Abstract
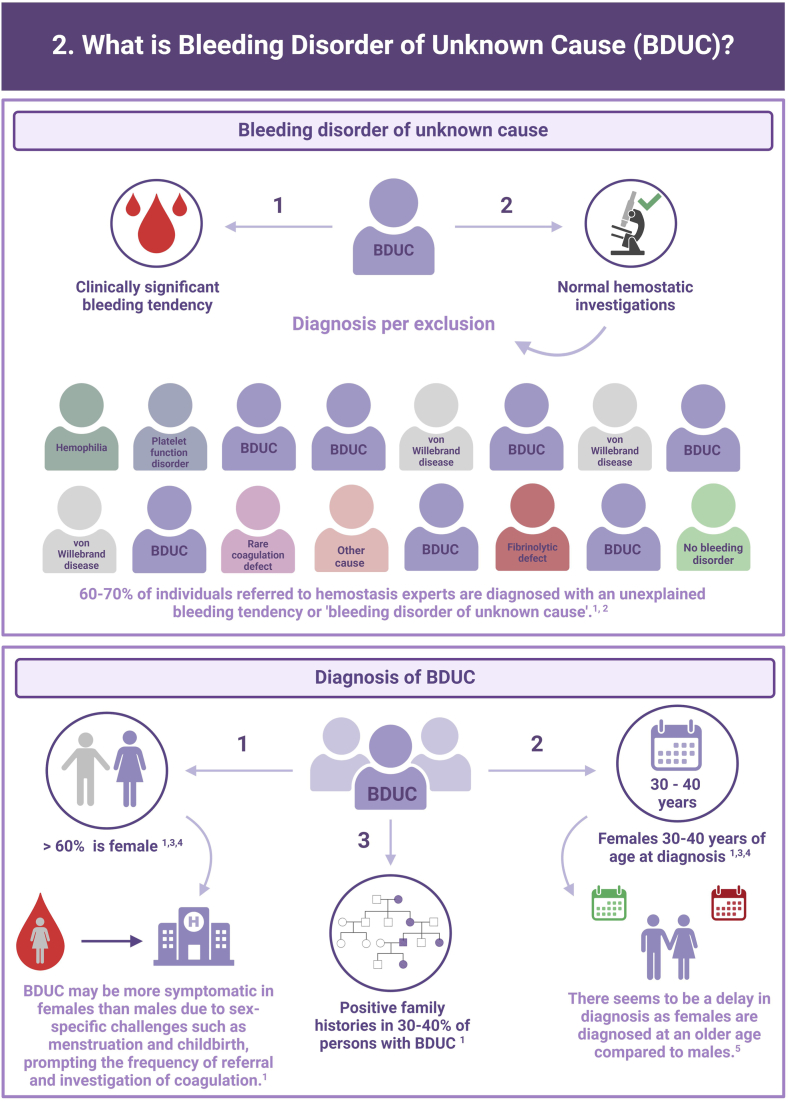
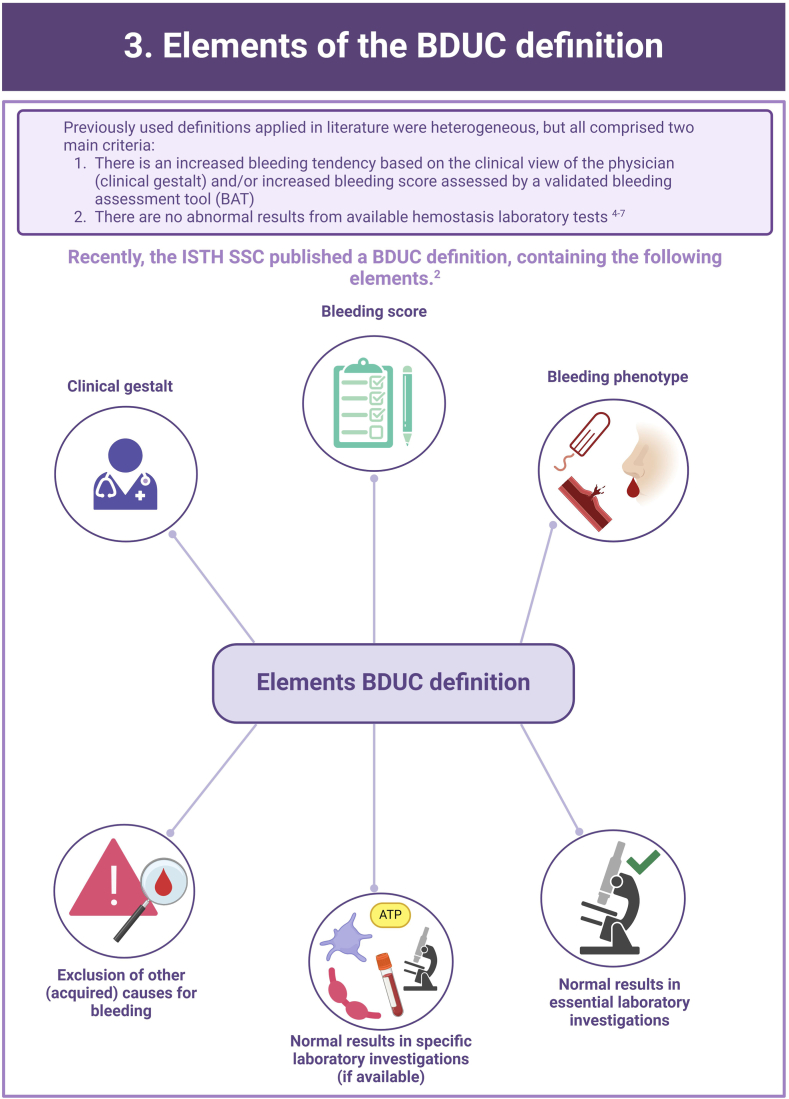
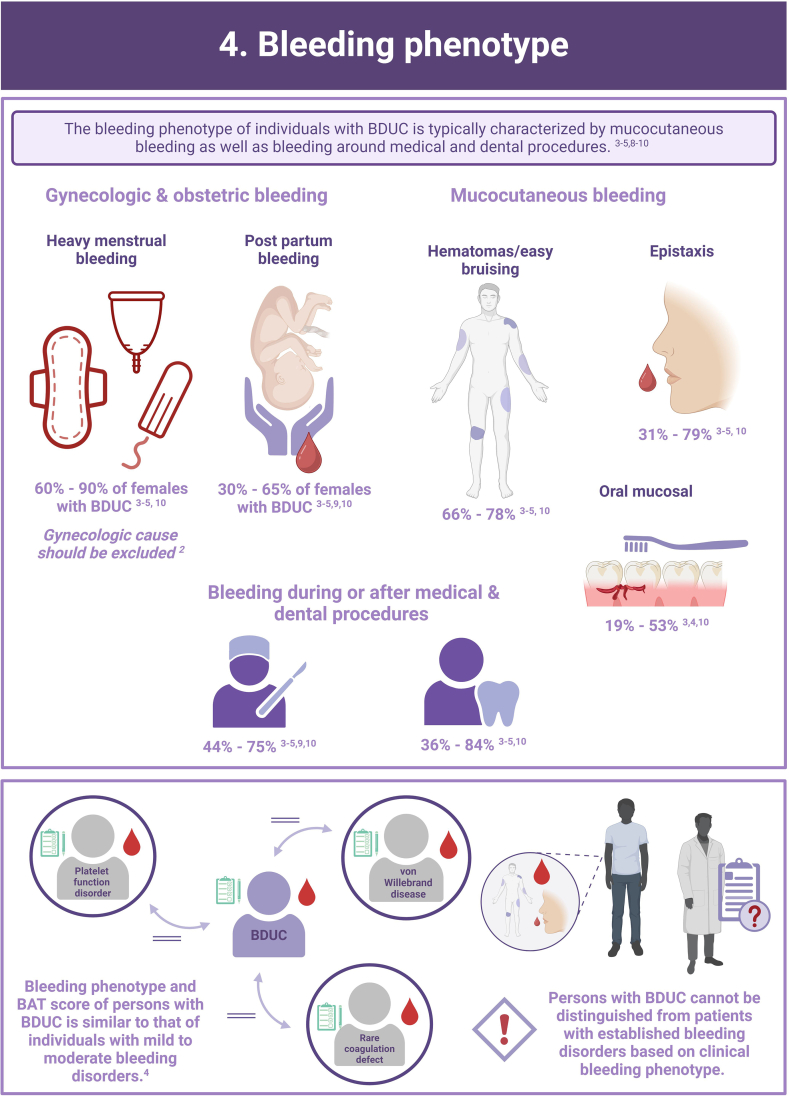
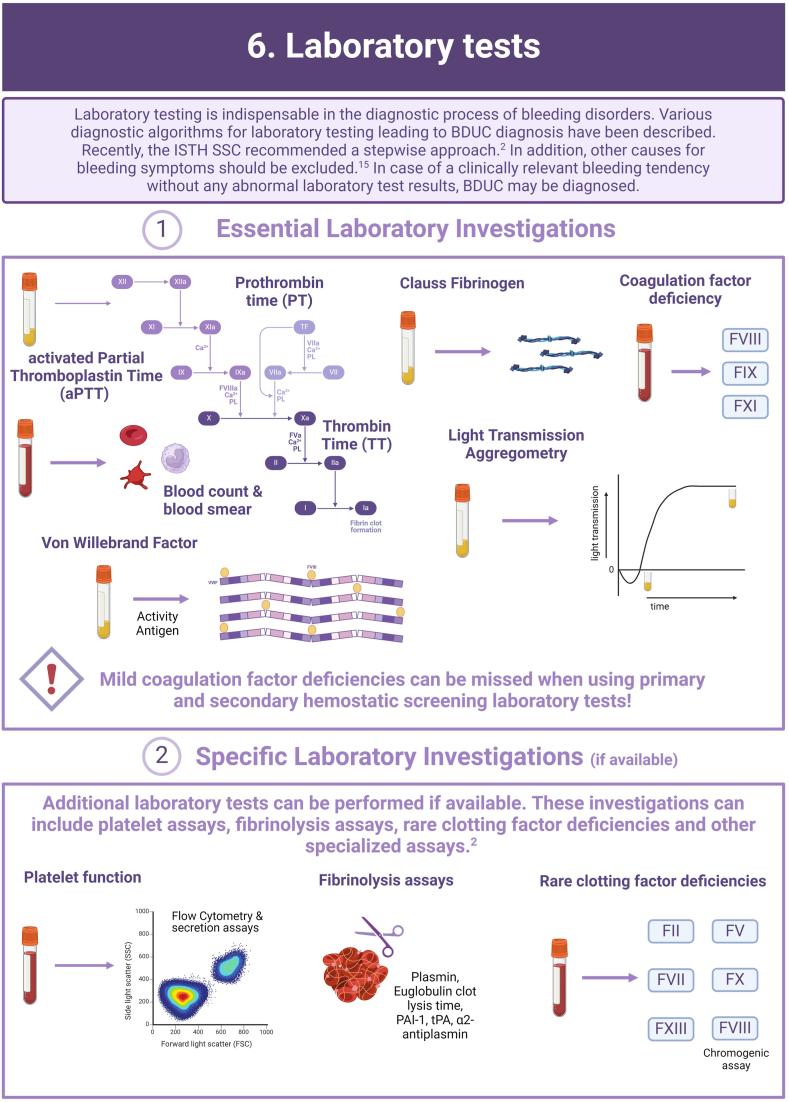
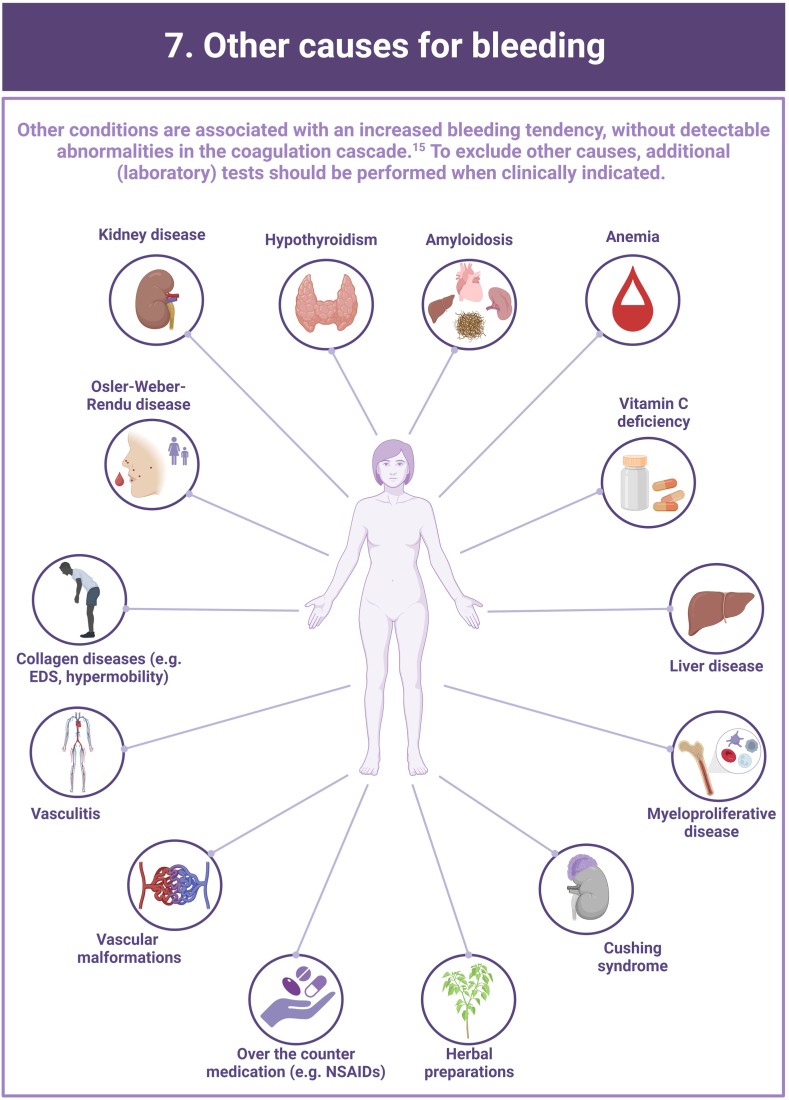
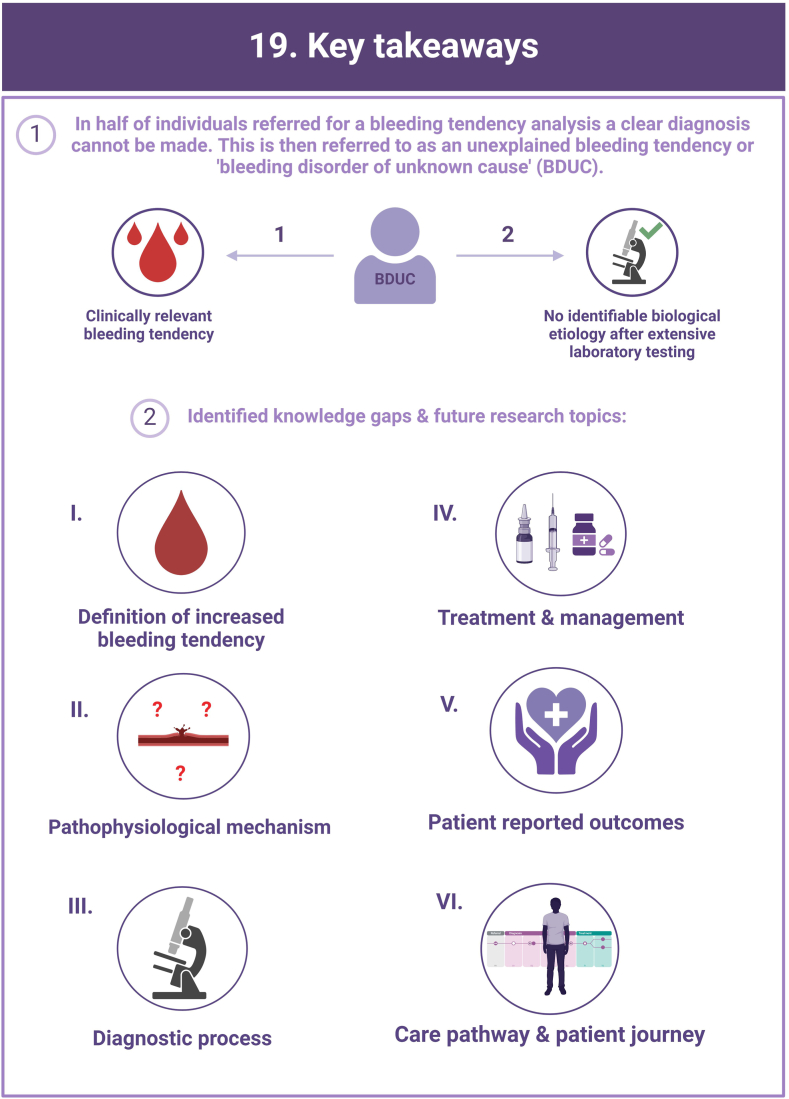
In more than half of the individuals with a clinically relevant bleeding tendency who are referred to hemostasis experts, no biological etiology can be found after extensive laboratory testing. These persons are diagnosed with an unexplained bleeding tendency or “bleeding disorder of unknown cause” (BDUC). The mucocutaneous bleeding phenotype of individuals with BDUC is generally comparable to that of individuals with inherited bleeding disorders such as von Willebrand disease or platelet function disorders. BDUC definitions applied in literature are heterogeneous, but all comprise 2 main criteria: (1) there is an increased bleeding tendency based on the clinical view of the physician and/or an increased bleeding score; (2) no abnormalities are found with available hemostasis laboratory tests. This is reflected in the recent published BDUC definition by the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis, stating that BDUC is a diagnosis of exclusion, characterized by normal hemostatic investigations despite a clinically significant bleeding tendency. Importantly, other nonhemostatic and acquired causes of bleeding should be excluded, but details on exclusion criteria and associated diagnostic testing remain undefined. Patients and health care providers are challenged by the uncertainty and lack of formal diagnosis particularly as there is no clear consensus regarding treatment. Research on the diagnostic value of new laboratory tests in individuals with BDUC has not yet been productive. In this illustrative review, the current practice and knowledge gaps in BDUC are addressed, previous research on BDUC is outlined and future directions with outstanding questions for future research in BDUC are highlighted.
