Abstract
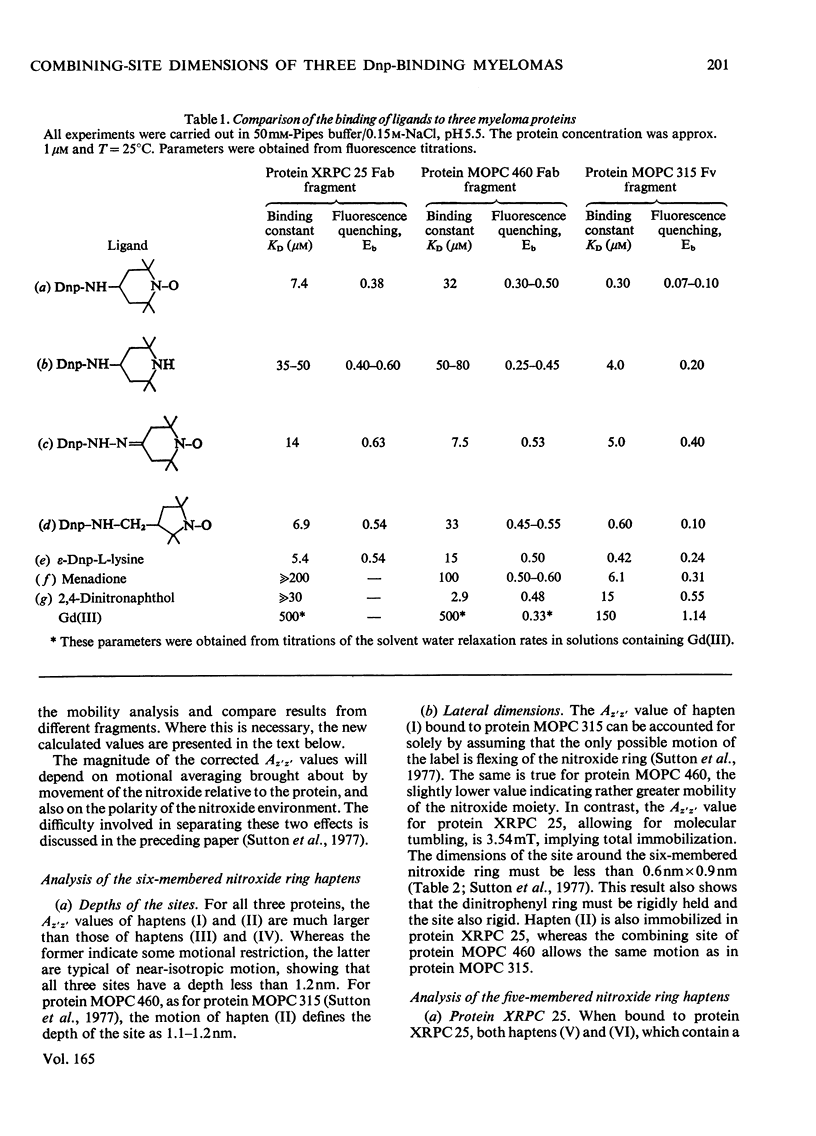
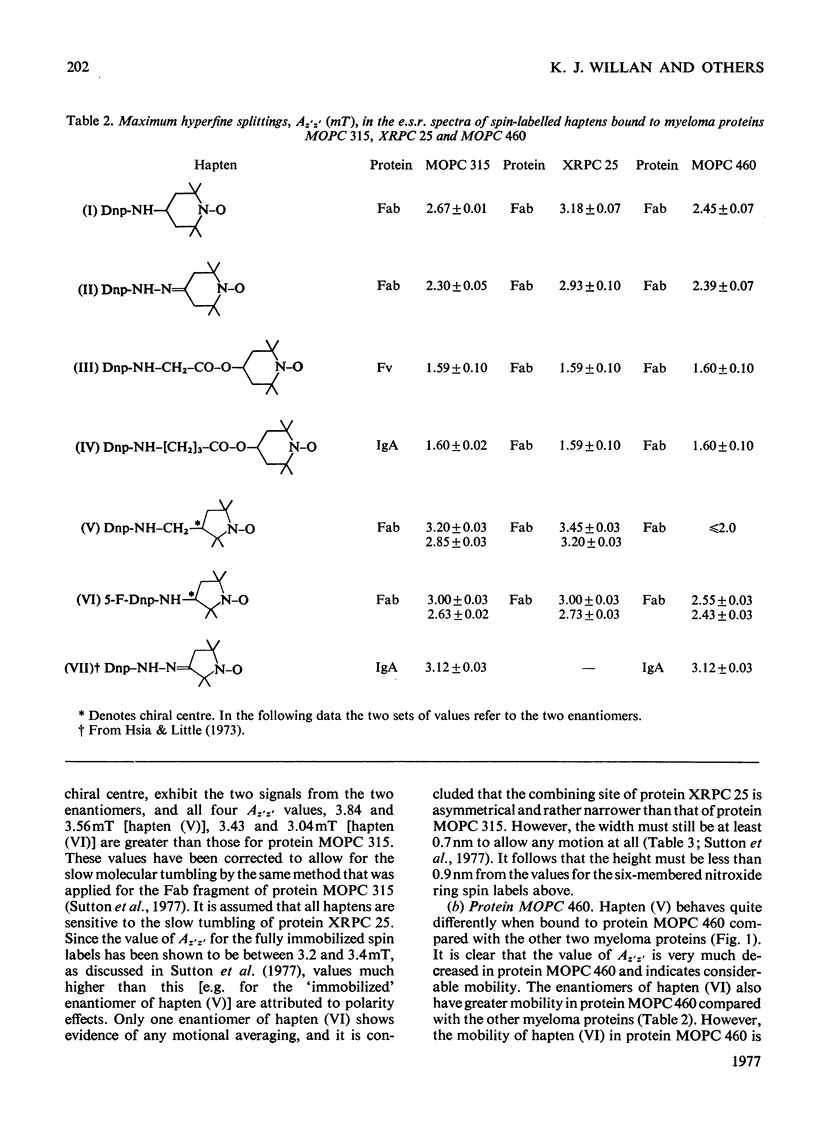
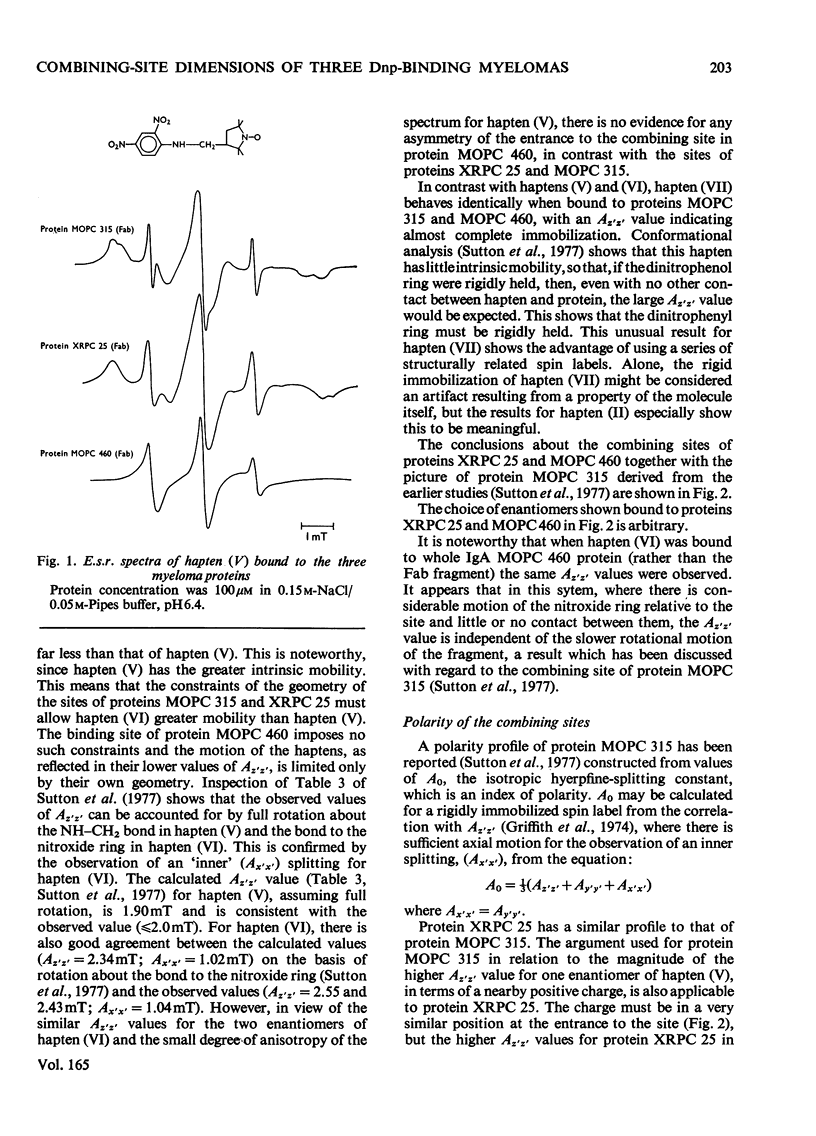
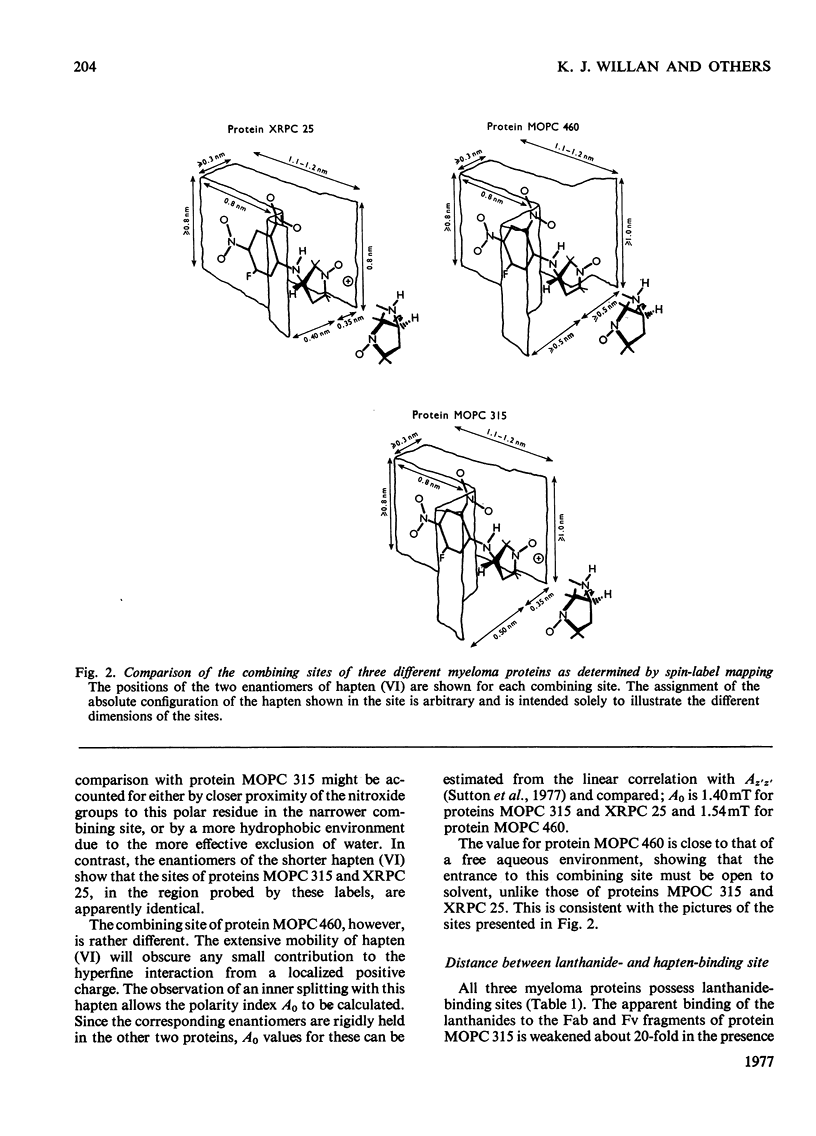
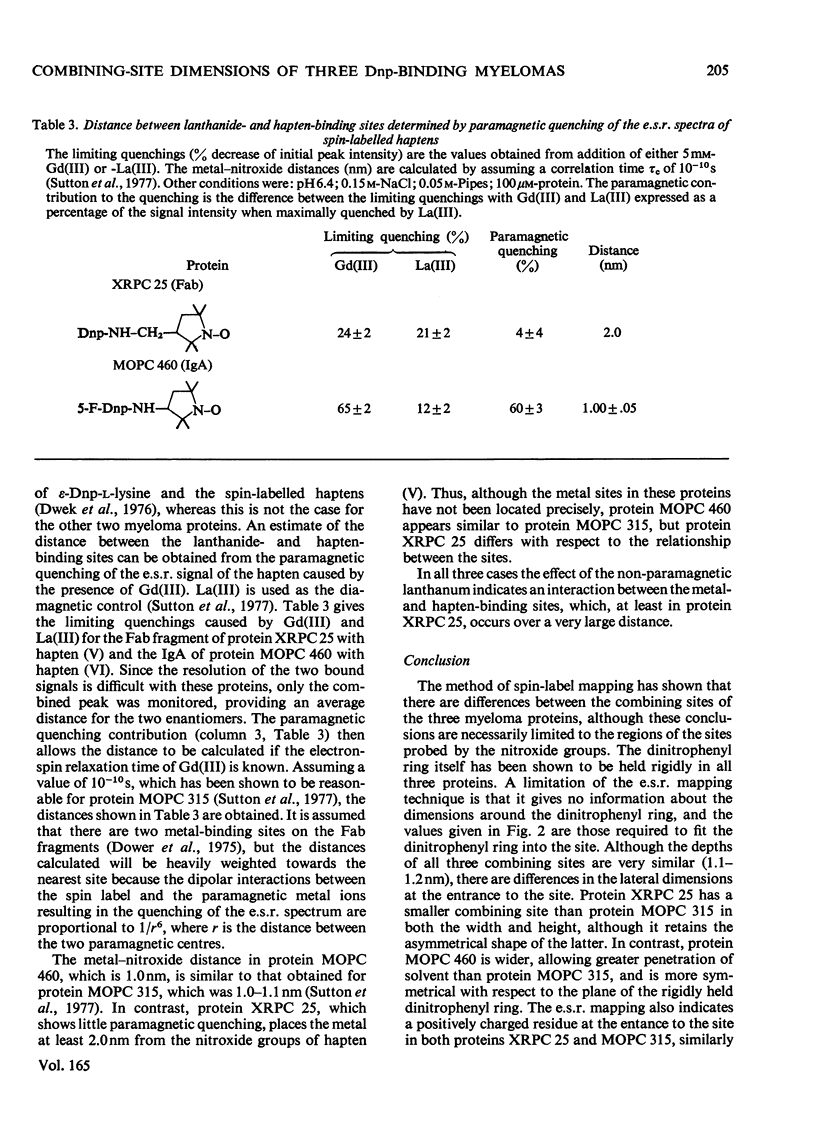
The mouse immunoglobulin A myeloma proteins MOPC 315, MOPC 460 and XRPC 25 all possess dinitrophenyl (Dnp)-binding activity. Differences in specificities were shown by measuring the affinities of a variety of haptens. By using a series of Dnp-spin-labelled haptens, the dimensions of the binding sites of the three myeloma proteins were compared by the method described for protein MOPC 315 [Sutton, Gettins, Givol, Marsh, Wain-Hobson, Willan & Dwek (1977) Biochem. J. 165, 177–197]. The dinitrophenyl ring is rigidly held in all three sites. The depths of the sites are all 1.1–1.2nm, but there are differences in the lateral dimensions at the entrance to the sites. For protein XRPC 25 these dimensions are 0.75nm×0.8nm, which may be compared with 0.85nm×1.1nm for protein MOPC 315 and ≥1.0nm×1.1nm for protein MOPC 460. The site in