation for the plant (Zhang et al., 2005). It is generally believed that shade-tolerant plants have an LCP below 20 µmol·m−2·s−1 and an LSP of 500–1,000 µmol·m−2·s−1 or lower (Jiang, 2004). The results of this study indicate that the LCP of mature trees is 2.69 µmol·m−2·s−1, and the LSP is 1,185.31 µmol·m−2·s−1. The LCP of seedlings is 4.70 µmol·m−2·s−1, and the LSP is 403.45 µmol·m−2·s−1 (Table 2). These values demonstrate typical characteristics of shade-tolerant plants, indicating that H. hainanensis seedlings require a certain degree of shading for growth. The difference between LCP and LSP in mature trees is greater than that in seedlings, with mature plants having a higher LCP and a lower LSP compared to seedlings, suggesting that mature plants have a stronger adaptability to light environments than seedlings. Similar observations have been made in other plants such as Argentina anserina, Populus, and Bletilla striata (Li et al., 2022; Chen et al., 2022; Luo et al., 2019). Sheng et al. (2020) found that larger stomatal apertures are one of the characteristics enabling plants to adapt to low light conditions. According to the correlation analysis in this study, stomatal conductance shows a strong correlation with photosynthetic-light response parameters, with seedlings exhibiting significantly larger stomatal conductance than mature trees, consistent with the previous findings of seedlings adapted to low light environments. Therefore, in the cultivation of H. hainanensis, it is recommended to provide certain shading treatment during the seedling stage and gradually reduce the shading as the plants grow.
CO2 is one of the primary raw materials for photosynthesis in plants. Sufficient CO2 can promote the net photosynthetic rate of plants, while insufficient CO2 can inhibit the accumulation of organic compounds. As CO2 concentration gradually increases, it can enhance carboxylase activity while inhibiting ribulose-1,5-bisphosphate carboxylase, thereby enhancing photosynthesis (Li et al., 2016; Sun et al., 2010). The Amax of mature trees is significantly higher than that of seedlings (Table 3), indicating that mature trees have a higher capacity to utilize CO2 compared to seedlings. The α of seedlings is significantly higher than that of mature trees, suggesting that seedlings have a stronger adaptation ability in a low CO2 environment. Meanwhile, the range of CSP is relatively large, indicating that H. hainanensis has a broad range of adaptability to CO2 concentrations. Due to the lower Pmax compared to Amax, insufficient CO2 supply in the environment is one of the main limiting factors for its photosynthetic rate. Therefore, planting mature trees and seedlings of H. hainanensis in an environment with higher CO2 concentration is beneficial for their growth and development. Stomata, unique structures on the leaf surface, control gas exchange and water loss in leaves. According to the correlation analysis in this study, there is a significant correlation between stomatal density, stomatal conductance, CCP, CSP, and Ci. Both stomatal density and stomatal conductance are higher in seedlings compared to mature trees, indicating that seedlings have a stronger ability for gas exchange and are more efficient in absorbing more CO2 in a low CO2 environment, demonstrating their stronger adaptability to such conditions, consistent with the previous results. However, due to factors such as higher chlorophyll content and more mature development of photosynthetic organs in mature trees, they have a stronger capacity to utilize CO2, resulting in generally higher net photosynthetic rates (Kinose et al., 2020).