Keywords: bleeding disorder of unknown cause, diagnosis, hemostasis, review, treatment
Essentials
-
•
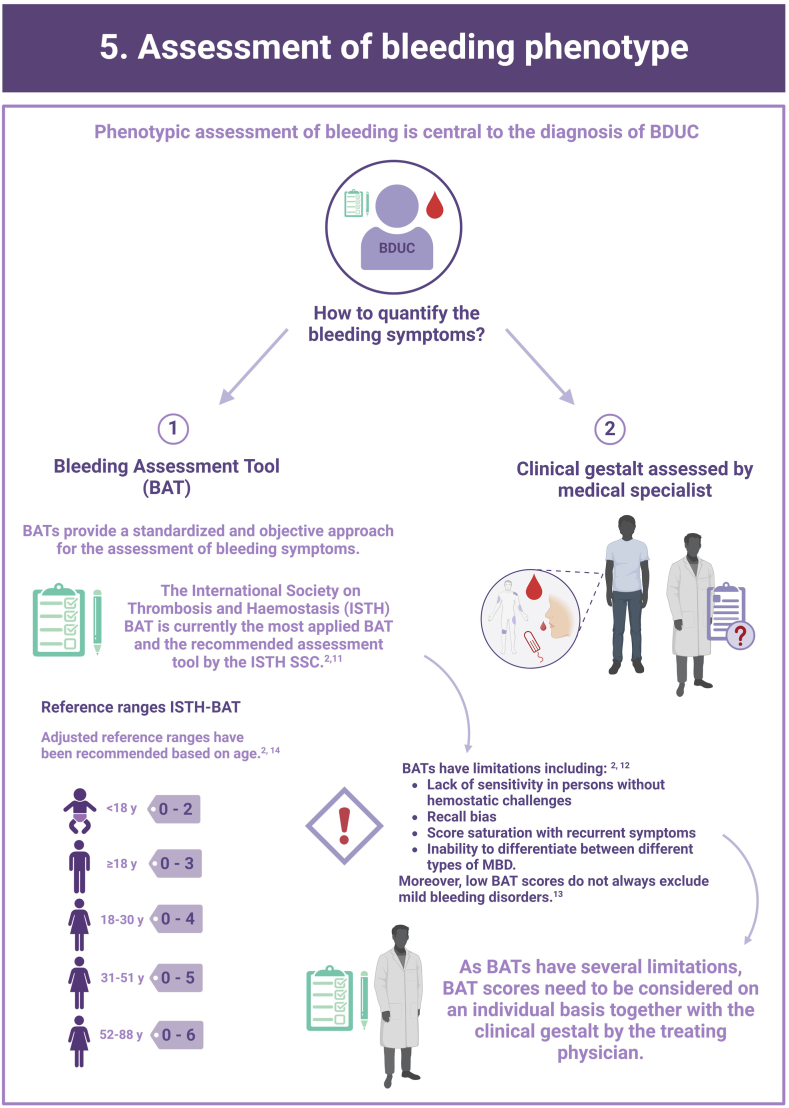
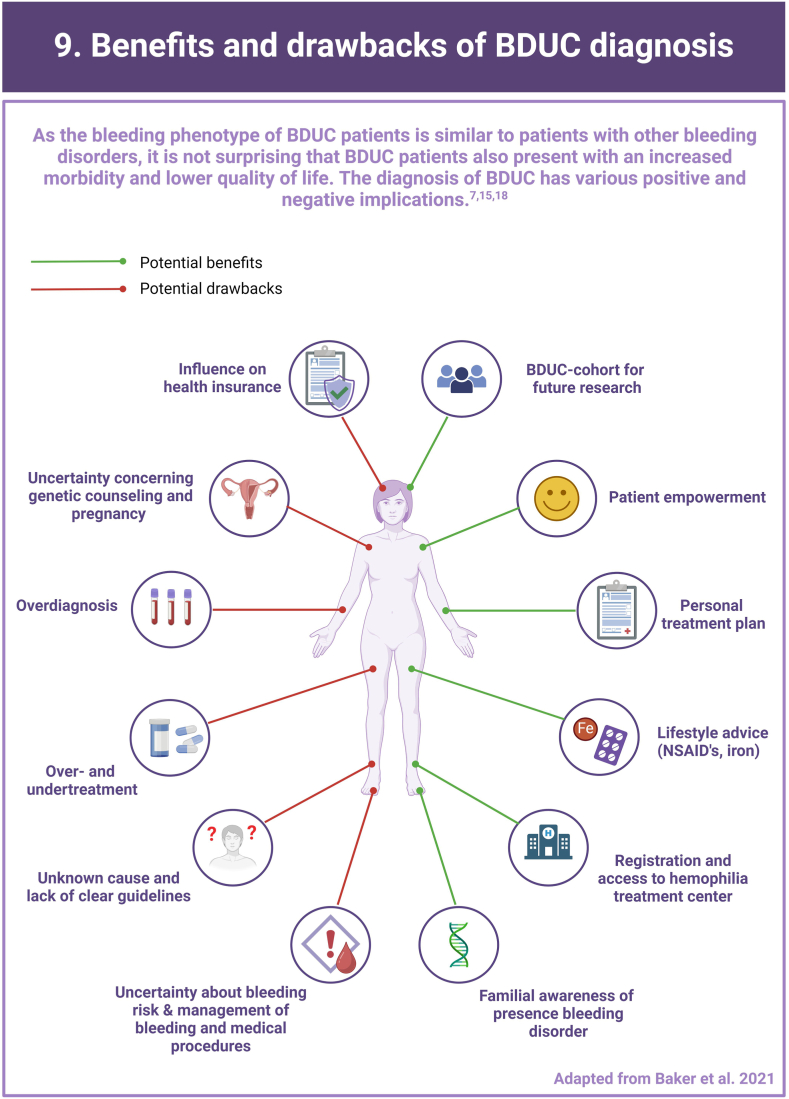
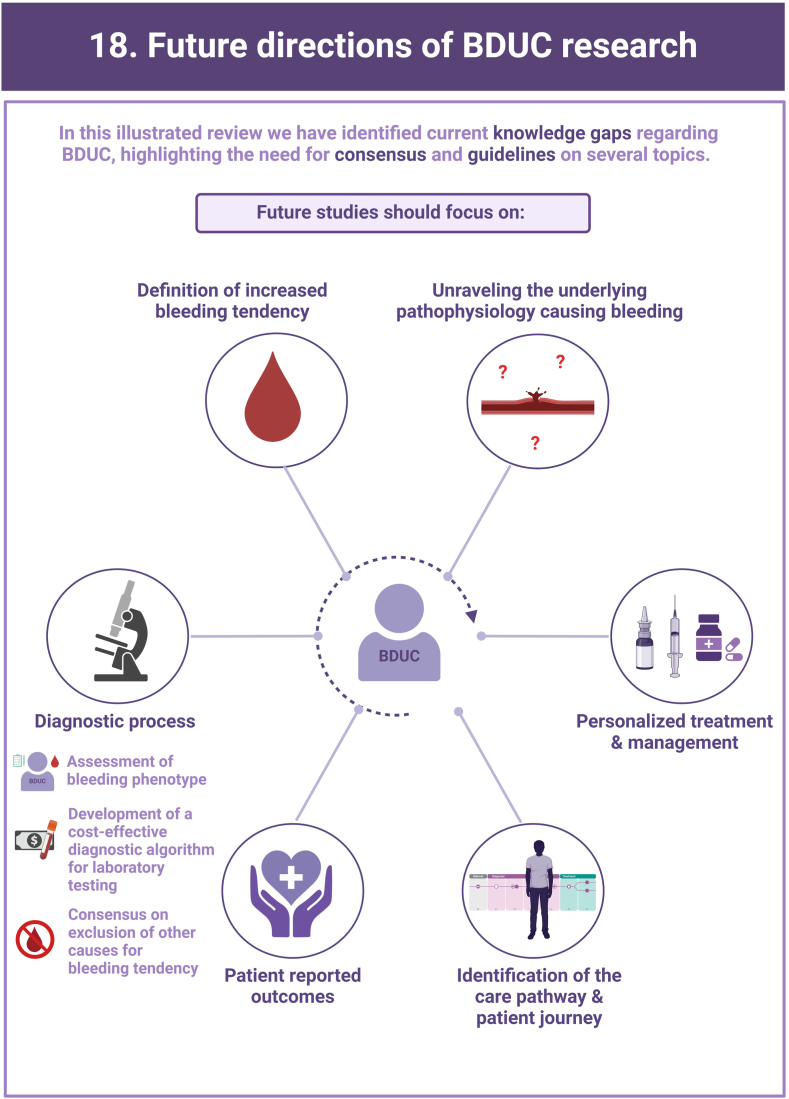
Bleeding Disorder of Unknown Cause (BDUC) is defined by a positive personal and/or family history of bleeding with normal laboratory test results.
