Abstract
Microplastics (MPs), less than 5 mm in length, have become a major environmental issue due to their hazardous physical and chemical properties. The research investigated 54 sediment samples collected from three different zones of the beaches, namely the wrack line, beach face, and swash zone. This study aims to enumerate the number and polymeric variety of microplastics found in beach sediments from coastal islands of Bangladesh, including Sandwip, Kutubdia, and Saint Martin's Island in the northeastern Bay of Bengal. NaCl solution with the density of 1.2 g/cm3 was used as a density-separating solvent. Microplastics were extracted using conventional protocols, yielding an average of 193 ± 68.9, 175.5 ± 63.1, and 266.3 ± 232 particles per kg from the collected samples of Sandwip, Kutubdia, and Saint Martin's Island respectively, with five morphotypes: fiber, film, fragment, foam, and pellet, where fiber dominated each island. White microplastics were most spread in both Sandwip and Saint Martin's Island, whereas translucent and blue were most abundant in Kutubdia. Moreover, polypropylene (PP) was shown to be the greatest number of polymer groups among those analyzed microplastic particles using ATR-FTIR (Attenuated total reflectance-Fourier transform infrared) spectrometer. Using scanning electron microscopy (SEM), it was also possible to detect surface degradation, rupture, or fracture that was probably caused by the environment. The study emphasizes the critical need for continued research and monitoring to better understand the dynamics of microplastic pollution and its long-term impacts. By tackling the underlying causes and implementing effective management practices, we can achieve a cleaner and more sustainable future for coastal communities and marine ecosystems.
Keywords: Microplastics, Coastal island, Shoreline, Anthropogenic factors, Bay of Bengal
1. Introduction
Microplastics have become an escalating issue in contemporary times due to their pervasive presence in environments closely connected to human activities, posing risks to ecosystems, wildlife, and human health. Their widespread occurrence, persistence, and potential toxicity make them a serious threat, necessitating increased attention and action to mitigate their impact on natural systems and public health [1]. They can be introduced in the environment either as water-insoluble readymade micro-pellets, microbeads, microfibers, and other forms those are intentionally produced by mankind, known as primary sources [2,3] and the tiny breakage part of large plastic matters produced in the environment known as secondary sources [4,5]. Daily commodities, cosmetic accessories, or industrial sludges contribute huge amount of primary microplastics [6] while the majority of microplastics originate from secondary sources [7]. In fact, all kinds of sizable plastics end up into microplastics through physical, chemical, or biological degradation process in the surrounding [7,8]. Apart from being on terrain, plastics enter the ocean environment by three ways: wind, soil, and river pathways [[9], [10], [11]] and a large amount of microplastics have already been discovered there [[12], [13], [14]].
Once in the ocean, microplastics can remain suspended or floated in the water column [9,15] or translocated both horizontally and vertically by numerous forces like currents, waves, winds, or other else [16,17]. Eventually they settle down in either an ocean basin or sediments, which is a significant sink of microplastics, although they can again be exposed back to the water or deepen in the sediment layer if sediment gets stirred [18,19]. Benthic organisms can act as the carriers of microplastics to the next level of the food chain [[20], [21], [22]].
Organisms being confused with food particles consume microplastics, while minute microplastics can enter the tissues, bloodstream, or other internal organs of aquas organisms directly and thus enter into the food web [23]. Such small microplastics' physical components and their poisonous compounds can cause health risks for the organisms, such as retardation in growth, infertility, blockage of body tracts, and so on [[24], [25], [26], [27]]. Micro/nanoplastics negatively impacted seed germination, particularly at high concentrations, and may have also caused the chlorophyll content of Arabidopsis thaliana to drop [28]. In addition, microplastics inhibit the growth of Chlorella sp., a primary producer, which has an impact on the food chain and oxygen generation [29]. Numerous dangerous organic substances, including diazinon [30], as well as bacteria, viruses, antibiotics, and other inorganic particles, such as heavy metals [31], tetracycline [32] and dyes [33] are absorbed by microplastics. Concerning levels of the aforementioned contaminants have been found in the Bay of Bengal [[34], [35], [36]]. Research has also revealed that microplastics' capacity to attract and release organic and inorganic pollutants can be enhanced by higher temperatures [33].
Ultimately, microplastics can be exposed to the top most predator of the food web, humans, and cause numerous diseases like cardiovascular disease, cancer, obesity, oxidative stress, neurotoxic, respiratory disease, metabolic effects, reproductive and developmental disorder, etc. [28,37]. The global presence of microplastics in both aquatic and land ecosystems makes them ubiquitous contaminants and silent threats to the ecosystem [38]. However, amidst this dire situation, the message of hope is that studies have demonstrated that Aspergillus sp. [39]. can break down a variety of plastic polymers, while Spirulina platensis [30] and Saccharomyces cerevisiae [39] may be used to remove specifically polystyrene (PS) from the environment. Another inexpensive option can be oak powder containing tannin components; it is excellent at removing PS microplastics through charge neutralization and surface adsorption methods [40]. Significant amounts of microplastics have been found around the Indian Ocean coast, including the shores of Bangladesh [[41], [42], [43], [44]], India [[45], [46], [47], [48], [49], [50], [51]], Myanmar [52], Thailand, and a few other islands [47,[53], [54], [55]]. The Bay of Bengal, a marginal bay of the Indian Ocean, contains a large number of offshore islands, but only one, Saint Martin's Island, had undergone repeated studies [42,44] for microplastics just because of being an important tourist destination. Nonetheless, other non-touristic islands, i.e., Sandwip and Kutubdia, should also receive attention because of their substantial populations who are directly or indirectly connected to the beach and thus involved in the marine ecosystem. It is necessary to determine the quantity of microplastics before removing them from the Bay of Bengal's aquas environment, which is why the current study was required, and this is the first of its type to provide light on the amount, polymeric diversity, and distribution pattern of microplastics on the beaches of the studied coastal islands. Furthermore, disposable face masks facilitate microplastics to escape into the environment [56], which highlights the need for more research on the quantity of microplastics in the region under study—as it was carried out shortly after the COVID-19 epidemic when an excessive amount of disposable face masks were released into the environment. This study aims to analyze the types, polymers, and shapes of microplastics and assess their color distribution across the shorelines of the three coastal islands. Additionally, surface degradation of MPs will be assessed by using a scanning electron microscope (SEM) in order to better understand the indication of environmental wear and breakdown. This study may be vital to understand the uprising trend and extends of microplastics for legislators, environmental conservators, and policymakers and to take the necessary steps to mitigate the environmental, ecological, and biological diversity in and around the study area.
2. Materials and methods
2.1. Study area
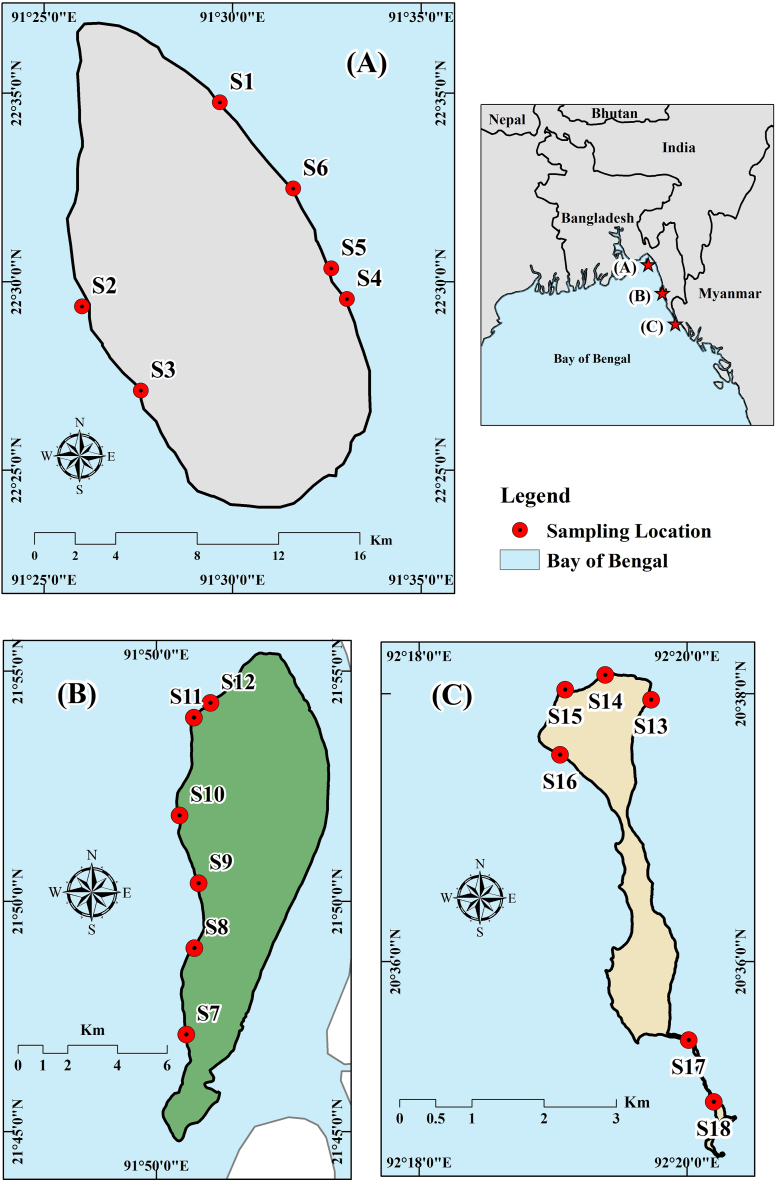
Studied three islands are located in the north-eastern Bay of Bengal, Bangladesh. One of the three studied islands is Sandwip (Fig. 1A), situated between latitudes 22°22′ and 22°34′ N and longitudes 91°26′ and 91°34′ E. Geographically, the island is bordered to the north by the Bamuni River, to the west by the Meghna River and Hatia Island, to the east by the Sandwip Channel, and to the south by the Bay of Bengal [57]. Another island is Kutubdia (Fig. 1B), which lies between latitude 21° 44′23″ to 21° 55′24″ N and longitude 91°50′40″ to 91°54′04″ E, situated in the south-eastern part of Bangladesh and having an area of 215.8 km2 and separated from the main land by the Kutubdia channel. This terrain has an elevation that ranges from 0 to 25 feet (7.6 m) above the water level. Lastly, only coral bearing and one of Bangladesh's tiniest but most beautiful islands, Saint Martin's Island (Fig. 1C), is situated in the country's most southeasterly region [[58], [59]]. In the Bay of Bengal, this island, with a dumbbell shape, is situated around 9 km south of the Cox's Bazar-Teknaf Peninsula [60].
Fig. 1.
Study area and sediment collection points – (A) Sandwip; (B) Kutubdia; and (C) Saint Martin's Island.