protein MOPC 460 is more symmetrical with respect to the plane of the dinitrophenyl ring than in either of the other two myeloma proteins and also allows greater penetration of solvent. In protein XRPC 25 a positively charged residue was located at the entrance to the site, similarly positioned to that reported for protein MOPC 315 [Sutton, Gettins, Givol, Marsh, Wain-Hobson, Willan & Dwek (1977) Biochem.J. 165, 177–197]. All three proteins possess lanthanide-binding sites, but only in protein MOPC 315 is there antagonism between lanthanide and hapten binding. However, the effects of the diamagnetic La(III) on the electron-spin-resonance spectra of bound Dnp spin labels in both proteins MOPC 460 and XRPC 25 suggest an interaction between the two sites. Comparison of this effect with that caused by the addition of the paramagnetic Gd(III) enables the distance between the lanthanide- and hapten-binding sites to be calculated. In both proteins MOPC 460 and MOPC 315 the metal site is approx. 1.0nm from the nitroxide moiety of the spin-labelled hapten, but in protein XRPC 25 this distance is at least 2.0nm.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dower S. K., Dwek R. A., McLaughlin A. C., Mole L. E., Press E. M., Sunderland C. A. The binding of lanthanides to non-immune rabbit immunoglobulin G and its fragments. Biochem J. 1975 Jul;149(1):73–82. doi: 10.1042/bj1490073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower S. K., Wain-Hobson S., Gettins P., Givol D., Jackson W. R., Perkins S. J., Sunderland C. A., Sutton B. J., Wright C. E., Dwek R. A. The combining site of the dinitrophenyl-binding immunoglobulin A myeloma protein MOPC 315. Biochem J. 1977 Aug 1;165(2):207–223. doi: 10.1042/bj1650207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R. A., Givol D., Jones R., McLaughlin A. C., Wain-Hobson S., White A. I., Wright C. Interactions of the lanthanide- and hapten-binding sites in the Fv fragment from the myeloma protein MOPC 315. Biochem J. 1976 Apr 1;155(1):37–53. doi: 10.1042/bj1550037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwek R. A. Structural studies in solution on the combining site of the myeloma protein MOPC 315. Contemp Top Mol Immunol. 