During the diurnal variation of photosynthesis (Fig. 1), as the Beijing time approaches noon, the light intensity becomes excessively high, the environmental temperature gradually increases while humidity decreases. This leads to enhanced transpiration in plant leaves. Faced with environmental water scarcity and rapid internal water loss, the leaves opt to reduce stomatal conductance or even close stomata to decrease transpiration, reduce water loss, and simultaneously decrease CO2 uptake. This results in both mature trees and seedlings displaying low values of Pn. When the “midday depression” phenomenon occurs, Ci shows an upward trend, by adjusting Gs to fix the CO2 level in the environment to the maximum extent and further reducing leaf water loss, the impact of high temperature can be mitigated. Similar observations were made by Xu et al. (2024) in three species of Geodorum plants. We found that PAR may influence environmental factors such as temperature and humidity. Around 10:00 a.m., light intensity gradually increases, while temperature and humidity enter suitable ranges. This adjustment modulates Gs, promoting stomatal opening and gas exchange, raising leaf temperature, increasing the vapor pressure difference inside and outside the leaf, thus accelerating transpiration rate, ultimately enhancing photosynthetic rate. Subsequently, as PAR decreases, environmental temperature and humidity exceed optimal ranges, causing leaf Gs to decline gradually, thus reducing the photosynthetic rate. The average daily net photosynthetic rate of mature trees is significantly higher than that of seedlings, indicating that seedlings may face difficulties in accumulating substances and experience developmental constraints. This puts them at a disadvantage in intense population competition, resulting in slow population renewal and gradually entering a “death spiral.” Jiang (2018) also found that weak adaptability and low survival rate of H. hainanensis seedlings are among the main reasons for their endangered status. Similar phenomena have been observed in endangered plants such as Vatica guangxiensis (Xiao et al., 2023), Abies ziyuanensis (Li, 2024), and Glyptostrobus pensilis (Zheng, 2021).
The relationship between leaf anatomy and photosynthetic characteristics
This study found both upper and lower epidermises consist of a single layer of cells. In mature trees, most of the cells in the upper and lower epidermis are nearly rectangular, while in seedlings, they are irregularly elliptical. The palisade tissue in mature trees consists of 5–7 layers of cells, mostly rectangular in shape, arranged in an orderly and compact manner. The palisade tissue in seedlings comprises 4–5 layers of cells, which are irregularly elliptical, loosely arranged with some intercellular spaces, and the cells are shorter. In comparison to seedlings, the spongy tissue in mature trees exhibits a denser arrangement, more layers, and smaller intercellular spaces. Mature trees have well-developed vascular bundles, with 5–7 rows of conduits in the xylem. Abundant heterogeneous cells are mainly distributed near the vascular bundles and the xylem. The water storage tissue cells are small in size, numerous, and densely arranged. In contrast, the vascular bundles in seedlings are less developed, with 4–6 rows of conduits in the xylem. There are fewer heterogeneous cells compared to mature trees, primarily distributed near the vascular bundles and the xylem. The water storage tissue cells are larger in size and fewer in number. The parenchyma tissue cells are larger and plump, with some cells differentiating into water storage tissue, providing moisture for normal physiological activities of the seedlings (Fig. 5). The leaf is one of the vital organs for photosynthesis in plants, and the structural parameters of the UET, LET, PPT and SPT in leaves can all be influenced by external environmental factors, thereby altering the plant’s photosynthetic characteristics. The cellular structural features of leaves adapted to low light conditions include smaller upper and lower epidermis thickness, larger leaf thickness, and well-developed palisade and spongy tissues (Li et al., 2020a; Chen et al., 2020). In this study, the leaf thickness of mature trees was significantly greater than that of seedlings, while the thickness of both the upper and lower epidermis was significantly lower than that of seedlings. Additionally, both the palisade and spongy parenchyma thicknesses were significantly higher in mature trees compared to seedlings (Table 4), indicating that mature trees are better adapted to growth under low light environments. The palisade-to-spongy tissue ratio is an indicator reflecting the development of palisade parenchyma in plant leaves, where a higher value indicates more developed palisade parenchyma (Liang et al., 2014). Moreover, plant photosynthesis is significantly influenced by the palisade-to-spongy tissue ratio (Dong et al., 2022). A tightly arranged palisade parenchyma with cells closer to a rectangular shape can effectively increase the distribution density of chloroplasts within palisade cells, while a greater thickness of spongy parenchyma with a looser arrangement is favorable for gas exchange and photosynthesis in leaves (Xue, 2020). In mature trees of H. hainanensis, the palisade parenchyma cells are mostly rectangular in shape compared to seedlings, and the palisade-to-spongy tissue ratio is significantly higher, indicating that the palisade parenchyma in mature trees is more developed, with significantly higher RPPT than in seedlings. Consequently, the arrangement is more compact, and chloroplast distribution within palisade parenchyma cells is more concentrated, indicating that the photosynthetic activity of mature H. hainanensis trees is stronger than that of seedlings.