2.2. Samples collection
For sampling, eighteen stations were selected, and three surface (up to 2 cm depth) sediment samples were collected from each station during low tide across the beaches in order to incorporate the wrack line, beach face, and swash zone (total point = 54) between March 2021 and February 2022. Fig. 1 provides a brief overview of the sample locations. Surface sediments were dug by spoon and kept in the zipper polythene bags, sealed and leveled with a permanent marker. Distilled water was used to wash the spoon after sampling at every point. After sampling, the samples were taken to the Fibre & Polymer Research Division of the Bangladesh Council of Scientific and Industrial Research (BCSIR) to analyze. The exact locations of sampling points were taken by the GPS tracker, which was calibrated to be accurate. For avoiding contamination, during the whole process some control measures were taken, as mentioned in Masura et al. (2015) [61].
2.3. Sample pretreatment
The samples were prepared for sieving through a 5 mm sieve to discard the substances greater than 5 mm. Samples were dried in an oven at 90 °C for 1 day to dry the moisture completely. After drying, the sediments were crushed for homogenization and prepared for the sieving. For preparing a solvent (brine solution), 500 mL distill water was taken in a glass beaker with NaCl (as much as 40 % of this water) and stirred for 15–20 min to mix them properly. The mixture was kept at rest for 30 min and measured by density meter (Model: DMA 5000M) at 25 °C temperature, and the density of the mixture was found to be 1.2 gcm−3. By density separation, microplastics were floating on the surface of the mixture, which were less dense than 1.2 gcm−3. 100 gm of dried homogenized sediments were taken to be mixed with the readied saturated brine solution and sonicated for 5 min at 30 °C. After sonication, the mixture was kept at rest for 12 h so that microplastics could float at the surface of the mixture for density variation. All the floating microplastics were transferred to the filter paper. For serving the purpose, density separated upper clear supernatant was decanted and digested by 20 mL of hydrogen peroxide (30 % H2O2) to remove the organic debris. The solution was kept on a magnetic stirrer at 30 °C and 300 rpm rotation for 6 h to mix it well. The hydrogen peroxide solution was diluted with plenty of distill water and filtered through 0.45 μm cellulose nitrate filter paper with the help of a suction pump.
2.4. Enumeration and observation of the external surface of microplastics
The number of the microplastics was counted consciously and observed their morphology at 4 magnification with the help of a microscope (Leica EZ4) and pictures were taken. A scanning electronic microscope (SEM) was used to examine the material surface phenomena and visualize them [62]. The selected plastic particles were scanned in a SEM with a high-energy electron, and the resulting electrons/X-rays were analyzed. These emitted electrons/X-rays provide information on a material's topography, morphology, composition, grain orientation, crystallographic information, and so on.
2.5. Polymeric type identification
ATR-FTIR (Attenuated total reflectance Fourier transform infrared) is a powerful analytical technique that determines a compound's absorbance spectrum. By using this method, plastic particles were identified by the type of polymer that they were made from.
2.6. Quality control
As the sediment samples were kept in polyethylene bags, proper concern was there to carry them to the lab as soon as possible. To avoid microplastic contamination from the outside, all the liquids were filtered through a 0.45 μm sieve. To avoid external contamination, each solution was wrapped in aluminum foil [63]. Throughout the treatment, all kinds of measures were taken, such as not wearing polyester clothing and constantly wearing hand gloves along with a lab apron. Along with all precautions, blank tests were also performed for every batch of samples and considered the count of microplastics accordingly to validate the result.
2.7. Statistical analysis
The abundance of microplastics was provided as a mean value with a standard deviation. The Shapiro-Wilk test was used to determine the distribution pattern. Python and ArcGIS 10.5 were used to create visual data representations and maps, respectively.
3. Results
3.1. Microplastics abundance along the shore
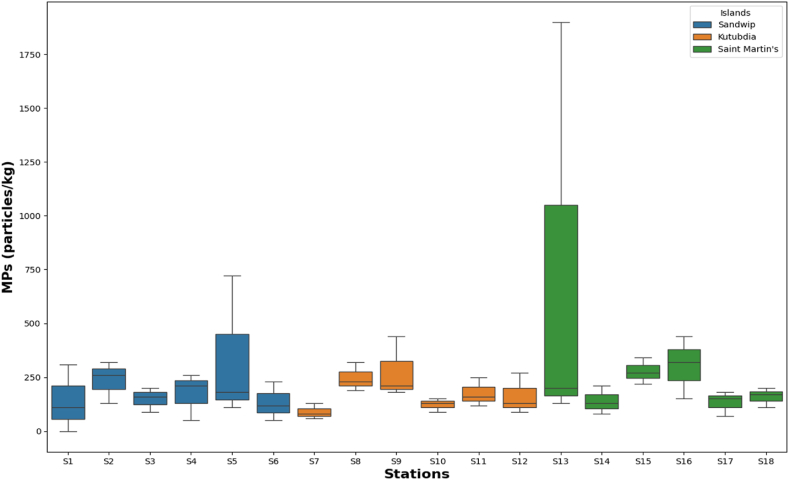
The highest and lowest abundance among eighteen stations were observed in S13 at Saint Martin's Island Jettyghat and S7 at Kutubdia Island, respectively. Particularly in Sandwip, Kutubdia, and Saint Martin's Island, the highest concentrations of microplastics (particles/kg) were 720, 440, and 1900, respectively (Fig. 2).
Fig. 2.
Average microplastic particle abundance per kilogram at each location. Each box has a thick center line that represents the median, a box height that displays the interquartile range, and whiskers that show the lowest and highest values.
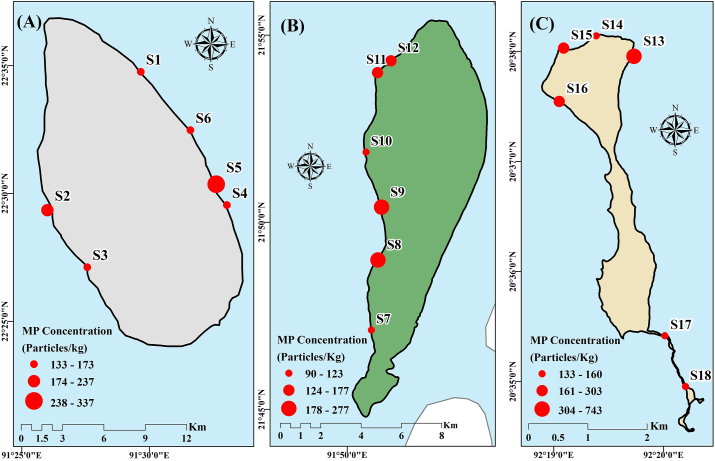
All the stations, located on Sandwip, exhibited a uniform distribution (p-value = 0.098 which is greater than 0.05) of microplastics along shoreline, having the highest abundance in site S5 at Sandwip Jettyghat (Fig. 3A). As our control site S6 experiences the least amount of human interaction, it has the lowest MPs overall. Sediments of S1, S3, and S4 contain the moderate level of MPs where boat anchoring or other occasional activities were observed.
Fig. 3.
MPs concentration along the shoreline of the studied islands: (A) Sandwip; (B) Kutubdia; (C) Saint Martin's Island.
Kutubdia Island also experienced a homogenous microplastic distribution (p-value = 0.754 which is greater than 0.05) which means no significant variation in the number of microplastics along the shoreline with the highest value of microplastics in S9 (Fig. 3B). Station S7 (Wind power mill, moderately popular site with sea wall) contained the least number of MPs in this island, whereas the most abundant site was S9, which is a local beach, and no sea wall/polder was observed there during sampling. Huge human intervention, fish drying, and other commercial activities were reported from the site; no dredging was seen along the beach. The polders or sea walls prevent the continuous flow of runoff from the mainland with debris, so absence of polder can be the reason for the highest number of MPs in any site. However, the numbers of MPs in S8 are closer to those of S9. S8 is the only beach where the few resorts are located. Apart from that, the level of MPs in the sediment of sites S10 and S12 were moderate.
This study found a heterogenous distribution of microplastics (p-value = 0.018 which is less than 0.05) suggesting a significant variation in the number of microplastics along the shoreline of Saint Martin's Island (Fig. 3C). Among the sites, the highest number of MPs were observed from S13, which serves as the main entry point to the island (Jettyghat) for tourists and locals. This location is the busiest area on the island, featuring the highest density of restaurants, hotels, local markets, ships, and boats. This area consequently attracts a significant number of tourists. Consequently, high levels of pollution may stem from various sources, including tourist and local activities, fragments from ships and boats, fishing nets, and untreated waste runoff. With an abundance of hotels, restaurants, and transport vessels, this high-traffic area plays a significant role in contributing to pollution on the island.
On the other hand, site S17, situated in the Chhera Dwip, that place goes under water periodically and thus gets washed up regularly, which can be the reason behind having the lowest number of microplastics, though macroplastics were abundant over there. Apart from them, S15 and S16 contained a moderate level of MPs in their correspondent sediments.
3.2. Microplastics distribution across the shore
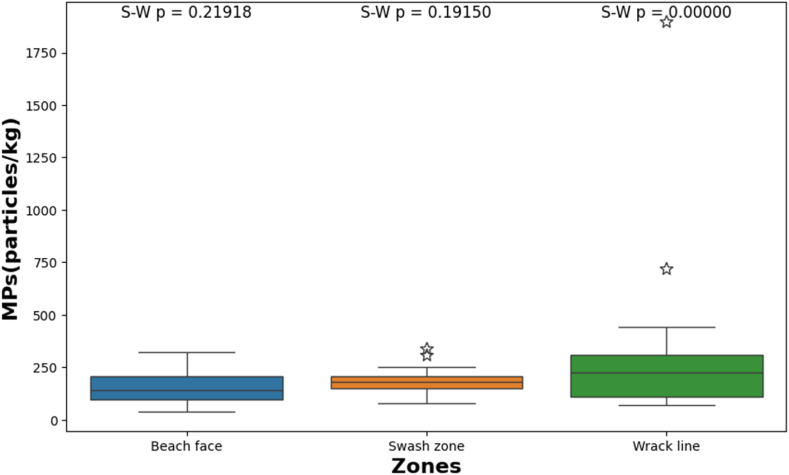
Fig. 4 shows that microplastic (MP) concentrations varied across different beach zones, with the wrack line exhibiting higher MP occurrences than the swash and beach face zones. In this study, the number of MPs observed from the wrack line was 1670, 1370, and 2820 particles/kg in Sandwip, Kutubdia, and Saint Martin's Island, respectively. In contrast, the swash zone and beach face contained 1150 and 730, 980 and 880, and 1250 and 1200 particles per kg, respectively from Sandwip, Kutubdia, and Saint Martin's Island. From Fig. 4 it can be seen that the beach face and swash zone follow the normal distribution while wrack line exhibits a statistically significant difference (p-value = 0.000 which is less than 0.05) among the number of MPs. This study found that MPs were most concentrated in the wrack line, typically the high tide line, where litter tends to accumulate during low tide [43,44].
