Abstract
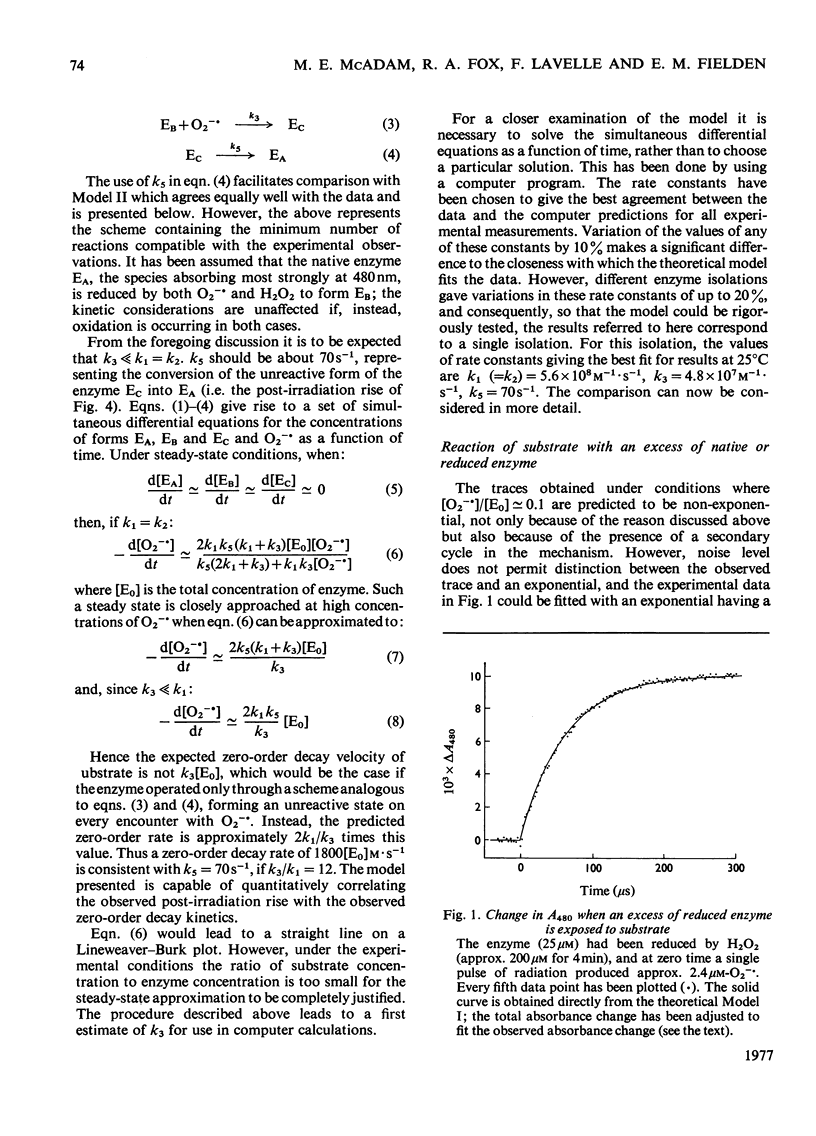
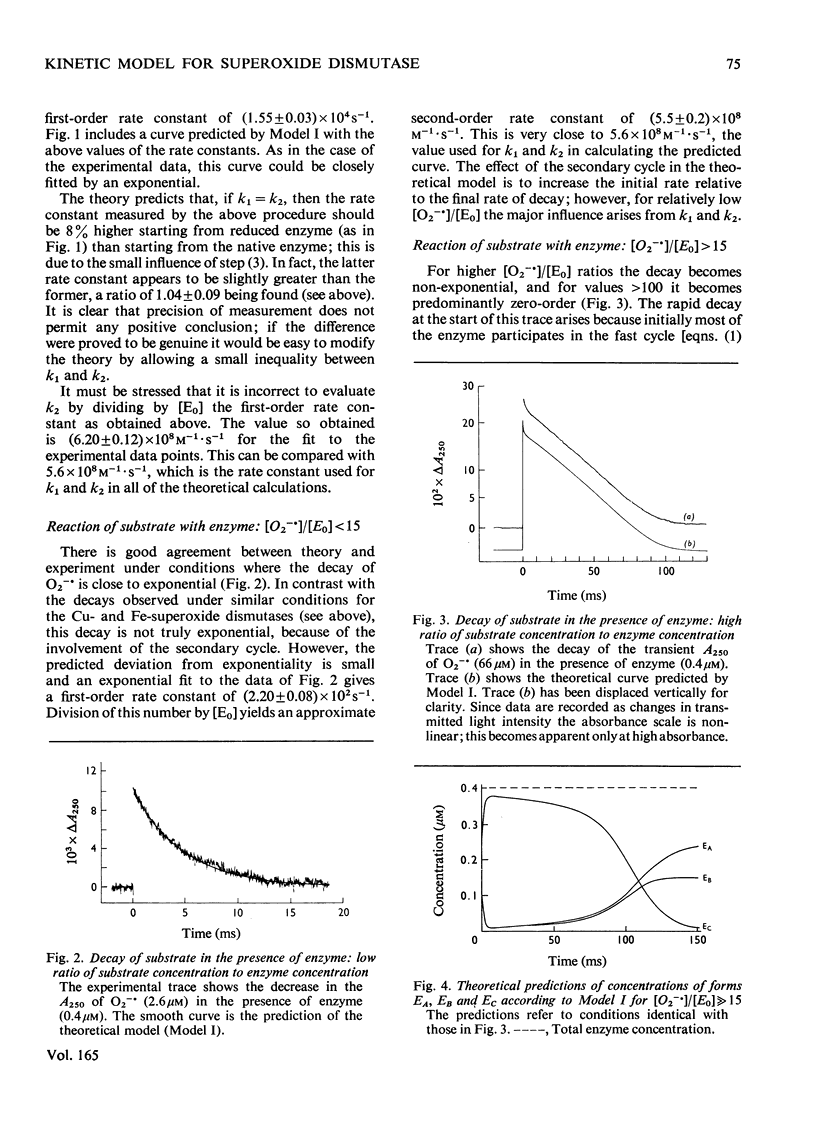
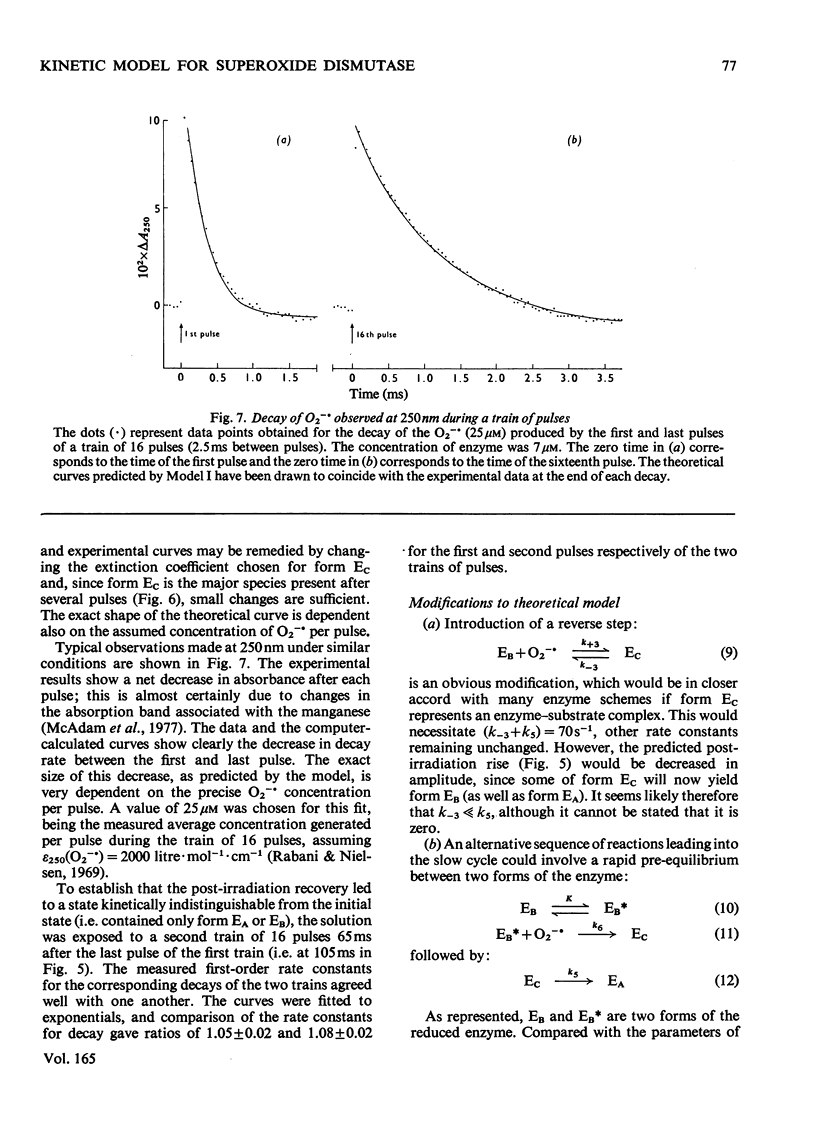
The enzymic reaction mechanism of a manganese-containing superoxide dismutase from Bacillus stearothermophilus was studied by using pulse radiolysis. During catalysis (pH 8.9; 25 degrees C), changes occurring in the kinetics of substrate disappearance and in the visible absorption of the enzyme at 480 nm established that the simple two-step mechanism found for copper- and iron-containing superoxide dismutases is not involved. At a low ratio (less than 15) of substrate concentration to enzyme concentration the decay of O2--is close to exponetial, whereas at much higher ratios (greater than 100) the observed decay is predominantly zero-order. The simplest interpretation of the results invokes a rapid one-electron oxidation-reduction cycle ('the fast cycle') and, concurrently, a slower reaction giving a form of the enzyme that is essentially unreactive towards O2-- but which undergoes a first-order decay to yield fully active native enzyme ('the slow cycle'). The fast cycle involves the native enzyme EA and a form of the enzyme EB which can be obtained also by treating the form EA with H2O2. Computer calculations made with such a simple model predict behaviour in excellent agreement with the observed results.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister J. V., Bannister W. H., Bray R. C., Fielden E. M., Roberts P. B., Rotilio G. The superoxide dismutase activity of human erythrocuprein. FEBS Lett. 1973 Jun 1;32(2):303–306. doi: 10.1016/0014-5793(73)80859-2. [DOI] [PubMed] [Google Scholar]
