Abstract
Blue light controls the development of sporangiophores in the zygomycete Phycomyces blakesleeanus Burgeff. Light represses the production of microsporangiophores and enhances the development of macrosporangiophores. Inhibition of the biosynthesis of tetrahydrobiopterin, a cofactor of NO synthase, inhibits this photomorphogenesis. Light induces production of citrulline from arginine in the mycelium and in sporangiophores. The citrulline-forming activity is dependent on NADPH, independent of calcium, and inhibited by NO synthase inhibitors. It is reduced in tetrahydrobiopterin-depleted mycelium. Light induces emission of NO from the developing fungus in the same order of magnitude as citrulline formation from arginine. The NO donor sodium nitroprusside can replace the light effect on sporangiophore development, and inhibitors of NO synthase repress it. We suggest that a fungal NO synthase is involved in sporangiophore development and propose its participation in light signaling.
Nitric oxide synthases (NOS) catalyze the formation of l-citrulline and NO from l-Arg and oxygen. Tetrahydrobiopterin (BH4) is a cofactor for certain monooxygenases and an essential factor for the function of NOS as enzymatic studies of BH4-free expressed enzymes (Gorren et al., 1996; Hurshman et al., 1999) and the crystal structure of mammalian NOS have shown (Crane et al., 1998; Raman et al., 1998; Fischmann et al., 1999). Recent experiments with antipterins have demonstrated that BH4 of NOS is not participating in a dielectronic redox cycle as in BH4-dependent monooxygenases (Bömmel et al., 1998; Riethmüller et al., 1999). Most likely a BH3 radical is formed after a one-electron transfer from BH4 to the heme ferrous-dioxygen complex, and the BH3 subsequently is reduced by the NOS flavins (Crane et al., 1998; Hurshman et al., 1999).
BH4 is present in the ascomycete Neurospora crassa Shear et Dodge at low concentrations (up to 10 pmol g−1 mycelia) and at much higher concentrations in sporangiophores and mycelia of the zygomycete Phycomyces blakesleeanus Burgeff (up to 2 nmol g−1; Maier and Ninnemann, 1995a). Because fungi lack BH4-dependent monooxygenases, the function of BH4 in these organisms is unknown. The biosynthesis of BH4 starts from GTP, which is converted by GTP-cyclohydrolase I, 6-pyruvoyl-5,6,7,8-tetrahydopterin synthase, and sepiapterin reductase to BH4 (for review, see Duch and Smith, 1991; Thöny et al., 2000). This pathway is known in animals and was also shown for bacteria (Son and Rosazza, 2000), cyanobacteria (Lee et al., 1999), Physarum polycephalum (Werner-Felmayer et al., 1994), and by us for Euglena gracilis and the fungi N. crassa and P. blakesleeanus (Maier and Ninnemann, 1995a).
Measurement of citrulline formation from 3H-labeled Arg showed an NOS-like activity present in the fungi N. crassa and P. blakesleeanus (Ninnemann and Maier, 1996). Such NOS-like activities were also found in eubacteria (Chen and Rosazza, 1995; Chen et al., 1996; Morita et al., 1997; Son and Rosazza, 2000), the slime mold Physarum polycephalum (Werner-Felmayer et al., 1994), and in several species of higher plants (Cueto et al., 1996; Ninnemann and Maier, 1996; Delledonne et al., 1998; Durner et al., 1998; Barroso et al., 1999; Ribeiro et al., 1999). NO production may also result from nitrite reductase in bacteria (Chen et al., 1996) or from nitrate reductase in plants (Rockel et al., 1996; Wildt et al., 1997; Yamasaki et al., 1999; Yamasaki and Sakihama, 2000).
No NOS gene from higher plants or fungi has been cloned and no studies showed a dependence of NO or citrulline formation on BH4. Calcium-independent NOS with biochemical features closely resembling those of mammalian-inducible NOS was purified from the slime mold P. polycephalum (Werner-Felmayer et al., 1994) and was recently cloned (Golderer et al., 2001).
In N. crassa and P. blakesleeanus, the synthesis of BH4 is inhibited by 2,4-diamino-6-hydroxypyrimidine (DAHP), an inhibitor of GTP-cyclohydrolase I, the first enzyme in folic acid and BH4 biosynthesis (Maier and Ninnemann, 1995a). In both fungi, blocking the synthesis of pteridines has a strong effect on vegetative spore development: In N. crassa the production of conidia is reduced, and in P. blakesleeanus BH4 depletion affects photomorphogenesis of sporangiophores. In P. blakesleeanus, two types of sporangiophores exist: up to 10-cm-long macrosporangiophores with sporangia containing some 100,000 spores, and 100-μm microsporangiophores with several hundred spores in each sporangium. The development of both types is differently controlled by blue light, macrosporangiophores being promoted by light and microsporangiophores photoinhibited (Corrochano and Cerdá-Olmedo, 1988; Corrochano and Cerdá-Olmedo, 1992). The development of macro- and microsporangiophores in P. blakesleeanus is controlled by different photoreceptors and different light signal transduction pathways (Corrochano et al., 1988; Flores et al., 1998). Inhibition of BH4 synthesis prevents the blue light-enhanced development of macrosporangiophores and suspends the blue light-suppressed development of microsporangiophores partly (Maier and Ninnemann, 1995b). We found an NOS-like activity in mycelia and macrosporangiophores of P. blakesleeanus, which was higher in irradiated than in dark macrosporangiophores (Ninnemann and Maier, 1996).
