Abstract
Background
Patients with recent acute coronary syndrome (ACS) commonly experience chest pain, which affects quality of life even when not due to recurrence of ACS. This post hoc analysis of ODYSSEY OUTCOMES assessed the effect of alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor, on the incidence of chest pain not due to recurrent ACS.
Methods
Patients with recent ACS (n = 18,894) and elevated atherogenic lipoprotein levels despite optimized statin therapy were randomized to subcutaneous alirocumab or matching placebo every 2 weeks. Alirocumab dose was adjusted to target low-density lipoprotein cholesterol (LDL-C) 25–50 mg/dL (0.6–1.3 mmol/L) and to avoid consecutive LDL-C <15 mg/dL (0.39 mmol/L). Non-hospitalized chest pain adverse events and chest pain events requiring hospitalization but negatively adjudicated for recurrent ACS were assessed.
Results
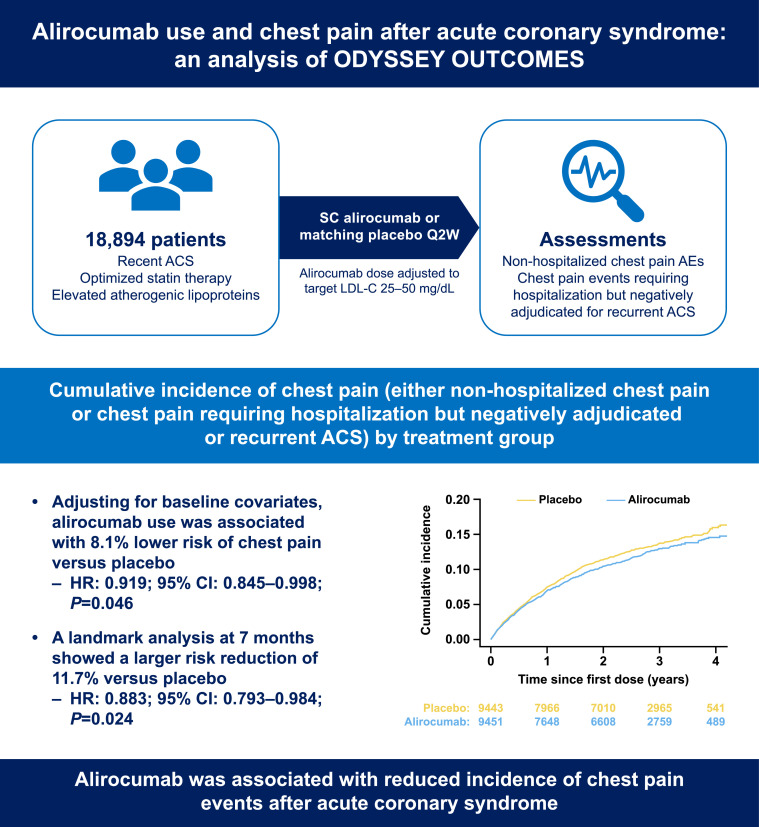
Chest pain not requiring hospitalization was reported as an adverse event in 1490 patients, including 7.5 % and 8.3 % of alirocumab and placebo groups, respectively. Hospitalization for chest pain negatively adjudicated for recurrent ACS occurred in 952 patients, including 4.8 % and 5.3 % of alirocumab and placebo groups, respectively. Adjusting for baseline covariates, alirocumab use was associated with 8.1 % lower risk of chest pain (either non-hospitalized or hospitalized events) versus placebo (HR: 0.919; 95 % CI: 0.845–0.998; P = 0.046); a landmark analysis at 7 months showed a larger, 11.7 % risk reduction (HR: 0.883; 95 % CI: 0.793–0.984; P = 0.024).
Conclusions
Alirocumab use is associated with reduced incidence of chest pain events after ACS, including those not requiring hospitalization and those requiring hospitalization but not adjudicated as recurrent ACS.
