ABSTRACT
Background
A low-protein diet (LPD) is recommended to patients with advanced chronic kidney disease (CKD), whereas geriatric guidelines recommend a higher amount of protein. The aim of this study was to evaluate the safety of LPD treatment in older adults with advanced CKD.
Methods
The EQUAL study is a prospective, observational study including patients ≥65 years of age with an incident estimated glomerular filtration rate <20 ml/min/1.73 m2 in six European countries with follow-up through 6 years. Nutritional status was assessed by a 7-point subjective global assessment (SGA) every 3–6 months. Prescribed diet (g protein/kg of bodyweight) was recorded on every study visit; measured protein intake was available in three countries. Time to death and decline in nutritional status (SGA decrease of ≥2 points) were analysed using marginal structural models with dynamic inverse probability of treatment and censoring weights.
Results
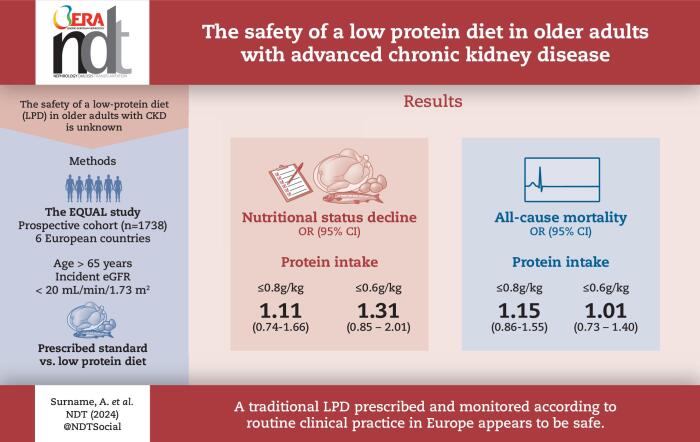
Of 1738 adults (631 prescribed LPD at any point during follow-up), there were 1319 with repeated SGA measurements, of which 267 (20%) decreased in SGA ≥2 points and 565 (32.5%) who died. There was no difference in survival or decrease in nutritional status for patients prescribed a LPD ≤0.8 g/kg ideal bodyweight {odds ratio [OR] for mortality 1.15 [95% confidence interval (CI) 0.86–1.55)] and OR for decrease in SGA 1.11 [95% CI 0.74–1.66]} in the adjusted models. In patients prescribed a LPD <0.6 g/kg ideal bodyweight, the results were similar. There was a significant interaction with LPD and older age >75 years, lower SGA and higher comorbidity burden for both mortality and nutritional status decline.
Conclusions
In older adults with CKD approaching end-stage kidney disease, a traditional LPD prescribed and monitored according to routine clinical practice in Europe appears to be safe.
Keywords: age, CKD, malnutrition, nutrition, survival analysis
Graphical Abstract
Graphical Abstract.
KEY LEARNING POINTS.
What was known:
The challenges of nutritional care in older adults with advanced chronic kidney disease (CKD) are many and current nutritional guidelines are contradictory.
CKD guidelines suggest the use of a low-protein diet (LPD), whereas geriatric guidelines recommend a higher amount of protein to reduce the risk of sarcopenia and malnutrition.
This study adds:
This study suggests that LPD, prescribed and monitored according to routine clinical practice in Europe, is a safe treatment for older adults with CKD.
In patients with a high-risk profile, such as the very old and those with a large comorbidity burden, the benefits of LPD should be weighed against the risks of accelerated nutritional status decline.
Potential impact:
The results from this large, European study may contribute to improved nutritional management in older adults with CKD and underscore the importance of nutritional monitoring regardless of dietary regimen.
INTRODUCTION
A low-protein diet (LPD) is recommended to patients with advanced chronic kidney disease (CKD) to delay kidney decline progression and improve quality of life [1]. A LPD needs to be prescribed under careful considerations to avoid protein energy wasting (PEW), since a decrease in appetite, development of a poor nutritional status and weight loss begin relatively early in the course of CKD [2–5].