Fig. 4.
Microplastic particle abundance per kilogram at different zones. Each box has a thick center line that represents the median, a box height that displays the interquartile range, and whiskers that show the lowest and highest values.
3.3. Shapes and colors of microplastics
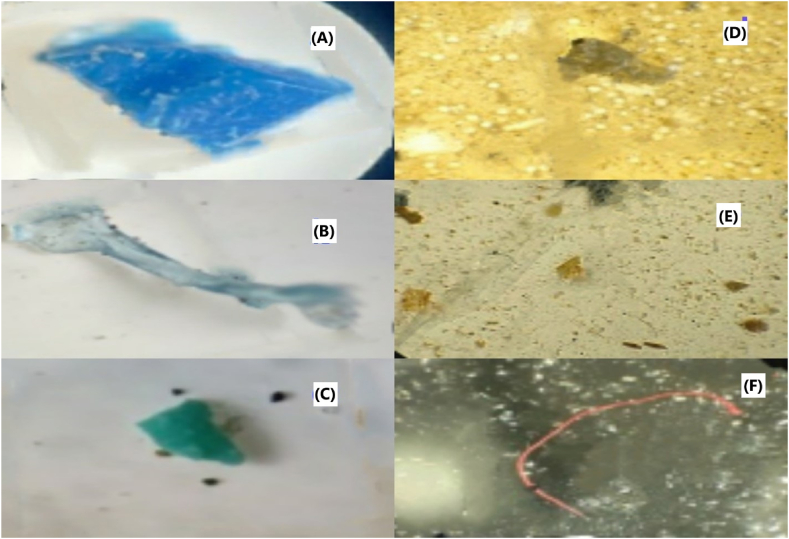
Fig. 5 displays the various shapes and colors of microplastics that observed under the microscope during visual identification. These particles showed a range of distinct shapes, including fragments (Fig. 5A,B&C), fibers (Fig. 5F), and films (Fig. 5D and E), which were categorized based on their unique structural characteristics. The colors of the microplastics varied widely, with dominant hues such as blue, red, transparent, and turquoise, reflecting a diverse composition of materials. The identification of these shapes and colors aids in understanding the potential sources and environmental impact of the microplastics.
Fig. 5.
Images of microplastics; (A) blue fragment; (B) bluish fragment; (C) turquoise fragment; (D)+(E) transparent film; (F) red fiber.
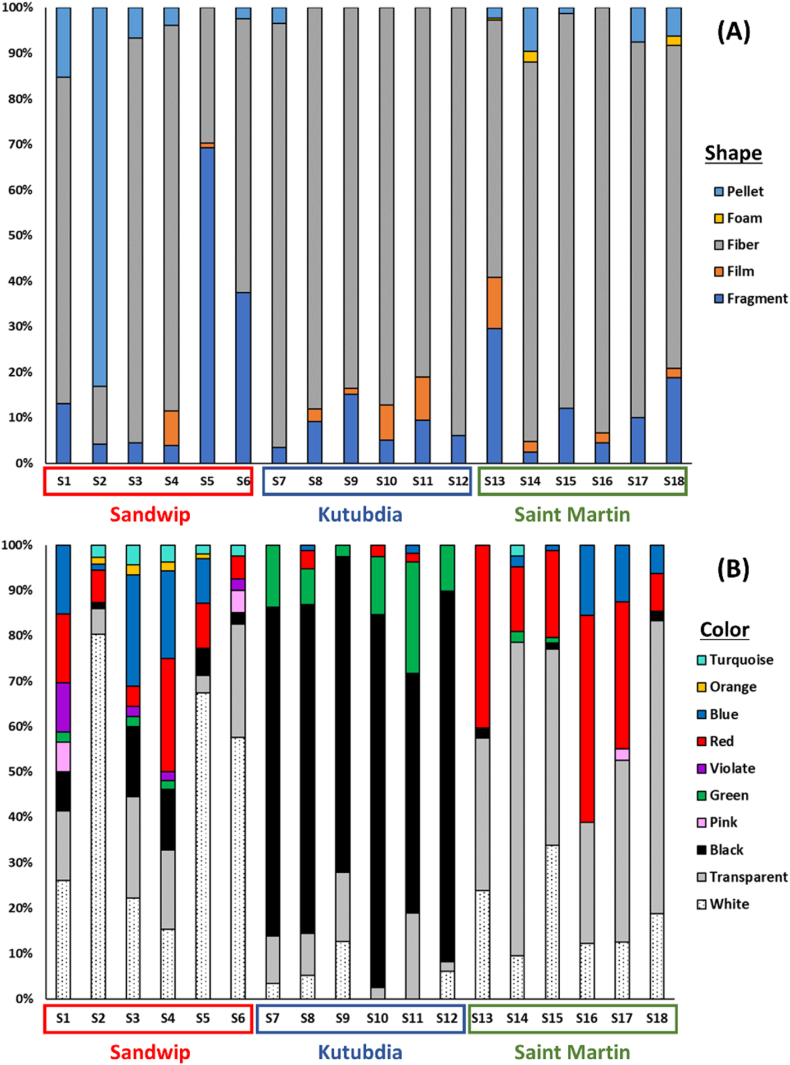
Fig. 6A illustrates the distribution of microplastic shapes along the shorelines of three offshore islands of Bangladesh. In Sandwip Island, the microplastic composition consisted of 50.57 % fibers, 27.71 % fragments, 20.29 % pellets, and 1.43 % films. In Kutubdia Island, fibers were most dominant at 87.12 %, followed by 9.2 % fragments, 3.37 % films, and 0.31 % pellets. In contrast, Saint Martin's Island had 73 % fibers, 17.87 % fragments, 5.51 % films, 3.04 % pellets, and 0.57 % foams (Fig. 6A).
Fig. 6.
Percentage of (A) shapes and (B) colors of microplastics found from the islands.
Regarding color distribution, microplastics in Sandwip Island were predominantly white (60.87 %), followed by red (10.43 %), translucent (8.70 %), blue (6.96 %), violet and black (4.35 % each), pink (2.61 %), and green and orange (0.87 %). In Kutubdia, 22.11 % were translucent and blue, 18.95 % white, 15.79 % red, 4.74 % black, with 2.11 % each for violet, orange, and green. On Saint Martin's Island, microplastics were primarily white (65.94 %), with additional colors including translucent (10.14 %), red (8.7 %), blue (7.25 %), black (5.07 %), pink (1.45 %), and turquoise (0.72 %) (Fig. 6B).
3.4. ATR-FTIR analysis
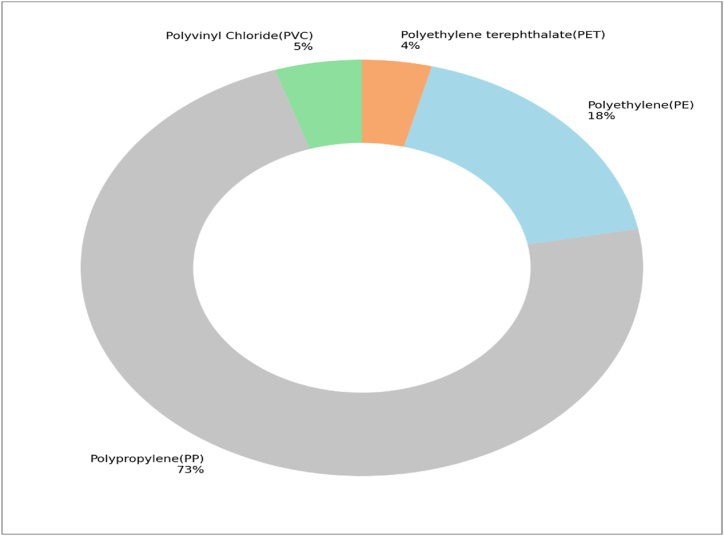
A total of four types of polymers were identified and characterized from the 23 microplastic particles using ATR-FTIR Spectrophotometry in this study. The identified polymers include Polypropylene (PP), PET (polyethylene terephthalate) Polyvinyl Chloride (PVC), and Polyethylene (PE) where PP > PE > PVC > PET (Fig. 7). However, due to limitations in analyzing each microplastic fragment, not all collected particles could undergo polymer identification process, as many were too small to participate in the process. As a result, only touchable microplastics (<5 mm) were analyzed for polymer composition. A total of 23 particles were selected for ATR-FTIR spectroscopic identification, and all were confirmed to be microplastics.
Fig. 7.
Relative abundances of the extracted polymer types and diameter of the microplastics from Sandwip, Kutubdia, and Saint Martin's Island, Bay of Bengal.
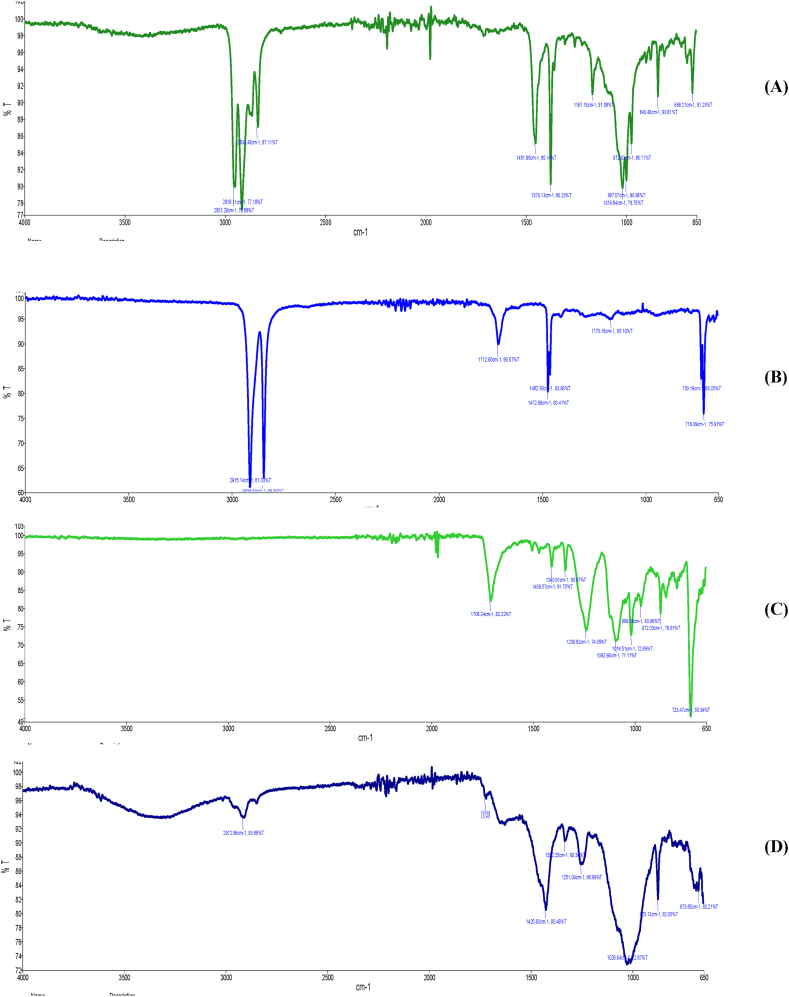
ATR-FTIR reveals the most abundant polymer as polypropylene (PP) (72.7 %) that can come from ropes, twine, tape, carpets, upholstery, clothing and camping equipment. These findings are aligned with previous other studies making it stronger [50,64,65]. Because of its waterproof properties it is mostly used in marine section [66]. Though PP imposes low polymeric risk to environment, that should not be overlooked if the site incorporates huge amount of it [67]. Fig. 8A shows that the CH2-group vibration in the PP polymer chain is represented by four large peaks in the absorption wave number range of 3000-2800 cm−1. The peaks at 2953.29 and 2873 cm−1 are ascribed to CH3 asymmetric and symmetric stretching vibrations, respectively, while the peaks at 2918.11 and 2838.48 cm−1 are ascribed to CH2 asymmetric and symmetric stretching vibrations, respectively [68,69]. Two strong peaks can also be seen in the ATR-FTIR spectra at 1460 and 1378 cm−1. CH3 symmetric deformation vibrations are responsible for the peak at 1378 cm−1, whereas CH3 asymmetric deformation vibrations or CH2 scissor vibrations are responsible for the peak at 1460 cm−1. In the wavenumber range of 1200–750 cm−1, untreated PP film also exhibits a large number of tiny peaks. C–C asymmetric stretching, CH3 asymmetric rocking, and C–H wagging vibrations are responsible for the peak at 1167 cm−1, whereas CH3 asymmetric rocking vibrations are responsible for the peak at 998 cm−1. CH3 asymmetric
Fig. 8.