In this study we investigated whether NO-releasing substances will substitute for NOS activity and substitute for the effect of light; then NOS inhibitors should reduce the effects of light on sporangiophorogenesis. We also investigated whether NOS activity can be stimulated by light in vivo and in vitro and whether this light activation depends on BH4. We compared citrulline production and NO emission of developing fungi quantitatively.
RESULTS
Effect of an NO Generator and of NOS Inhibitors on Sporangiophore Photomorphogenesis
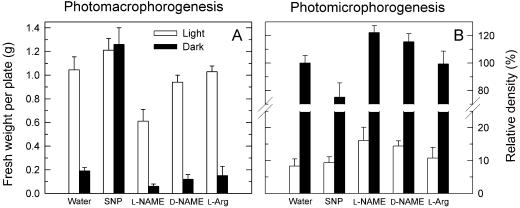
We investigated the effects of the NO-releasing substance sodium nitroprusside (SNP) and of the NOS inhibitor l-NG-nitro-Arg methylester (l-NAME) on the development of macro- and microsporangiophores (Fig. 1) to test whether the inhibition of photostimulation of macrosporangiophore development and photodepression of microsporangiophore density due to the lowered BH4 content (Maier and Ninnemann, 1995b) is caused by a deficiency of NOS. In dark controls (water), 0.2 g of macrosporangiophores was produced (Fig. 1A) and irradiation caused a 5-fold increase in its yield. With NO-releasing SNP the macrosporangiophore yield in darkness reached the same levels as in light. SNP only weakly stimulated an additional growth of macrosporangiophores on irradiated mycelia, where its yield was nearly maximal. Thus, NO has the same effect as light for induction of macrosporangiophore development. Application of the inhibitor l-NAME reduced macrosporangiophore yield to 60% on irradiated mycelia and to 40% on dark ones. The stereoisomer d-NAME lowered sporangiophore yield under both conditions, but at a reduced extent than that by l-NAME. l-Arg had no effect.
Figure 1.
Effects of the NO generator SNP and the NOS inhibitor l-NAME on sporangiophore development in P. blakesleeanus. A, Effect on photomacrophorogenesis. B, Effect on photomicrophorogenesis. Water, d-NAME, and l-Arg were used as controls. Data were calculated from three independent experiments with five parallel plates in each treatment. Error bars: ses.
The development of microsporangiophores was suppressed by blue light (Fig. 1B). In darkness the density of microsporangiophores reached several thousand per centimeter−2; on irradiated plates it was reduced by 90%. In light, SNP had no effect on microsporangiophores, but reduced them in darkness to 80% of the control levels. l-NAME enhanced microsporangiophores in the dark to 120% and doubled them on light-treated mycelia. d-NAME also enhanced them slightly, whereas l-Arg had no effect. l-NG-monomethyl-Arg (l-NMMA) and d-NMMA had no effect on photomorphogenesis (data not shown).
Effect of NOS Inhibitors and Light on Citrulline-Forming Activity
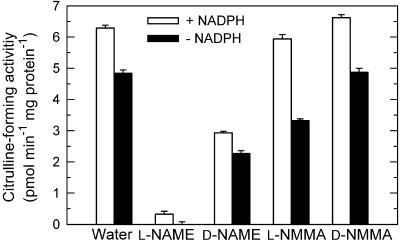
We investigated the effect of NOS inhibitors (Fig. 2) and light (Fig. 3) on the enzyme activity of NOS, to compare the results of these photobiological experiments with the corresponding NOS activities. The BH4-dependent NOS produce NO and citrulline from Arg and oxygen, which requires electron flow from NADPH via FAD, FMN, and heme to the substrates. We measured the formation of 3H-citrulline from 3H-Arg (Bredt and Snyder, 1990; Ninnemann and Maier, 1996). About 70% of citrulline-forming activity was NADPH independent (Fig. 2), presumably due to alternative electron sources in crude extracts. This is a common observation for NOS activities in unpurified enzyme extracts from various sources. In extracts from irradiated mycelium the citrulline-forming activity reached 6 pmol min−1 mg−1 protein at pH 7. Removal of Ca2+ from the assay mixture had no effect on the activity (102% ± 4% of control levels). Citrulline formation was nearly completely inhibited by 5 mm l-NAME, 50% inhibited by 5 mm d-NAME, but only slightly inhibited by 5 mm l-NMMA and not by 5 mm d-NMMA. These inhibitory effects on enzyme activity was in correlation with the inhibitory effect of l-NAME in vivo on photomorphogenesis (Fig. 1) and with little effect of l-NMMA and d-NMMA.
Figure 2.
Inhibition of citrulline-forming activity in P. blakesleeanus. Activities were measured by conversion of 3H-Arg to 3H-citrulline at pH 7.0 with and without 1 mm NADPH in crude extracts prepared from irradiated submerged mycelia. Inhibitors and control substances were 5 mm. Error bars are ses (n = 3).