Research has shown that plant photosynthesis is highly sensitive to arid environments, primarily affecting plant photosynthesis by inhibiting the photosynthetic system and reducing the content of photosynthetic enzymes (Liu et al., 2023). This study found that both types of leaves have a higher number of abnormal cells surrounding leaf veins, while the distribution of upper and lower epidermis is relatively sparse (Fig. 5). Abnormal cells have a higher osmotic potential and stronger water absorption capacity, allowing them to store water. When facing a drought environment, they can at least temporarily provide water to other cells, enhancing resistance (Yang et al., 2007). The vascular bundle is one of the important factors influencing the efficiency of water transport in plants. The more developed the vascular bundle, the stronger the efficiency of water transport, and the degree of vascular bundle development can reflect the plant’s drought resistance (Ma et al., 2020). The xylem is mainly responsible for the transport of water and inorganic salts. The more developed the xylem, the better the plant’s water and inorganic salt transport (Li et al., 2023). The content of abnormal cells in mature trees is higher than in seedlings, and the vascular bundles and xylem are well-developed, indicating abundant water storage cells. Therefore, it can be inferred that mature trees have stronger drought resistance than seedlings, and similar phenomena have been observed in plants such as Populus euphratica and Populus pruinosa (Zhao, 2016). Even in arid environments, leaves are still able to temporarily provide water and carry out normal photosynthesis to provide energy for themselves.
The relationship between photosynthetic pigments and photosynthetic characteristics
Chlorophyll is one of the essential components of photosynthetic pigments in plants, and its content and ratio are key indicators of a plant’s adaptive capacity to the environment (Rodriguez-Calcerrada et al., 2008). The Chl a/b ratio of mature H. hainanensis trees and seedlings is consistently below 3 (Table 6), indicating shade-tolerant characteristics. However, mature trees exhibit higher chlorophyll content and lower Chl a/b ratio, suggesting their enhanced utilization of light energy (Huang et al., 2016; Hoflacher & Bauer, 1982). Correlation analysis results demonstrate a significant positive relationship between chlorophyll and Pn, as well as leaf structure. Based on the actual growth conditions, it is speculated that seedlings generally occupy lower positions within the community, where abundant light is intercepted by mature trees and other tall plants. In response to low light conditions, seedlings may adapt by reducing chlorophyll content to avoid photoinhibition caused by excessive chlorophyll levels. However, in actual community environments, excessive shading of seedlings often results in insufficient light absorption, exacerbating cellular and tissue underdevelopment and chlorophyll deficiency, leading to decreased Pn and forming a vicious cycle. These findings align with the diurnal variations in photosynthesis and leaf structure mentioned earlier. Pan et al. (2024b) also discovered similar survival challenges among seedlings of Vatica guangxiensis.
Conclusions
In summary, there are differences in the photosynthetic characteristics between mature H. hainanensis trees and seedlings, and these differences are primarily attributed to variations in photosynthetic pigments and leaf structure. While mature trees exhibit a broader range of light adaptability, they are more suited to grow under low light intensity. Seedlings, on the other hand, display typical shade-tolerant characteristics and are only suitable for growth in environments with high shading and sufficient moisture. The underdeveloped photosynthetic organs and poor adaptability in seedlings, insufficient natural light and low survival rates during wild growth are likely one of the reasons for H. hainanensis endangered status. Therefore, during in-site conservation, it is possible to promote the growth and development of seedlings by appropriately employing artificial “windowing” or shading methods based on the actual growth environment of the seedlings. It is recommended to provide appropriate shade for seedlings during artificial cultivation of H. hainanensis, while ensuring adequate water and CO2 levels. As the plants grow, the degree of shading can be gradually reduced. Once the plants reach maturity, they can be transplanted to environments with lower shading. This study aims to provide a theoretical basis for the in situ conservation and artificial cultivation of H. hainanensis through fundamental research on the photosynthetic physiology, leaf structure, and photosynthetic pigments of mature trees and seedlings.