1977;6:1–52. doi: 10.1007/978-1-4684-2841-4_1. [DOI] [PubMed] [Google Scholar]
- Eisen H. N., Simms E. S., Potter M. Mouse myeloma proteins with antihapten antibody acitivity. The protein produced by plasma cell tumor MOPC-315. Biochemistry. 1968 Nov;7(11):4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- Griffith O. H., Dehlinger P. J., Van S. P. Shape of the hydrophobic barrier of phospholipid bilayers (evidence for water penetration in biological membranes). J Membr Biol. 1974;15(2):159–192. doi: 10.1007/BF01870086. [DOI] [PubMed] [Google Scholar]
- Hsia J. C., Little J. R. Structural properties of the ligand binding sites of murine myeloma proteins. FEBS Lett. 1973 Apr 1;31(1):80–84. doi: 10.1016/0014-5793(73)80077-8. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Simms E. S., Eisen H. N. Specificity and structure of the myeloma protein produced by mouse plasmacytoma MOPC-460. Biochemistry. 1971 Apr 27;10(9):1693–1699. doi: 10.1021/bi00785a029. [DOI] [PubMed] [Google Scholar]
- Lancet D., Pecht I. Kinetic evidence for hapten-induced conformational transition in immunoglobin MOPC 460. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3549–3553. doi: 10.1073/pnas.73.10.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards F. F., Konigsberg W. H., Rosenstein R. W., Varga J. M. On the specificity of antibodies. Science. 1975 Jan 17;187(4172):130–137. doi: 10.1126/science.46122. [DOI] [PubMed] [Google Scholar]
- Sharon J., Givol D. Preparation of Fv fragment from the mouse myeloma XRPC-25 immunoglobulin possessing anti-dinitrophenyl activity. Biochemistry. 1976 Apr 6;15(7):1591–1594. doi: 10.1021/bi00652a033. [DOI] [PubMed] [Google Scholar]
- Sutton B. J., Gettins P., Givol D., Marsh D., Wain-Hobson S., Willan K. J., Dwek R. A. The gross architecture of an antibody-combining site as determined by spin-label mapping. Biochem J. 1977 Aug 1;165(2):177–197. doi: 10.1042/bj1650177. [DOI] [PMC free article] [PubMed] [Google Scholar]