-
•
More than half of patients with bleeding symptoms seen in hemostasis clinics are diagnosed with BDUC.
-
•
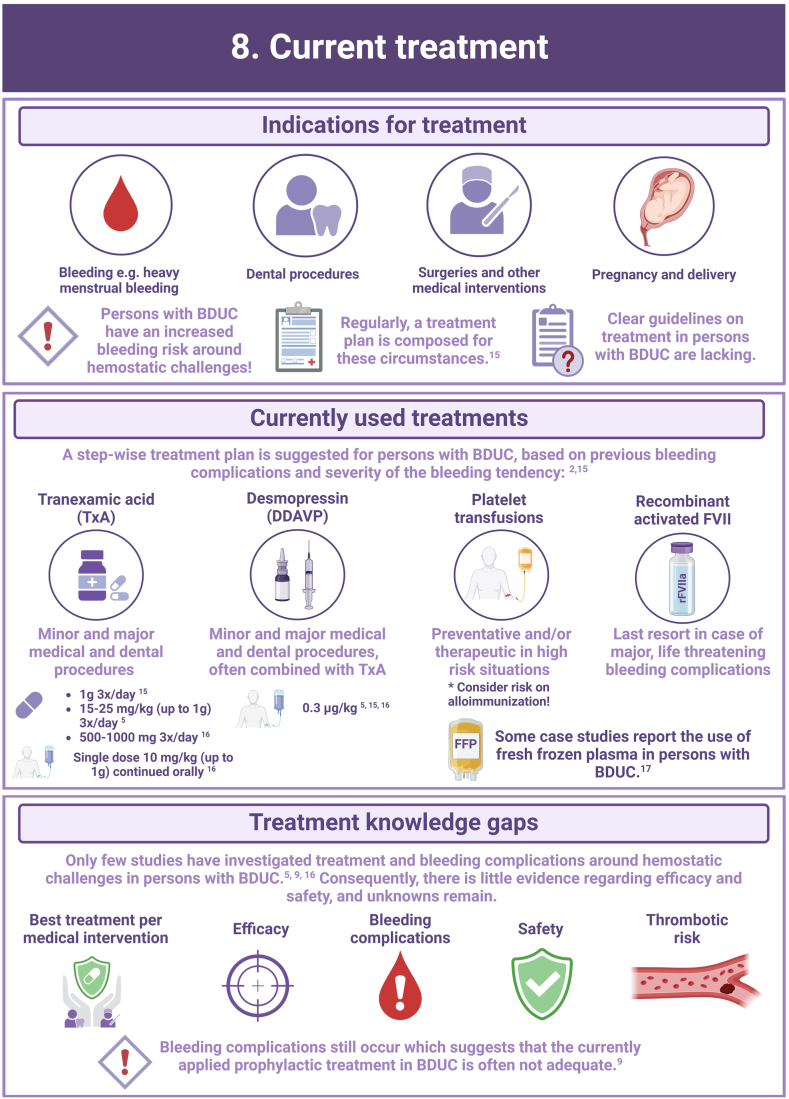
There are major knowledge gaps and lack of consensus on the approach to treatment.
-
•
Research is critically required to better understand the impact and determinants of BDUC.
Acknowledgments
Figures are created in BioRender.com
Funding
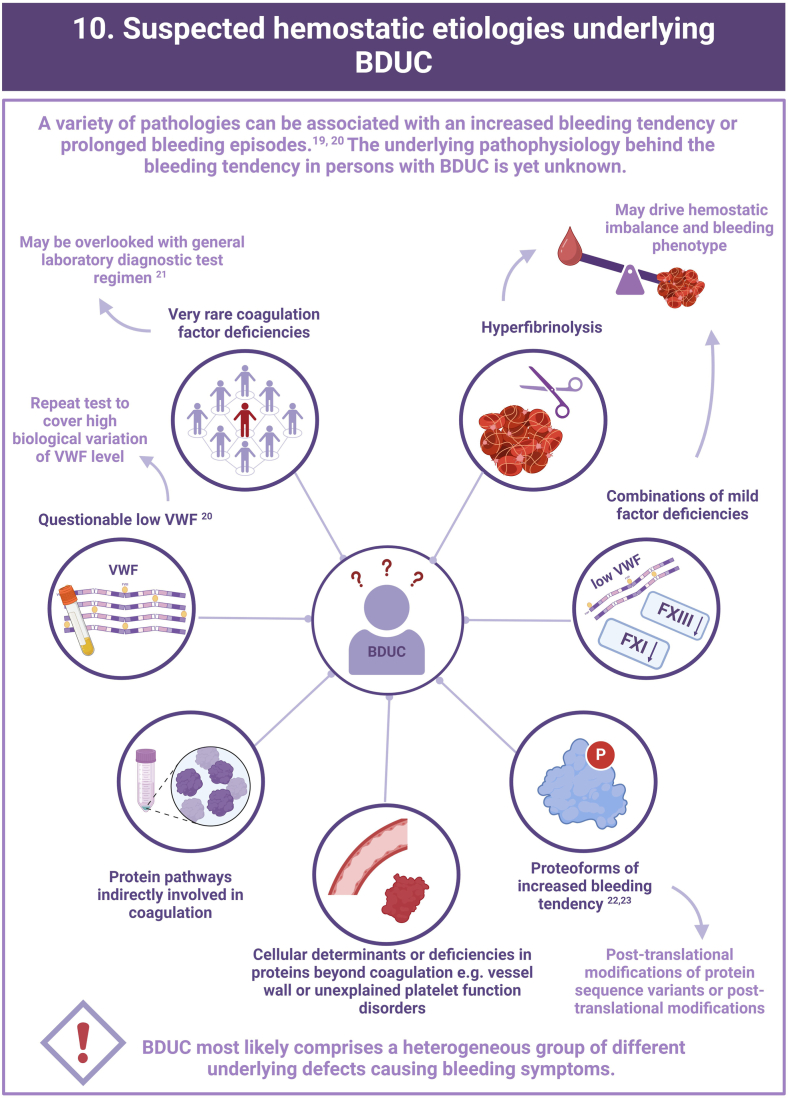
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author contribution
A.L.L.M. and C.M.A.M. share first authorship and wrote original draft, designed the capsule, and reviewed the process. T.T.v.D., M.J.H.A.K., Y.M.C.H., M.v.d.B., R.E.G.S., S.E.M.S., K.J.F., K.M., P.L.d.E., L.N., I.v.M., R.I.B., and J.S.O’D. reviewed and edited the manuscript. M.H.C. and F.C.J.I.H-M. reviewed, edited, and supervised the study.
Relationship disclosure
TTvD has received research funding from the Bertus Kem Stipendium (GNGH).
MJHAK has received an investigator-initiated research grant from Dutch Research Council (NWO), The Netherlands Organisation for Health Research and Development (ZonMw), Netherlands thrombosis foundation and Sobi, and speaker fees from Roche, Sobi and BMS. All payments go to the Erasmus MC as an institution. YMCH is professor of clinical chemistry, in particular hemostasis. In this position she collaborates with and tests reagents and equipment from IVD companies in the field of hemostasis (Werfen, Siemens, Roche, Nodia, Stago). She is also an advisor of Promicol. REGS has received research funding from Bayer, CSL Behring, Hemab, NovoNordisk, Novartis, Octapharma, Sanofi, and Sobi. All payments go to the institution. MHC has received researcher initiated research and travel grants from the Dutch Research Council (NWO), the Netherlands Organization for Health Research and Development (ZonMw), the Dutch Healthcare Insurers Innovation Fund, Pfizer, Baxter/Baxalta/Shire/Takeda, Bayer Schering Pharma, CSL Behring, Sobi, Novo Nordisk, Novartis, Nordic Pharma, Roche and Octapharma and has served as a board member for Roche and Bayer. All scholarships, prizes and reimbursements go to Erasmus MC as an institution. FCJIH-M has received a research grant from Octapharma. All other authors have no conflict of interest to disclose.
