Abstract
The morphological classification of nucleated blood cells is fundamental for the diagnosis of hematological diseases. Many Deep Learning algorithms have been implemented to automatize this classification task, but most of the time they fail to classify images coming from different sources. This is known as “domain shift”. Whereas some research has been conducted in this area, domain adaptation techniques are often computationally expensive and can introduce significant modifications to initial cell images. In this article, we propose an easy-to-implement workflow where we trained a model to classify images from two datasets, and tested it on images coming from eight other datasets. An EfficientNet model was trained on a source dataset comprising images from two different datasets. It was afterwards fine-tuned on each of the eight target datasets by using 100 or less-annotated images from these datasets. Images from both the source and the target dataset underwent a color transform to put them into a standardized color style. The importance of color transform and fine-tuning was evaluated through an ablation study and visually assessed with scatter plots, and an extensive error analysis was carried out. The model achieved an accuracy higher than 80% for every dataset and exceeded 90% for more than half of the datasets. The presented workflow yielded promising results in terms of generalizability, significantly improving performance on target datasets, whereas keeping low computational cost and maintaining consistent color transformations. Source code is available at: https://github.com/mc2295/WBC_Generalization
Keywords: Deep learning, Classification, White blood cell, Few shot learning, Domain adaptation
Graphical abstract
Highlights
-
•
Our study introduces a workflow for classifying blood cells from different domains.
-
•
The model adapts to eight datasets with minimal annotations (less than 100 images each).
-
•
We implement color standardization in lαβ space for consistent visual style.
-
•
Results show good generalization, with accuracy exceeding 90% for many datasets.
Introduction
Context
The study of blood smears is a routine laboratory test that follows automated complete blood cell count and leucocyte differential count. It provides essential information about the patient health condition. Nucleated blood cells are divided into different categories including white blood cells and occasionally nucleated red blood cells (or erythroblasts). Differential count is essential to reveal disorders that affect specific types of cells. Therefore, the classification of white blood cells and nucleated red blood cells is a key stage for the diagnosis of various pathological conditions, including malignancies such as leukemia and lymphomas. Usually, this analysis is performed by experts who visually assess microscopic images, which is error-prone and time-consuming.1 Deep learning solutions have gained significant importance in recent years, as they can automate processes and alleviate the increasing pressure on hospitals.2,3
A well-known problem with such an approach, however, is that it lacks generalizability capacity, and whereas a computer vision algorithm can give good results when trained on a given source dataset, it will most probably fail to obtain similar performance when applied on images from a different target dataset.4 Having images from different origins for training and testing can cause serious limitations. This phenomenon is called domain shift and is caused by variations in data distribution originating from different sources.5 This situation is very common; the visual aspect of images from various healthcare centers can vary greatly due to variations in lighting conditions, camera characteristics, backgrounds etc. and thus there is a need for algorithms that operate on data from any source. Domain adaptation is defined as the process of making an algorithm able to perform well on a new domain.
There has been a growing appeal for few-shot learning strategies applied to clinical data because the number of health-related labeled samples is most often scarce.6, 7, 8 In few-shot learning, the model is trained by using only a few annotated images. It can use previous knowledge learnt from another task, this process being defined as transfer learning. So far, only a few articles have tackled the matter of generalizability in the classification of white blood cells, and to our knowledge, no study has yielded an interpretable and simple-to-implement workflow for the classification of cell images coming from an unknown dataset.
In this article, we enhance the generalizability capacity of a classifier model on a new dataset, using a few labeled images. We propose a general workflow to classify images at low computational cost. We trained a neural network to classify white blood cell images from two source public datasets. Then, we applied the model to eight target datasets, by fine-tuning the model with no more than 100 labeled images from these datasets. For each dataset, a visual transformation was applied to standardize the color appearance to a common style. We also conducted extensive experiments to evaluate and understand the role of each step of our process. We visually assessed the benefits of our approach, and we carried out an error analysis. This article studies a large number of data sources, some of the aforementioned datasets having never been studied for generalizability performances before. This shows that a clinical center willing to classify a local dataset of images can obtain good performance with little annotation effort.
Related work
Until recently, the early diagnosis of hematological disorders was mainly based on clinician visual assessment of blood smears. The digitization of images, however, has paved the way for automated processes.1
At first, the automation of image treatment solely relied on machine learning principles such as SVM or random forest.9,10 This approach needed consequent pre-processing of the images, to extract relevant features before training models. In particular, the segmentation of the cell or the nucleus was often necessary.11,12
More recently, deep learning solutions have gained increasing popularity due to their ability to automatically extract relevant features without the need for manual pre-processing.2,3,13 Many studies have explored the classification of different cell types from peripheral blood or from bone marrow.4,8,14, 15, 16, 17 Classification was also employed for the differentiation between healthy and pathological cells.6,18,19
Most of the previously mentioned studies utilized uniform datasets, with both the training and testing phases employing images from the identical dataset. Nevertheless, a few articles addressed the issue of model generalizability, with most of them employing solely two or three different datasets. Among them, some researchers have dealt with generalizability by producing new images. In Refs,20, 21, 22, 23 generative adversarial network (GAN) models were designed to generate different-looking images and to augment the size of the dataset. Furthermore, data augmentation methods were employed, but mainly with random augmentation techniques, such as color jittering or random crop.24 In Baydilli et al.,20 and Claro et al.,24 10 datasets and 18 datasets were employed, respectively, in order to enhance the diversity of images. To the best of our knowledge, these are the only articles employing more than five datasets for the classification of white blood cell types.
At a feature level, other studies have tried to extract domain-independent features from images. In Refs,25, 26, 27 machine learning and deep learning techniques were combined to extract more robust features. This needed, however, further preprocessing treatment. Other articles trained models to specifically extract generalizable features in parallel with classification; in this case, the adversarial loss was used to make feature vectors indistinguishable.28,29 Nevertheless, this approach was still specific to the domains under study.
Combining feature and image focus, another approach was developed in Pandey et al.30; a variational autoencoder was trained to reconstruct an image from a wide latent space and classify it. When a new dataset was considered, the closest clone was chosen in the latent space thanks to structural similarity index, and the reconstructed image of this clone was classified.
These approaches presented several limitations. First, in order to generalize well, some of these models needed images from the test dataset for training. Second, the computational cost was high. Finally, whether it was with GAN or image transforms, the creation of new images did not ensure they were realistic looking.
Material and methods
Overview
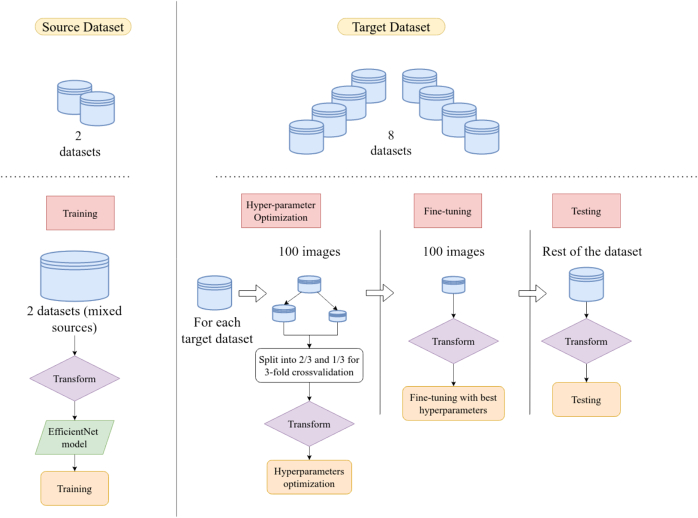
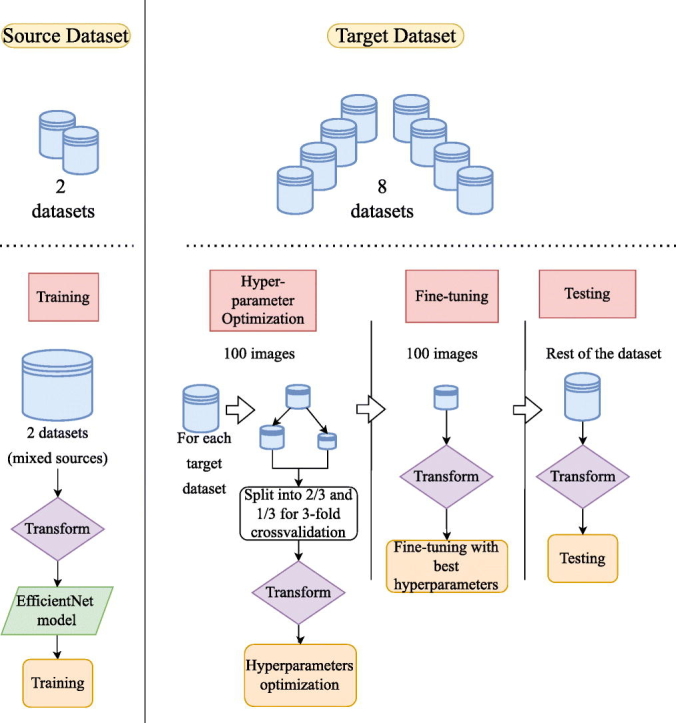
The overall workflow of our study is illustrated in Fig. 1, and consists in using different datasets for training and test, in order to evaluate the out-of-domain performances of our model.