Trial registration
Keywords: Acute coronary syndrome, Alirocumab, Cardiovascular disease, Chest pain, Proprotein convertase subtilisin/kexin type 9, Low-density lipoprotein cholesterol
1. Introduction
The ODYSSEY OUTCOMES trial investigated the effect of alirocumab, a proprotein convertase subtilisin/kexin type 9 inhibitor versus placebo on cardiovascular outcomes after an acute coronary syndrome (ACS) in patients receiving maximal statin therapy [1]. The primary endpoint was a composite of death from coronary heart disease, nonfatal myocardial infarction, fatal or nonfatal ischemic stroke, or unstable angina requiring hospitalization. The risk of recurrent ischemic cardiovascular events was lower among alirocumab-treated patients, with a composite primary endpoint event occurring in 9.5 % patients vs 11.1 % among placebo (hazard ratio [HR], 0.85; 95 % confidence interval [CI], 0.78–0.93; P < 0.001) [2]. Among patients with ACS, complaints of chest pain are common and significantly affect quality of life even when not due to recurrent ACS [3]. This post hoc analysis of the ODYSSEY OUTCOMES trial investigated the effect of alirocumab on the incidence of treatment-emergent chest pain events, including those reported as adverse events (AE) that did not require hospitalization and events of chest pain requiring hospitalization that were negatively adjudicated as either unstable angina or myocardial infarction according to protocol definition.
2. Materials and methods
2.1. ODYSSEY OUTCOMES trial design
The ODYSSEY OUTCOMES trial design and primary results have been previously published [1,2]. Briefly, ODYSSEY OUTCOMES was a multicenter, double-blind, placebo-controlled trial that randomized patients with a recent ACS (defined as myocardial infarction or unstable angina) and who had elevated atherogenic lipids despite stable optimized statin therapy. Patients received either subcutaneous alirocumab 75 mg every 2 weeks or matching placebo. The dose of alirocumab was adjusted under blinded conditions to target an LDL-C level of 25–50 mg/dL (0.6–1.3 mmol/L) and to avoid consecutive LDL-C levels below 15 mg/dL (0.39 mmol/L). The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All sites obtained Institutional Review Board or Ethics Committee approval as per local and national guidelines. All patients provided written informed consent.
2.2. Assessments / endpoints
This post hoc analysis evaluated chest pain events including non-hospitalized events reported as adverse events, and events requiring hospitalization that were negatively adjudicated as unstable angina or myocardial infarction. Analyses were performed for time from first study dose to first chest pain occurrence and included patients with complete first and last dose dates, grouped by treatment assignment. Safety population (those receiving ≥1 dose or part of dose) was assessed for chest pain events during the treatment-emergent adverse event (TEAE) period, defined for each patient as the interval between the first study dose and 70 days after the last study dose (or death if earlier).
2.3. Identification of chest pain
Chest pain events included those requiring hospitalization but negatively adjudicated for unstable angina or myocardial infarction according to protocol [1]. Also included in the analysis were non-hospitalized chest pain events identified from TEAEs under one of the preferred terms outlined in Supplementary Table 1, reviewed by a blinded expert investigator and confirmed by a second blinded expert investigator.
2.4. Statistical analysis
Kaplan-Meier cumulative incidence of chest pain events was assessed by study treatment. Cox regression model was conducted to evaluate the effect of alirocumab on chest pain incidence adjusting for baseline demographic covariates and medical history. Proportional hazard assumption was assessed globally and for each covariate, and the violation of the covariate proportionality assumption was resolved by stratification. Adjusted landmark analysis (≤7 months vs >7 months) was also conducted. The landmark of 7 months was empirically selected based on survival curves summarizing MACE endpoints from the main study results [2]. A sensitivity 1-year landmark analysis was also conducted. In addition, single and multiple chest pain events in the same individual were analyzed separately. The adjusted alirocumab effect was further evaluated by sex. Same analyses were applied for non-hospitalized chest pain and negatively adjudicated ACS requiring hospitalization individually. A supplementary analysis was performed using logistic regression to determine the occurrence of chest pain, after adjusting for the follow-up time of the patients. All analyses were done in R version 4.3.0.