Supplemental Information
The net photosynthetic rate of mature trees is significantly higher than that of seedlings.
Funding Statement
This study was supported by the Subproject of National Key R&D Program (2022YFF1300703); the Guangxi Forestry Science and Technology Promotion Demonstration Project ([2022]GT23); and the Hechi City Science and Technology Plan Project (Hechi Science AC231113). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Rong Zou, Email: zr@gxib.cn.
Xiao Wei, Email: wx@gxib.cn.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Jianwang Xu conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, and approved the final draft.
Jianmin Tang conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Haolong Jiang analyzed the data, prepared figures and/or tables, and approved the final draft.
Rong Zou conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Xiao Wei conceived and designed the experiments, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.
References
- Chai et al. (2021).Chai CN, Hou QX, Ci XQ, Xiao JH, Zhang CY, Li J. Genetic diversity of Horsfieldia hainanensis: an endangered species with extremely small populations. Journal of Tropical and Subtropical Botany. 2021;29(05):547–555. doi: 10.11926/jtsb.4364. [DOI] [Google Scholar]
- Chen et al. (2020).Chen B, Liu XW, Xu C, Li HY, Xia B, Yang Y, He M. Effects of different photosynthetically active radiation on physiological characteristics and ultrastructure of two species of Commelinaceae. Journal of Northeast Forestry University. 2020;48(11):14–22. doi: 10.13759/j.cnki.dlxb.2020.11.003. [DOI] [Google Scholar]
- Chen et al. (2022).Chen XH, Liu HQ, Zhang XJ, Chen XJ, Yang MS, Zhang J. Comparative analysis of photosynthetic and physiological characteristics of red-leafed ‘quanhong’ and ‘xuanhong’ poplare cultivars. Acta Horticulturae Sinica. 2022;49(2):437–447. doi: 10.16420/j.issn.0513-353x.2020-0759. [DOI] [Google Scholar]
- Chondrogiannis et al. (2023).Chondrogiannis C, Kotsi K, Grammatikopoulos G, Petropoulou Y. Seasonal differences in leaf photoprotective potential between adults and juveniles of two mediterranean perennials with distinct growth forms: a comparative field study. Plants. 2023;12(17):3110. doi: 10.3390/plants12173110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong et al. (2022).Dong MY, Wang JX, Wu M, Zhou ZY, Cheng S, Li YH. Leaf structure and photosynthetic characteristics of two species of Hesperis. Acta Prataculturae Sinica. 2022;31(07):172–184. doi: 10.11686/cyxb2021425. [DOI] [Google Scholar]
- He (2013).He GZ. Research on the rapid propagation techniques of endangered southern forest tree species, namely Horsfieldia hainanensis and Toona ciliata. Guangxi, China: Qinzhou Forestry Science Research Institute, Guangxi Zhuang Autonomous Region; 2013. [Google Scholar]
- Huang et al. (2016).Huang CJ, Wei G, Jie YC, Xu JJ, Anjum SA, Tanveer M. Effect of shade on plant traits, gas exchange and chlorophyll content in four ramie cultivars. Photosynthetica. 2016;54(3):390–395. doi: 10.1007/s11099-016-0186-x. [DOI] [Google Scholar]
- Hoflacher & Bauer (1982).Hoflacher H, Bauer H. Light acclimation in leaves of the juvenile and adult life phases of ivy (Hedera helix) Physiologia Plantarum. 1982;56(2):177–182. doi: 10.1111/j.1399-3054.1982.tb00321.x. [DOI] [Google Scholar]