Fig. 1.
Overview of the general workflow. Firstly, the model was trained on two source datasets. Secondly, for each target dataset, it was fine-tuned on 100 or less images and tested on the remaining images. All images underwent visual transform.
First, a model employing the EfficientNet architecture was trained on two source datasets: it constituted the source dataset. Images were collected and made public by Barcelona and Munich clinical centers.31,32 Afterwards, we fine-tuned the model on the eight other datasets, called the target datasets. For each target dataset, fine-tuning hyperparameters such as learning rate or batch size were optimized by dividing the fine-tuning dataset into train and valid subsets, and employing cross-validation. We report the numbers of images selected for fine-tuning in Table 3. The rest of the target datasets were used for testing purpose. A transform was applied both to the source and the target images to ensure they all followed the same visual “style”.
Table 3.
Number of images used for fine-tuning. 100 images were taken from large datasets, 1/10th of the total number of images were taken for smaller datasets. No images were needed to fine-tune the model on JSLH, because images were already well classified without fine-tuning.
| Fine-tuning set | Fine-tuning set size | Remaining images |
|---|---|---|
| Rabin | 100 | 16,533 |
| Munich 2021 | 100 | 103,366 |
| Lisc | 20 | 222 |
| Jslh | 0 | 72 |
| Jin woo choi | 20 | 230 |
| Jiangxi tecom | 25 | 275 |
| Bccd | 100 | 8511 |
| Tianjin | 100 | 2371 |
Description of the datasets
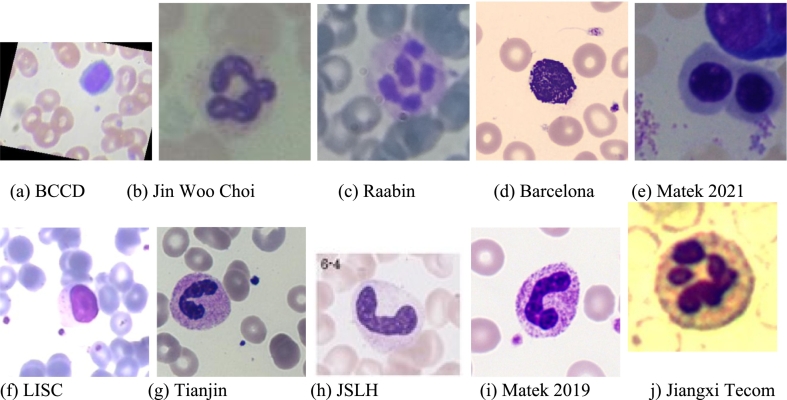
After a review of existing datasets in literature, we collected images from 12 studies. From these datasets, we discarded Cella Vision Blog which was very small and contained several annotation mistakes, as well as Ruinjing dataset which was only composed of lymphocytes, lymphoma, and blasts. Finally, we used 10 datasets, 2 of them being used for training, and the rest for fine-tuning and testing. The description of the final datasets is given in Table 1, and sample images from each dataset are shown in Fig. 2. The visual aspects of the images varied among the different datasets due to resolution, capture method, and magnification power. Details about the acquisition conditions for each dataset are provided in Appendix A. Both peripheral blood smears and bone marrow smears are represented in datasets and they were treated the same by the algorithm. For this study, only six WBC classes were considered, namely eosinophils, basophils, neutrophils, monocytes, erythroblasts, and lymphocytes. These classes were chosen as to maximize the number of classes shared among datasets, regardless of where cells were from. In some cases, classes had higher granularity; for example, neutrophils could be described as band neutrophil or segmented neutrophils. We classified these images into the main category they belonged to; in this case, they would both be labeled as neutrophils.
Table 1.
Description of the datasets with the links to the datasets. Tianjin dataset is available upon request. Classes used for this study are bolded. Only basophils, eosinophils, erythroblasts, lymphocytes, monocytes, and neutrophils were kept for study. If these classes were described more precisely (e.g., band neutrophils and segmented neutrophils) only the main label (e.g., neutrophil) was given to the cell.
| Name | Nb images | Classes |
|---|---|---|
| Barcelona31 | 17,092 | NEU, EOS, BAS, LYT, MON, PMO, MYB, EBO, PLA, THO |
| Jiangxi Tecom33 | 300 | BAS, EOS, LYT, NEU, MON |
| Jin Woo Choi34 | 2174 | NGB, NGS, ERB, MMZ, MYO, MYB, ERO, ERP, PMO, PBL |
| BCCD35 | 3500 | EOS, LYT, MON, NEU |
| LISC36 | 257 | BAS, EOS, LYT, NEU, MON |
| Munich 20214 | 171,373 | NGB, NGS, LYT, MON, EOS, BAS, MMZ, MYB, PMO, BLA, PLM, KSC, OTH, ART, NIF, PEB, EBO, HAC, ABE, LYI, FGC |
| Munich 201932 | 18,365 | BAS, EBO, EOS, KSC, LYT, LYA, MMZ, MYO, MON, NGB, NGS, PMB |
| Raabin37 | 4514 | EOS, LYT, MON, NEU |
| JSLH38 | 148 | NGB, ERB,MMZ,MYO, ERO, ERP, PEB, PMO, NGS |
| Tianjin39 | 8564 | BAS, EOS, LYT, NEU, MON |
Fig. 2.
Images from every dataset. Images come from 10 different dataset, both of bone marrow and of peripheral blood cells.
Table 2 details the number of available images. The names of cells categories are given by a three-letter abbreviation. The corresponding full names can be found in the Glossary.
Table 2.
The distribution of cell classes for every dataset.
| Dataset | Basophils | Eosinophils | Erythroblasts | Lymphocytes | Monocytes | Neutrophils |
|---|---|---|---|---|---|---|
| Munich 2021 | 441 | 5891 | 27,395 | 26,307 | 4040 | 39,392 |
| Rabin | 301 | 1066 | 3609 | 795 | 10,862 | |
| Munich 2019 | 79 | 424 | 78 | 3948 | 1789 | 8593 |
| Lisc | 53 | 39 | 52 | 48 | 50 | |
| Jslh | 56 | 24 | ||||
| Jin woo choi | 150 | 100 | ||||
| Jiangxi tecom | 1 | 22 | 48 | 53 | 176 | |
| Bccd | 3133 | 3108 | 3095 | 3171 | ||
| Tianjin | 302 | 1098 | 1863 | 1201 | 4100 | |
| Barcelona | 1218 | 3117 | 1551 | 1214 | 1420 | 3329 |
Data processing
Images from Tianjin and LISC datasets were not initially centered around the cell. Thus, these images were first cropped to have a 250 × 250 window around the cell. Masks were provided with LISC images, and bounding boxes with Tianjin images.
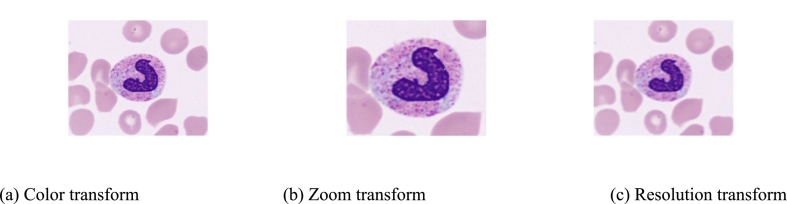
We applied a color transform to standardize the color style of each image (Fig. 3). Color-based transformation was chosen, and more precisely conversion with lαβ space – also known as CIELAB color space –40 following the method described in Reinhard et al.41 In addition, to better represent the variety of zooms and of resolutions, images from Barcelona that were of better quality in the training set were augmented with degradation of image resolution and random zoom (Fig. 4). After these transformations, all the augmented images were resized to 224 × 224 size as input of the neural network.
Fig. 3.
Color transform: all images were put in the same style with Lab-space transformation.
Fig. 4.
Successive transforms applied to training set.
Training strategy
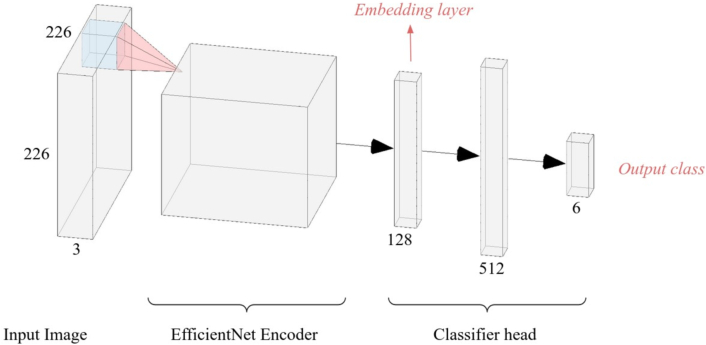
A preliminary comparison was drawn between the following architectures: VGG16, ResNet101, EfficientNet-B0, ViT, and Inception V3, in order to determine the model that would exhibit the best performances on out-of-domain images. This evaluation was based on a small subset of images. EfficientNet architecture was selected for an overall stronger ability to generalize across diverse datasets. The model was divided into two parts: the encoder was designed to extract features from images, and was implemented according to EfficientNet-B0 architecture. At the end of the encoder, a 128-node linear layer was added. We referred to the output of this layer as the image embedding. Then, a classifier head was positioned at the top of the model, comprising two fully connected linear layers, with 512 and 6 nodes, corresponding to the number of classes. The classifier head also incorporated Batch Normalization and Dropout techniques, with the activation function ReLU applied (Fig. 5).