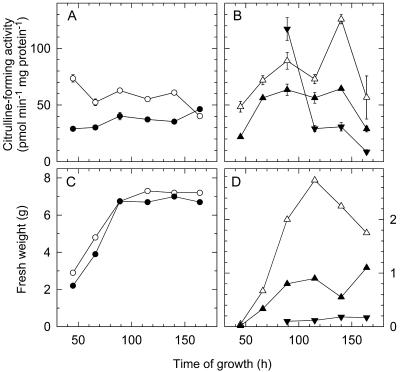
Figure 3.
Light effect on citrulline-forming activity (measured at pH 8.3) during growth of mycelia in liquid culture (A) and during development of sporangiophores (B). The time course of growth of mycelia mass in one flask (C) and sporangiophore yield of one plate (D) are shown for comparison with the respective changes in citrulline-forming activity (A and B). Mycelia in light (○) or darkness (●); macrosporangiophores in light (▵) or darkness (▴) and microsporangiophores in darkness (▾), they were too few in light for analysis. Error bars are ses (n = 3).
Light enhanced the citrulline-forming activity two to three times in mycelia grown in liquid culture (Fig. 3A). At the end of the logarithmic phase the difference disappeared. The macrosporangiophores showed an enhanced citrulline-forming activity in light (Fig. 3B). The increase in light was highest in nearly ripe and declined in older macrosporangiophores. In young microsporangiophores the activity was high in darkness and declined rapidly with age (Fig. 3B). The data show that in developmentally important growth phases (young mycelia and sporangiophores) NOS activity was higher in light than in the dark. Irradiation of extracts from dark-grown mycelia showed no effect on the citrulline-forming activity (data not shown).
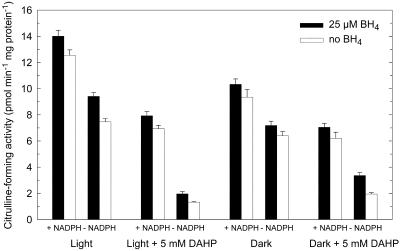
Light Stimulation of Citrulline-Forming Activity Is Dependent on BH4 in Vivo
Exogenous BH4 had only a small effect on the citrulline-forming activity in vitro (Fig. 4). Because the cofactor is firmly bound to NOS, it cannot be removed during desalting procedures. This behavior is also known from metazoan NOS, where BH4 dependence could unequivocally be shown only after expression in BH4-free Escherichia coli cells (Gorren et al., 1996). To investigate the effect of depletion of BH4 in vivo on the fungal citrulline-forming activity we grew P. blakesleeanus on a medium containing 5 mm DAHP. In irradiated mycelia, the BH4 content was reduced from 1.3 to 0.2 nmol g−1, and in dark-grown mycelia from 1.2 to 0.1 nmol g−1. We measured the citrulline-forming activity with and without BH4 in the assay mixture (Fig. 4). DAHP treatment reduced the citrulline-forming activity of mycelia grown in light and darkness. In vivo inhibition of BH4 synthesis by growing in DAHP blocked nearly all light-enhanced NOS activity.
Figure 4.
Effect of in vivo inhibition of BH4 synthesis during growth (with/without 5 mm DAHP) and/or addition of BH4 on mycelial citrulline formation. Mycelia of P. blakesleeanus were grown on DAHP (5 mm) in liquid culture in light or darkness. Activities were measured in enzyme extracts at pH 7 by conversion of 3H-Arg to 3H-citrulline with and without added NADPH to the reaction mixture. Error bars are ses (n = 3).
Determination of NO in Citrulline-Forming Assays
During the NOS reaction, NO is formed from Arg in equimolar amounts with citrulline. Because the magnitude of the citrulline-forming activity was in the range of 1 to 10 pmol min−1 mg−1, one expects hundreds of picomoles of NO from 1 mg protein within 60 min. The produced NO was rapidly oxidized to nitrite within the assay mixture, which accumulated to concentrations of several hundred nanomolar. For measurement of nitrite we reduced it to NO in a stirring solution of KI/HCl and measured the released NO in the gas phase by chemiluminescence detection following the method described by Archer et al. (1995). Addition of nitrite to the enzyme extract and buffer solution before starting the incubation had shown that the nitrite was destroyed during incubation with a rate of 2 to 10 pmol min−1 mg−1, a value comparable to the expected NO/nitrite formation. We found 0% to 5% of the expected NO formation in the enzyme assays as nitrite. Because of the variable rates of nitrite destruction it was difficult to compare samples with each other quantitatively. However, protein extracts of mycelia grown on DAHP, with BH4 levels reduced to 15% of control levels, produced residual nitrite levels below the detection limit, whereas in assays performed with extracts of mycelium grown without inhibitor a small amount of nitrite was detected.