3. Results
3.1. Baseline characteristics according to chest pain status
Overall, 18,894 participants from the safety population were included in the analysis (alirocumab, n = 9451; placebo, n = 9443). The median follow-up time was 2.6 (Q1: 2.1; Q3: 3.3) years for this analysis. During the study, 1490 patients reported chest pain as AE, and 952 patients were hospitalized for chest pain that subsequently was negatively adjudicated for ACS (Supplementary Table 1). A total of 211 patients experienced both types of events. Patients from Western Europe and North America reported a higher rate of chest pain AE than patients from the rest of the world. A higher proportion of patients who experienced chest pain during the study had prior (before index event) medical history of revascularization versus patients who did not experience chest pain (30.1 % vs 18.4 %) (Supplementary Table 2).
3.2. Incidence of chest pain by treatment group
In the alirocumab and placebo groups, respectively, 7.5 % and 8.3 % of patients reported chest pain as AE, and 4.8 % and 5.3 % were hospitalized with chest pain that was negatively adjudicated as ACS. The observed incidence of combined chest pain events was 11.2 % and 12.5 % for the alirocumab and placebo groups, respectively (Table 1). Kaplan-Meier estimated cumulative incidence of chest pain by treatment group is provided in the Central Illustration and Supplementary Figure 1. After adjusting for baseline covariates, the risk of having chest pain was 8.1 % lower (HR: 0.919; 95 % CI: 0.845–0.998; P = 0.046; Table 2) on alirocumab compared to placebo. When looking at the two types of events separately, the adjusted risk was reduced by 6.4 % for non-hospitalized chest pain (HR: 0.936; 95 % CI: 0.845–1.036; P = 0.202) and by 8.5 % for negatively adjudicated ACS requiring hospitalization (HR: 0.915; 95 % CI: 0.806–1.040; P = 0.173). A 7-month landmark analysis showed that the reduction in the risk of experiencing chest pain with alirocumab, compared to placebo, was 11.7 % (>7 months, HR: 0.883; 95 % CI: 0.793–0.984; P = 0.024; Supplementary Table 3). A sensitivity 1-year landmark analysis gave similar though non-significant results (HR: 0.901; 95 % CI: 0.792–1.026; P = 0.116). The incidence of chest pain requiring hospitalization and each individual preferred term for non-hospitalized chest pain are summarized in Supplementary Table 1. Irrespective of treatment group, most patients experienced a single chest pain event during the study (Supplementary Table 4). Kaplan-Meier estimated cumulative incidence by treatment group and sex is provided in Supplementary Figure 2 and adjusted results are provided in Table 2. The incidence of chest pain by treatment group, the number of chest pain events and sex is summarized in Supplementary Table 5. The supplementary analysis model showed similar results, indicating that the odds of experiencing chest pain was 10 % lower for patients on alirocumab compared to those on a placebo (odds ratio: 0.899; 95 % CI: 0.822–0.984; P = 0.020). Interestingly, the rate of MACE was nearly double among those who experienced chest pain compared with those who did not (389/2231, or 17.4 % vs 1562/16663, or 9.4 %, respectively).
Table 1.
Incidence of chest pain and number of chest pain events during the TEAE period by treatment group and overall
| Alirocumab (n = 9451) | Placebo (n = 9443) | Overall (N = 18,894) | Unadjusted relative risk alirocumab vs placebo | |
|---|---|---|---|---|
| Combineda | ||||
| Total number of events (patients with events, n) | 1439 (1055) | 1554 (1176) | 2993 (2231) | |
| Risk (95 % CI) | 0.112 (0.105–0.118) | 0.125 (0.118–0.131) | 0.118 (0.114–0.123) | 0.896 |
| Non-hospitalized chest pain events as AE | ||||
| Total number of events (patients with events, n) | 892 (710) | 949 (780) | 1841 (1490) | |
| Risk (95 % CI) | 0.075 (0.070–0.080) | 0.083 (0.077–0.088) | 0.079 (0.075–0.083) | 0.910 |
| Hospitalized chest pain events negatively adjudicated for unstable angina or myocardial infarction | ||||
| Total number of events (patients with events, n) | 547 (449) | 605 (503) | 1152 (952) | |
| Risk (95 % CI) | 0.048 (0.043–0.052) | 0.053 (0.049–0.058) | 0.050 (0.047–0.054) | 0.892 |
Combined: Non-hospitalized chest pain events as AE or hospitalized chest pain events negatively adjudicated for unstable angina or myocardial infarction.