Many of CKD patients are elderly; >50% of all European patients on maintenance dialysis treatment are >65 years of age [6, 7]. Older adults have several comorbid conditions and a high prevalence of poor nutritional status [3, 8]. Nutrition guidelines for older adults are contradictory. While CKD guidelines in general suggest the use of LPD in CKD stages 3–5, geriatric guidelines recommend a daily amount of at least 1.0 g protein/kg [9] to reduce the risk of sarcopenia and malnutrition. If dialysis treatment is initiated, the protein requirement increases. The recommended intake is 1.0–1.2 g/kg/day to maintain a stable nutritional status and to compensate for losses during the dialysis procedure [1]. The use of a LPD differs between countries and in regions in the same country [10, 11]. A LPD can be tailored and provided in several ways. The traditional LPD is based on mixed protein sources (0.6–0.8 g/kg/day) and often consists of regional cuisines. Very-low-protein diets (0.3–0.4 g/kg/day) should be supplemented with amino acid or ketoacid supplements to reach the requirements for essential amino acids [1]. Recently published studies showed potential benefits of plant-dominant LPDs in CKD [12, 13]. An adequate energy intake is essential regardless of the protein level or type of protein source. Usually patients who are prescribed a LPD are carefully monitored by nephrologists and dietitians, who also consider the total energy intake, assess signs of PEW and evaluate dietary adherence [10, 11, 14–16]. The challenges of nutritional care in older adults with CKD are many [17] and the Kidney Disease Outcomes Quality Initiative (KDOQI) nutrition guideline have raised concern about the safety of a LPD in older adults.
Very few studies have investigated the association between LPDs and changes in nutritional status and mortality in older adults with CKD at risk of malnutrition. The aim of this study was to evaluate the safety of LPD treatment in older adults with advanced CKD, approaching end-stage kidney disease. For this purpose, we used a large European inception cohort of carefully phenotyped patients with stages 4–5 CKD and >65 years of age with repeated follow-up visits up to 6 years in routine nephrology care.
MATERIALS AND METHODS
Study design and study population
The EQUAL study is a multicentre, prospective observational cohort study involving six European countries (Germany, Italy, The Netherlands, Poland, Sweden and the UK). Inclusion criteria are people >65 years of age with an incident estimated glomerular filtration rate (eGFR) <20 ml/min/1.73 m2 under nephrology care. The patients received routine medical care as provided by the nephrology clinics in each country; the study visits took place every 6 months until the eGFR decreased to <10 ml/min/1.73 m2, after which the interval was every 3 months. At each study visit, the nephrologist completed a questionnaire with extensive clinical data and information on the prescribed diet. All standardized data were collected repeatedly, including comorbidities, nutritional status assessed by a 7-point subjective global assessment (SGA), medication and routine blood and urine biochemistry. Patients were followed up to 6 years. A full description of the study protocol has been published elsewhere [18]. For this study, we included participants who had entered the study before 26 August 2020 and for whom we had information regarding prescribed diet (Supplementary Fig. S1). All the study participants signed a written informed consent and the EQUAL study was approved by the ethical review board in all participating countries.
LPD
On every follow-up visit, the nephrologist indicated whether the patient was prescribed a protein restriction and the specified amount in g/kg bodyweight. In the main analysis, we defined any prescribed protein intake ≤0.8 g/kg bodyweight as a LPD. To evaluate adherence to the diet regime, we used measured urea appearance from 24-hour urinary collections (patients in Sweden, Italy and The Netherlands). The dietary protein intake was then calculated according to the Maroni formula [19] and was normalized to an adjusted body mass index (BMI) of 23 kg/m2 [1] for each period when the patient was prescribed a standard diet or LPD until they started kidney replacement therapy. To test the robustness of the results, we additionally used a lower cut-off level of ≤0.6 g protein/kg bodyweight as our definition of a LPD. To evaluate the outcomes stratified by dietary adherence we divided a period when a patient was prescribed a standard diet into either ‘standard diet—adherent’ if the measured protein intake was >0.8 g/kg or ‘spontaneously low protein intake’ if the measured protein intake was ≤0.8 g/kg. The period when the patient was prescribed a LPD was categorized similarly into ‘LPD ≤0.8 g/kg adherent’, ‘LPD ≤0.8 g/kg non-adherent’ and ‘LPD ≤0.6 g/kg adherent’.