- Bray R. C., Cockle S. A., Fielden E. M., Roberts P. B., Rotilio G., Calabrese L. Reduction and inactivation of superoxide dismutase by hydrogen peroxide. Biochem J. 1974 Apr;139(1):43–48. doi: 10.1042/bj1390043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgen J., Harris J. I., Kolb E. Superoxide dismutase from Bacillus stearothermophilus: crystallization and preliminary x-ray diffraction studies. J Mol Biol. 1976 Aug 5;105(2):333–335. doi: 10.1016/0022-2836(76)90116-9. [DOI] [PubMed] [Google Scholar]
- Bridgen J., Harris J. I., Northrop F. Evolutionary relationships in superoxide dismutase. FEBS Lett. 1975 Jan 1;49(3):392–395. doi: 10.1016/0014-5793(75)80793-9. [DOI] [PubMed] [Google Scholar]
- Brock C. J., Harris J. I., Sato S. Superoxide dismutase from Bacillus stearothermophilus. Preparation of stable apoprotein and reconstitution of fully active Mn enzyme. J Mol Biol. 1976 Oct 25;107(2):175–178. doi: 10.1016/s0022-2836(76)80025-3. [DOI] [PubMed] [Google Scholar]
- Fielden E. M., Roberts P. B., Bray R. C., Lowe D. J., Mautner G. N., Rotilio G., Calabrese L. Mechanism of action of superoxide dismutase from pulse radiolysis and electron paramagnetic resonance. Evidence that only half the active sites function in catalysis. Biochem J. 1974 Apr;139(1):49–60. doi: 10.1042/bj1390049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):35–97. doi: 10.1002/9780470122860.ch2. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Annu Rev Biochem. 1975;44:147–159. doi: 10.1146/annurev.bi.44.070175.001051. [DOI] [PubMed] [Google Scholar]