Fig. 5.
Model architecture. The EfficientNet Encoder extracts the features from the image. These features are represented in the 128-layer, which we will refer as the image embeddings. The three fully connected linear layers are called head of the model and perform classification.
The training dataset was made of images from Barcelona and Munich 2019.31,32 The model was trained with batches made of images from these two datasets. The batch size was 16, the learning rate was 10−4 and the number of epochs was 15. LabelSmoothingCrossEntropy was chosen as a loss, and Adam optimizer was used. Computations were performed using Pytorch and Fastai library.
Fine-tuning strategy
To reproduce the situation of a new clinical center coming with a few annotated images, 100 or less images were selected from every target dataset, with the same proportion of images of each class. Fine-tuning was performed by retraining the model using only these images, as if the clinical center had only this small subset as ground-truth. The number of images used was reported in Table 3. No images were needed to fine-tune the model on JSLH dataset because images were good enough to be classified without fine-tuning.
The choice of fine-tuning parameters, principally the learning rate, the batch size and the number of epochs, plays a critical role in the performance of the model. Given the limited size of the fine-tuning subsets and the important differences between images from different datasets, parameters had to be carefully set to prevent overfitting. Thus, the fine-tuning set was partitioned into training and validation subsets, and hyper parameter optimization was carried on using Optuna library. 3-fold cross-validation was utilized to mitigate the specificity of the results to the very small fine-tuning validation set.
Results and discussion
Classification of target datasets
The overall accuracy is defined as the number of rightly predicted images over the total number of images. Precision and recall are relative to a class.
We first trained the model on source datasets. We split the two source datasets in 80/20 to evaluate the performances of the EfficientNet model after initial training, and obtained accuracy, macro precision and macro recall of 0.88, 0.76, 0.77 for Munich 2018 and 0.98, 0.98, 0.98 for Barcelona, respectively.
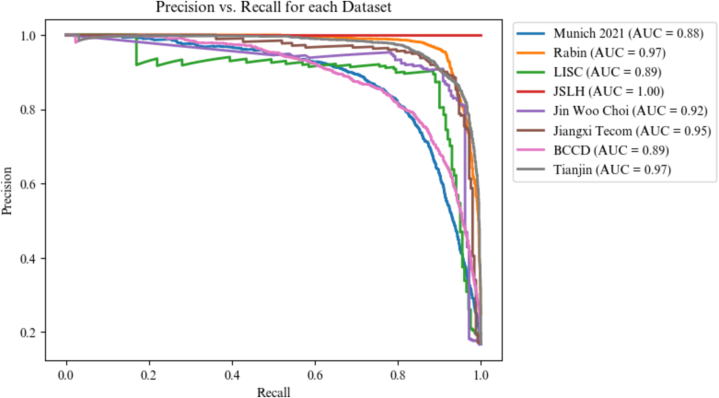
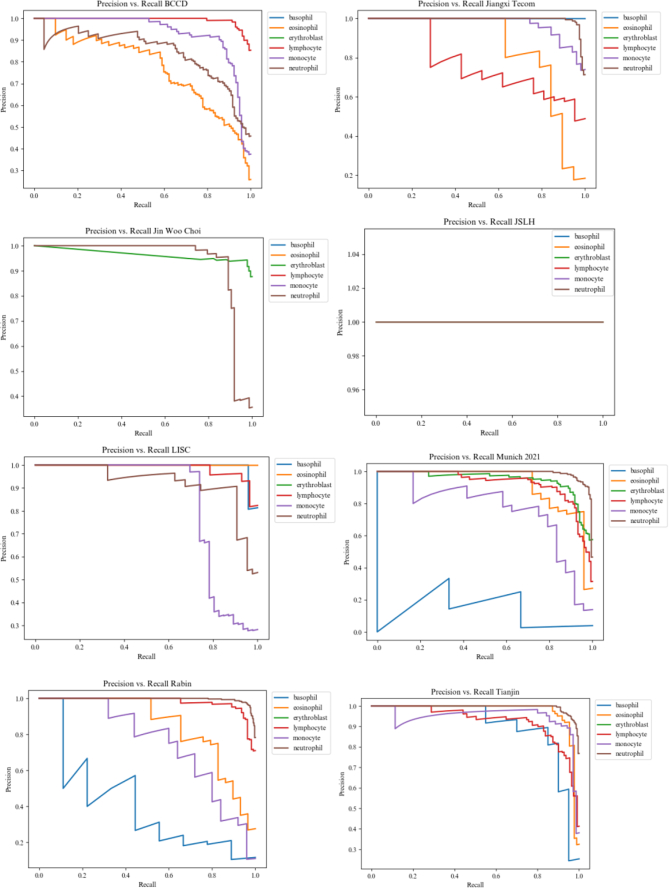
Afterwards, the model was fine-tuned on each target dataset. The classification results of every fine-tuned model on its target dataset are reported in Fig. 6, and average Precision vs. Recall curves of each target dataset are plotted in Fig. 7. Detailed Precision vs. Recall curves can be found in Appendix B.
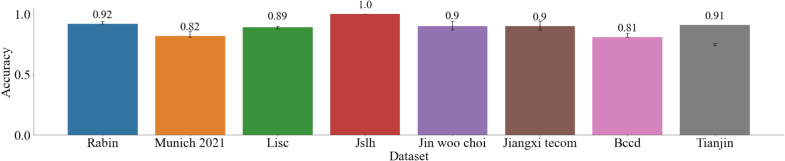
Fig. 6.
Accuracy per dataset, the error bars are the interval of confidence obtained with bootstrapping.
Fig. 7.
Precision vs Recall curve of the model predictions after fine-tuning on each dataset. Each curve averages the results over all classes.
Bootstrapping techniques were employed on the model predictions to obtain confidence intervals of the results.42
The model accuracy is higher than 0.8 for each target dataset, showcasing its generalization capabilities despite the utilization of fewer than 100 annotated images per dataset. These results are supported by the Precision vs. Recall plot, where AUC is superior to 0.85 for all dataset.
Recall and precision per class are also presented in Table 4. Neutrophils are consistently classified with high precision and recall, whereas monocytes are often classified as lymphocytes and neutrophils. Recall is particularly low for basophils in the Munich 2018, 2021, and Rabin datasets. This result was expected, basophils being the rarest class.
Table 4.
Precision (Pre) and Recall (Rec) per class after fine-tuning the model for every target dataset. Precision and recall are computed, respectively, to their class.
| Dataset | Bas |
Eos |
Ery |
Lym |
Mon |
Neu |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Rec | Pre | Rec | Pre | Rec | Pre | Rec | Pre | Rec | Pre | Rec | |
| Rabin | 0.88 | 0.36 | 0.78 | 0.72 | 0.87 | 0.97 | 0.73 | 0.79 | 0.96 | 0.98 | ||
| Munich 2021 | 0.64 | 0.13 | 0.75 | 0.80 | 0.78 | 0.93 | 0.76 | 0.88 | 0.91 | 0.28 | 0.89 | 0.96 |
| Lisc | 0.96 | 1.0 | 1.0 | 0.70 | 0.96 | 0.93 | 0.70 | 0.97 | 0.88 | 0.91 | ||
| Jslh | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||
| Jin woo choi | 1.0 | 0.87 | 0.72 | 1.0 | ||||||||
| Jiangxi tecom | 0.58 | 1.0 | 0.80 | 0.60 | 0.88 | 0.85 | 0.96 | 0.97 | ||||
| Bccd | 0.70 | 0.67 | 0.94 | 0.94 | 0.81 | 0.92 | 0.77 | 0.71 | ||||
| Tianjin | 0.95 | 0.73 | 0.81 | 0.99 | 0.77 | 0.94 | 0.93 | 0.92 | 0.98 | 0.98 | ||
Each class is represented in at least five datasets, except for erythroblasts which are only in three datasets. For this class, recall and precision are higher than 0.8 over all three datasets, including Munich 2021 dataset which contains 27,395 erythroblasts from 700 patients. We thus conclude that generalization capacity of our workflow also applies to erythroblasts.
Interestingly, performances of the model are not the highest for the Munich 2021 dataset, even if one of the source datasets comes from the same laboratory. This can be explained by various factors: cells come from bone marrow in one dataset and from peripheral blood in the other, and the conditions of acquisition are significantly different (camera, magnification power, resolution, format of the image, date). This contributed to make the visual aspects of these two datasets drastically different.
Ablation study
To better understand the contribution of each step of our approach, we carried out a series of complementary experiments. The comparison was drawn between the following experimental settings.
-
1)
Without source train; we directly trained a new model on each dataset, without any pretraining on the source dataset.
-
2)
Without fine-tuning; the model that was trained on the source dataset was directly tested on images from the target dataset.
