Abstract
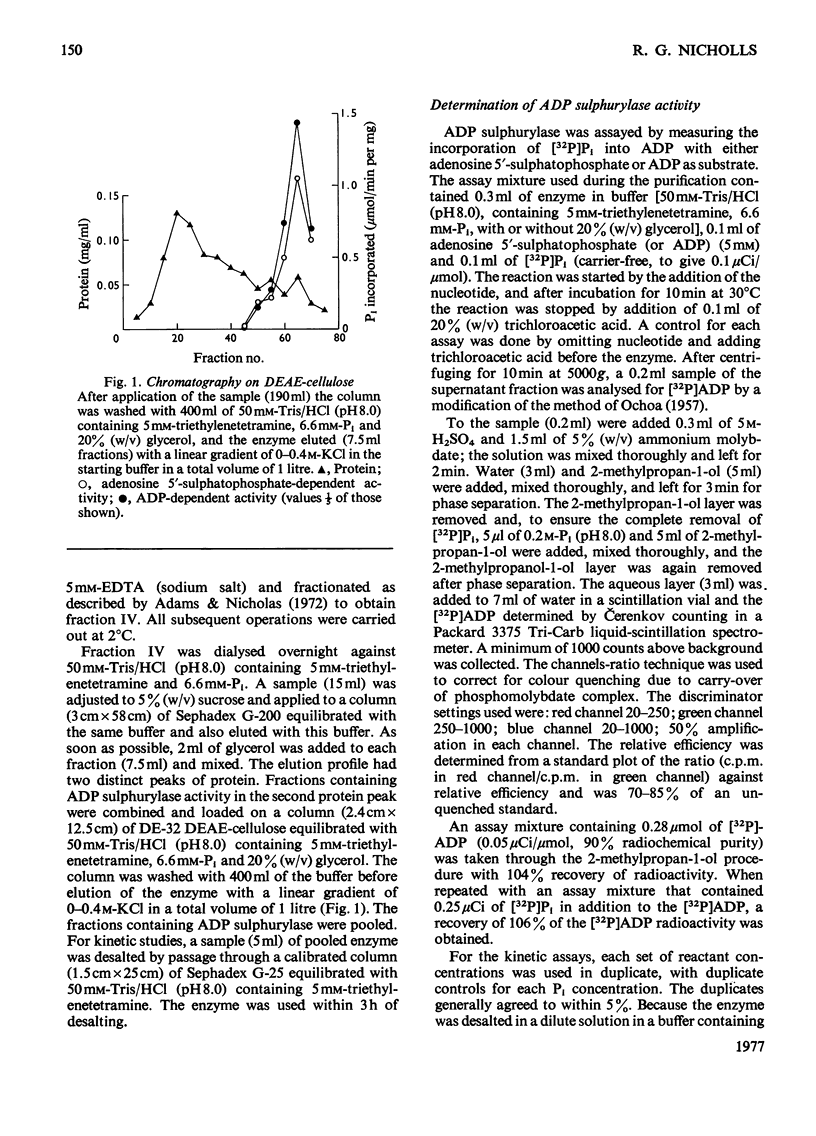
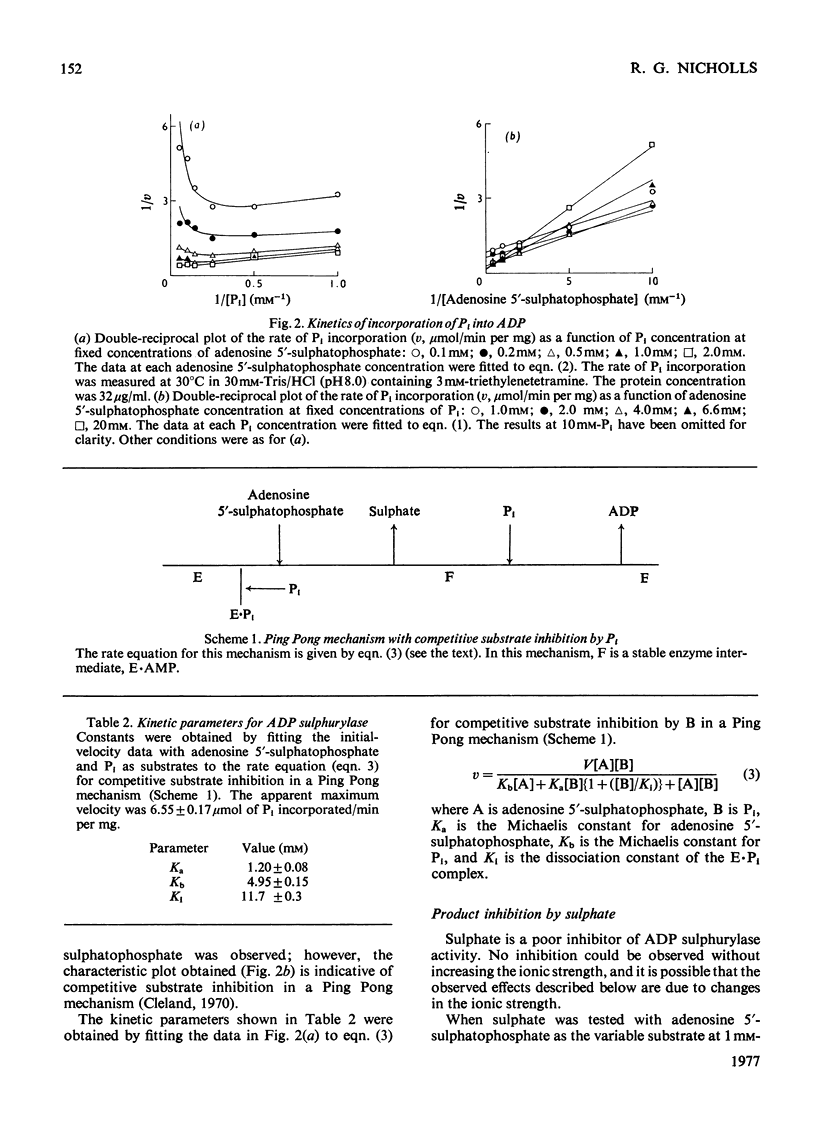
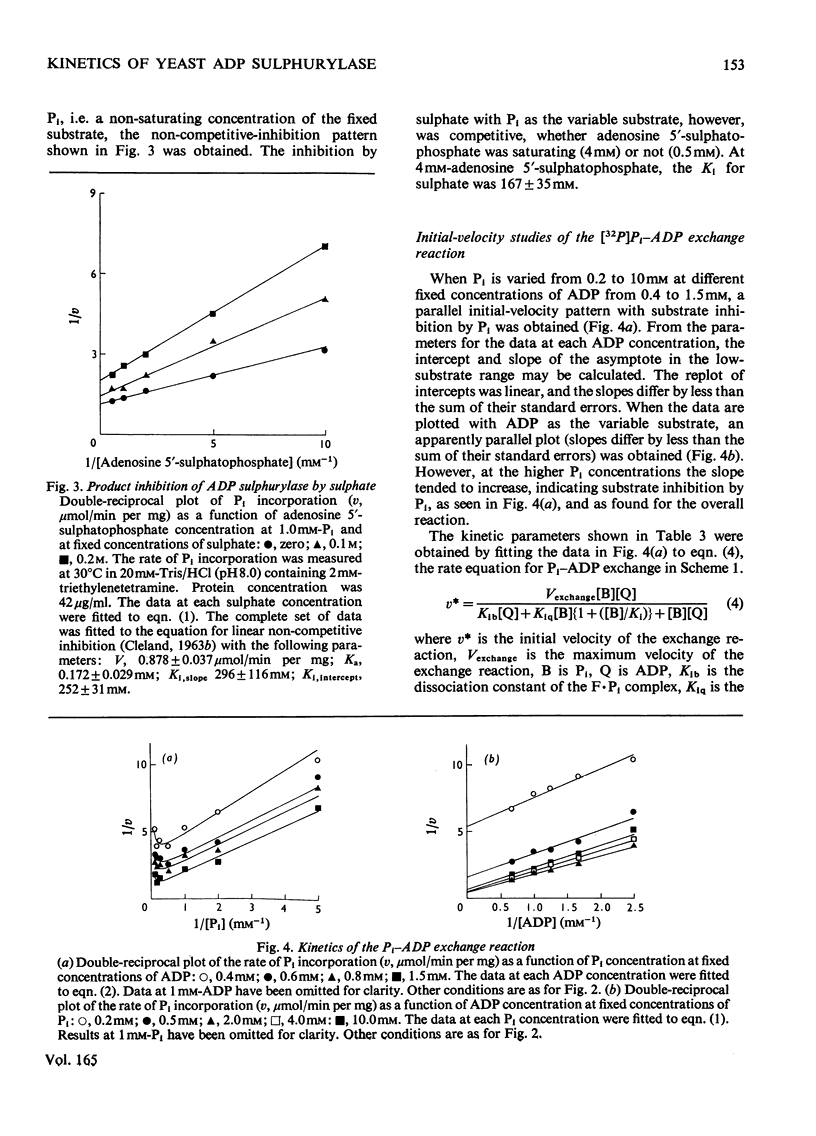
ADP sulphurylase (EC 2.7.7.5) was purified by chromatography on Sephadex G-200 and DEAE-cellulose. The enzyme was assayed by measuring the incorporation of [32P]Pi into ADP in the presence of the substrate for the reverse reaction, adenosine 5'-sulphatophosphate. In the concentration ranges investigated, by using initial-velocity, product-inhibition and isotope-exchange studies, the data were consistent with a Ping Pong reaction mechanism, with Km for adenosine 5'-sulphatophosphate of 1.20 +/- 0.08 mM and a Km for Pi of 4.95 +/- 0.15 mM. Competitive substrate inhibition by Pi (Ki = 11.7 +/- 0.3 mM) was found. ADP sulphurylase catalyses a sulphate-independent Pi-ADP exchange reaction, the kinetics of which are consistent with the kinetics of the overall reaction, inconsistent with the assay of Burnell & Anderson [(1973) Biochem. J. 133, 417-428], which is based on a sulphate-dependent Pi-ADP exchange reaction.