Types of polymers of plastics found among the studied samples: A = PP; B= PE, C= PET, D = PVC.
rocking and C–C asymmetric stretching vibrations are responsible for the peak at 974 cm−1, whereas CH3 asymmetric rocking and C–C asymmetric and symmetric stretching vibrations are responsible for the peak at 901 cm−1. CH2 rocking vibrations are the cause of the peaks at 844 and 810 cm−1 [68]. In Fig. 8B, from 2850 cm−1 to 3000 cm−1, the stretching frequency was acute and significant, displaying –CH2 symmetric stretching. For the ethylene groups from PE, this resulted from sp3 C-H stretching (Fig. 8B) [70]. According to the spectrum, the carbonyl group associated with the ester bond exhibited a high C=O stretch at an average of 1712 cm−1. At about 1462 cm−1, the spectra showed –CH2 bending deformation. C-O-C stretching is visible in the ester vibration at maxima of 1170 cm−1. Furthermore, for every spectrum, every band in the 700–900 cm−1 frequency range was attributed to C–H out-of-plane bending vibrations.
The -CH2- deformation band and C(O)-O stretching of ester groups are attributed to the two peaks at 1408 and 1238.92 cm−1 (Fig. 8C), respectively, which indicates it to be PET (polyethylene terephthalate).
The spectrum of poly vinyl chloride (PVC) reveals a high absorption peak C-CL stretching around 673 cm−1, methylene groups (-CH-C) wagging at 1425.80 cm−1, and -C-H extending from the CH-Cl structure at 1251.04 cm−1 (Fig. 8D). Furthermore, it displays a significant vibration peak at 1727 cm−1, which might be attributable to the carbonyl band from the phthalate plasticizer, which is usually utilized in PVC.
4. Discussion
4.1. Microplastics are ubiquitous
Microplastic particles were detected in all sediment samples obtained from Sandwip, Kutubdia, and Saint Martin's Islands, demonstrating profound microplastic contamination in these regions, and this finding can be supported by prior literature [47,49,50,53,55,[71], [72], [73], [74]]. The present study revealed that there was a significantly higher abundance of microplastics in the sediment of a highly populated environment (Saint Martin's Island Jettyghat) compared to other places that are less human interrupted. Jettyghat (S13), the access point to Saint Martin's Island, is the island's most frequently visited beach, containing a market, hotels, ships, boats, fishing trawlers, and an array of other vehicles. The concentration of infrastructure at this site is significantly higher than that of other sampling stations on the island. Activities and structures on beaches influenced the amount of microplastics over the study area; for instance, the sea wall can facilitate reducing the influx of microplastics by restricting the land runoff [44] which is seen in S7 (Kutubdia). Among five polymeric types, polypropylene (PP) was the most abundant microplastic that was found from all the islands and this is aligned with other study conducted on Saint Martin's Island [44].
Microplastics in Sandwip (p-value = 0.098) and Kutubdia (p-value = 0.754) showed the homogenous (as p-value> 0.05) distribution along the shoreline, while Saint Martin's Island (p-value = 0.018) showed heterogeneous distribution (as p-value< 0.05) indicating highly variable values of microplastics from overwhelmed touristic spots to relatively calm sites [75,76]. But the beaches of Sandwip and Kutubdia are mostly dominated by locals rather than tourists (as populations of these two islands are higher than Saint martin's island) [77] where natives produce a significant number of plastic wastes, which may be responsible for the production of microplastics there.
In Sandwip, the mean abundance of MPs (193 ± 68.9 items kg−1) was higher than that of the MPs in the Barra Beach, Portugal, and South China, where they have reported 100 particles kg−1 [64] and 170 particles kg−1 [78] respectively. This increased amount of microplastics might be attributed to cumulatively the natives and Feni River, that runs through several industrial areas and empties into the Bay of Bengal, where microplastics can ultimately end up on the island's beaches. The MPs observed in Tuticorin Beach was 181 particles kg−1 and another study of India [51] found closer to the mean abundance of MPs in Sandwip. However, MPs from different beaches like East Frisian Islands, North Sea (49600 particles kg−1) [79]; South Andaman (414.35 ± 87.4 particles kg−1), India [47]; Karnataka (264 ± 62 to 1002 ± 174 particles kg−1), India [50] Rayong province of Eastern Thailand (568.33 ± 153.05 particles kg−1) [74] Cox's Bazar, Bangladesh (368.68 ± 10.65 particles kg−1) [43] were significantly higher than what this study found.
In Kutubdia, the mean abundance of MPs (175.5 ± 63.1 particles kg−1) was higher than that of the MPs in the Baja California Peninsula (135 ± 92 particles kg−1), Mexico [73]; Barra Beach (100 particles kg−1), Portugal [64] and South China (170 particles kg−1) [78]. The MPs observed in Phuket Coastline (188.3 ± 34.5 items kg−1), Thailand [55]; Tuticorin Beach (181 particles kg−1), India [51] found closer to the mean abundance of MPs in Kutubdia. However, MPs from different beaches like Rhode Island (varies from 40 micro plastic particles to 4.6 million from 100 g sediment), USA [80]; East Frisian Islands, North Sea (49600 particles kg−1) [79]; Virginia and North Carolina (600–2200 particles kg−1), USA [81]; South Andaman (414.35 ± 87.4 particles kg−1), India; Cox's Bazar, Bangladesh (368.68 ± 10.65 particles kg−1) (Hossain et al., 2021) were significantly higher than what this study found here. The amount of microplastics may rise as urbanization becomes more significant near this island and a new deep sea port (Matarbari Port) is under construction [82]. As a result, precautions must be introduced to preserve this island from excessive plastic contamination further.
In Saint Martin's Island, the mean abundance of MPs (266.3 ± 232 particles kg−1) was higher than that of the MPs in the Phuket Coastline (188.3 ± 34.5 items kg−1), Thailand [55]; Tuticorin Beach (181 particles kg−1), India [51]; Baja California Peninsula (135 ± 92 particles kg−1), Mexico [73]; Barra Beach (100 particles kg−1), Portugal [64]; and South China (170 particles kg−1) [78]. The MPs observed in Odisha (258.7 ± 90.0 particles kg−1), southeastern coast of India [83]; Andaman (249.82 ± 105.78 particles kg−1), India [47,51,84]; Karnataka (264 ± 62–1002 ± 174 particles kg−1), India [50] found closer to the mean abundance of MPs in Saint Martin's Island. Furthermore, very recent studies for the investigation of MP abundance were conducted in Saint Martin's Island where Tajwar et al. (2022) found the mean abundance of MPs was about 208 particles kg−1 [44] and Al Nahian et al. (2022) found the range of MPs abundance was 0.33–317.67 particles kg−1 [42]. These values slightly differ from the present study and which may be explained as due to the differences in site choice, sampling time, or patterns. Thus, recent two studies apparently justify the present study.
However, MPs from different beaches like East Frisian Islands, North Sea (49600 particles kg−1); Virginia and North Carolina (600–2200 particles kg−1) [71], USA; South Andaman (414.35 ± 87.4 particles kg-1), India [47]; Rayong province of Eastern Thailand (568.33 ± 153.05 particles kg−1) [74]; Cox's Bazar, Bangladesh (368.68 ± 10.65 particles kg−1) [43] were significantly higher than what this study found here.
As the wrack line receives organic or inorganic debris from both the sea and land and debris is deposited due to wave action, the highest number of MPs was expected to be along the wrack line. The present finding could be supported by the results of Rahman et al. (2020) [41] and Turra et al. (2014) [85]. Again, the second-highest number of MPs was reported from the swash zone. Undoubtedly, the swash zone is the area which is always submerged and gets enough time for the settlement of MPs over there in the sediments [86]. Though the average abundance of MPs is higher along the wrack line, there exists a few anomalies having the highest value in the swash zone, like locations S1, S3, S10, S11, S14, S15, S17, and S18, which can be explained by the direct exposure of the area to the turbulence of sea water and the level of pollution by tourists [41] The reason behind the lower abundance along the wrack line could be that the sands were evacuated from the wrack line for making the pavement along the beach, and locations S10 and S11 are prominently used for the anchoring of the fishing trawlers along the swash zone, and it is well known that fishing activities contribute to the MPs abundance. So direct disturbance in the swash zone was the reason for having a greater number of MPs than the wrack line for those two sites. Swash zone of site S14 was covered by rocks and boulders, which influenced the zone to have a greater number of MPs over there [87,88], S15 site experiences gathering of fishing boats at the swash zone regularly, which can be the reason behind the higher abundance of MPs in the correspondent sediments [41].
4.2. Diverse shaped microplastics were abundant in the sampling sites
Definitely, the shapes of microplastics are used to describe their origin [51]. For instance, the majority (70.3 %) of microplastic particles observed from our samples were found to be fiber, which can be assumed to be from fishing nets, ropes, or clothes [78,86]. This finding emphasizes fishermen, natives, or tourists to be much more careful in their contribution to the marine litters [73].