-
3)
Head only fine-tuning; only the head of the model was fine-tuned, the encoder was frozen.
-
4)
Mixed sources; the model was fine-tuned with images from mixed sources, i.e., from both the target dataset and the source dataset (100 + 100 images from Barcelona + Tianjin).
-
5)
Fine-tuning without color transform; the model was fine-tuned without applying color transform on target images.
-
6)
Proposed workflow; the model was fine-tuned under the previously described experimental conditions (c.f. workflow presented in “Methods”).
The ablation study was conducted for each dataset, and the average performances are presented in Table 5. Detailed results can be found in Appendix C. We calculated p-values using a pairwise Wilcoxon test, demonstrating that the differences in accuracy observed between two experimental conditions were significant. The outcomes of this study confirm the superiority of our workflow over other experimental setups. Furthermore, it can be noticed that performance improvement comes principally from fine-tuning. The addition of color transform only slightly influences results; in general, fine-tuning is more important than color transform to improve classification on images from a new dataset, even if their coloration is different.
Table 5.
Classification results under various experimental conditions. The ablation study was performed for every dataset and then averaged. Accuracy is defined as the overall accuracy; computed with the total number of rightly classified images. Precision and recall are the average of precision and recall over the six classes (i.e., macro-measures). p-values are computed between every pair of successive experimental conditions (Condition 1 with Condition 2, Condition 2 with Condition 3…etc.). p-values lower than 1e-5 are approximated as ∼0.
| Experimental conditions | Accuracy | Precision | Recall | p value |
|---|---|---|---|---|
| 1.Without source train | 0.48 | 0.38 | 0.34 | |
| 2. Without fine-tuning | 0.59 | 0.48 | 0.50 | ∼0 |
| 3. Head only fine-tuning | 0.71 | 0.65 | 0.60 | ∼0 |
| 4. Mixed sources fine-tuning | 0.74 | 0.62 | 0.58 | 0.024 |
| 5. Fine-tuning without color transform | 0.84 | 0.83 | 0.76 | ∼0 |
| 6. Proposed workflow | 0.89 | 0.85 | 0.84 | ∼0 |
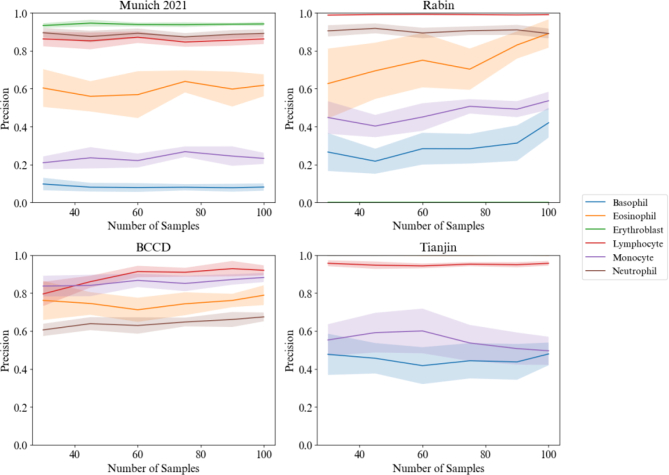
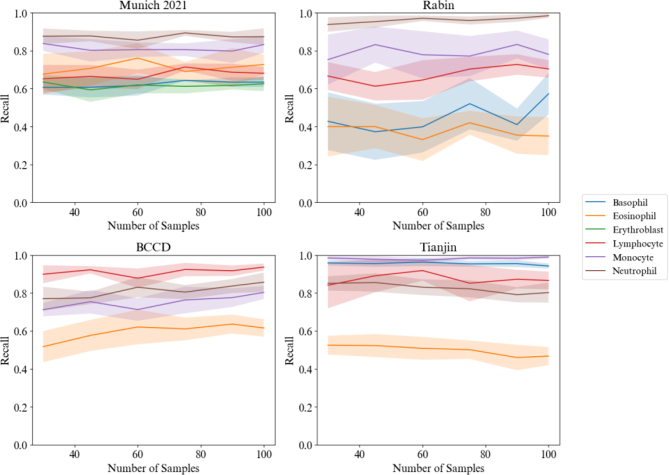
Additionally, a study on the influence of the fine-tuning sample size on accuracy, precision, and recall is provided in Appendix D. This analysis was conducted on the four largest datasets, each containing more than 1000 images, where 100 images were used for fine-tuning. We observed that the number of samples had only a small effect on final accuracy. However, it positively impacted the precision and recall of rare classes, such as eosinophils and basophils, in the Rabin dataset. In conclusion, we selected a sample size of 100 images for this study. Increasing the number would have significantly prolonged the annotation process, whereas reducing it would have lowered the diversity of images in certain classes. For the smaller datasets, we had no choice but to use 1/10th of the dataset for fine-tuning while preserving enough images for evaluation.
Relevance of color transformation
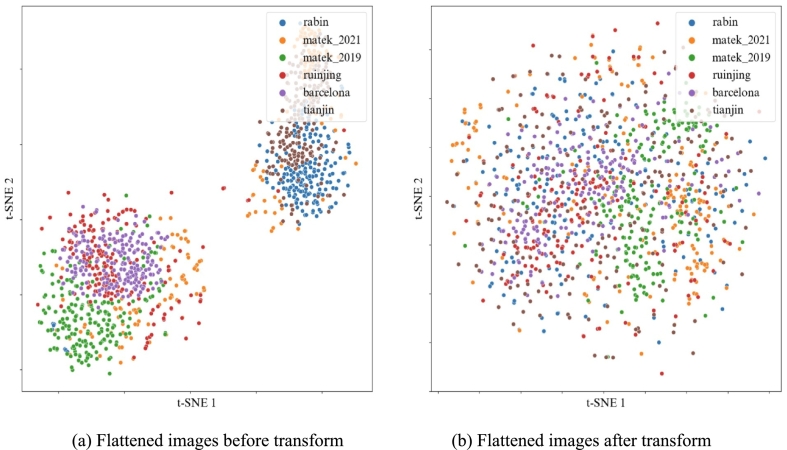
Visual transformation aimed to reduce color disparities resulting from the variety of possible imaging conditions. This transform presented several advantages, including computational efficiency, alignment with color standards familiar to clinicians, and the prevention of image distortion. The representation of images from each dataset is shown in Fig. 8, which evidenced the impact of transform. Each point corresponded to a flattened image after t-SNE dimensionality reduction.43 After transform, the distributions of pixels in 2D overlapped better among different datasets.
Fig. 8.
t-SNE representation of images from different datasets before and after transform. Each point is obtained by flattening the corresponding image and performing dimensionality reduction. For each dataset, only 200 images are represented.
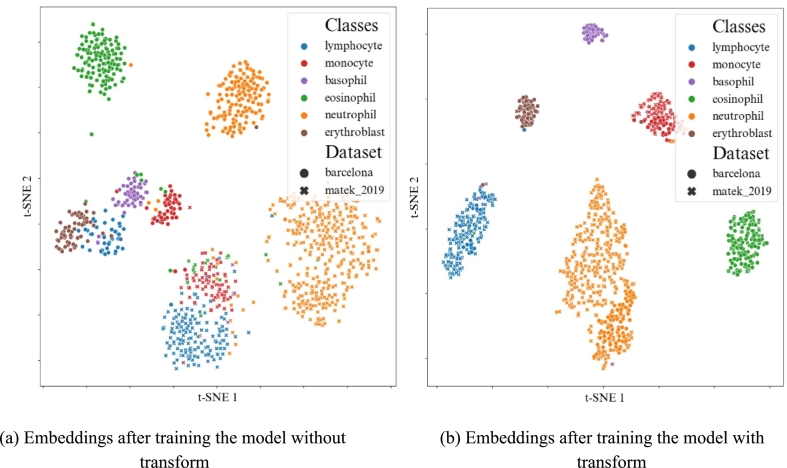
In general, color transform was efficient at reducing domain shift, at a very low cost. In Fig. 9, the embeddings of the images from the source dataset were visualized following a t-SNE dimensionality reduction. The plots illustrated how the transform function could bring images from different datasets closer together when they belonged to the same class. Thus, the use of a transform yielded tighter clusters of classes irrespective of the image's source dataset. The workflow was a form of domain adaptation method.
Fig. 9.
t-SNE of the embeddings output by the encoder of the model, after the model was trained with or without the color transform. Barcelona images are represented with dots, whereas Munich 2018 images are represented with crosses. The transform brings together images from different datasets when they belong to the same class.