- Houter & Pons (2012).Houter NC, Pons TL. Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia. 2012;169(1):33–45. doi: 10.1007/s00442-011-2175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang (2004).Jiang GM. Plant physiological ecology. Beijing: Higher Education Press; 2004. [Google Scholar]
- Jiang (2018).Jiang YH. Ecological characteristics and endangered reason analysis of Horsfieldia hainanensis Merr, as an extremely small population. Hunan, China: Central South University of Forestry and Technology; 2018. [Google Scholar]
- Jiang et al. (2016).Jiang YH, Xiang WH, Jiang Y, He YH, Lin JY. Floristic composition, structure and phytogeographic characteristics of Horsfieldia hainanensis community in Guangxi. Journal of Beijing Forestry University. 2016;38(01):74–82. doi: 10.13332/j.1000-1522.20150102. [DOI] [Google Scholar]
- John et al. (2007).John R, Dalling JW, Harms KE, Yavitt JB, Stallard RF, Mirabello M, Hubbell SP, Valencia R, Navarrete H, Vallego M, Foster RB. Soil nutrients influence spatial distributions of tropical tree species. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(3):864–869. doi: 10.1073/pnas.0604666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinose et al. (2020).Kinose K, Fukamachi Y, Watanabe M, Izuta T. Ozone-induced change in the relationship between stomatal conductance and net photosynthetic rate is a factor determining cumulative stomatal ozone uptake by Fagus crenata seedlings. Trees. 2020;34(2):445–454. doi: 10.1007/s00468-019-01927-1. [DOI] [Google Scholar]
- Li et al. (2020a).Li DL, Jin YQ, Cui MF, Huang LX, Pei WH. Growth, photosynthesis and ultrastructure of mesophyll cells for Cercidiphyllum japonicum seedlings with shading in summer. Journal of Zhejiang Agriculture and Forestry University. 2020a;37(03):496–505. doi: 10.11833/j.issn.2095-0756.20190370. [DOI] [Google Scholar]
- Li et al. (2020b).Li J, Zhou XY, Zhou JP, Shang R, Wang Y, Jing P. Comparative study on several determination methods of chlorophyll content in plants. IOP Conference Series: Materials Science and Engineering. 2020b;730(1):012066. doi: 10.1088/1757-899x/730/1/012066. [DOI] [Google Scholar]
- Li et al. (2016).Li LX, Liu JM, Liao XF, Xiong X, Luo C, Liu JJ. Effect of Drepanostachyum luodianense photosynthesis on characteristic of response to CO2. Journal of Northeast Forestry University. 2016;44(08):18–23. doi: 10.13759/j.cnki.dlxb.2016.08.004. [DOI] [Google Scholar]
- Li (2024).Li S. Study on the seedling competition and suitable habitat of plant species with extremely small populations Abies ziyuanensis. Guangxi, China: Guangxi Normal University; 2024. [Google Scholar]
- Li et al. (2023).Li X, Wu QS, Xu SQ, Li S, Liu YH, Zhang YM. Leaf morphological structure and drought resistance evaluation of three species of Crassulaceae. Journal of Northeast Normal University (Natural Science Edition) 2023;55(03):114–121. doi: 10.16163/j.cnki.dslkxb202108030001. [DOI] [Google Scholar]
- Li et al. (2022).Li XQ, Lu YM, Huang AM, Yuan RB, Li JR, Hu DD, Zhong QL, Chen DL. Light response model fitting and photosynthetic characteristics of ten different fern species in subtropics. Journal of Ecology. 2022;42(08):3333–3344. doi: 10.5846/stxb202104181006. [DOI] [Google Scholar]
- Li et al. (2019).Li YN, Yan W, Luo LJ, Lv YL, Huang JX. Preparation of paraffin sections of plant leaves with a low-toxic transparent agent. Chinese Journal of Tropical Agriculture. 2019;39(04):58–61. [Google Scholar]