NO Emission of Phycomyces blakesleeanus Growing on Solid Medium
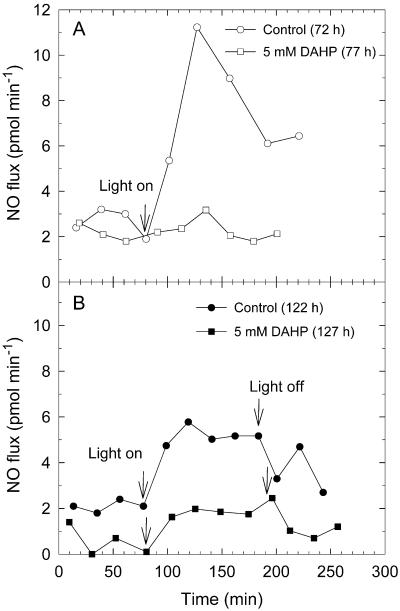
The chemiluminescence detector system is able to detect NO added to purified air down to a concentration of 10 pL L−1 in the gas phase. The citrulline-forming assay suggested that the mycelium of P. blakesleeanus produces 1 to 10 pmol NO min−1 mg−1 extractable protein during development. We measured the NO emission of P. blakesleeanus at different ages (3 d old, 72–77 h, Fig. 5A; 5 d old, 121.5–126.5 h, Fig. 5B). The NO flux was about 2 to 2.5 pmol min−1 in the dark at both ages. In 3-d-old, fungus NO emissions reached 11 pmol min−1 within 40 min after starting irradiation, about five times the dark level, and dropped again to 6 pmol min−1 during continued illumination within the next 2 h. Irradiated fungi grown on DAHP showed nearly no increase of NO emission when compared with the illuminated controls. Figure 5B shows an experiment with 5-d-old fungus. Illumination raised the NO emission by three times, whereas the DAHP-treated irradiated fungus emitted only 30% of the illuminated inhibitor-free mycelium. After ending the illumination, the NO emission reached dark levels within 1 h. Fungi, which were 3 to 5 d old and grown on solid medium, contained 2 to 5 mg extractable protein in a growth area of 100 cm2. Therefore, the NO flux can be estimated to originate from an NO-forming process with a minimum activity of 1 pmol min−1 mg−1 extractable protein in irradiated P. blakesleeanus.
Figure 5.
NO emission from controls and BH4-depleted (5 mm inhibitor DAHP) mycelia growing on two plates with 14.5-cm diameter (A) and on two plates with 14.5- and 8.5-cm diameters (B). At the start of the respective experiments the mycelia had grown for 72 h (A, control), 77 h (A, DAHP), 122 h (B, control), and 127 h (B, DAHP). Start and stop of illumination are indicated by arrows.
DISCUSSION
The data of this study suggest that a fungal NOS is involved in sporangiophore development in P. blakesleeanus. The citrulline-forming activity of protein extracts from mycelium and the NO-forming activity in developing fungus on solid medium were in the same range of 1 to 10 pmol min−1 mg−1. Citrulline formation and NO emission were enhanced by light and inhibited at the same degree, if the fungi were grown on medium containing DAHP (Figs. 4 and 5). Addition of BH4 to the citrulline-forming assay had only a small effect on the activity, presumably because the cofactor is firmly bound to the enzyme and cannot be removed by gel filtration. If grown in presence of DAHP, BH4 levels were reduced to 15% of controls. Lowered BH4 levels could cause an incorrect assembly of the NOS dimers and accumulation of inactive enzyme, which cannot be reactivated by addition of BH4 to the citrulline-forming assay (Fig. 4). Measurement of NO in the assay mixture of the citrulline-forming assay was not possible quantitatively because the NO was rapidly oxidized to nitrite, which was further destroyed in an NADPH-enhanced reaction to unknown products. Measurement of residual nitrite showed that its production was dependent on BH4 during growth of the fungus.
The NO emission was enhanced by light. This was confirmed by citrulline formation of protein extracts of mycelia and sporangiophores at different ages (Fig. 3) and by light-enhanced NO flux of developing fungi (Fig. 5). The time resolution of our NO emission measurements was 20 min. NO emission reached maximum activity after 40 min, adapted to somewhat reduced levels during continuous illumination, and dropped again to dark levels within 1 h after ending the illumination. The kinetics suggest an induction by enhancement of protein synthesis of NOS itself or of an activating factor, followed by adaptation after reaching maximum levels and down-regulation by reduced expression after ending of illumination. This behavior is typical for elements of signaling chains. Because the citrulline-forming activity was not activated by light in vitro in crude extracts, the photoactivation of NO emission presumably was established by a signal transduction chain originating in different still unknown flavoprotein photoreceptor(s) of P. blakesleeanus.
The citrulline-forming activity is not enhanced by Ca2+. The inducible NOS of animals are also Ca2+ independent and are mainly controlled by transcriptional regulation. Also, the NOS of P. polycephalum is Ca2+ independent (Werner-Felmayer et al., 1994). A receptor for NO of fungi might be a soluble guanylate cyclase as in metazoans. An involvement of cyclic nucleotides in light signaling for transient increase of sporangiophore growth was suggested (Cohen, 1974; Cohen and Atkinson, 1978), but the findings could not be repeated (Leutwiler and Brandt, 1983). No investigations on the role of cyclic nucleotides in photomorphogenesis of sporangiophores were published.
Depletion of BH4 during development by growth on DAHP inhibited light-enhanced growth of macrophores more than it released light-dependent suppression of microsporangiophore density (Maier and Ninnemann, 1995b). Also, NO donors and NOS inhibitors had a more pronounced effect on macrosporangiophores than on microphores (Fig. 1). These observations suggest that NOS affects the signaling events controlling macrophorogenesis more than those controlling microphorogenesis. The action spectra for the high and low fluence responses of photomacrophorogenesis and photomicrophorogenesis suggest different but related photoreceptors, perhaps even four different receptors (Corrochano et al., 1988). A recent study has shown that the kinase inhibitor staurosporine can replace the effect of light in photophorogenesis (Tsolakis et al., 1999), suggesting that a kinase is inhibited during light signaling in photophorogenesis.