AE, adverse events; CI, confidence interval; TEAE, treatment-emergent adverse event.
Central Illustration.
Cumulative incidence of chest pain event (either non-hospitalized chest pain or hospitalized chest pain negatively adjudicated for unstable angina or myocardial infarction) by treatment group using Kaplan-Meier estimation
Table 2.
Cox proportional hazard regression estimateda hazard ratio of incidence of chest pain between alirocumab and placebo
| Types of chest pain events | Population | HR (95 % CI), P-value | P-value for interactionc |
|---|---|---|---|
| Combinedb | Overall | 0.919 (0.845–0.998), 0.046 | |
| Males | 0.916 (0.830–1.011) | 0.958 | |
| Females | 0.923 (0.790–1.078) | ||
| Non-hospitalized chest pain events as AE | Overall | 0.936 (0.845-1.036), 0.202 | |
| Males | 0.924 (0.819–1.042) | 0.698 | |
| Females | 0.969 (0.801–1.172) | ||
| Hospitalized chest pain events negatively adjudicated for unstable angina or myocardial infarction | Overall | 0.915 (0.806–1.040), 0.173 | |
| Males | 0.921 (0.791–1.071) | 0.851 | |
| Females | 0.895 (0.706–1.135) |
Cox regression model adjusted covariates included age, sex, race, ethnicity, BMI, medical history of chest pain since qualifying ACS event and medical history of revascularization.
Combined: Non-hospitalized chest pain events as AE or hospitalized chest pain events negatively adjudicated for unstable angina or myocardial infarction.
P-value for testing the interaction between sex and treatment arms.
ACS, acute coronary syndrome AE, adverse events; BMI, body mass index; CI, confidence interval; HR, hazard
4. Conclusions
Patients with recent ACS commonly experience chest pain, which affects quality of life [[4], [5], [6]]. This post hoc analysis of the ODYSSEY OUTCOMES trial provides evidence for a reduction in the incidence of chest pain among participants treated with alirocumab compared with placebo, which was particularly evident after 7 months in the study.
The primary endpoint of ODYSSEY OUTCOMES included positively adjudicated unstable angina requiring hospitalization. The treatment effect on unstable angina was significantly beneficial (HR: 0.61) even though the number of events was small (60 on placebo and 37 on treatment) and occurred in fewer than 1 % of the study cohort [2]. In this analysis, we focused on chest pain events that were not captured in the definition of MACE, and show that patients frequently experienced an adverse event of chest pain without hospitalization (alirocumab 7.5 % and placebo 8.3 %) or were hospitalized for chest pain that was then negatively adjudicated for ACS according to study protocol (alirocumab 4.8 % and placebo 5.3 %), and that lower rates occurred for those randomized to alirocumab versus those on placebo in each category.
Coronary microvascular dysfunction, often found in association with acute coronary syndrome [7], is identified more often in women than in men [[8], [9], [10]]. Lipid-lowering therapy with a PCSK9 inhibitor added to statin has not been shown to improve coronary microvascular dysfunction [11]. These observations raise the possibility that symptoms due to microvascular dysfunction, and therefore unresponsive to alirocumab, contributed to the numerically smaller treatment effect of alirocumab on chest pain in women versus men in the current study. The fact that alirocumab reduced chest pain events in parallel with reducing rates of acute myocardial infarction and hospitalization for unstable angina suggests that the beneficial effect on chest pain was not simply the result of a clinical downgrading of MACE. Finally, the nearly double rate of MACE among those reporting chest pain suggests the potential utility of this symptom in reducing cohort size and follow-up length to adequately power outcome trials in ACS. Overall, the effect of alirocumab on chest pain has the potential to benefit quality of life and may positively affect acceptance of, and adherence to, treatment.