A noticeable number of fragments (18 %) were also observed from the beaches, clearly indicating their origin as macroplastics, implying that they are primarily created by the degradation or breakage of large plastics. This finding focuses on the significance of preventing the entrance of plastic or microplastic litter by any of the activities of professionals, natives, or tourists, and should take the initiative of cleaning the marine or beach environment regularly. Since the islands do not contain any plastics industries, all the microplastics are assumed to be from the sludge, whether from the islands or from the sediment and runoff from nearby river systems and the ocean [89].
4.3. Color distribution of MPs along the shorelines of the coastal islands
A wide range of colors of found microplastics were observed. Since clear and transparent materials have been attributed to polypropylene, white plastic to polyethylene, and opaque colors to LDPE, color has been utilized as a first step in identifying the chemical makeup [90]. Lastly, because the degree of yellowing or darkening was linked to an increase in the carbonyl index, which in turn indicated the level of photooxidation or ageing, color has also been utilized as an indicator of residency time at the sea surface or weathering [91]. Pigmented pellets often get lighter and lose part of their original color [92]. Therefore, an assumption can easily be made that Sandwip and Saint Martin's Island were dominated by the polyethylene as white MPs were highest in number there. Furthermore, the source of MPs has also been identified using color. Castro et al. [93] connected the high proportion of blue-colored MPs (60 %) found in water samples from Jurujuba (Nitero'i, RJ, Brazil) to the deterioration of blue gallon bottles used to support mussel farming as well as hygiene goods. In the light of this knowledge, it can be assumed that the highest proportion of the blue colored microplastic particles were mainly from the fragments of blue colored polyethene which were being used in the process of drying fish on the beaches by the native in Kutubdia Island.
4.4. Scrutinization of the surficial extent of microplastics
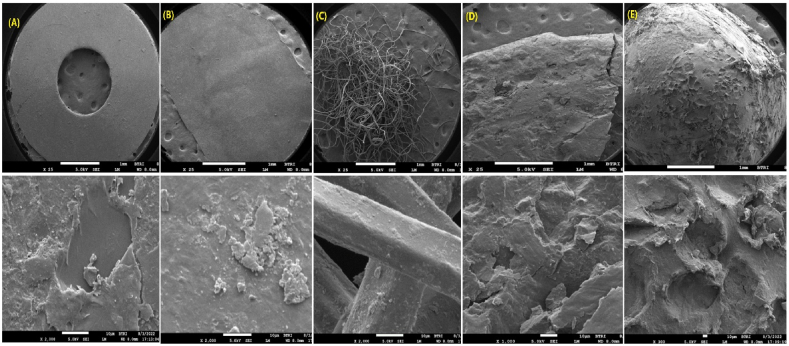
The scanning electron microscope (SEM) images demonstrate valuable insights into the structural characteristics and degradation patterns of microplastics [94]. These images reveal how small microplastic particles can serve as carriers for other particles, possibly enhancing the spread of contaminants across marine environments [95]. SEM images (Fig. 9) also highlighted the rupture, fracture, or deterioration that is occurring as a result of long-term exposure to natural conditions. The damage is particularly visible in the form of erosion, cracks, and surface roughness, which indicates the gradual degradation of the microplastics presented in the natural environment.
Fig. 9.
Surficial images of microplastics by SEM; (A) disk; (B) film; (C) microfiber; (D) fragment; (E) pellet [overall view (upper row) with a corresponding closer view (lower row)].
Fig. 9 illustrates the overall view (upper row of figure) with a corresponding closer view (lower row of figure) of them, which showcases distinct structural details of different shapes where A, B, C, D, and E of Fig. 9 represent disk, film, microfiber, fragment, and pellet, respectively. On closer inspection, the extent of degradation is visible, with damage likely due to factors such as sun exposure, wave action, mechanical pressure, and temperature fluctuations [[96], [97], [98]]. Microplastics can become fragmented and weathered under these conditions, increasing their surface area and, therefore, their exposure to other pollutants [[98], [99], [100]]. Observations such as these highlight the durability and persistence of microplastics in marine environments, as well as their potential role in facilitating contaminants' spread [101,102].
4.5. MPs polymers induce threat to the environment
The primary cause of the deterioration of polymers found on beaches or the water's surface is photo-degradation, which is brought on by exposure to UV light [103,104]. This mechanism produces oxidation reactions that set off the breakdown of the polymer chain's C-H chemical bonds and the production of free radicals. These will combine with oxygen to produce radicals called peroxides [105]. Furthermore, when exposed to ultraviolet (UV) light, polymers like PE and PP become yellow, however the underlying processes are still unclear. The breakdown of additives included in the polymer matrix, such as phenolic antioxidants, whose breakdown products contain quinoid structures that might result in this coloration, is another possible source of the yellowing phenomena [106]. In addition to photodegradation, biodegradation—the result of microorganisms—hydrolysis, and, lastly, erosion by mechanical processes—are also, albeit to a lesser degree, accountable for the breakdown of polymers [[107], [108], [109]]. The polymers' constituent chemical constituents have the potential to contaminate the environment [67,110]. A combination of monomers linked to catalysts and initiators required for the polymerization process is known as a polymer matrix. The mixture is then supplemented with additives to give the polymers the required characteristics. During the seldom finished polymerization events or during polymer breakdown, all of these chemical components can be found freely in the polymer matrix. Being free, they have the ability to move throughout the polymer life cycle and land in aquatic environments [[111], [112], [113]]. Many food products, including table salt [[114], [115], [116]] beer, canned food [117], tap water [118], and bottles of water, contain plastic particles. Twelve brands of beer, twelve brands of table salt [114] and 159 tap water samples were analyzed. The results indicate that each person consumes 5800 plastic particles annually from these goods. Furthermore, due to seafood contamination [119], an average consumer who regularly eats a lot of seafood is thought to swallow 11,000 plastic particles yearly. Additionally, there's a chance of inhalation ingestion, particularly with synthetic microfibers, which are particularly flammable due to their tiny size and easy entry into the respiratory system [[120], [121], [122]]. A human being would absorb between 74,000 and 121,000 plastic particles annually if the various pathways of ingestion (such as food and inhalation) were totaled [123].
5. Conclusion
This study highlights the pervasive presence of microplastics in the beach sediments of Sandwip, Kutubdia, and Staint Martin's Island. The findings reveal significant contamination across all sampled zones. Fibers were the most prevalent of the five MP types across all islands, with percentages of 50.57 %, 87.12 %, and 73 % in Sandwip, Kutubdia, and Saint Martin's Island, respectively. Using ATR-FTIR spectroscopy, four types of polymers were identified out of 23 selected pieces of plastic, where polypropylene (PP) [73 %] was the most found polymer, followed by polyethylene (PE) [14 %], polyester (PES) [8 %], and polyvinyl chloride (PVC) [5 %]. The higher concentration of MPs in the wrack lines points to substantial land-based sources, likely exacerbated by local and tourist activities. The variance in MP concentration along the sampling sites indicates that, while there was no discernible difference in the abundance of microplastics between the studied sites in Sandwip and Kutubdia, the designated public tourist spots on Saint Martin's Island are characterized by a significantly higher microplastic composition. Though not a popular tourist destination like Saint Martin's Island, a significant amount of microplastics from Sandwip and Kutubdia were discovered there. Furthermore, the degraded and rough surficial state was observed with the help of a scanning electron microscope (SEM), which led to anticipate about the damage under certain environmental conditions. Addressing this issue requires immediate interdisciplinary collaboration and evidence-based approaches to mitigate microplastic pollution. Protecting the ecological integrity and resilience of coastal habitats is crucial for ensuring a sustainable future for both the environment and local communities. Implementing comprehensive coastal management strategies, focusing on pollution sources and promoting sustainable tourism practices, is imperative to tackle the root causes of microplastic contamination and promote environmental sustainability.
CRediT authorship contribution statement
Kamrunnahar Kanak: Writing – review & editing, Writing – original draft, Visualization, Software, Resources, Methodology, Investigation, Data curation, Conceptualization. Md. Kawser Ahmed: Writing – review & editing, Visualization, Supervision, Resources, Methodology. Muhammad Saiful Islam: Writing – review & editing, Visualization, Resources, Methodology, Data curation, Conceptualization. Mahmudul Hasan: Writing – original draft, Visualization, Validation, Supervision, Resources, Methodology, Data curation, Conceptualization. K. M. Azam Chowdhury: Visualization, Supervision. Kazi Belayet Hossain: Writing – review & editing, Visualization, Software.
Data availability
Data will be made available on request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Kamrunnahar Kanak reports equipment, drugs, or supplies and travel were provided by Government of the People's Republic of Bangladesh Ministry of Science and Technology. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors gratefully acknowledge the National Science and Technology (NST) Fellowship 2020–21 provided by the Ministry of Science and Technology, Government of the People's Republic of Bangladesh, as well as support from the International Finance Investment and Commerce Bank (IFIC) and the International Centre for Ocean Governance (ICOG). Special thanks are extended to the staff at the Bangladesh Council of Scientific and Industrial Research (BCSIR) for their invaluable technical assistance and laboratory facilities.