Outcomes
Nutritional status was assessed during every follow-up visit with the 7-point SGA [20], where a score of 6–7 corresponds to good nutritional status, 3–5 is moderate malnutrition and <3 is severe malnutrition. To minimize the risk of misclassification, we regarded a decrease in SGA of at least 2 points as a decline in nutritional status. Vital status and cause of death were collected as part of the study protocol.
Covariates
Information regarding demographics (age, sex, country), clinical information (primary renal disease, comorbidity), socio-economic status (level of education, marital status) and lifestyle (alcohol intake, smoking habits) were collected at baseline, whereas laboratory values [haemoglobin, plasma albumin, sodium, potassium, phosphorous, calcium, parathyroid hormone, urea, standard bicarbonate, eGFR (using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation) [21] and cholesterol] and clinical data [blood pressure (BP), BMI, kidney replacement therapy] were collected during the entire follow-up.
Statistical analyses
The covariates were described as means, medians and proportions stratified by LPD according to their underlying distribution and compared by non-parametric statistics. Education was categorized into four classes (low, intermediate, high and other); smoking habits were categorized as current smoker, former smoker or never smoker; and alcohol consumption was categorized into four categories based on the average number of units of alcohol per week. Patients with no information were categorized into a separate category. Patients were followed from baseline until the end of the follow-up period or death. We performed two separate analyses for our main outcomes of a decrease in SGA and mortality. For the survival analysis we included all patients with information regarding the prescribed diet. Once a patient had started a LPD, the patient remained in that group until the end of follow-up (intention to treat). Since we hypothesized that the potential effects of diet not only could influence the risk of immediate outcomes, but also future risk, we followed up regardless of whether the patient started kidney replacement therapy or not. However, the probability of treatment was only computed for the patients as long as they were not on dialysis. For the SGA analysis we excluded patients with fewer than two SGA measurements and followed them until the date of a decrease in SGA of at least 2 units from baseline or death.
Marginal structural survival models (pooled logistic regression models) were used to investigate the relationship between the treatment and our two outcomes [22, 23]. The stabilized inverse probability weights for receiving or not receiving treatment with a LPD were computed over the follow-up period using logistic regression models including information on age, sex, country, all relevant comorbidities, Charlson comorbidity index score, level of education, marital status, alcohol, smoking, BP and laboratory measurements (haemoglobin, albumin, potassium, urea, phosphate, eGFR) at baseline and all time-updated laboratory measurements and BP measurements. For the mortality analyses we additionally included SGA at baseline and over time. To account for informative censoring, we then computed the stabilized censoring weights similarly. The stabilized weights were centred around 1.0 with a low standard deviation; there were no extreme weights, and no truncation was therefore applied. To account for non-linear effects, we modelled time as a natural cubic spline with three knots. The outcome model included the baseline covariates described above. The interactions between prescribed diet and several pre-defined subgroups [age >75 and <75 years, high (≥6) versus low comorbidity score, diabetes mellitus, sex and normal versus lower (<6) SGA] were investigated and if statistically significant (P < .05) we performed stratified analyses. Missing values are reported in Supplementary Table S1.
We evaluated risk associated with adherence to a LPD in a restricted subgroup analyses where information on measured protein intake was available. For these analyses we first compared patients with a ‘standard diet—adherent’ to ‘LPD ≤0.8 g/kg adherent’ and to ‘LPD ≤0.8 g/kg non-adherent’. We subsequently compared patients with a ‘spontaneously low protein intake’ to patients with ‘LPD ≤0.8 g/kg adherent’ and ‘LPD ≤0.8 g/kg non-adherent’. For descriptive purposes, we additionally compared ‘spontaneously low protein intake’ to ‘standard diet—adherent’ using time-dependent Cox proportional hazards models and cumulative incidence curves adjusting for age, sex, eGFR and country.
We also performed several sensitivity analyses. First, we repeated both main outcomes and subgroups for patients prescribed ≤0.6 g protein/kg bodyweight. Second, we repeated the analyses after imputing the missing laboratory values with everyone's mean, followed by the population mean. Third, we analysed the measured protein intake by actual weight instead of ideal bodyweight. All analyses were performed with Stata 15 (StataCorp, College Station, TX, USA).