Footnotes
Handling Editor: Michelle Sholzberg
Amaury L.L. Monard and Caroline M.A. Mussert share the first authorship and contributed equally to this work.
References
- 1.Thomas W., Downes K., Desborough M.J.R. Bleeding of unknown cause and unclassified bleeding disorders; diagnosis, pathophysiology and management. Haemophilia. 2020;26:946–957. doi: 10.1111/hae.14174. [DOI] [PubMed] [Google Scholar]
- 2.Baker R.I., Choi P., Curry N., Gebhart J., Gomez K., Henskens Y., et al. Standardization of definition and management for bleeding disorder of unknown cause: communication from the SSC of the ISTH. J Thromb Haemost. 2024;22:2059–2070. doi: 10.1016/j.jtha.2024.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Gebhart J., Hofer S., Panzer S., Quehenberger P., Sunder-Plassmann R., Hoermann G., et al. High proportion of patients with bleeding of unknown cause in persons with a mild-to-moderate bleeding tendency: results from the Vienna Bleeding Biobank (VIBB) Haemophilia. 2018;24:405–413. doi: 10.1111/hae.13422. [DOI] [PubMed] [Google Scholar]
- 4.Quiroga T., Goycoolea M., Panes O., Aranda E., Martinez C., Belmont S., et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92:357–365. doi: 10.3324/haematol.10816. [DOI] [PubMed] [Google Scholar]
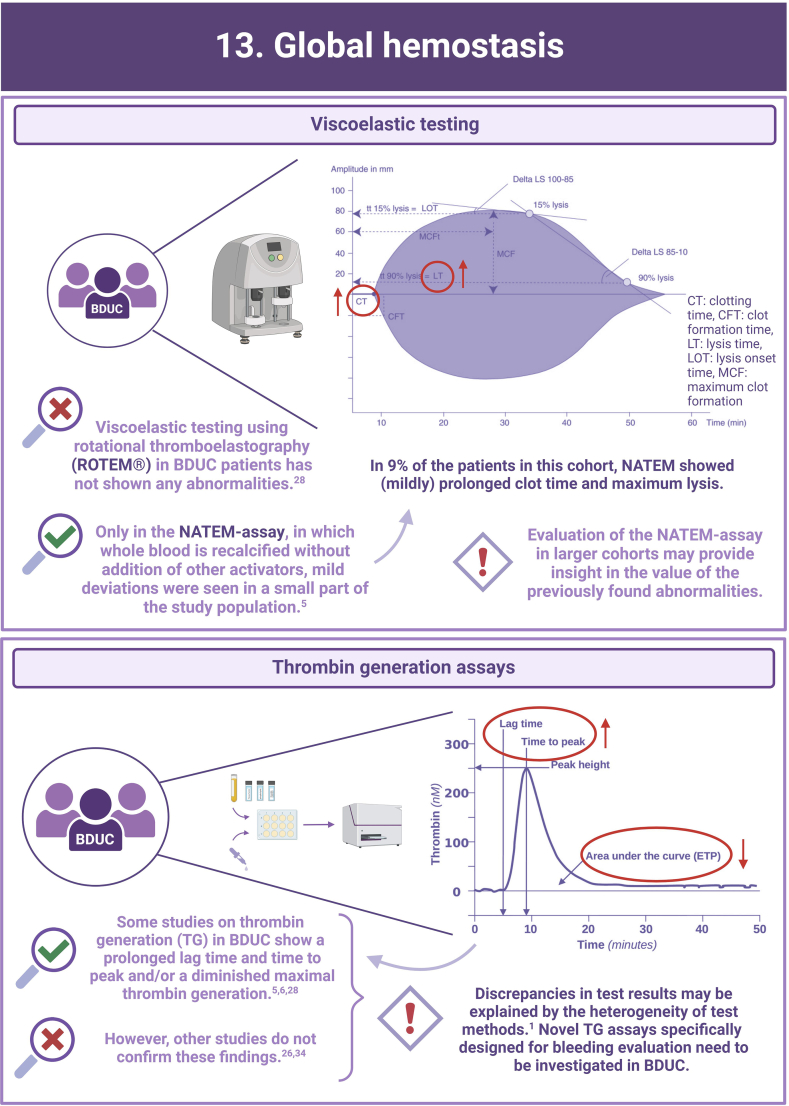
- 5.MacDonald S., Wright A., Beuche F., Downes K., Besser M., Symington E., et al. Characterization of a large cohort of patients with unclassified bleeding disorder; clinical features, management of haemostatic challenges and use of global haemostatic assessment with proposed recommendations for diagnosis and treatment. Int J Lab Hematol. 2020;42:116–125. doi: 10.1111/ijlh.13124. [DOI] [PubMed] [Google Scholar]
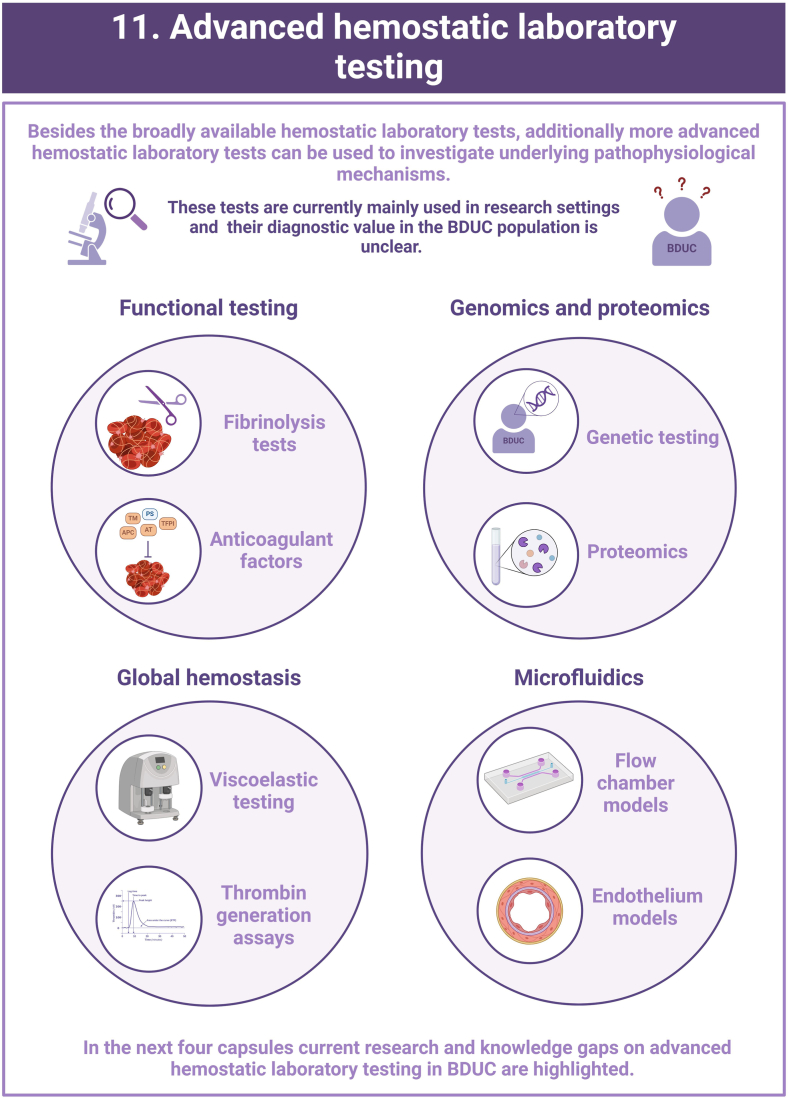
- 6.Hofer S., Ay C., Rejto J., Wolberg A.S., Haslacher H., Koder S., et al. Thrombin-generating potential, plasma clot formation, and clot lysis are impaired in patients with bleeding of unknown cause. J Thromb Haemost. 2019;17:1478–1488. doi: 10.1111/jth.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Relke N., Kuthiala S., Grabell J., Hopman W.M., James P. The bleeding score: useful in predicting spontaneous bleeding events in adults with bleeding of unknown cause? Haemophilia. 2020;26:e31–e33. doi: 10.1111/hae.13775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeghiero F., Pabinger I., Ragni M., Abdul-Kadir R., Berntorp E., Blanchette V., et al. Fundamentals for a systematic approach to mild and moderate inherited bleeding disorders: an EHA consensus report. Hemasphere. 2019;3:e286. doi: 10.1097/HS9.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Veen C.S.B., Huisman E.J., Romano L.G.R., Schipaanboord C.W.A., Cnossen M.H., de Maat M.P.M., et al. Outcome of surgical interventions and deliveries in patients with bleeding of unknown cause: an observational study. Thromb Haemost. 2021;121:1409–1416. doi: 10.1055/s-0041-1726344. [DOI] [PubMed] [Google Scholar]
- 10.Mehic D., Neubauer G., Janig F., Kaider A., Ay C., Pabinger I., Gebhart J. Risk factors for future bleeding in patients with mild bleeding disorders: longitudinal data from the Vienna Bleeding Biobank. J Thromb Haemost. 2023;21:1757–1768. doi: 10.1016/j.jtha.2023.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Rodeghiero F., Tosetto A., Abshire T., Arnold D.M., Coller B., James P., et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8:2063–2065. doi: 10.1111/j.1538-7836.2010.03975.x. [DOI] [PubMed] [Google Scholar]