References
- 1.Hidalgo-Ruz V., Gutow L., Thompson R.C., Thiel M. Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ. Sci. Technol. 2012;46:3060–3075. doi: 10.1021/ES2031505. [DOI] [PubMed] [Google Scholar]
- 2.Wang C., Zhao J., Xing B. Environmental source, fate, and toxicity of microplastics. J. Hazard Mater. 2021;407 doi: 10.1016/j.jhazmat.2020.124357. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann M., Gutow L., Klages M. Marine anthropogenic litter. 2015. [DOI]
- 4.Li J., Qu X., Su L., Zhang W., Yang D., Kolandhasamy P., Li D., Shi H. Microplastics in mussels along the coastal waters of China. Environ. Pollut. 2016;214:177–184. doi: 10.1016/j.envpol.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 5.Gasperi J., Wright S.L., Dris R., Collard F., Mandin C., Guerrouache M., Langlois V., Kelly F.J., Tassin B. Microplastics in air: are we breathing it in? Curr. Opin. Environ. Sci. Heal. 2018;1:1–5. doi: 10.1016/J.COESH.2017.10.002. [DOI] [Google Scholar]
- 6.Yurtsever M. Glitters as a source of primary microplastics: an approach to environmental responsibility and ethics. J. Agric. Environ. Ethics. 2019;32:459–478. doi: 10.1007/s10806-019-09785-0. [DOI] [Google Scholar]
- 7.Welden N.A., Lusher A. Elsevier Inc.; 2020. Microplastics: from Origin to Impacts. [DOI] [Google Scholar]
- 8.da Costa J.P., Duarte A.C., Rocha-Santos T.A.P. Elsevier Ltd; 2017. Microplastics – Occurrence, Fate and Behaviour in the Environment. [DOI] [Google Scholar]
- 9.Waldschläger K., Lechthaler S., Stauch G., Schüttrumpf H. The way of microplastic through the environment – application of the source-pathway-receptor model. Sci. Total Environ. 2020;713 doi: 10.1016/j.scitotenv.2020.136584. submitted for publication. [DOI] [PubMed] [Google Scholar]
- 10.Alfonso M.B., Arias A.H., Ronda A.C., Piccolo M.C. Continental microplastics: presence, features, and environmental transport pathways. Sci. Total Environ. 2021;799 doi: 10.1016/j.scitotenv.2021.149447. [DOI] [PubMed] [Google Scholar]
- 11.Friot D., Boucher J. IUCN Library System; 2017. Primary Microplastics in the Oceans.https://portals.iucn.org/library/node/46622 [Google Scholar]
- 12.Van Cauwenberghe L., Devriese L., Galgani F., Robbens J., Janssen C.R. Microplastics in sediments: a review of techniques, occurrence and effects. Mar. Environ. Res. 2015;111:5–17. doi: 10.1016/j.marenvres.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Harris P.T. The fate of microplastic in marine sedimentary environments: a review and synthesis. Mar. Pollut. Bull. 2020;158 doi: 10.1016/j.marpolbul.2020.111398. [DOI] [PubMed] [Google Scholar]
- 14.Claessens M., De Meester S., Van Landuyt L., De Clerck K., Janssen C.R. Occurrence and distribution of microplastics in marine sediments along the Belgian coast. Mar. Pollut. Bull. 2011;62:2199–2204. doi: 10.1016/j.marpolbul.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Nel H.A., Dalu T., Wasserman R.J. Sinks and sources: assessing microplastic abundance in river sediment and deposit feeders in an Austral temperate urban river system. Sci. Total Environ. 2018;612:950–956. doi: 10.1016/j.scitotenv.2017.08.298. [DOI] [PubMed] [Google Scholar]
- 16.Porter A. The movement of plastics through marine ecosystems and the influences on bioavailability and uptake into marine biota. 2019:1–182. https://ore.exeter.ac.uk/repository/handle/10871/37482 [Google Scholar]
- 17.Du S., Zhu R., Cai Y., Xu N., Yap P.S., Zhang Y., He Y., Zhang Y. Environmental fate and impacts of microplastics in aquatic ecosystems: a review. RSC Adv. 2021;11:15762–15784. doi: 10.1039/d1ra00880c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Constant M., Kerhervé P., Mino-Vercellio-Verollet M., Dumontier M., Sànchez Vidal A., Canals M., Heussner S. Beached microplastics in the northwestern mediterranean sea. Mar. Pollut. Bull. 2019;142:263–273. doi: 10.1016/j.marpolbul.2019.03.032. [DOI] [PubMed] [Google Scholar]
- 19.Bellasi A., Binda G., Pozzi A., Galafassi S., Volta P., Bettinetti R. Microplastic contamination in freshwater environments: a review, focusing on interactions with sediments and benthic organisms. Environ. - MDPI. 2020;7:1–27. doi: 10.3390/environments7040030. [DOI] [Google Scholar]
- 20.Costa E., Piazza V., Lavorano S., Faimali M., Garaventa F., Gambardella C. Trophic transfer of microplastics from copepods to jellyfish in the marine environment. Front. Environ. Sci. 2020;8:1–7. doi: 10.3389/fenvs.2020.571732. [DOI] [Google Scholar]
- 21.Cverenkárová K., Valachovičová M., Mackul’ak T., Žemlička L., Bírošová L. Microplastics in the food chain. Life. 2021;11:1–18. doi: 10.3390/life11121349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen L.-E., Kellermann M.Y., Schupp P.J. Secondary metabolites of marine microbes: from natural products chemistry to. Chem. Ecol. 2020 doi: 10.1007/978-3-030-20389-4_8. [DOI] [Google Scholar]
- 23.Elizalde-Velázquez G.A., Gómez-Oliván L.M. Microplastics in aquatic environments: a review on occurrence, distribution, toxic effects, and implications for human health. Sci. Total Environ. 2021;780 doi: 10.1016/j.scitotenv.2021.146551. [DOI] [PubMed] [Google Scholar]
- 24.Bhuyan M.S. Effects of microplastics on fish and in human health. Front. Environ. Sci. 2022;10:1–17. doi: 10.3389/fenvs.2022.827289. [DOI] [Google Scholar]
- 25.Khan M.T., Cheng Y.L., Hafeez S., Tsang Y.F., Yang J., Nawab A. Microplastics in wastewater: environmental and health impacts. Detec. Remed. Strat. 2022 doi: 10.1007/978-3-030-39041-9_39. [DOI] [Google Scholar]
- 26.Godswill C., Gospel C. Impacts of plastic pollution on the sustainability of seafood value chain and human health. Int. J. Adv. Acad. Res. | Sci. 2019;5:2488–9849. [Google Scholar]
- 27.Bhuyan M.S. Effects of microplastics on fish and in human health. 2022. [DOI]
- 28.Yu Z., Xu X., Guo L., Yuzuak S., Lu Y. Physiological and biochemical effects of polystyrene micro/nano plastics on Arabidopsis thaliana. J. Hazard Mater. 2024;469 doi: 10.1016/j.jhazmat.2024.133861. [DOI] [PubMed] [Google Scholar]
- 29.Barari F., Gabrabad M.E., Bonyadi Z. Recent progress on the toxic effects of microplastics on Chlorella sp. in aquatic environments. Heliyon. 2024 doi: 10.1016/j.heliyon.2024.e32881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eydi Gabrabad M., Yari M., Bonyadi Z. Using Spirulina platensis as a natural biocoagulant for polystyrene removal from aqueous medium: performance, optimization, and modeling. Sci. Rep. 2024;14:2506. doi: 10.1038/s41598-024-53123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao X., Hassan I., Peng Y., Huo S., Ling L. Behaviors and influencing factors of the heavy metals adsorption onto microplastics: a review. J. Clean. Prod. 2021;319 [Google Scholar]
- 32.Zahmatkesh Anbarani M., Najafpoor A., Barikbin B., Bonyadi Z. Adsorption of tetracycline on polyvinyl chloride microplastics in aqueous environments. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-44288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khoshmanesh M., Sanati A.M., Shahcheragh S., Farjadfard S., Bonyadi Z., Ramavandi B. Recent advances in dyes uptake by microplastics in aquatic environments: influencing factors and ecotoxicological behaviors. Arab. J. Chem. 2024 [Google Scholar]
- 34.Sarma V., Krishna M.S., Srinivas T.N.R. Sources of organic matter and tracing of nutrient pollution in the coastal Bay of Bengal. Mar. Pollut. Bull. 2020;159 doi: 10.1016/j.marpolbul.2020.111477. [DOI] [PubMed] [Google Scholar]
- 35.Hasan M., Rahman M., Islam M.A., Hossain M.I.S., Kanak K., Azam O.R. Assessment of toxic heavy metals in surface water of the Meghna River estuary: an integrated statistical approach. Dhaka Univ. J. Earth Environ. Sci. 2021:143–155. [Google Scholar]
- 36.Islam M.S., Phoungthong K., Islam A.R.M.T., Ali M.M., Ismail Z., Shahid S., Kabir M.H., Idris A.M. Sources and management of marine litter pollution along the Bay of Bengal coast of Bangladesh. Mar. Pollut. Bull. 2022;185 doi: 10.1016/j.marpolbul.2022.114362. [DOI] [PubMed] [Google Scholar]
- 37.Sun K., Song Y., He F., Jing M., Tang J., Liu R. A review of human and animals exposure to polycyclic aromatic hydrocarbons: health risk and adverse effects, photo-induced toxicity and regulating effect of microplastics. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2021.145403. [DOI] [PubMed] [Google Scholar]
- 38.Worm B., Lotze H.K., Jubinville I., Wilcox C., Jambeck J. Plastic as a persistent marine pollutant. Annu. Rev. Environ. Resour. 2017;42:1–26. doi: 10.1146/annurev-environ-102016-060700. [DOI] [Google Scholar]
- 39.Zahmatkesh Anbarani M., Esmaeili Nasrabadi A., Bonyadi Z. Use of Saccharomyces cerevisiae as new technique to remove polystyrene from aqueous medium: modeling, optimization, and performance. Appl. Water Sci. 2023;13:166. [Google Scholar]
- 40.Esmaeili Nasrabadi A., Zahmatkesh Anbarani M., Bonyadi Z. Investigating the efficiency of oak powder as a new natural coagulant for eliminating polystyrene microplastics from aqueous solutions. Sci. Rep. 2023;13 doi: 10.1038/s41598-023-47849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahman S.M.A., Robin G.S., Momotaj M., Uddin J., Siddique M.A.M. Occurrence and spatial distribution of microplastics in beach sediments of Cox's Bazar, Bangladesh. Mar. Pollut. Bull. 2020;160 doi: 10.1016/J.MARPOLBUL.2020.111587. [DOI] [PubMed] [Google Scholar]