Relevance of fine-tuning
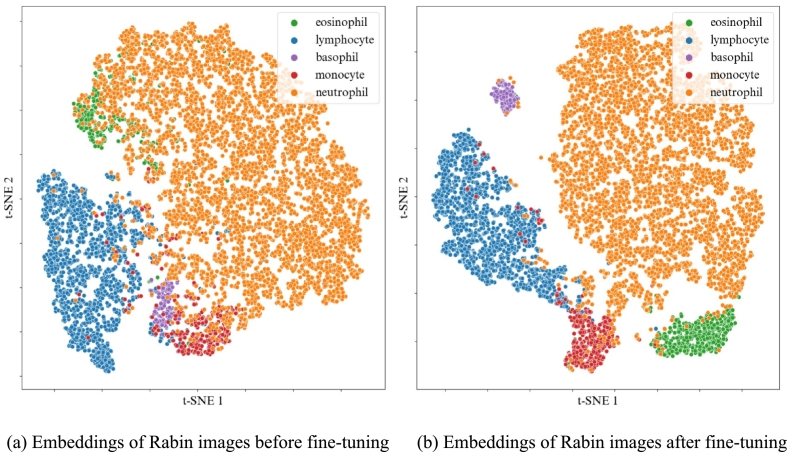
The fine-tuning strategy leveraged classification performances on the target datasets, enabling satisfactory results even with a limited number of annotated images. In Fig. 10, the visualization of embeddings from Rabin images illustrated how fine-tuning could improve classification performances when applied to a target dataset. Notably, fine-tuning allowed a better recognition of basophils, monocytes and eosinophils. Given that color plays a decisive role in identifying these cell categories, we can suppose that fine-tuning leads to a better comprehension of the coloration in the newly encountered dataset. Additionally, it allowed the classification to be very fast on large datasets, with the fine-tuning process completed quickly, and requiring the labeling of only 100 images by experts.
Fig. 10.
t-SNE of the embeddings output by the encoder of the model, before and after fine-tuning the model with 100 annotated images from the Rabin dataset.
Wrong-prediction analysis
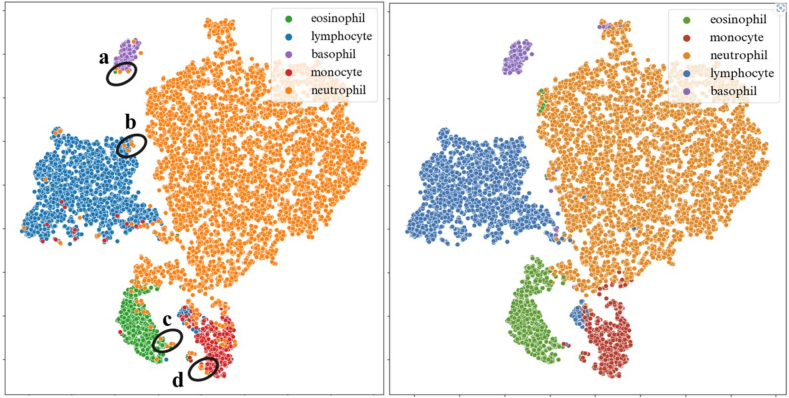
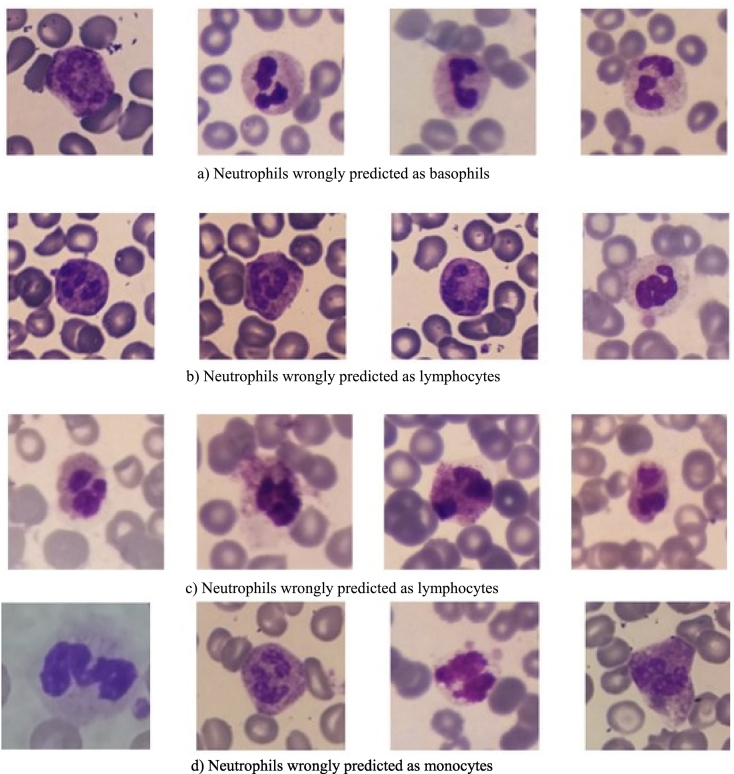
To have a deeper understanding of these results, we conducted an analysis of wrong predictions on Rabin dataset as depicted in Fig. 11, Fig. 12. Our focus was directed towards wrongly classified neutrophils. We showed their corresponding representation within the embedding space using a black circle. Beside the possible impact of nucleus shape, we observe that neutrophils showing purple granulation are inaccurately categorized as basophils, whereas those with more orange granulations are wrongly identified as eosinophils. Neutrophils featuring an indistinct nucleus and blurred granulation are often misclassified as lymphocytes. Additionally, neutrophils presenting a blue coloration in their cytoplasm frequently lead to mispredictions of monocytes. It is worth noticing that t-SNE clusters are not exactly aligned with the predictions, and there are a few exceptions of images belonging to a cluster but being predicted in another category (e.g., eosinophils in neutrophil cluster in Fig. 11 right).
Fig. 11.
Embedding space projected in 2D with t-SNE. Colors on the left correspond to ground truth labels, and colors on the right are rightly predicted labels. We studied wrongly predicted neutrophils from each dark circle region.
Fig. 12.
Analysis of images of neutrophils wrongly predicted in each category. Factors contributing to prediction errors in each category include image quality, granulation color, nucleus shape, and cytoplasm color.
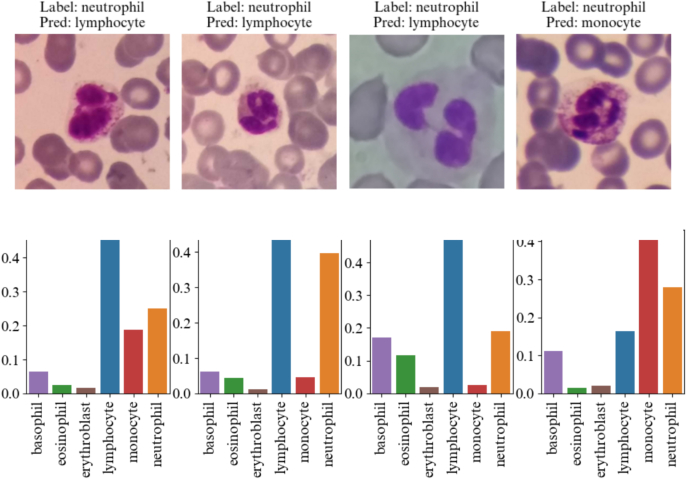
To conclude this analysis, we examined the output probability vector for incorrectly predicted neutrophils (Fig. 13). In the majority of wrong predictions, the second highest probability corresponds neutrophil, which aligns with the true class of the cell.
Fig. 13.
Output probability vector for wrongly predicted neutrophils by the model. Even if the highest probability is given to a wrong prediction label, the second highest probability corresponds neutrophil, which aligns with the true class of the cell.
Limitations and perspectives
The results of this study exhibit some limitations. Firstly, whereas the results were promising in terms of generalizability, further research would be necessary to improve classification performances for the clinical implementation of this algorithm. This could be achieved by using active learning: images where the model hesitates between different classes as in Fig. 13 could be re-annotated by the clinician to improve performances. Introducing human-in-the-loop annotation could overcome this limitation.
Secondly, the diverse datasets presented highly imbalanced cell distributions. Whereas some target datasets were large and exhibited a wide range of cell types, others consisted of only two classes or contained only a very limited number of cells. We did not discard small or heavily unbalanced datasets based on a minimum number of images criterion, as it appeared that they were accepted and used by the expert community.11,16,20,26 However, the representativeness of the results for these datasets should be studied to a greater extent. Although this diversity shows that our approach leads to a good capacity for generalization, some representations do not align with the diversity observed in real blood cell populations. In addition, further research should confirm these results with a broader system of classes, including earlier stages of white blood cell maturation that are commonly observed in bone marrow samples.
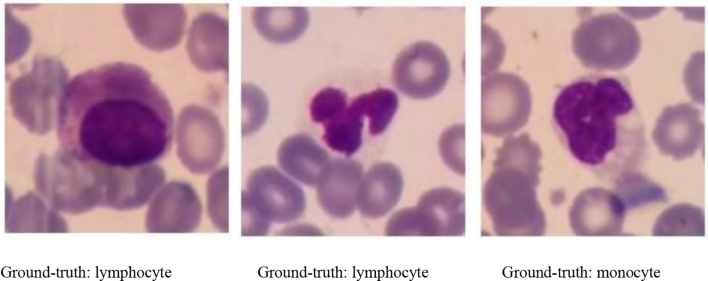
Lastly, the study of wrong predictions by the algorithm has underscored the importance of accurate annotation and high-quality images. The algorithm faced particular challenges when cells exhibited granulations that low resolution failed to display, or when there was there was an ambiguity about the cell's ground truth label (see Appendix E).