- 12.Rydz N., James P.D. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10:2223–2229. doi: 10.1111/j.1538-7836.2012.04923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moenen F., Nelemans P.J., Schols S.E.M., Schouten H.C., Henskens Y.M.C., Beckers E.A.M. The diagnostic accuracy of bleeding assessment tools for the identification of patients with mild bleeding disorders: a systematic review. Haemophilia. 2018;24:525–535. doi: 10.1111/hae.13486. [DOI] [PubMed] [Google Scholar]
- 14.Doherty D., Grabell J., Christopherson P.A., Montgomery R.R., Coller B.S., Lavin M., et al. Variability in International Society on Thrombosis and Haemostasis-Scientific and Standardization Committee endorsed Bleeding Assessment Tool (ISTH-BAT) score with normal aging in healthy females: contributory factors and clinical significance. J Thromb Haemost. 2023;21:880–886. doi: 10.1016/j.jtha.2022.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker R.I., O'Donnell J.S. How I treat bleeding disorder of unknown cause. Blood. 2021;138:1795–1804. doi: 10.1182/blood.2020010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obaji S., Alikhan R., Rayment R., Carter P., Macartney N., Collins P. Unclassified bleeding disorders: outcome of haemostatic challenges following tranexamic acid and/or desmopressin. Haemophilia. 2016;22:285–291. doi: 10.1111/hae.12811. [DOI] [PubMed] [Google Scholar]
- 17.Li L., Johnsen J.M., Doan C.X., Bollag L.A. Case report: anesthetic management for cesarean section in a parturient with unspecified inherited bleeding disorder. F1000Res. 2018;7:1482. doi: 10.12688/f1000research.16097.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanders Y.V., de Wee E.M., Meijer K., Eikenboom J., van der Bom J.G., Fijnvandraat C.J., et al. [Von Willebrand disease in the Netherlands: the WiN study] De ziekte van von Willebrand in Nederland: de WiN-studie. Ned Tijdschr Geneeskd. 2014;158 [PubMed] [Google Scholar]
- 19.Mehic D., Pabinger I., Gebhart J. Investigating patients for bleeding disorders when most of the "usual" ones have been ruled out. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.102242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehic D., Kraemmer D., Tolios A., Bücheler J., Quehenberger P., Haslacher H., et al. The necessity of repeat testing for von Willebrand disease in adult patients with mild to moderate bleeding disorders. J Thromb Haemost. 2024;22:101–111. doi: 10.1016/j.jtha.2023.09.010. [DOI] [PubMed] [Google Scholar]
- 21.Mehic D., Tolios A., Hofer S., Ay C., Haslacher H., Rejtö J., et al. Elevated levels of tissue factor pathway inhibitor in patients with mild to moderate bleeding tendency. Blood Adv. 2021;5:391–398. doi: 10.1182/bloodadvances.2020003464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James P.D., Connell N.T., Ameer B., Di Paola J., Eikenboom J., Giraud N., et al. ASH ISTH NHF WFH 2021 guidelines on the diagnosis of von Willebrand disease. Blood Adv. 2021;5:280–300. doi: 10.1182/bloodadvances.2020003265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cunha M.L., Bakhtiari K., Peter J., Marquart J.A., Meijers J.C., Middeldorp S. A novel mutation in the F5 gene (factor V Amsterdam) associated with bleeding independent of factor V procoagulant function. Blood. 2015;125:1822–1825. doi: 10.1182/blood-2014-08-592733. [DOI] [PubMed] [Google Scholar]
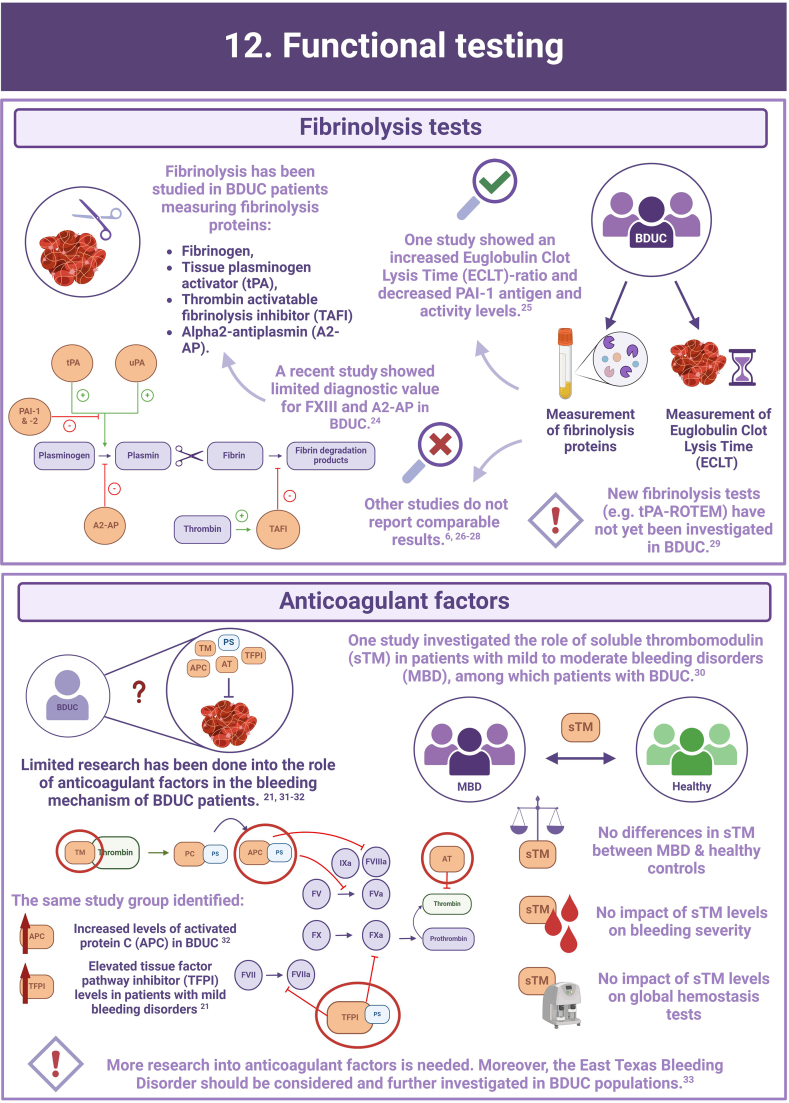
- 24.Ariëns S., Huisman A., Hovinga I., Urbanus R.T., van Galen K.P.M., van Vulpen L.F.D., et al. Limited value of testing for factor XIII and α2-antiplasmin deficiency in patients with a bleeding disorder of unknown cause. Haemophilia. 2024;30:998–1002. doi: 10.1111/hae.15059. [DOI] [PubMed] [Google Scholar]