MATERIALS AND METHODS
Strains and Media
Phycomyces blakesleeanus Burgeff wild type strain NRRL 1555(−) was grown on minimal medium (Thornton, 1973; Maier and Ninnemann, 1995b), containing 13.3 mm l-Asn and 0.5 μm thiamine. Liquid cultures were grown from 105 heat-activated spores (48°C, 15 min) in batches of 0.2 L in 2-L glass flasks with baffles for improved aeration at 20°C and 80 rpm agitation. Pteridine synthesis was inhibited by adding 5 mm sterile filtered DAHP (20-mm stock solution). Irradiation was by white fluorescence light at 100 μmol m−2 s−1. Dark controls were wrapped in light-tight aluminum foil and black clothes. Mycelia were harvested under red safety light by filtration through a nylon mesh, washed with water, and frozen in liquid nitrogen and stored at −70°C.
Photomorphogenesis Experiments
Sporangiophore development (Fig. 1) was monitored as described (Corrochano and Cerdá-Olmedo, 1988, 1992; Corrochano et al., 1988; Maier and Ninnemann, 1995b). Spores were obtained from pooled stage V macrosporangia, suspended in water, and heat activated at 48°C for 15 min. 105 spores were mixed with 3 mL minimal agar (7 g L−1 agar, held at 50°C) and layered on a 9-cm plate containing 20 mL of solidified minimal agar (15 g L−1 agar). Plates were left unpiled and unsealed in the dark at 20°C and at saturated humidity. After 48 h, 200 μL of freshly prepared, sterile filtered solutions of SNP (1 mm), NOS inhibitors (5 mm), l-Arg (5 mm), or water were plated under red safety light in a sterile working bench on the surface of the agar. The mycelia had already germinated from the spores and were grown to a dense lawn of hyphae still not elevating above the agar surface. The plates were irradiated through a milk glass window for 6 h with diffuse white light from a 200-W halogen lamp mounted 1.5 m above the plates in a reflecting metal housing. The energy fluence rate was adjusted to 4 W m−2 at the agar surface, corresponding to a blue light energy fluence rate of 1 W m−2 (measured through a no. 5562 Corning broadband blue filter, maximum at 430 nm). This energy fluence rate corresponded to a blue light (430 nm) photon fluence rate of 4 μmol m−2 s−1 and is just below the saturation level for induction of macrophorogenesis (Corrochano et al., 1988; Corrochano and Cerdá-Olmedo, 1992; Maier and Ninnemann, 1995b). The temperature inside the plates was monitored and stable at 20°C. After illumination all plates were incubated for 90 h in the dark. Macrophores were harvested with forceps, frozen in liquid nitrogen, and weighed. For microphore analysis we photographed the agar surface at 20× magnification with a videocamera and counted microphores at 10 randomly chosen areas on each plate using image processing software (Sigmascan 2.0, SPSS Science, Erkrath, Germany). Reported values are means with ses of three independent experiments, with five parallel series of irradiated and dark plates. Treatments were compared with ANOVA.
Analysis of Citrulline-Forming Activity
NOS activity (Figs. 2, 3, and 4) was measured by the conversion of 3H-Arg to 3H-citrulline (Bredt and Snyder, 1990; Hofmann and Schmidt, 1995; Ninnemann and Maier, 1996). For preparation of enzyme extracts, the frozen mycelia were powdered under liquid nitrogen, suspended in three volumes of 50 mm triethanolamine hydrochloride and 0.5 mm EDTA (pH 7.0) containing 1 μm leupeptin, 1 μm pepstatin A, 7 mm dithiotreitol, 0.2 mm phenylmethylsulfonyl fluoride, and 10% (v/v) glycerol, and disrupted with a glass homogenizer and a Teflon pestle. The homogenates were cleared by centrifugation at 4°C (30 min, 48,000g, and 60 min, 85,000g). The endogenous Arg and soluble cofactors were removed by gel filtration using Sephadex G25 columns. The triplicate incubations were started by addition of 50 μL of the extract to the reaction mixture containing 0.05 μm calmodulin (bovine brain), 226 μm CaCl2, 477 μm MgCl2, 0.5 mm EDTA, 5 μm FAD, 5 μm FMN, 25 μm BH4, 100 μm l-Arg (including 37 kBq l-[2,3,4,5-3H]Arg), 3.5 mm dithiotreitol, and 1 mm NADPH in 50 mm triethanolamine-hydrochloride buffer (pH 7.0 or pH 8.3) and 10% (v/v) glycerol. After 30 min at 37°C the reaction was stopped with 0.89 mL 0.2 m sodium acetate, 2 mm EDTA, 2 mm EGTA, and 2 mm citrulline (pH 5.5). The incubation mixture was applied to 1-mL columns of Dowex AG50 WX-8 (H+ form), and eluted with 1 mL of water. The cation-exchange resin binds all products that are positively charged at pH 5.5; the non-charged products like l-citrulline flow through. The produced l-[3H]citrulline was quantified by liquid scintillation counting. Protein concentrations were determined by the Coomassie Blue dye assay using bovine serum albumin as a standard.