Nonetheless, this study introduced an easy-to-implement workflow to obtain robust classification performances. This was the first study to explore the generalizability capacity of models at low computational cost, and with so many datasets. The workflow was elaborated by taking into consideration the diversity of coloration images could take. Furthermore, it was specifically designed to be fast in computations, and to cut down on the necessary annotation time. Hence, this article provides an approach that can be used on images with various visual aspect and was applied to a local dataset with good performances.
Conclusion
In this study, we proposed a workflow to enable a classification EfficientNet network to accurately label cells from 10 different sources with low computational cost. The key aspect of our approach lies in combining a color transformation to standardize the visual style of images with a fine-tuning technique. Fine-tuning significantly improved classification performance, resulting in an overall accuracy exceeding 80% for all datasets. Furthermore, the benefits of fine-tuning and color transformation were visually confirmed by plotting the embeddings of the images generated by the model, highlighting the model's improved ability to cluster images by cell classes.
Further research should be conducted, firstly to enhance classification accuracy for specific datasets and secondly to create larger and more diverse datasets of cell images.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Acknowledgments
We would like to acknowledge Klara Lunte and Grégoire Salas for their important preliminary work that paved the way for this study. We also acknowledge the clinical centers that answered our request and gave us access to their data, without which we could not have carried out our comparison. Among them, a special thanks to Tianjin Medical University Affiliated Medical Center, Japan Society of Diagnostics Hemtaology, Ruijing Hospital, Shanghai Jiaotong University School of Medicine, Razi Hospital in Rasht, Gholhak Laboratory, Shahr-e-Qods Laboratory, and Takht-e Tavous Laboratory in Tehran, Institute of Computational Biology, Helmholtz Zentrum München–German Research Center for Environmental Health, Hematology-Oncology and BMT Research Center of Imam Khomeini hospital in Tehran, Roboflow in Singapore, Seoul National University Hospital, Jiangxi Tecom Science Corporation, Core Laboratory the Hospital Clinic, Barcelona.
Glossary
- Abbreviation
Meaning
- ABE
Abnormal eosinophil
- ART
Artifact
- BAS
Basophil
- BLA
Blast
- BMS
Bone marrow smear
- EBO
Erythroblast
- EOS
Eosinophil
- ERA
Acidophilic erythroblast
- ERB
Basophilic erythroblast
- ERO
Orthochromatic erythroblast
- ERP
Polychromatic erythroblast
- FGC
Faggott cell
- GAN
Generative adversarial network
- HAC
Hairy cell
- KSC
Smudge cell
- LYA
Lymphocyte (atypical)
- LYA
Lymphoma
- LYI
Immature lymphocyte
- LYT
Lymphocyte
- MGG
May-Grünwald Giemsa
- MMZ
Metamyelocyte
- MON
Monocyte
- MYB
Myelocyte
- MYO
Myeloblast
- NEU
Neutrophil
- NGB
Band neutrophil
- NGS
Segmented neutrophil
- NIF
Not identifiable
- OTH
Other cell
- PBL
Pronormoblast
- PBS
Peripheral blood smear
- PEB
Proerythroblast
- PLA
Platelet
- PLM
Plasma cell
- PMB
Promyelocyte (bilobed)
- PMO
Promyelocyte
- THO
Thrombocyte
- WBC
White blood cell
Appendix A. Appendices
Table A.1.
Dataset detailed description.
| Name | Centre | Nb | Classes | Imaging system and format | Magnification | PBS or BMS | Healthy or pathological |
|---|---|---|---|---|---|---|---|
| Barcelona31 | Core Laboratory the Hospital Clinic, Barcelona | 17,092 | NEU, EOS, BAS, LYT, MON, PMO, MYB, EBO, PLA, THO | CellaVision DM96 (.jpg) | 0.1 μm per pixel (360 × 363 pixels) | PBS | Healthy |
| Cella Vision Blog33 | 100 | BAS, EOS, LYT, NEU, MON | CellaVision (.bmp) | (300 × 300 pixels) | |||
| Jiangxi Tecom33 | Jiangxi Tecom Science Corporation | 300 | BAS, EOS, LYT, NEU, MON | Motic Moticam Pro 252A optical microscope camera (.bmp) | (120 × 120) | ||
| Jin Woo Choi34 | Seoul National University Hospital. | 2174 | NGB, NGS, ERB, MMZ, MYO, MYB, ERO, ERP, PMO, PBL | (.jpg) | x1000 magnification, (144 × 144 pixels) | BMS | |
| BCCD35 | Roboflow | 3500 | EOS LYT, MON, NEU | (.jpeg) | (320 × 240 pixels) | PBS | |
| LISC36 | Hematology-Oncology and BMT Research Center of Imam Khomeini hospital in Tehran | 257 | BAS, EOS, LYT, NEU, MON | Light microscope (Microscope-Axioskope 40) and digital camera Sony Model No. SSCDC50AP(.png) | x100 (250 × 250 pixels) | PBS | Healthy |
| Munich 20214 | Helmholtz Zentrum München–German Research Center for Environmental Health, | 171,373 | NGB, NGS, LYT, MON, EOS, BAS, MMZ, MYB, PMO, BLA, PLM, KSC, OTH, ART, NIF, PEB, EBO, HAC, ABE, LYI, FGC | CCD camera mounted on a brightfield microscope (Zeiss Axio Imager Z2) (.jpg) | x40 (250 × 250 pixels) | BMS | Pathological |
| Munich 201932 | Helmholtz Zentrum München–German Research Center for Environmental Health, | 18,365 | BAS, EBO, EOS, KSC, LYT, LYA, MMZ, MYO, MON, NGB, NGS, PMB | M8 digital microscope Scanner (Precipoint GmbH, Freising) (.tiff) | x100 (400 × 400 pixels) | PBS | Pathological |
| Raabin37 | Razi Hospital in Rasht, Gholhak Laboratory, Shahr-e-Qods Laboratory, and Takht-e Tavous Laboratory in Tehran, | 16,633 | EOS, LYT, MON, NEU | Smartphones mounted to occular lenses of microscopes (Olympus CX18 and Zeiss) (.jpg) | x100 (575 × 575 pixels) | PBS | Both |
| Ruinjing44 | Ruijing Hospital, Shanghai Jiaotong University School of Medicine | 1647 | BLA, LYT, LYA | Cella Vision (.jpg) | x100 (360 × 363) | PBS | Pathological |
| JSLH38 | Japan Society of Diagnostics Hemtaology | 148 | NGB, ERB, MMZ, MYO, ERO, ERP, PEB, PMO, NGS | (.png) | (85 × 74 pixels) | ||
| Tianjin39 | Tianjin Medical University Affiliated Medical Center | 6228 | BAS, EOS, LYT, NEU, MON | Nikon DS-Ri2 Color Camera (.png) | x100 (250 × 250 pixels) | PBS |
Appendix B. Precision vs. Recall Curve per class for each dataset
Fig. B.1.
Precision–recall curve per class for each dataset.
Appendix C. Ablation study per dataset
Table C.1.
Results under various experimental conditions for each dataset.
| Experimental conditions | Dataset | Accuracy | Precision | Recall |
|---|---|---|---|---|
| 1) no_source_train | Munich 2021 | 0.31 | 0.34 | 0.29 |
| 2) no fine_tune | Munich 2021 | 0.75 | 0.69 | 0.63 |
| 3) head_only | Munich 2021 | 0.77 | 0.74 | 0.62 |
| 4) mixed_train | Munich 2021 | 0.78 | 0.74 | 0.63 |
| 5) no_transform | Munich 2021 | 0.83 | 0.78 | 0.65 |
| 6) our_workflow | Munich 2021 | 0.82 | 0.79 | 0.66 |
| 1) no_source_train | Rabin | 0.49 | 0.48 | 0.34 |
| 2) no fine_tune | Rabin | 0.65 | 0.47 | 0.46 |
| 3) head_only | Rabin | 0.82 | 0.85 | 0.67 |
| 4) mixed_train | Rabin | 0.80 | 0.70 | 0.55 |
| 5) no_transform | Rabin | 0.92 | 0.76 | 0.68 |
| 6) our_workflow | Rabin | 0.92 | 0.85 | 0.76 |
| 1) no_source_train | LISC | 0.56 | 0.46 | 0.50 |
| 2) no fine_tune | LISC | 0.46 | 0.38 | 0.45 |
| 3) head_only | LISC | 0.67 | 0.68 | 0.67 |
| 4) mixed_train | LISC | 0.71 | 0.71 | 0.70 |
| 5) no_transform | LISC | 0.93 | 0.93 | 0.93 |
| 6) our_workflow | LISC | 0.89 | 0.90 | 0.90 |
| 1) no_source_train | JSLH | 0.45 | 0.18 | 0.25 |
| 2) no fine_tune | JSLH | 1.00 | 1.00 | 1.00 |
| 3) head_only | JSLH | 1.00 | 1.00 | 1.00 |
| 4) mixed_train | JSLH | 1.00 | 1.00 | 1.00 |
| 5) no_transform | JSLH | 1.00 | 1.00 | 1.00 |
| 6) our_workflow | JSLH | 1.00 | 1.00 | 1.00 |
| 1) no_source_train | Jin Woo Choi | 0.47 | 0.16 | 0.22 |
| 2) no fine_tune | Jin Woo Choi | 0.57 | 0.18 | 0.31 |
| 3) head_only | Jin Woo Choi | 0.81 | 0.55 | 0.54 |
| 4) mixed_train | Jin Woo Choi | 0.78 | 0.32 | 0.33 |
| 5) no_transform | Jin Woo Choi | 0.88 | 0.89 | 0.86 |
| 6) our_workflow | Jin Woo Choi | 0.90 | 0.86 | 0.94 |
| 1) no_source_train | Jiangxi Tecom | 0.69 | 0.64 | 0.47 |
| 2) no fine_tune | Jiangxi Tecom | 0.16 | 0.20 | 0.21 |
| 3) head_only | Jiangxi Tecom | 0.85 | 0.51 | 0.47 |
| 4) mixed_train | Jiangxi Tecom | 0.54 | 0.34 | 0.31 |
| 5) no_transform | Jiangxi Tecom | 0.77 | 0.71 | 0.52 |
| 6) our_workflow | Jiangxi Tecom | 0.90 | 0.85 | 0.88 |
| 1) no_source_train | BCCD | 0.53 | 0.35 | 0.36 |
| 2) no fine_tune | BCCD | 0.25 | 0.17 | 0.17 |
| 3) head_only | BCCD | 0.41 | 0.40 | 0.40 |
| 4) mixed_train | BCCD | 0.41 | 0.40 | 0.41 |
| 5) no_transform | BCCD | 0.84 | 0.83 | 0.85 |
| 6) our_workflow | BCCD | 0.81 | 0.81 | 0.81 |
| 1) no_source_train | Tianjin | 0.33 | 0.41 | 0.27 |
| 2) no fine_tune | Tianjin | 0.85 | 0.69 | 0.77 |
| 3) head_only | Tianjin | 0.82 | 0.86 | 0.77 |
| 4) mixed_train | Tianjin | 0.91 | 0.76 | 0.71 |
| 5) no_transform | Tianjin | 0.56 | 0.73 | 0.62 |
| 6) our_workflow | Tianjin | 0.91 | 0.74 | 0.76 |
Appendix D. Evolution of the results with the number of fine-tuning samples
Fig. D.1.