Study limitations, beyond those intrinsic to any post hoc analysis, include the observation period limited to treatment exposure, the incompleteness of adverse event reporting, and no formal adjudication for non-hospitalized chest pain events. However, it is reasonable to expect a low level of missingness and a high level of accuracy in the reporting of chest pain as adverse event, given that patients were in a study of cardiac protection and adverse events reporting was done by cardiovascular providers. To this point, it is important to note that the rate of MACE was nearly double among those who experienced chest pain compared with those who did not, supporting a clinically meaningful value of these adverse events in heralding the highest risk for the worst cardiovascular outcomes.
In conclusion, treatment with alirocumab reduced the rate of chest pain events among patients with recent ACS. These results are in line with the main effect of alirocumab on the rate of MACE.
Funding
This analysis was funded by Regeneron Pharmaceuticals, Inc.
Disclosures
Sergio Fazio, Ruifeng Chen, Kasturi Talapatra, Gregory P Geba, Taylor Brackin, Kusha A Mohammadi, Robert Pordy and Garen Manvelian are all employees of and shareholders in Regeneron Pharmaceuticals, Inc.
David J Maron reports consulting fees from Regeneron Pharmaceuticals, Inc.
Gregory G Schwartz reports research support to the University of Colorado from AstraZeneca, Resverlogix, Roche, Sanofi, and The Medicines Company; he is also coinventor of pending US patent 62/806,313 (“Methods for Reducing Cardiovascular Risk”) assigned in full to the University of Colorado.
Michael Szarek receives salary support from CPC, a non-profit academic research organization affiliated with the University of Colorado that receives research grant/consulting funding from Abbott, Agios, Alexion Pharma, Alnylam, Amgen, Angionetics, ARCA Biopharma, Array, AstraZeneca, Atentiv, Audentes, Bayer, Better Therapeutics, Brigham and Women's Hospital, Bristol-Myers Squibb, Cardiol Therapeutics, CellResearch, Cook Medical, Cook, CSL Behring, Eidos Therapeutics, EP Trading Co, Esperion Therapeutics, Everly Health, Faraday, Fortress Biotech, HDL Therapeutics, Heartflow, Hummingbird Bioscience, Insmed, Janssen, Kowa Research, Lexicon, Merck, MedPace, Medtronic, Moderna, Novate Medical, NovoNordisk, Pfizer, PhaseBio, PPD Development, Prairie Education and Research, Prothena Biosciences, Regeneron Pharmaceuticals, Inc., Regio Biosciences, Sanifit Therapeutics, Sanofi, Smith and Nephew, Stealth BioTherapeutics, University of Colorado, University of Pittsburgh, Worldwide Clinical Trials, Wraser, and Yale Cardiovascular Research Group; has received fees for performing analyses, steering committee fees, and travel support from Sanofi and Regeneron Pharmaceuticals, Inc.; has received consulting fees from CiVi, Lexicon, Amarin, and Esperion; has received Data Safety and Monitoring Board membership fees from Resverlogix and Janssen; and is a member of JACC editorial board.
Ph. Gabriel Steg reports grants and nonfinancial support (cochair of the ODYSSEY OUTCOMES trial; as such, he received no personal fees, but his institution has received funding for the time he has devoted to trial coordination, and he has received support for travel related to trial meetings) from Sanofi; research grants and personal fees from Bayer (Steering Committee MARINER, grant for epidemiological study), Merck (speaker fees, grant for epidemiological studies), Sanofi (cochair of the ODYSSEY OUTCOMES trial; cochair of the SCORED trial; consulting, speaking), Servier (Chair of the CLARIFY registry; grant for epidemiological research), and Amarin (executive steering committee for the REDUCE-IT trial [Disease Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial]; consulting); and personal fees from Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Pfizer, Idorsia, Myokardia, Novo Nordisk, Novartis, Regeneron Pharmaceuticals, Inc., and AstraZeneca. He also has a European application number/patent number, issued on October 26, 2016 (no. 15712241.7), for a method for reducing cardiovascular risk, all royalties assigned to Sanofi.