Pteridine isolation and quantification were performed as described previously (Maier and Ninnemann, 1995a).
Measurement of NO Emission from Developing Mycelia
NO emitted by developing mycelia was measured in the air by chemiluminescence detection. Plates with developing mycelium at different stages were transferred to a sealed glass vessel (6.2-L volume), closed at the bottom by a Teflon plate. Purified air was passed through the vessel with a constant rate of 2 L min−1. Background concentration of NO in the inflowing air was less than 50 pL L−1. The concentration of NO in the air was measured by chemiluminescence detection (CLD AL ppt 770, ECO-Physics, Durnten, Switzerland; detection limit 20 pL L−1; 5-s time resolution). NO fluxes were calculated from the concentration differences between the inlet and outlet of the chamber (Wildt et al., 1997). Plates were placed in the chamber without lid. Because continuous NO emission of the mycelia in the dark was near the detection limit of the instrumental setting (reaching 50–100 pL L−1), the gas flow was interrupted for 5 min to accumulate the produced NO in the air volume inside the vessel. After reestablishing the gas flow, the chamber was continuously flushed for 15 min until NO concentration in the outflowing air was constant again. We summed up the NO emitted during these 15 min of flushing, which represents the NO emitted during a period of 20 min. During the 5 min of accumulation only a small amount of NO was destroyed by oxidation with oxygen. At a concentration of 500 pL L−1, NO has a very slow reaction rate with oxygen in clean air (5 × 10−7 pL L−1 s−1 with k = 1 × 10−11 m2 s−1; Fukuto, 1995). NO emission was followed continuously for 80 min in the dark. Then irradiation was started with white light by a halogen lamp at 250 μmol m−2 s−1 for 100 min. Infrared light emitted by the lamp was absorbed by a heat reflection filter (filter type IR3, Tempax naturblank, OptoChem Glass-Coating, Stromberg, Germany). The light induced NO release by photolysis from nitrite contaminations in the growth media. The release of trace amounts of NO from nitrite by light (295–410 nm) is known from experiments in seawater (Zafariou et al., 1980). We eliminated the NO release caused by photolysis using a blue light filter absorbing any light below 450 nm (Plexiglas XT 35270, yellow, 3 mm, Röhm, Darmstadt, Germany). Application of the filter to illuminated cultures immediately stopped the photolytic NO release, whereas the enzymatic NO release continued. Fifty percent of the NO release from illuminated cultures and 75% of the NO release of fungi growing on media containing DAHP was of photolytic origin. The photolytic NO release was substracted from the data.
Measurement of NO Emission from Enzyme Extracts
Initial measurements with the NO donor SNP added at different concentrations to the reaction mixture used in the citrulline-forming assay had shown that nearly all released NO is rapidly converted to nitrite. Because the low amounts of NOS activity deduced from the citrulline-forming assay (1–10 pmol min−1 mg−1) predicted only a nitrite accumulation of several 100 pmol in 1 mL-assays (up to 500 nm of nitrite), we had to use a very sensitive method for nitrite detection. We measured nitrite by a chemiluminescence assay using the experimental setting described above for NO measurement after nitrite was released from reaction mixtures by reduction to NO with potassium iodide in a stirred acidic solution (Archer et al., 1995). In this way, 20% to 40% of the produced nitrite could be released as NO from the reaction mixture and measured by the chemiluminescence detector. A plate (8.5-cm diameter) with 40 mL of 0.18 m HCl/0.5 m KI was placed into a glass vessel (0.74 L) connected to the chemiluminescence NO detector as described above. NOS assays were performed as described above for the citrulline-forming assay, but without addition of 3H-labeled Arg. After 1 h of incubation the assays were frozen immediately in liquid nitrogen and stored at −80°C until measurement. Addition of nitrite to the assay mixture before starting the incubation had shown that nitrite is stable during storage. For the measurements the assay mixtures were thawed and immediately added to the stirring KI/HCl solution inside the glass vessel through a small inlet, opened only for a few seconds. The released NO was accumulated for 45 s and then released into the chemiluminescence detector by flushing with clean air at 2 L min−1. After 5 to 15 min of continuous measurement the NO emission reached background levels and the next sample could be added. Up to nine samples could be measured with one batch of KI/HCl without a decrease of the chemiluminescence signal. After renewal of the KI/HCl solution, the solution had to stir for 5 min to reach background levels, and a further nine samples could be measured. The detection limit was 50 pmol nitrite. The chemiluminescence signal was linear with nitrite amounts higher than 100 pmol.
ACKNOWLEDGMENTS
We thank Ana Sierra for technical assistance with photobiological assays and Stefan Picker for help with pteridine and NOS analysis.