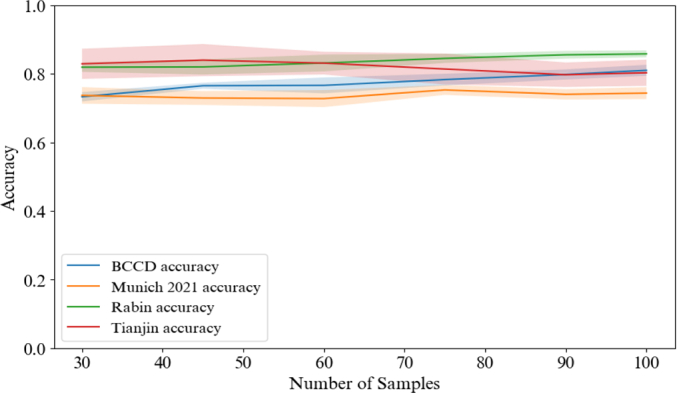
Evolution of the overall accuracy with the number of samples used for fine-tuning. For each sample size n [30, 45, 60, 75, 90, 100], we randomly picked n samples and reproduced the experiment 10 times. The curve corresponds to the mean accuracy, and the envelopes to the standard deviation of the results for each sample size n over the 10 experiments.
Fig. D.2.
Evolution of the precision per class with the number of samples used for fine-tuning. Each experiment has been performed 10 time, the envelopes correspond to the standard deviation of the results.
Fig. D.3.
Evolution of the recall per class with the number of samples used for fine-tuning. Each experiment has been performed 10 time, the envelopes correspond to the standard deviation of the results.
Appendix E. Examples of ambiguous ground-truth
Fig. E.1.
Examples of images from Rabin dataset with ambiguous ground-truth. According to the dataset ground-truth, these cells are lymphocytes and monocytes, but they present morphological properties of granulocytes (granulations, segmented nucleus).
References
- 1.Walter W., Pohlkamp C., Meggendorfer M., et al. Artificial intelligence in hematological diagnostics: game changer or gadget? Blood Rev. Oct. 2022;58:11. doi: 10.1016/j.blre.2022.101019. https://www.sciencedirect.com/science/article/pii/S0268960X22000935 p. 101019. issn: 0268-960X. url. (visited on 12/09/2022) [DOI] [PubMed] [Google Scholar]
- 2.Baghel N., Verma U., Kumar Nagwanshi K. WBCs-net: type identification of white blood cells using convolutional neural network. Multimedia Tools Appl. Dec. 2022;81(29):42131–42147. doi: 10.1007/s11042-021-11449-z. issn: 1380-7501. [DOI] [Google Scholar]
- 3.Acevedo A., Alférez S., Merino A., Puigvı L., Rodellar J. Recognition of peripheral blood cell images using convolutional neural networks. Comput. Methods Prog. Biomed. 2019;180 doi: 10.1016/j.cmpb.2019.105020. [DOI] [PubMed] [Google Scholar]
- 4.Matek C., Krappe S., Münzenmayer C., Haferlach T., Marr C. Highly accurate differentiation of bone marrow cell morphologies using deep neural networks on a large image data set. Blood. Nov. 2021;138(20):1917–1927. doi: 10.1182/blood.2020010568. issn: 0006-4971. (visited on 12/09/2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storkey A., Quinonero-Candela J., Sugiyama M., Schwaighofer A., Lawrence N. 2009. Dataset Shift in Machine Learning. [Google Scholar]
- 6.Abhishek A., Jha R.K., Sinha R., Jha K. Automated detection and classification of leukemia on a subject independent test dataset using deep transfer learning supported by Grad-CAM visualization. Biomed. Signal Process. Control. May 2023;83 doi: 10.1016/j.bspc.2023.104722. https://www.sciencedirect.com/science/article/pii/S1746809423001556 issn: 1746-8094. url. (visited on 05/26/2023) [DOI] [Google Scholar]
- 7.Pachitariu M., Stringer C. Cellpose 2.0: how to train your own model. Nat. Methods. Dec. 2022;19(12):1634–1641. doi: 10.1038/s41592-022-01663-4. https://www.nature.com/articles/s41592-022-01663-4 Publisher: Nature Publishing Group. issn: 1548-7105. url. (visited on 12/09/2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh R., Abdelpakey M.H., Shehata M.S., Mohamed M.M. Automated human cell classification in sparse datasets using few-shot learning. Scient. Rep. Feb. 2022;12(1):2924. doi: 10.1038/s41598-022-06718-2. https://www.nature.com/articles/s41598-022-06718-2 Publisher: Nature Publishing Group. issn: 2045-2322. url. (visited on 12/09/2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan S., Sajjad M., Hussain T., Ullah A., Imran A. A review on traditional machine learning and deep learning models for WBCs classification in blood smear images. IEEE Access. Dec. 2020 doi: 10.1109/ACCESS.2020.3048172. pp. 1–1. [DOI] [Google Scholar]
- 10.Sajjad M., Khan S., Shoaib M., et al. Dec. 2016. Computer Aided System for Leukocytes Classification and Segmentation in Blood Smear Images; pp. 99–104. [DOI] [Google Scholar]
- 11.New segmentation and feature extraction algorithm for classification of white blood cells in peripheral smear images Scient. Rep. url: https://www.nature.com/articles/s41598-021-98599-0 (visited on 02/10/2023). [DOI] [PMC free article] [PubMed]
- 12.Rawat J., Bhadauria H., Singh A., Virmani J. 2015 2nd International Conference on Computing for Sustainable Global Development (INDIACom) Mar. 2015. Review of leukocyte classification techniques for microscopic blood images; pp. 1948–1954. [Google Scholar]
- 13.Girdhar A., Kapur H., Kumar V. Classification of white blood cell using convolution neural network. Biomed. Signal Process. Control. 2022;71 doi: 10.1016/j.bspc.2021.103156. https://www.sciencedirect.com/science/article/pii/S1746809421007539 issn: 1746-8094. url. [DOI] [Google Scholar]
- 14.Tripathi S., Augustin A.I., Sukumaran R., Dheer S., Kim E. HematoNet: expert level classification of bone marrow cytology morphology in hematological malignancy with deep learning. Artif. Intel. Life Sci. Dec. 2022;2 doi: 10.1016/j.ailsci.2022.100043. https://www.sciencedirect.com/science/article/pii/S2667318522000137 issn: 2667-3185. url. (visited on 12/09/2022) [DOI] [Google Scholar]
- 15.Togacar M., Ergen B., Sertkaya M.E. Subclass separation of white blood cell images using convolutional neural network models. Elektronika ir Elektrotechnika. Oct. 2019;25(5):63–68. doi: 10.5755/j01.eie.25.5.24358. https://eejournal.ktu.lt/index.php/elt/article/view/24358 issn: 2029-5731. url. (visited on 12/09/2022) [DOI] [Google Scholar]
- 16.Kutlu H., Avci E., Ozyurt F. White blood cells detection and classification based on regional convolutional neural networks. Med. Hypotheses. Feb. 2020;135 doi: 10.1016/j.mehy.2019.109472. https://www.sciencedirect.com/science/article/pii/S0306987719310680 issn: 0306-9877. url. [DOI] [PubMed] [Google Scholar]
- 17.Patil A.M., Patil M.D., Birajdar G.K. White blood cells image classification using deep learning with canonical correlation analysis. IRBM. Oct. 2021;42(5):378–389. doi: 10.1016/j.irbm.2020.08.005. https://www sciencedirect.com/science/article/pii/S195903182030141X issn: 1959-0318. url. (visited on 12/09/2022) [DOI] [Google Scholar]
- 18.Eckardt J.-N., Middeke J.M., Riechert S., et al. Deep learning detects acute myeloid leukemia and predicts NPM1 mutation status from bone marrow smears. Leukemia. Jan. 2022;36(1):111–118. doi: 10.1038/s41375-021-01408-w. https://www.nature.com/articles/s41375-021-01408-w Publisher: Nature Publishing Group. issn: 1476-5551. url. (visited on 12/09/2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sahasrabudhe M., Sujobert P., Zacharaki E.I., et al. Deep multi-instance learning using multi-modal data for diagnosis of lymphocytosis. IEEE J. Biomed. Health Inform. June 2021;25(6):2125–2136. doi: 10.1109/JBHI.2020.3038889. https://ieeexplore.ieee.org/document/9263100/ issn: 2168-2194, 2168-2208. url. (visited on 01/10/2023) [DOI] [PubMed] [Google Scholar]