- 25.Valke L., Meijer D., Nieuwenhuizen L., Laros-van Gorkom B.A.P., Blijlevens N.M.A., van Heerde W.L., et al. Fibrinolytic assays in bleeding of unknown cause: improvement in diagnostic yield. Res Pract Thromb Haemost. 2022;6 doi: 10.1097/MBC.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alves G.S., Orsi F.A., Santiago-Bassora F.D., Quaino S.K., Montalvão S.A., Paula E.V., et al. Laboratory evaluation of patients with undiagnosed bleeding disorders. Blood Coagul Fibrinolysis. 2016;27:500–505. doi: 10.1097/MBC.0000000000000444. [DOI] [PubMed] [Google Scholar]
- 27.Gebhart J., Kepa S., Hofer S., Koder S., Kaider A., Wolberg A.S., et al. Fibrinolysis in patients with a mild-to-moderate bleeding tendency of unknown cause. Ann Hematol. 2017;96:489–495. doi: 10.1007/s00277-016-2893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veen C.S.B., Huisman E.J., Cnossen M.H., Kom-Gortat R., Rijken D.C., Leebeek F.W.G., et al. Evaluation of thromboelastometry, thrombin generation and plasma clot lysis time in patients with bleeding of unknown cause: a prospective cohort study. Haemophilia. 2020;26:e106–e115. doi: 10.1111/hae.13991. [DOI] [PubMed] [Google Scholar]
- 29.Heubel-Moenen F., Henskens Y.M.C., Verhezen P.W.M., Wetzels R.J.H., Schouten H.C., Beckers E.A.M. Fibrinolysis in patients with chemotherapy-induced thrombocytopenia and the effect of platelet transfusion. J Thromb Haemost. 2019;17:1073–1084. doi: 10.1111/jth.14465. [DOI] [PubMed] [Google Scholar]
- 30.Mehic D., Tolios A., Hofer S., Ay C., Haslacher H., Downes K., et al. Thrombomodulin in patients with mild to moderate bleeding tendency. Haemophilia. 2021;27:1028–1036. doi: 10.1111/hae.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehic D., Colling M., Pabinger I., Gebhart J. Natural anticoagulants: a missing link in mild to moderate bleeding tendencies. Haemophilia. 2021;27:701–709. doi: 10.1111/hae.14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehic D., Schramm T., Forstner-Bergauer B., Haslacher H., Ay C., Pabinger I., et al. Activated protein C and free protein S in patients with mild to moderate bleeding disorders. Thromb Res. 2024;235:98–106. doi: 10.1016/j.thromres.2024.01.018. [DOI] [PubMed] [Google Scholar]
- 33.Peterson J.A., Gupta S., Martinez N.D., Hardesty B., Maroney S.A., Mast A.E. Factor V east Texas variant causes bleeding in a three-generation family. J Thromb Haemost. 2022;20:565–573. doi: 10.1111/jth.15612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ay C., Haselböck J., Laczkovics C., Koder S., Pabinger I. Thrombin generation in patients with a bleeding tendency of unknown origin. Ann Hematol. 2011;90:1099–1104. doi: 10.1007/s00277-011-1201-8. [DOI] [PubMed] [Google Scholar]
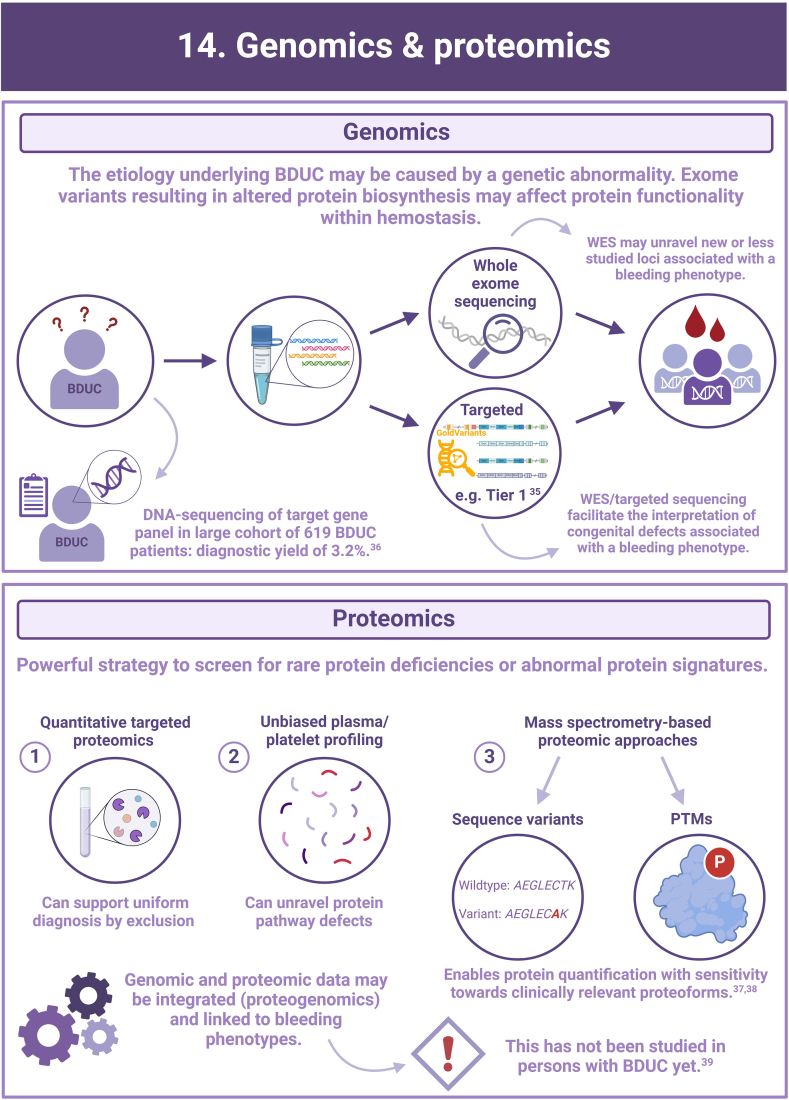
- 35.Megy K., Downes K., Morel-Kopp M.C., Bastida J.M., Brooks S., Bury L., et al. GoldVariants, a resource for sharing rare genetic variants detected in bleeding, thrombotic, and platelet disorders: communication from the ISTH SSC Subcommittee on Genomics in Thrombosis and Hemostasis. J Thromb Haemost. 2021;19:2612–2617. doi: 10.1111/jth.15459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Downes K., Megy K., Duarte D., Vries M., Gebhart J., Hofer S., et al. Diagnostic high-throughput sequencing of 2396 patients with bleeding, thrombotic, and platelet disorders. Blood. 2019;134:2082–2091. doi: 10.1182/blood.2018891192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gashash E.A., Aloor A., Li D., Zhu H., Xu X.Q., Xiao C., et al. An insight into glyco-microheterogeneity of plasma von willebrand factor by mass spectrometry. J Proteome Res. 2017;16:3348–3362. doi: 10.1021/acs.jproteome.7b00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kreft I.C., van Duijl T.T., van Kwawegen C., Atiq F., Phan W., Schuller M.B.P., et al. Variant mapping using mass spectrometry–based proteotyping as a diagnostic tool in von Willebrand disease. J Thromb Haemost. 2024;22:1894–1908. doi: 10.1016/j.jtha.2024.04.011. [DOI] [PubMed] [Google Scholar]