LITERATURE CITED
- Archer SL, Shultz PJ, Warren JB, Hampl V, Demaster EG. Preparation of standards and measurement of nitric oxide, nitroxyl, and related oxidation products. Methods. 1995;7:21–34. [Google Scholar]
- Barroso JB, Corpas FJ, Carreras A, Sandalio LM, Valderrama R, Palma JM, Lupianez JA, del Rio LA. Localization of nitric-oxide synthase in plant peroxisomes. J Biol Chem. 1999;274:36729–36733. doi: 10.1074/jbc.274.51.36729. [DOI] [PubMed] [Google Scholar]
- Bömmel HM, Reif A, Fröhlich LG, Frey A, Hofmann H, Marecak DM, Groehn V, Kotsonis P, La ML, Köster S. Anti-pterins as tools to characterize the function of tetrahydrobiopterin in NO synthase. J Biol Chem. 1998;273:33142–33149. doi: 10.1074/jbc.273.50.33142. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J-Y, Chang W-C, Chang T, Chang W-C, Liu M-Y, Payne WJ, LeGall J. Cloning, characterization, and expression of the nitric oxide-generating nitrite reductase and of the blue copper protein genes of Achromobacter cycloclastes. Biochem Biophys Res Commun. 1996;219:423–428. doi: 10.1006/bbrc.1996.0249. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Rosazza JPN. A bacterial nitric oxide synthase from a Nocardia species. Biochem Biophys Res Commun. 1995;203:1251–1258. doi: 10.1006/bbrc.1994.2317. [DOI] [PubMed] [Google Scholar]
- Cohen RJ. Cyclic AMP levels in Phycomyces during a response to light. Nature. 1974;251:144–146. doi: 10.1038/251144a0. [DOI] [PubMed] [Google Scholar]
- Cohen RJ, Atkinson MM. Activation of Phycomyces adenosine 3′,5′-monophosphate phosphodiesterase by blue light. Biochem Biophys Res Commun. 1978;83:616–621. doi: 10.1016/0006-291x(78)91034-3. [DOI] [PubMed] [Google Scholar]
- Corrochano LM, Cerdá-Olmedo E. Photomorphogenesis in Phycomyces: dependence on environmental conditions. Planta. 1988;174:309–314. doi: 10.1007/BF00959515. [DOI] [PubMed] [Google Scholar]
- Corrochano LM, Cerdá-Olmedo E. Sex, light and carotenes: the development of Phycomyces. Trends Genet. 1992;8:268–274. doi: 10.1016/0168-9525(92)90252-y. [DOI] [PubMed] [Google Scholar]
- Corrochano LM, Galland P, Lipson ED, Cerdá-Olmedo E. Photomorphogenesis in Phycomyces: fluence-response curves and action spectra. Planta. 1988;174:315–320. doi: 10.1007/BF00959516. [DOI] [PubMed] [Google Scholar]
- Crane BR, Arvai AS, Ghosh DK, Wu CQ, Getzoff ED, Stuehr DJ, Tainer JA. Structure of nitric oxide synthase oxygenase dimer with pterin and substrate. Science. 1998;279:2121–2126. doi: 10.1126/science.279.5359.2121. [DOI] [PubMed] [Google Scholar]
- Cueto M, Hernandez-Perera O, Martin R, Bentura ML, Rodrigo J, Lamas S, Golvano MP. Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett. 1996;398:159–164. doi: 10.1016/s0014-5793(96)01232-x. [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia YJ, Dixon RA, Lamb C. Nitric oxide functions as a signal in plant disease resistance. Nature. 1998;394:585–588. doi: 10.1038/29087. [DOI] [PubMed] [Google Scholar]
- Duch DS, Smith GK. Biosynthesis and function of tetrahydrobiopterin. J Nutr Biochem. 1991;2:411–423. [Google Scholar]
- Durner J, Wendehenne D, Klessig DF. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA. 1998;95:10328–10333. doi: 10.1073/pnas.95.17.10328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischmann TO, Hruza A, Niu XD, Fossetta JD, Lunn CA, Dolphin E, Prongay AJ, Reichert P, Lundell DJ, Narula SK. Structural characterization of nitric oxide synthase isoforms reveals striking active-site conservation. Nat Struct Biol. 1999;6:233–242. doi: 10.1038/6675. [DOI] [PubMed] [Google Scholar]
- Flores R, Cerdá-Olmedo E, Corrochano LM. Separate sensory pathways for photomorphogenesis in Phycomyces. Photochem Photobiol. 1998;67:467–472. [Google Scholar]
- Fukuto JM. Chemistry of nitric oxide: biologically relevant aspects. In: Ignarro L, Murad F, editors. Nitric Oxide: Biochemistry, Molecular Biology and Therapeutic Implications. San Diego: Academic Press; 1995. pp. 1–15. [DOI] [PubMed] [Google Scholar]
- Golderer G, Werner ER, Leitner S, Gröbner P, Werner-Felmayer G (2001) Nitric oxide synthase is induced in sporulation of Physarum polycephalum. Genes Dev (in press) [DOI] [PMC free article] [PubMed]
- Gorren ACF, List BM, Schrammel A, Pitters E, Hemmens B, Werner ER, Schmidt K, Mayer B. Tetrahydrobiopterin-free neuronal nitric oxide synthase: evidence for two identical highly anticooperative pteridine binding sites. Biochemistry. 1996;35:16735–16745. doi: 10.1021/bi961931j. [DOI] [PubMed] [Google Scholar]
- Hofmann H, Schmidt HHHW. Thiol dependence of nitric oxide synthase. Biochemistry. 1995;34:13443–13452. doi: 10.1021/bi00041a023. [DOI] [PubMed] [Google Scholar]