- 20.Baydilli Y.Y., Atila U., Elen A. Learn from one data set to classify all–a multi-target domain adaptation approach for white blood cell classification. Comput. Methods Programs Biomed. 2020;196 doi: 10.1016/j.cmpb.2020.105645. [DOI] [PubMed] [Google Scholar]
- 21.Hazra D., Byun Y.-C., Kim W.J. Enhancing classification of cells procured from bone marrow aspirate smears using generative adversarial networks and sequential convolutional neural network. Comput. Methods Programs Biomed. 2022;224 doi: 10.1016/j.cmpb.2022.107019. [DOI] [PubMed] [Google Scholar]
- 22.Barrera K., Merino A., Molina A., et al. Automatic generation of artificial images of leukocytes and leukemic cells using generative adversarial networks (synthetic cell GAN) Comput. Methods Programs Biomed. 2023;229 doi: 10.1016/j.cmpb.2022.107314. [DOI] [PubMed] [Google Scholar]
- 23.Almezhghwi K., Serte S. Improved classification of white blood cells with the generative adversarial network and deep convolutional neural network. Computat. Intel. Neurosci. 2020;2020 doi: 10.1016/j.cmpb.2022.107314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claro M.L., Veras R.d.M.S., Santana A.M., et al. Assessing the impact of data augmentation and a combination of CNNs on leukemia classification. Inform. Sci. Sep. 2022;609:1010–1029. doi: 10.1016/j.ins.2022.07.059. https://www.sciencedirect.com/science/article/pii/S0020025522007484 issn: 0020-0255. url. (visited on 05/26/2023) [DOI] [Google Scholar]
- 25.Abhishek A., Jha R.K., Sinha R., Jha K. Automated classification of acute leukemia on a heterogeneous dataset using machine learning and deep learning techniques. Biomed. Signal Process. Control. 2022;72 doi: 10.1016/j.bspc.2021.103341. [DOI] [Google Scholar]
- 26.Tavakoli S., Ghaffari A., Kouzehkanan Z.M. July 2021. Generalizability in White Blood Cells' Classification Problem. Pages: 2021.05.12.443717 Section: New Results. (visited on 05/26/2023) [DOI] [Google Scholar]
- 27.Salehi R., Sadafi A., Gruber A., et al. Medical Image Computing and Computer Assisted Intervention– MICCAI 2022: 25th International Conference, Singapore, September 18–22, 2022, Proceedings, Part III. Springer; 2022. Unsupervised cross-domain feature extraction for single blood cell image classification; pp. 739–748. [DOI] [Google Scholar]
- 28.Umer R.M., Gruber A., Boushehri S.S., Metak C., Marr C. Imbalanced Domain Generalization for Robust Single Cell Classification in Hematological Cytomorphology. Apr. 2023. arXiv:2303.07771 [cs]. (visited on 05/26/2023)
- 29.Zhang L., Fu Y., Yang Y., et al. Proceedings of the 2022 9th International Conference on Biomedical and Bioinformatics Engineering. 2022. Unsupervised cross-domain white blood cells classification using DANN; pp. 17–21. [DOI] [Google Scholar]
- 30.Pandey P., Kyatham V., Mishra D., Dastidar T.R. Target-independent domain adaptation for WBC classification using generative latent search. IEEE Trans Med Imaging. 2020;39(12):3979–3991. doi: 10.1109/TMI.2020.3009029. [DOI] [PubMed] [Google Scholar]
- 31.Acevedo A., Merino A., Alférez S., Molina A., Boldu L., Rodellar J. A dataset of microscopic peripheral blood cell images for development of automatic recognition systems. Data Brief. June 2020;30 doi: 10.1016/j.dib.2020.105474. issn: 2352-3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matek C., Schwarz S., Spiekermann K., Marr C. Human-level recognition of blast cells in acute myeloid leukaemia with convolutional neural networks. Nat. Mach. Intel. Nov. 2019;1(11):538–544. doi: 10.1038/s42256-019-0101-9. https://www.nature.com/articles/s42256-019-0101-9 Publisher: Nature Publishing Group. issn: 2522-5839. url. (visited on 12/09/2022) [DOI] [Google Scholar]
- 33.Zheng X., Wang Y., Wang G., Liu J. Fast and robust segmentation of white blood cell images by self-supervised learning. Micron. 2018;107:55–71. doi: 10.1016/j.micron.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Choi J.W., Ku Y., Yoo B.W., et al. White blood cell differential count of maturation stages in bone marrow smear using dual-stage convolutional neural networks. PLoS One. 2017;12(12) doi: 10.1371/journal.pone.0189259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.[dataset] Mohammad MahmudulAlam/Complete-Blood-Cell-Count-Dataset. Nov. 2022. https://github.com/MahmudulAlam/Complete-Blood-Cell-CountDataset original-date: 2018-04-28T18:57:10Z. url. (visited on 12/09/2022)
- 36.Rezatofighi S.H., Soltanian-Zadeh H. Automatic recognition of five types of white blood cells in peripheral blood. Comput Med Imaging Graph. 2011;35(4):333–343. doi: 10.1016/j.compmedimag.2011.01.003. [dataset] [DOI] [PubMed] [Google Scholar]
- 37.Kouzehkanan Z.M., Saghari S., Tavakoli S., et al. A large dataset of white blood cells containing cell locations and types, along with segmented nuclei and cytoplasm. Scient. Rep. Jan. 2022;12(1):1123. doi: 10.1038/s41598-021-04426-x. https://www.nature.com/articles/s41598-02104426-x Publisher: Nature Publishing Group. issn: 2045-2322. url. (visited on 02/10/2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tohyama K. Present status and perspective of laboratory hematology in Japan: on the standardization of blood cell morphology including myelodysplasia: on behalf of the Japanese Society for Laboratory Hematology. Int J Lab Hematol. 2018;40:120–125. doi: 10.1111/ijlh.12819. [DOI] [PubMed] [Google Scholar]
- 39.Li M., Lin C., Ge P., Li L., Song S., Zhang H. A deep learning model for detection of leukocytes under various interference factors. Scient. Rep. Feb. 2023;13(1):2160. doi: 10.1038/s41598-023-29331-3. https://www nature.com/articles/s41598-023-29331-3 Publisher: Nature Publishing Group. issn: 2045-2322. url. (visited on 05/26/2023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruderman D.L., Cronin T.W., Chiao C.-C. Statistics of cone responses to natural images: implications for visual coding. JOSA A. 1998;15(8):2036–2045. doi: 10.1364/JOSAA.15.002036. [DOI] [Google Scholar]
- 41.Reinhard E., Adhikhmin M., Gooch B., Shirley P. Color transfer between images. IEEE Comput. Graphics Appl. 2001;21(5):34–41. doi: 10.1109/38.946629. [DOI] [Google Scholar]
- 42.Dekking F.M., Kraaikamp C., Lopuhaä H.P., Meester L.E. Springer Nature; Feb. 19, 2007. A Modern Introduction to Probability and Statistics: Understanding why and how.https://www.ebook.de/de/product/3054516/f_m_dekking_c_kraaikamp_h_p_lopuhaae_l_e_meester_a_modern_introduction_to_probability_and_statistics_understanding_why_and_how.html 488 pp. isbn: 1852338962. url. [Google Scholar]
- 43.Van der Maaten L., Hinton G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008;9(11) http://jmlr.org/papers/v9/vandermaaten08a.html url. [Google Scholar]
- 44.Sheng B., Zhou M., Hu M., Li Q., Sun L., Wen Y. A blood cell dataset for lymphoma classification using faster R-CNN. Biotechnol. Biotechnological Equip. 2020;34(1):413–420. doi: 10.1080/13102818.2020.1765871. [DOI] [Google Scholar]