Data sharing
Qualified researchers may request access to study documents (including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan) that support the methods and findings reported in this manuscript. Individual anonymized participant data will be considered for sharing 1) once the product and indication has been approved by major health authorities (e.g., FDA, EMA, PMDA, etc) or development of the product has been discontinued globally for all indications on or after April 2020 and there are no plans for future development 2) if there is legal authority to share the data and 3) there is not a reasonable likelihood of participant re-identification. Submit requests to https://vivli.org/.
CRediT authorship contribution statement
Gregory P. Geba: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization. Ruifeng Chen: Writing – review & editing, Formal analysis, Conceptualization. Kasturi Talapatra: Writing – review & editing, Formal analysis, Conceptualization. Taylor Brackin: Writing – review & editing, Formal analysis, Conceptualization. Kusha A. Mohammadi: Writing – review & editing, Formal analysis, Conceptualization. Robert Pordy: Writing – review & editing, Formal analysis, Conceptualization. Garen Manvelian: Writing – review & editing, Formal analysis, Conceptualization. David J. Maron: Writing – review & editing, Formal analysis, Conceptualization. Gregory G. Schwartz: Writing – review & editing, Formal analysis, Conceptualization. Michael Szarek: Writing – review & editing, Formal analysis, Conceptualization. Ph. Gabriel Steg: Writing – review & editing, Formal analysis, Conceptualization. Sergio Fazio: Writing – review & editing, Writing – original draft, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank the patients, their families, all site investigators involved in this study, and the expert editorial assistance from Richa Attre of Regeneron Pharmaceuticals, Inc.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2024.100900.
Appendix. Supplementary materials
References
- 1.Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY OUTCOMES trial. Am Heart J. 2014;168:682–689. doi: 10.1016/j.ahj.2014.07.028. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 3.Gulati M, Levy PD, Mukherjee D, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 4.Rieckmann N, Neumann K, Feger S, et al. Health-related qualify of life, angina type and coronary artery disease in patients with stable chest pain. Health Qual Life Outcomes. 2020;18:140. doi: 10.1186/s12955-020-01312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox K, Garcia MA, Ardissino D, et al. Guidelines on the management of stable angina pectoris: executive summary: the task force on the management of stable angina pectoris of the european society of cardiology. Eur Heart J. 2006;27:1341–1381. doi: 10.1093/eurheartj/ehl001. [DOI] [PubMed] [Google Scholar]
- 6.Manolis AJ, Ambrosio G, Collins P, et al. Impact of stable angina on health status and quality of life perception of currently treated patients. The BRIDGE 2 survey. Eur J Intern Med. 2019;70:60–67. doi: 10.1016/j.ejim.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Del Buono MG, Montone RA, Camilli M, et al. Coronary microvascular dysfunction across the spectrum of cardiovascular diseases: JACC state-of-the-art review. J Am Coll Cardiol. 2021;78:1352–1371. doi: 10.1016/j.jacc.2021.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JJ, Park SJ, Choi DJ. Microvascular angina: angina that predominantly affects women. Korean J Intern Med. 2015;30:140–147. doi: 10.3904/kjim.2015.30.2.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel H, Aggarwal NT, Rao A, et al. Microvascular disease and small-vessel disease: The nexus of multiple diseases of women. J Womens Health (Larchmt) 2020;29:770–779. doi: 10.1089/jwh.2019.7826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaski JC, Crea F, Gersh BJ, Camici PG. Reappraisal of ischemic heart disease. Circulation. 2018;138:1463–1480. doi: 10.1161/CIRCULATIONAHA.118.031373. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara M, Asakura M, Hibi K, et al. Evolocumab for prevention of microvascular dysfunction in patients undergoing percutaneous coronary intervention: the randomised, open-label EVOCATION trial. EuroIntervention. 2022;18:e647–ee55. doi: 10.4244/EIJ-D-22-00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.