- Klug-Roth D., Fridovich I., Rabani J. Pulse radiolytic investigations of superoxide catalyzed disproportionation. Mechanism for bovine superoxide dismutase. J Am Chem Soc. 1973 May 2;95(9):2786–2790. doi: 10.1021/ja00790a007. [DOI] [PubMed] [Google Scholar]
- Klug D., Rabani J., Fridovich I. A direct demonstration of the catalytic action of superoxide dismutase through the use of pulse radiolysis. J Biol Chem. 1972 Aug 10;247(15):4839–4842. [PubMed] [Google Scholar]
- Lavelle F., McAdam M. E., Fielden E. M., Roberts P. B. A pulse-radiolysis study of the catalytic mechanism of the iron-containing superoxide dismutase from Photobacterium leiognathi. Biochem J. 1977 Jan 1;161(1):3–11. doi: 10.1042/bj1610003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam M. E., Levelle F., Fox R. A., Fielden E. M. A pulse-radiolysis study of the manganese-containing superoxide dismutase from Bacillus stearothermophilus. Biochem J. 1977 Jul 1;165(1):81–87. doi: 10.1042/bj1650081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick M., Rabani J., Yost F., Fridovich I. The catalytic mechanism of the manganese-containing superoxide dismutase of Escherichia coli studied by pulse radiolysis. J Am Chem Soc. 1974 Nov 13;96(23):7329–7333. doi: 10.1021/ja00830a026. [DOI] [PubMed] [Google Scholar]
- Rotilio G., Bray R. C., Fielden E. M. A pulse radiolysis study of superoxide dismutase. Biochim Biophys Acta. 1972 May 12;268(2):605–609. doi: 10.1016/0005-2744(72)90359-2. [DOI] [PubMed] [Google Scholar]