- Liang et al. (2014).Liang WB, Zhao LJ, Li JX, Xiao J. Leaf comparative anatomy of Styrax (Styracaceae) in Hunan. Plant Research. 2014;34(02):148–158. doi: 10.7525/j.issn.1673-5102.2014.02.002. [DOI] [Google Scholar]
- Liu et al. (2023).Liu CL, Huang WJ, Tang ZS, Song ZS, Huang PW, Li B, Zhang YS, Wang CL, Wang YP. Effects of drought stress on seed germination and seedling photosynthetic physiological characteristics of three species of licorice. Chinese Wild Plant Resources. 2023;42(08):10–17. doi: 10.3969/j.issn.1006-9690.2023.08.002. [DOI] [Google Scholar]
- Luo et al. (2019).Luo M, Liu H, Yang PF, Zhang JX, Yang ZG, Xia Y, Wu MK. Study on the characteristics of photosynthetic light response in leaves of Bletila striata seedlings at different acclimation stages. Seed. 2019;38(9):52–56. doi: 10.16590/j.cnki.1001-4705.2019.09.052. [DOI] [Google Scholar]
- Mahmud et al. (2018).Mahmud K, Medlyn BE, Duursma RA, Campany C, De Kauwe MG. Inferring the effects of sink strength on plant carbon balance processes from experimental measurements. Biogeosciences. 2018;15(13):4003–4018. doi: 10.5194/bg-15-4003-2018. [DOI] [Google Scholar]
- Ma et al. (2020).Ma HY, Lv XX, Ji YN, Li XW. Leaf anatomical structure and drought resistance of 17 Caragana species. Research on Soil and Water Conservation. 2020;27(01):340–346. doi: 10.13869/j.cnki.rswc.2020.01.047. [DOI] [Google Scholar]
- Pan et al. (2024a).Pan LP, Tang JM, Jiang HD, Zou R, Chai SF, Wei X. Comparison of photosynthesis and structure of leaves between Manglietia aromatica seedlings and adult plants. Molecular Plant Breeding. 2024a:1–11. [Google Scholar]
- Pan et al. (2024b).Pan XF, Zou R, Tang JM, Wei X, Jiang HD, Yang YS. Comparative study on photosynthetic characteristics and leaf microstructure of Vatica guangxiensis seedling and adult trees. Guihaia. 2024b:1–15. [Google Scholar]
- Rodriguez-Calcerrada et al. (2008).Rodriguez-Calcerrada J, Reich PB, Rosenqvist E, Pardos JA, Cano FJ, Aranda I. Leaf physiological versus morphological acclimation to high-light exposure at different stages of foliar development in oak. Tree Physiology. 2008;28(5):761–771. doi: 10.1093/treephys/28.5.761. [DOI] [PubMed] [Google Scholar]
- Sheng et al. (2020).Sheng JY, Cui WX, Zhang EJ, Yu XR, Xiong F. Morphological structure observation and photosynthetic characteristics analysis of taro leaf. Journal of Biology. 2020;37(02):61–64. [Google Scholar]
- Sun et al. (2010).Sun CX, Hao JJ, Wang J, Miu L, Chen ZH, Qi H. Responses of photosynthetic physiological characteristics of two transgenic cotton (Gossypium hirsutum L) varieties to CO2, concentration. Journal of Ecology. 2010;30(02):504–510. [Google Scholar]
- Vona et al. (2018).Vona V, Di Martino Rigano V, Andreoli C, Lobosco O, Caiazzo M, Martello A, Rigano C. Comparative analysis of photosynthetic and respiratory parameters in the psychrophilic unicellular green alga Koliella antarctica, cultured in indoor and outdoor photo-bioreactors. Physiology and Molecular Biology of Plants. 2018;24(6):1139–1146. doi: 10.1007/s12298-018-0595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen (1997).Wen ZD. Recent studies on plant water use efficiency under elevated atmospheric concentrations of carbon dioxide. Journal of Tropical and Subtropical Botany. 1997;03:83–90. [Google Scholar]
- Wu et al. (2000).Wu GH, Tian LS, Hu SX, Wang NA. Physical geography. Third Edition. Beijin: Higher Education Press; 2000. [Google Scholar]