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams C. A., Nicholas D. J. Adenosine 5'-pyrophosphate sulphurylase in baker's yeast. Biochem J. 1972 Jul;128(3):647–654. doi: 10.1042/bj1280647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams C. A., Warnes G. M., Nicholas D. J. Preparation of labeled adenosine 5'-phosphosulfate using APS reductase from Thibacillus denitrificans. Anal Biochem. 1971 Jul;42(1):207–213. doi: 10.1016/0003-2697(71)90028-5. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine diphosphate sulphurylase activity in leaf tissue. Biochem J. 1973 Jul;133(3):417–428. doi: 10.1042/bj1330417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. II. Inhibition: nomenclature and theory. Biochim Biophys Acta. 1963 Feb 12;67:173–187. doi: 10.1016/0006-3002(63)91815-8. [DOI] [PubMed] [Google Scholar]
- Grunberg-Manago M., Del Campillo-Campbell A., Dondon L., Michelson A. M. ADP-sulfurylase de levure catalysant un échange entre l'orthophosphate et le phosphate terminal des nucleosides diphosphates. Biochim Biophys Acta. 1966 Jul 20;123(1):1–16. [PubMed] [Google Scholar]
- Nicholls R. G., Jerfy A., Roy A. B. The determination of the initial velocity of enzyme-catalysed reactions. Anal Biochem. 1974 Sep;61(1):93–100. doi: 10.1016/0003-2697(74)90336-4. [DOI] [PubMed] [Google Scholar]
- OCHOA S. Enzymic synthesis of polynucleotides. III. Phosphorolysis of natural and synthetic ribopolynucleotides. Arch Biochem Biophys. 1957 Jul;69:119–129. doi: 10.1016/0003-9861(57)90479-4. [DOI] [PubMed] [Google Scholar]
- Reid M. S., Bieleski R. L. A simple apparatus for vertical flat-sheet polyacrylamide gel electrophoresis. Anal Biochem. 1968 Mar;22(3):374–381. doi: 10.1016/0003-2697(68)90278-9. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]