- 42.Al Nahian S., Rakib M.R.J., Haider S.M.B., Kumar R., Mohsen M., Sharma P., Khandaker M.U. Occurrence, spatial distribution, and risk assessment of microplastics in surface water and sediments of Saint Martin Island in the Bay of Bengal. Mar. Pollut. Bull. 2022;179 doi: 10.1016/J.MARPOLBUL.2022.113720. [DOI] [PubMed] [Google Scholar]
- 43.Hossain M.B., Banik P., Nur A.A.U., Rahman T. Abundance and characteristics of microplastics in sediments from the world's longest natural beach, Cox's Bazar, Bangladesh. Mar. Pollut. Bull. 2021;163 doi: 10.1016/J.MARPOLBUL.2020.111956. [DOI] [PubMed] [Google Scholar]
- 44.Tajwar M., Shreya S.S., Gazi M.Y., Hasan M., Saha S.K. Microplastic contamination in the sediments of the Saint Martin's Island, Bangladesh. Reg. Stud. Mar. Sci. 2022;53 doi: 10.1016/J.RSMA.2022.102401. [DOI] [Google Scholar]
- 45.Balasubramaniam M., Phillott A.D. Preliminary observations of microplastics from beaches in the Indian ocean. Indian Ocean Turt. Newsl. (IOTN) 2016:13–16. https://www.iotn.org/wp-content/uploads/2017/05/23-05-PRELIMINARY-OBSERVATIONS-OF-MICROPLASTICS-FROM-BEACHES-IN-THE-INDIAN-OCEAN.pdf [Google Scholar]
- 46.Dewan A., Corner R., Saleem A., Rahman M.M., Haider M.R., Rahman M.M., Sarker M.H. Assessing channel changes of the Ganges-Padma River system in Bangladesh using Landsat and hydrological data. Geomorphology. 2017;276:257–279. doi: 10.1016/J.GEOMORPH.2016.10.017. [DOI] [Google Scholar]
- 47.Patchaiyappan A., Ahmed S.Z., Dowarah K., Jayakumar S., Devipriya S.P. Occurrence, distribution and composition of microplastics in the sediments of South Andaman beaches. Mar. Pollut. Bull. 2020;156 doi: 10.1016/j.marpolbul.2020.111227. [DOI] [PubMed] [Google Scholar]
- 48.Sambandam M., Dhineka K., Sivadas S.K., Kaviarasan T., Begum M., Hoehn D., Sivyer D., Mishra P., Murthy M.V.R. Occurrence, characterization, and source delineation of microplastics in the coastal waters and shelf sediments of the central east coast of India, Bay of Bengal. Chemosphere. 2022;303 doi: 10.1016/J.CHEMOSPHERE.2022.135135. [DOI] [PubMed] [Google Scholar]
- 49.Sarkar D.J., Das Sarkar S., Das B.K., Manna R.K., Behera B.K., Samanta S. Spatial distribution of meso and microplastics in the sediments of river Ganga at eastern India. Sci. Total Environ. 2019;694 doi: 10.1016/J.SCITOTENV.2019.133712. [DOI] [PubMed] [Google Scholar]
- 50.Yaranal N.A., Subbiah S., Mohanty K. Distribution and characterization of microplastics in beach sediments from Karnataka (India) coastal environments. Mar. Pollut. Bull. 2021;169 doi: 10.1016/J.MARPOLBUL.2021.112550. [DOI] [PubMed] [Google Scholar]
- 51.Tiwari M., Rathod T.D., Ajmal P.Y., Bhangare R.C., Sahu S.K. Distribution and characterization of microplastics in beach sand from three different Indian coastal environments. Mar. Pollut. Bull. 2019;140:262–273. doi: 10.1016/J.MARPOLBUL.2019.01.055. [DOI] [PubMed] [Google Scholar]
- 52.Thiemann T. Microplastic in the marine environment of the indian ocean. J. Environ. Prot. (Irvine,. Calif). 2023;14:297–359. [Google Scholar]
- 53.Tun T.Z., Mon E.E., Nakata H. Microplastics distribution in sediments collected from Myanmar. Arch. Environ. Contam. Toxicol. 2024;86:1–12. doi: 10.1007/s00244-023-01042-w. [DOI] [PubMed] [Google Scholar]
- 54.Curren E., Kuwahara V.S., Yoshida T., Leong S.C.Y. Marine microplastics in the ASEAN region: a review of the current state of knowledge. Environ. Pollut. 2021;288 doi: 10.1016/j.envpol.2021.117776. [DOI] [PubMed] [Google Scholar]
- 55.Akkajit P., Tipmanee D., Cherdsukjai P., Suteerasak T., Thongnonghin S. Occurrence and distribution of microplastics in beach sediments along Phuket coastline. Mar. Pollut. Bull. 2021;169 doi: 10.1016/J.MARPOLBUL.2021.112496. [DOI] [PubMed] [Google Scholar]
- 56.Barari F., Bonyadi Z. Evaluation of the leaching of microplastics from discarded medical masks in aquatic environments: a case study of Mashhad city. Appl. Water Sci. 2023;13:229. [Google Scholar]
- 57.Islam M.A., Hasan M., Peas M.H., Naime M.A., Gazi M.Y., Rahman M.M. Shoreline vulnerability assessment in an offshore island (Sandwip), Bangladesh–an appraisal of geospatial techniques. Dhaka Univ. J. Earth Environ. Sci. 2016;5:51–60. [Google Scholar]
- 58.Alam M.J., Kamal A.S.M.M., Ahmed M.K., Rahman M., Hasan M., Rahman S.A.R. Nutrient and heavy metal dynamics in the coastal waters of St. Martin's island in the Bay of Bengal. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Habib M.H.R., Rahman M., Uddin M.M., Shimu N.J., Hasan M., Alam M.J., Islam M.S. Application of AHP and geospatial technologies to assess ecotourism suitability: A case study of Saint Martin’s Island in Bangladesh. Reg. Stud. Mar. Sci. 2024;70:103357. [Google Scholar]
- 60.Das Karmaker K., Hasan M., Parvin A., Parvin A., Hossain M.S., Rahman M., Shaikh M.A.A., Haque M.I.-M., Hossain M.K. Holistic perilous index-based environmental appraisal of Metal (oid) s in the sole coral-bearing island of northeastern bay of Bengal. Chemosphere. 2024;359 doi: 10.1016/j.chemosphere.2024.142245. [DOI] [PubMed] [Google Scholar]
- 61.Masura J., Baker J., Foster G., Arthur C. Laboratory methods for the analysis of microplastics in the marine environment. NOAA Mar. Debris Progr. Natl. 2015:1–31. https://marinedebris.noaa.gov/sites/default/files/publications-files/noaa_microplastics_methods_manual.pdf [Google Scholar]
- 62.Tajwar M., Shreya S.S., Hasan M., Hossain M.B., Gazi M.Y., Sakib N. Assessment of microplastics as contaminants in a coal mining region. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e11666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tajwar M., Hasan M., Shreya S.S., Rahman M., Sakib N., Gazi M.Y. Risk assessment of microplastic pollution in an industrial region of Bangladesh. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chouchene K., Prata J.C., da Costa J., Duarte A.C., Rocha-Santos T., Ksibi M. Microplastics on Barra beach sediments in aveiro, Portugal. Mar. Pollut. Bull. 2021;167 doi: 10.1016/J.MARPOLBUL.2021.112264. [DOI] [PubMed] [Google Scholar]
- 65.Sathish N., Jeyasanta K.I., Patterson J. Abundance, characteristics and surface degradation features of microplastics in beach sediments of five coastal areas in Tamil Nadu, India. Mar. Pollut. Bull. 2019;142:112–118. doi: 10.1016/J.MARPOLBUL.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 66.Jemec Kokalj A., Dolar A., Drobne D., Marinšek M., Dolenec M., Škrlep L., Strmljan G., Mušič B., Škapin A.S. Environmental hazard of polypropylene microplastics from disposable medical masks: acute toxicity towards Daphnia magna and current knowledge on other polypropylene microplastics. Microplastics and Nanoplastics. 2022;2:1–15. doi: 10.1186/s43591-021-00020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lithner D., Larsson A., Dave G. Environmental and health hazard ranking and assessment of plastic polymers based on chemical composition. Sci. Total Environ. 2011;409:3309–3324. doi: 10.1016/J.SCITOTENV.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 68.Socrates G. John Wiley & Sons; 2004. Infrared and Raman Characteristic Group Frequencies: Tables and Charts. [Google Scholar]
- 69.Sciarratta V., Vohrer U., Hegemann D., Müller M., Oehr C. Plasma functionalization of polypropylene with acrylic acid. Surf. Coating. Technol. 2003;174:805–810. [Google Scholar]
- 70.Hamim F.A.R., Ghani S.A., Zainudin F. Properties of recycled high density polyethylene (RHDPE)/Ethylene vinyl acetate (EVA) blends: the effect of blends composition and compatibilisers. J. Phys. Sci. 2016;27 [Google Scholar]
- 71.Dodson G.Z., Shotorban A.K., Hatcher P.G., Waggoner D.C., Ghosal S., Noffke N. Microplastic fragment and fiber contamination of beach sediments from selected sites in Virginia and North Carolina, USA. Mar. Pollut. Bull. 2020;151 doi: 10.1016/J.MARPOLBUL.2019.110869. [DOI] [PubMed] [Google Scholar]
- 72.Hengstmann E., Tamminga M., vom Bruch C., Fischer E.K. Microplastic in beach sediments of the Isle of Rügen (Baltic Sea) - implementing a novel glass elutriation column. Mar. Pollut. Bull. 2018;126:263–274. doi: 10.1016/J.MARPOLBUL.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 73.Piñon-Colin T. de J., Rodriguez-Jimenez R., Pastrana-Corral M.A., Rogel-Hernandez E., Wakida F.T. Microplastics on sandy beaches of the Baja California Peninsula, Mexico. Mar. Pollut. Bull. 2018;131:63–71. doi: 10.1016/J.MARPOLBUL.2018.03.055. [DOI] [PubMed] [Google Scholar]
- 74.Prarat P., Thanayotmethi T., Sriboonyapirat T., Donsomchit P., Hongsawat P., Chouychai B. Preliminary study of abundance and characteristics of microplastics on beach sediment along the coast of Rayong province, Thailand. IOP Conf. Ser. Earth Environ. Sci. 2020;581 doi: 10.1088/1755-1315/581/1/012033. [DOI] [Google Scholar]
- 75.Franco A.A., Iglesias-Arroyo D., Egea-Corbacho Á., Martín-García A.P., Quiroga J.M., Coello M.D. Influence of tourism on microplastic contamination at wastewater treatment plants in the coastal municipality of Chiclana de la Frontera. Sci. Total Environ. 2023;900 doi: 10.1016/j.scitotenv.2023.165573. [DOI] [PubMed] [Google Scholar]
- 76.Gül M.R. Short-term tourism alters abundance, size, and composition of microplastics on sandy beaches. Environ. Pollut. 2023;316 doi: 10.1016/j.envpol.2022.120561. [DOI] [PubMed] [Google Scholar]
- 77.B. Sadar, B. Sadar, B. Sadar, B. Sadar, B. Sadar, B. Sadar, B. Sadar, No Title, (n.d.).