- 39.Eldjarn G.H., Ferkingstad E., Lund S.H., Helgason H., Magnusson O.T., Gunnarsdottir K., et al. Large-scale plasma proteomics comparisons through genetics and disease associations. Nature. 2023;622:348–358. doi: 10.1038/s41586-023-06563-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
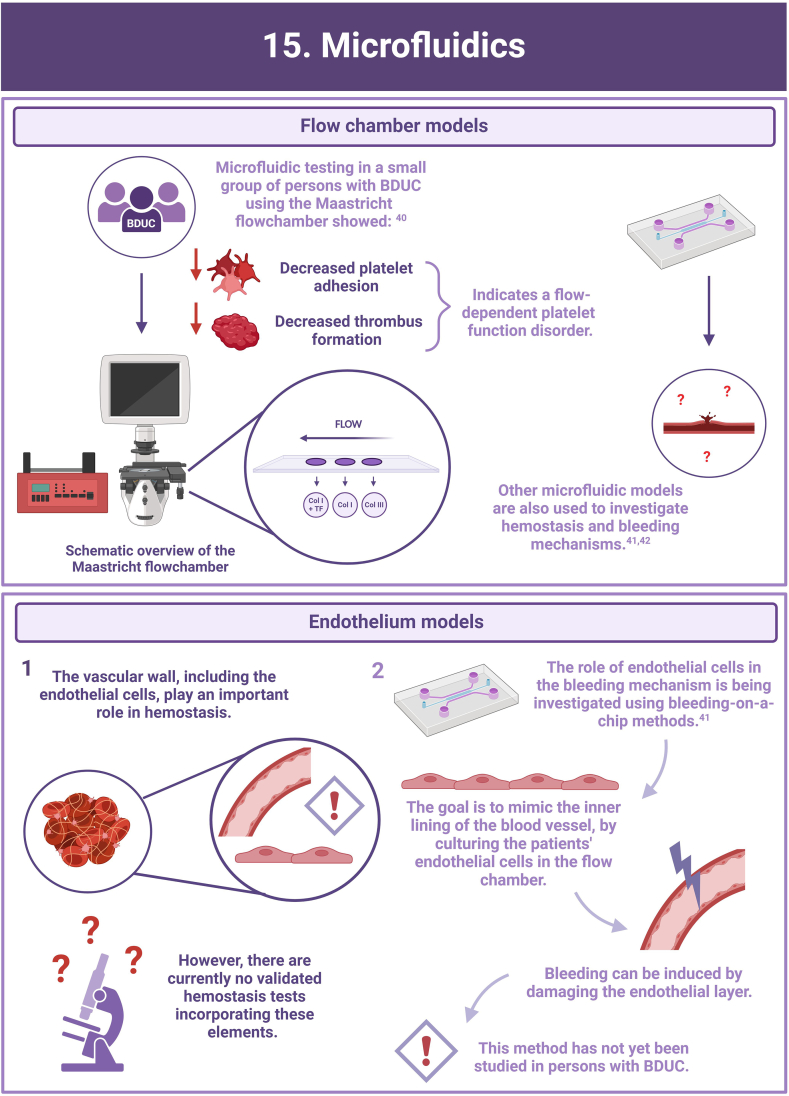
- 40.Heubel-Moenen F., Brouns S.L.N., Herfs L., Boerenkamp L.S., Jooss N.J., Wetzels R.J.H., et al. Multiparameter platelet function analysis of bleeding patients with a prolonged platelet function analyser closure time. Br J Haematol. 2022;196:1388–1400. doi: 10.1111/bjh.18003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakurai Y., Hardy E.T., Ahn B., Tran R., Fay M.E., Ciciliano J.C., et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nat Commun. 2018;9:509. doi: 10.1038/s41467-018-02990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cnossen M.H., van Moort I., Reitsma S.H., de Maat M.P.M., Schutgens R.E.G., Urbanus R.T., et al. SYMPHONY consortium: orchestrating personalized treatment for patients with bleeding disorders. J Thromb Haemost. 2022;20:2001–2011. doi: 10.1111/jth.15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
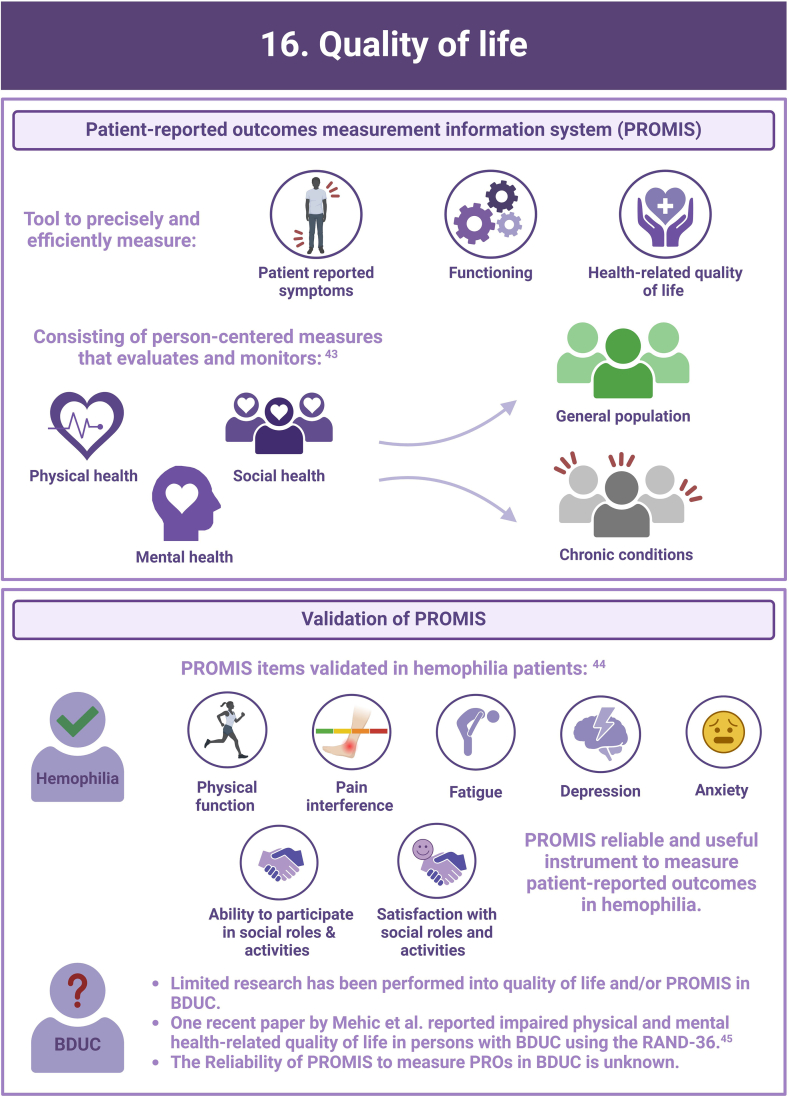
- 43.Cella D., Riley W., Stone A., Rothrock N., Reeve B., Yount S., et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuijlaars I.A.R., Teela L., van Vulpen L.F.D., Timmer M.A., Coppens M., Gouw S.C., et al. Generic PROMIS item banks in adults with hemophilia for patient-reported outcome assessment: feasibility, measurement properties, and relevance. Res Pract Thromb Haemost. 2021;5 doi: 10.1002/rth2.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mehic D., Schwarz S., Shulym I., Ay C., Pabinger I., Gebhart J. Health-related quality of life is impaired in bleeding disorders of unknown cause: results from the Vienna Bleeding Biobank. Res Pract Thromb Haemost. 2023;7 doi: 10.1016/j.rpth.2023.102176. [DOI] [PMC free article] [PubMed] [Google Scholar]
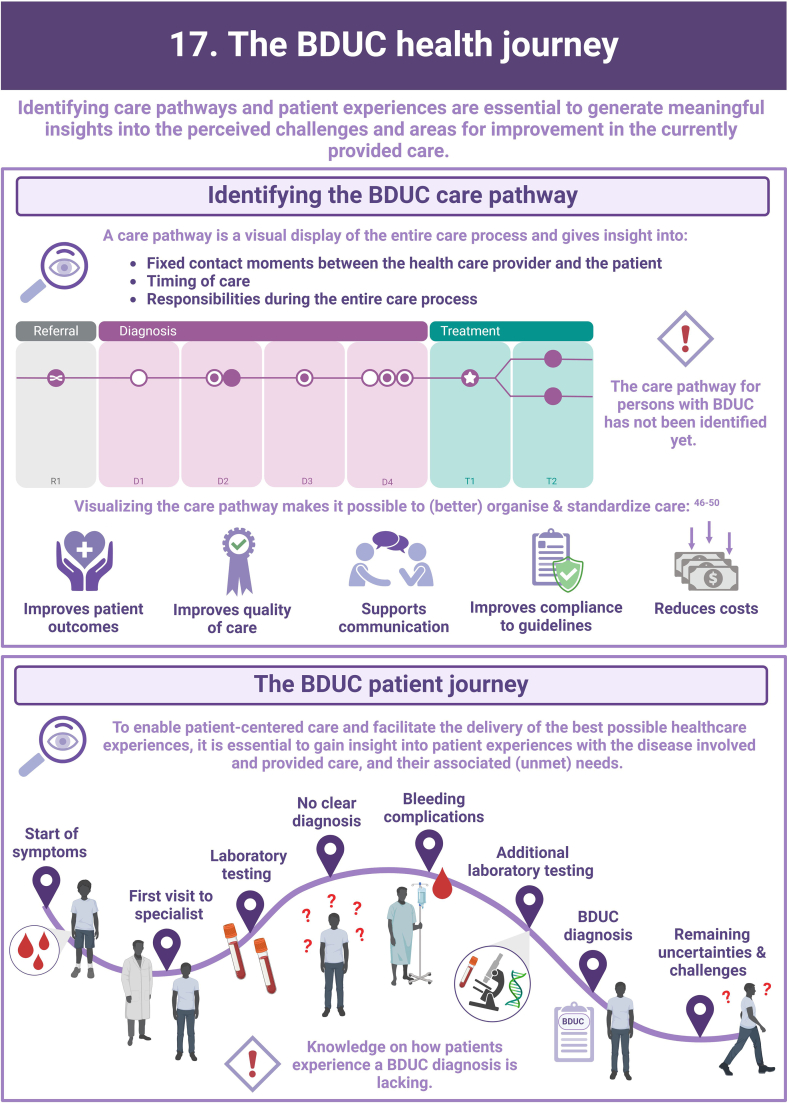
- 46.Jayaram A., Nagel R.W., Jasty R. Impact of clinical pathway on quality of care in sickle cell patients. J Pediatr Hematol Oncol. 2010;32:537–539. doi: 10.1097/MPH.0b013e3181e7570a. [DOI] [PubMed] [Google Scholar]
- 47.Lawal A.K., Rotter T., Kinsman L., Machotta A., Ronellenfitsch U., Scott S.D., et al. What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Med. 2016;14:35. doi: 10.1186/s12916-016-0580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schrijvers G., van Hoorn A., Huiskes N. The care pathway: concepts and theories: an introduction. Int J Integr Care. 2012;12:e192. doi: 10.5334/ijic.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shoji F., Yano T., Haro A., Yoshida T., Ito K., Morodomi Y., et al. Assessing a clinical pathway to improve the quality of care in pulmonary resections. Surg Today. 2011;41:787–790. doi: 10.1007/s00595-010-4483-x. [DOI] [PubMed] [Google Scholar]
- 50.Vanhaecht K., Ovretveit J., Elliott M.J., Sermeus W., Ellershaw J., Panella M. Have we drawn the wrong conclusions about the value of care pathways? Is a Cochrane review appropriate? Eval Health Prof. 2012;35:28–42. doi: 10.1177/0163278711408293. [DOI] [PubMed] [Google Scholar]