- Xiao et al. (2023).Xiao YX, He ZX, Su QZ, Tang X, Huang GL, Liu M, Yu WB, Tang JW. Current distribution and conservation strategy of Vatica guangxiensis (Dipterocarpaceae), a rare and endangered species with extremely small population. Western Forestry Science. 2023;52(03):1–7. doi: 10.16473/j.cnki.xblykx1972.2023.03.001. [DOI] [Google Scholar]
- Xu et al. (2024).Xu AZ, Jiang HD, Pu QK, Wei X, Wei YJ, Luo YJ, Chai SF. Comparative study on leaf anatomical structures and photosynthetic characteristics of three Geodorum species. Guihaia. 2024;44(01):113–125. doi: 10.11931/guihaia.gxzw202302011. [DOI] [Google Scholar]
- Xue (2020).Xue L. Photosynthetic characteristics and leaf anatomical structure of five precious tree species under shading condition. Changsha, Hunan, China: Central South University of Forestry and Technology; 2020. [Google Scholar]
- Yang et al. (2007).Yang KJ, Zhang XP, Zhang XW, Zhang ZX, Cao JJ. Study on anatomical structure of leaves of rare plant Emmenopterys henryi Oliv. Plant Research. 2007;02:195–198. doi: 10.3969/j.issn.1673-5102.2007.02.016. [DOI] [Google Scholar]
- Ye (2010).Ye ZP. A review on modeling of responses of photosynthesis to light and CO2. Journal of Plant Ecology. 2010;34(06):727–740. doi: 10.3773/j.issn.1005-264x.2010.06.012. [DOI] [Google Scholar]
- Ye, Yu & Kang (2012).Ye ZP, Yu Q, Kang HJ. Evaluation of photosynthetic electron flow using simultaneous measurements of gas exchange and chlorophyll fluorescence under photorespiratory conditions. Photosynthetica. 2012;50(3):472–476. doi: 10.1007/s11099-012-0051-5. [DOI] [Google Scholar]
- Yokoya et al. (2007).Yokoya NS, Necchi O, Martins AP, Gonzalez SF, Plastino EM. Growth responses and photosynthetic characteristics of wild and phycoerythrin-deficient strains of Hypnea musciformis (Rhodophyta) Journal of Applied Phycology. 2007;19(3):197–205. doi: 10.1007/s10811-006-9124-9. [DOI] [Google Scholar]
- Zhang et al. (2005).Zhang WF, Fan DY, Xie ZQ, Jiang XH. The seasonal photosynthetic responses of seedlings of the endangered plant Cathaya argyrophylla to different growth light environments. Biological Diversity. 2005;05:387–397. doi: 10.3321/j.issn:1005-0094.2005.05.003. [DOI] [Google Scholar]
- Zhao (2016).Zhao PY. The individual leaf morphological and anatomical features of Populus euphratica and Populus pruinosa and its relationship with the different developmental stages. Xinjiang, China: Tarim University; 2016. [Google Scholar]
- Zheng (2021).Zheng XH. Population structure characteristics and protection strategies of natural Glyptostrobus pensilis forests. Southern Agriculture. 2021;15(36):107–110. doi: 10.19415/j.cnki.1673-890x.2021.36.033. [DOI] [Google Scholar]
- Zhong et al. (2018).Zhong SY, Chen GD, Qiu MH, Liu MH, Lin L, Lin ZW. Investigation on the geographical distribution and habitat characteristics of Horsfieldia hainanensis in Hainan island. Fujian Forestry Science and Technology. 2018;45(01):82–86. doi: 10.13428/j.cnki.fjlk.2018.01.018. [DOI] [Google Scholar]
- Zong et al. (2022).Zong D, Wang JM, Zhang Y, Ma DX, Jiang FR, Zhang XL, He CZ. Comparison of photosynthetic characteristics of nine poplar species in southwest China in autumn. Journal of Northwest Forestry University. 2022;37(4):57–63. doi: 10.3969/j.issn.1001-7461.2022.04.08. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The net photosynthetic rate of mature trees is significantly higher than that of seedlings.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data is available in the Supplemental Files.