- 78.Dou P.C., Mai L., Bao L.J., Zeng E.Y. Microplastics on beaches and mangrove sediments along the coast of South China. Mar. Pollut. Bull. 2021;172 doi: 10.1016/J.MARPOLBUL.2021.112806. [DOI] [PubMed] [Google Scholar]
- 79.Liebezeit G., Dubaish F. Microplastics in beaches of the east Frisian islands Spiekeroog and Kachelotplate. Bull. Environ. Contam. Toxicol. 2012;89:213–217. doi: 10.1007/S00128-012-0642-7/TABLES/1. [DOI] [PubMed] [Google Scholar]
- 80.Cashman M.A., Langknecht T., El Khatib D., Burgess R.M., Boving T.B., Robinson S., Ho K.T. Quantification of microplastics in sediments from Narragansett Bay, Rhode Island USA using a novel isolation and extraction method. Mar. Pollut. Bull. 2022;174 doi: 10.1016/j.marpolbul.2021.113254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kadhum S.A., Abed S.A., Ewaid S.H., Al-Ansari N., Naji A. J. Phys. Conf. Ser. IOP Publishing; 2020. Microplastic contamination of surface sediment of Euphrates River, Iraq: a preliminary study. [Google Scholar]
- 82.Strokal M., Bai Z., Franssen W., Hofstra N., Koelmans A.A., Ludwig F., Ma L., van Puijenbroek P., Spanier J.E., Vermeulen L.C. Urbanization: an increasing source of multiple pollutants to rivers in the 21st century. Npj Urban Sustain. 2021;1:1–13. [Google Scholar]
- 83.Ranjani M., Veerasingam S., Venkatachalapathy R., Mugilarasan M., Bagaev A., Mukhanov V., Vethamony P. Assessment of potential ecological risk of microplastics in the coastal sediments of India: a meta-analysis. Mar. Pollut. Bull. 2021;163 doi: 10.1016/j.marpolbul.2021.111969. [DOI] [PubMed] [Google Scholar]
- 84.Sathish M.N., Patterson J. Comparative study on the status of microplastics in different functional areas of Tuticorin, Southeast coast of India. Sci. Total Environ. 2023;894 doi: 10.1016/j.scitotenv.2023.164904. [DOI] [PubMed] [Google Scholar]
- 85.Turra A., Manzano A.B., Dias R.J.S., Mahiques M.M., Barbosa L., Balthazar-Silva D., Moreira F.T. Three-dimensional distribution of plastic pellets in sandy beaches: shifting paradigms. Sci. Rep. 2014;4:4435. doi: 10.1038/srep04435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Islam M.S., Hossin M.S., Uddin M.R., Islam M.T., Antu U.B., Islam A.R.M.T., Hasan M., bin Hossain M.T., Ali M.M., Ismail Z. Sources and impacts of microplastic on the world's longest sea beach of the Bay of Bengal coasts: a review on microplastic management. Chem. Ecol. 2024:1–25. [Google Scholar]
- 87.Bissen R., Chawchai S. Microplastics on beaches along the eastern Gulf of Thailand – a preliminary study. Mar. Pollut. Bull. 2020;157 doi: 10.1016/J.MARPOLBUL.2020.111345. [DOI] [PubMed] [Google Scholar]
- 88.Wu P., Lin S., Cao G., Wu J., Jin H., Wang C., Wong M.H., Yang Z., Cai Z. Absorption, distribution, metabolism, excretion and toxicity of microplastics in the human body and health implications. J. Hazard Mater. 2022;437 doi: 10.1016/j.jhazmat.2022.129361. [DOI] [PubMed] [Google Scholar]
- 89.Wilson D.R., Godley B.J., Haggar G.L., Santillo D., Sheen K.L. The influence of depositional environment on the abundance of microplastic pollution on beaches in the Bristol Channel, UK. Mar. Pollut. Bull. 2021;164 doi: 10.1016/J.MARPOLBUL.2021.111997. [DOI] [PubMed] [Google Scholar]
- 90.Rodríguez-Seijo A., Pereira R. Compr. Anal. Chem. Elsevier; 2017. Morphological and physical characterization of microplastics; pp. 49–66. [Google Scholar]
- 91.Stolte A., Forster S., Gerdts G., Schubert H. Microplastic concentrations in beach sediments along the German Baltic coast. Mar. Pollut. Bull. 2015;99:216–229. doi: 10.1016/j.marpolbul.2015.07.022. [DOI] [PubMed] [Google Scholar]
- 92.Turner A., Holmes L. Occurrence, distribution and characteristics of beached plastic production pellets on the island of Malta (central Mediterranean) Mar. Pollut. Bull. 2011;62:377–381. doi: 10.1016/j.marpolbul.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 93.Castro R.O., Silva M.L., Marques M.R.C., V de Araújo F. Evaluation of microplastics in Jurujuba Cove, Niterói, RJ, Brazil, an area of mussels farming. Mar. Pollut. Bull. 2016;110:555–558. doi: 10.1016/j.marpolbul.2016.05.037. [DOI] [PubMed] [Google Scholar]
- 94.Maddison C., Sathish C.I., Lakshmi D., Wayne O., Palanisami T. An advanced analytical approach to assess the long-term degradation of microplastics in the marine environment. npj Mater. Degrad. 2023;7:59. [Google Scholar]
- 95.Kinigopoulou V., Pashalidis I., Kalderis D., Anastopoulos I. Microplastics as carriers of inorganic and organic contaminants in the environment: a review of recent progress. J. Mol. Liq. 2022;350 [Google Scholar]
- 96.Chubarenko I., Esiukova E., Bagaev A., Isachenko I., Demchenko N., Zobkov M., Efimova I., Bagaeva M., Khatmullina L. Microplastic Contam. Aquat. Environ. Elsevier; 2018. Behavior of microplastics in coastal zones; pp. 175–223. [Google Scholar]
- 97.Belioka M.-P., Achilias D.S. The effect of weathering conditions in combination with natural phenomena/disasters on microplastics' transport from aquatic environments to agricultural soils. Microplastics. 2024;3:518–538. [Google Scholar]
- 98.Belioka M.-P., Achilias D. 2024. How Natural Phenomena and Disasters Together with the Weathering Conditions Affect Microplastics and Nanoplastics in Agricultural Soils and in Farmlands. [Google Scholar]
- 99.Liu P., Zhan X., Wu X., Li J., Wang H., Gao S. Effect of weathering on environmental behavior of microplastics: properties, sorption and potential risks. Chemosphere. 2020;242 doi: 10.1016/j.chemosphere.2019.125193. [DOI] [PubMed] [Google Scholar]
- 100.Corcoran P.L. Handb. Microplastics Environ. Springer; 2022. Degradation of microplastics in the environment; pp. 531–542. [Google Scholar]
- 101.Mammo F.K., Amoah I.D., Gani K.M., Pillay L., Ratha S.K., Bux F., Kumari S. Microplastics in the environment: interactions with microbes and chemical contaminants. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140518. [DOI] [PubMed] [Google Scholar]
- 102.Hale R.C., Seeley M.E., La Guardia M.J., Mai L., Zeng E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean. 2020;125 [Google Scholar]
- 103.Benítez A., Sánchez J.J., Arnal M.L., Müller A.J., Rodríguez O., Morales G. Abiotic degradation of LDPE and LLDPE formulated with a pro-oxidant additive. Polym. Degrad. Stabil. 2013;98:490–501. [Google Scholar]
- 104.Cooper D.A., Corcoran P.L. Effects of mechanical and chemical processes on the degradation of plastic beach debris on the island of Kauai, Hawaii. Mar. Pollut. Bull. 2010;60:650–654. doi: 10.1016/j.marpolbul.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 105.Ziani K., Ioniță-Mîndrican C.-B., Mititelu M., Neacșu S.M., Negrei C., Moroșan E., Drăgănescu D., Preda O.-T. Microplastics: a real global threat for environment and food safety: a state of the art review. Nutrients. 2023;15:617. doi: 10.3390/nu15030617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Endo S., Takizawa R., Okuda K., Takada H., Chiba K., Kanehiro H., Ogi H., Yamashita R., Date T. Concentration of polychlorinated biphenyls (PCBs) in beached resin pellets: variability among individual particles and regional differences. Mar. Pollut. Bull. 2005;50:1103–1114. doi: 10.1016/j.marpolbul.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 107.Bher A., Mayekar P.C., Auras R.A., Schvezov C.E. Biodegradation of biodegradable polymers in mesophilic aerobic environments. Int. J. Mol. Sci. 2022;23 doi: 10.3390/ijms232012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gilani I.E., Sayadi S., Zouari N., Al-Ghouti M.A. Plastic waste impact and biotechnology: exploring polymer degradation, microbial role, and sustainable development implications. Bioresour. Technol. Rep. 2023 [Google Scholar]
- 109.Mehmood C.T. 2017. AN INVESTIGATION INTO CATALYTIC PHOTO-BIODEGRADATION OF POLYTHENE FILMS. [Google Scholar]
- 110.Yuan Z., Nag R., Cummins E. Ranking of potential hazards from microplastics polymers in the marine environment. J. Hazard Mater. 2022;429 doi: 10.1016/j.jhazmat.2022.128399. [DOI] [PubMed] [Google Scholar]
- 111.Horton A.A., Dixon S.J. Microplastics: an introduction to environmental transport processes. Wiley Interdiscip. Rev. Water. 2018;5:e1268. [Google Scholar]
- 112.Issac M.N., Kandasubramanian B. Effect of microplastics in water and aquatic systems. Environ. Sci. Pollut. Res. 2021;28:19544–19562. doi: 10.1007/s11356-021-13184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vivekanand A.C., Mohapatra S., Tyagi V.K. Microplastics in aquatic environment: challenges and perspectives. Chemosphere. 2021;282 doi: 10.1016/j.chemosphere.2021.131151. [DOI] [PubMed] [Google Scholar]
- 114.Kosuth M., Mason S.A., V Wattenberg E. Anthropogenic contamination of tap water, beer, and sea salt. PLoS One. 2018;13 doi: 10.1371/journal.pone.0194970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pironti C., Ricciardi M., Motta O., Miele Y., Proto A., Montano L. Microplastics in the environment: intake through the food web, human exposure and toxicological effects. Toxics. 2021;9:224. doi: 10.3390/toxics9090224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danopoulos E., Jenner L., Twiddy M., Rotchell J.M. Microplastic contamination of salt intended for human consumption: a systematic review and meta-analysis. SN Appl. Sci. 2020;2:1–18. doi: 10.1289/EHP7171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Udovicki B., Andjelkovic M., Cirkovic-Velickovic T., Rajkovic A. Microplastics in food: scoping review on health effects, occurrence, and human exposure. Int. J. Food Contam. 2022;9:7. [Google Scholar]
- 118.Danopoulos E., Twiddy M., Rotchell J.M. Microplastic contamination of drinking water: a systematic review. PLoS One. 2020;15 doi: 10.1371/journal.pone.0236838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Cauwenberghe L., Janssen C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014;193:65–70. doi: 10.1016/j.envpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 120.Koelmans A.A., Redondo-Hasselerharm P.E., Nor N.H.M., de Ruijter V.N., Mintenig S.M., Kooi M. Risk assessment of microplastic particles. Nat. Rev. Mater. 2022;7:138–152. [Google Scholar]
- 121.Jones L.R., Wright S.J., Gant T.W. A critical review of microplastics toxicity and potential adverse outcome pathway in human gastrointestinal tract following oral exposure. Toxicol. Lett. 2023 doi: 10.1016/j.toxlet.2023.08.011. [DOI] [PubMed] [Google Scholar]
- 122.Thompson R.C., Moore C.J., Vom Saal F.S., Swan S.H. Plastics, the environment and human health: current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2153–2166. doi: 10.1098/rstb.2009.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Belontz S.L. North America; 2021. An Assessment of the Spatial and Temporal Distribution of Microplastics in Surface and Subsurface Sediment of Lake Huron. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.