- Hurshman AR, Krebs C, Edmondson DE, Huynh BH, Marletta MA. Formation of a pterin radical in the reaction of the heme domain of inducible nitric oxide synthase with oxygen. Biochemistry. 1999;38:15689–15696. doi: 10.1021/bi992026c. [DOI] [PubMed] [Google Scholar]
- Lee SW, Lee HW, Chung HJ, Kim YA, Kim YJ, Hahn Y, Chung JH, Park YS. Identification of the genes encoding enzymes involved in the early biosynthetic pathway of pteridines in Synechocystis sp. PCC 6803. FEMS Microbiol Lett. 1999;176:169–176. doi: 10.1111/j.1574-6968.1999.tb13658.x. [DOI] [PubMed] [Google Scholar]
- Leutwiler LS, Brandt M. Absence of significant light-induced changes in cAMP levels in sporangiophores of Phycomyces blakesleeanus. J Bacteriol. 1983;153:555–557. doi: 10.1128/jb.153.1.555-557.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier J, Ninnemann H. Biosynthesis of pteridines in Neurospora crassa, Phycomyces blakesleeanus and Euglena gracilis: detection and characterization of biosynthetic enzymes. Photochem Photobiol. 1995a;61:43–53. doi: 10.1111/j.1751-1097.1995.tb09241.x. [DOI] [PubMed] [Google Scholar]
- Maier J, Ninnemann H. Inhibition of light-dependent photomorphogenesis of sporangiophores from Phycomyces blakesleeanus by application of pteridine biosynthesis inhibitors. Photochem Photobiol. 1995b;61:206–209. doi: 10.1111/j.1751-1097.1995.tb09241.x. [DOI] [PubMed] [Google Scholar]
- Morita H, Yoshikawa H, Sakata R, Nagata Y, Tanaka H. Synthesis of nitric oxide from the two equivalent guanidino nitrogens of L-arginine by Lactobacillus fermentum. J Bacteriol. 1997;179:7812–7815. doi: 10.1128/jb.179.24.7812-7815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninnemann H, Maier J. Indications for the occurrence of nitric oxide synthases in fungi and plants and the involvement in photoconidiation of Neurospora crassa. Photochem Photobiol. 1996;61:393–398. doi: 10.1111/j.1751-1097.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- Raman CS, Li HY, Martasek P, Kral V, Masters BSS, Poulos TL. Crystal structure of constitutive endothelial nitric oxide synthase: a paradigm for pterin function involving a novel metal center. Cell. 1998;95:939–950. doi: 10.1016/s0092-8674(00)81718-3. [DOI] [PubMed] [Google Scholar]
- Ribeiro EA, Cunha FQ, Tamashiro WMSC, Martins IS. Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett. 1999;445:283–286. doi: 10.1016/s0014-5793(99)00138-6. [DOI] [PubMed] [Google Scholar]
- Riethmüller C, Gorren ACF, Pitters E, Hemmens B, Habisch HJ, Heales SJR, Schmidt K, Werner ER, Mayer B. Activation of neuronal nitric-oxide synthase by the 5-methyl analog of tetrahydrobiopterin: functional evidence against reductive oxygen activation by the pterin cofactor. J Biol Chem. 1999;274:16047–16051. doi: 10.1074/jbc.274.23.16047. [DOI] [PubMed] [Google Scholar]
- Rockel P, Rockel A, Wildt J, Segschneider H-J. Nitric oxide (NO) emission by higher plants. In: Van Cleemput O, Hofmann G, Vermoesen A, editors. Progress in Nitrogen Cycling Studies. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1996. pp. 603–606. [Google Scholar]
- Son JK, Rosazza JPN. Cyclic guanosine-3′,5′-monophosphate and biopteridine biosynthesis in Nocardia sp. J Bacteriol. 2000;182:3644–3648. doi: 10.1128/jb.182.13.3644-3648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thöny B, Auerbach G, Blau N. Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J. 2000;347:1–16. [PMC free article] [PubMed] [Google Scholar]
- Thornton RM. New photoresponses of Phycomyces. Plant Physiol. 1973;51:570–576. doi: 10.1104/pp.51.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolakis G, Parashi E, Galland P, Kotzabasis K. Blue light signaling chains in Phycomyces: phototransduction of carotenogenesis and morphogenesis involves distinct protein kinase/phosphatase elements. Fungal Genet Biol. 1999;28:201–213. doi: 10.1006/fgbi.1999.1175. [DOI] [PubMed] [Google Scholar]
- Werner-Felmayer G, Golderer G, Werner ER, Gröbner P, Wachter H. Pteridine biosynthesis and nitric oxide synthase in Physarum polycephalum. Biochem J. 1994;304:105–111. doi: 10.1042/bj3040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildt J, Kley D, Rockel A, Rockel P, Segschneider H-J. Emission of NO from several higher plant species. J Geophys Res D. 1997;102:5919–5927. [Google Scholar]
- Yamasaki H, Sakihama Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000;468:89–92. doi: 10.1016/s0014-5793(00)01203-5. [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y, Takahashi S. An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci. 1999;4:128–129. doi: 10.1016/s1360-1385(99)01393-x. [DOI] [PubMed] [Google Scholar]
- Zafariou O, McFarland M, Bromund R. Nitric oxide in seawater. Science. 1980;207:637–639. doi: 10.1126/science.207.4431.637. [DOI] [PubMed] [Google Scholar]