RESULTS
Patient characteristics
In total, we included 1738 individuals, of which 1319 had at least two SGA measurements and were retained in the analysis of SGA decline (Supplementary Fig. S1). Patient characteristics, stratified on the prescribed diet regimen, are presented in Table 1, and baseline characteristics for patients by measured protein intake are presented in Supplementary Table S2. The median age was 76 years and 65% were male. At baseline, 737 patients (43%) had a good nutritional status, 953 (55%) were moderately malnourished and 34 (2%) were classified with severe malnutrition. Over a median follow-up time of 2.1 years [interquartile range (IQR) 0.9–6.5], 500 started dialysis and 75 individuals were kidney transplanted.
Table 1:
Patient characteristics at baseline stratified by prescription of a LPD during any time over the follow-up period.
| Standard diet | Low protein diet | ||
|---|---|---|---|
| Characteristics | (n = 1107) | (n = 631) | P-value |
| Female, n (%) | 413 (37) | 191 (30) | .01 |
| Age (years), median (IQR) | 76 (71–81) | 76 (70–81) | .65 |
| Country, n (%) | <.001 | ||
| Germany | 146 (13) | 8 (1) | |
| Italy | 124 (11) | 289 (46) | |
| Netherlands | 119 (11) | 144 (23) | |
| Poland | 93 (8) | 9 (1) | |
| Sweden | 124 (11) | 181 (29) | |
| UK | 501 (45) | 0 (0) | |
| Primary renal disease, n (%) | .86 | ||
| Glomerular disease | 91 (9) | 34 (8) | |
| Tubulointerstitial disease | 80 (8) | 40 (9) | |
| Systemic disease | 21 (2) | 5 (1) | |
| Diabetes | 197 (20) | 88 (20) | |
| Hypertension, renovascular diseases | 304 (33) | 175 (39) | |
| Hereditary disease | 30 (3) | 9 (2) | |
| Other specified disorders | 46 (5) | 14 (3) | |
| Unknown | 186 (20) | 80 (18) | |
| Clinical data | |||
| eGFR (ml/min/1.73 m2), mean (SD) | 17.6 (5.6) | 16.7 (5.2) | <.001 |
| Systolic BP (mmHg), median (IQR) | 141 (130–158) | 140 (126–154) | .01 |
| Diastolic BP (mmHg), median (IQR) | 73 (66–80) | 74 (68–80) | .40 |
| BMI (kg/m2), medina (IQR) | 28.3 (25–32) | 27.2 (24–31) | <.001 |
| SGA overall score, mean (SD) | 5.9 (1.0) | 6.1 (1.0) | <.001 |
| Supplement amino acids, n (%) | 4 (0.4) | 44 (7) | .02 |
| Start kidney replacement therapy, n (%) | 300 (27) | 200 (32) | .11 |
| Laboratory measurements, mean (SD) | |||
| Haemoglobin (g/l) | 129 (17) | 130 (16) | .17 |
| Sodium (mmol/l) | 140 (3) | 140 (3) | .13 |
| Potassium (mmol/l) | 4.7 (0.6) | 4.6 (0.6) | .01 |
| Phosphate (mmol/l) | 1.3 (0.3) | 1.3 (0.3) | .98 |
| Urea (mmol/l) | 20.1 (8.2) | 22.6 (11.0) | <.001 |
| Albumin (g/l) | 38 (6.0) | 37 (5.5) | .01 |
| Cholesterol (mmol/l) | 4.6 (1.4) | 4.5 (1.2) | .87 |
| Comorbidity, n (%) | |||
| Charlson comorbidity index, mean (SD) | 7.1 (1.8) | 7.2 (2.0) | .14 |
| Diabetes mellitus | 443 (40) | 266 (42) | .53 |
| Cerebrovascular disease | 159 (14) | 99 (16) | .28 |
| Coronary artery disease | 351 (32) | 202 (32) | .21 |
| Malignancy | 211 (19) | 139 (22) | .53 |
| Heart failure | 180 (16) | 119 (19) | .41 |
| Education, n (%) | |||
| Low | 266 (32) | 160 (29) | .31 |
| Intermediate | 407 (49) | 267 (49) | |
| High | 112 (13) | 99 (18) | |
| Other | 49 (6) | 20 (4) | |
| Marital status, n (%) | .05 | ||
| Married/partner | 515 (62) | 371 (68) | |
| Divorced/widowed/single | 318 (38) | 174 (32) | |
| Lifestyle | |||
| Smoker | .87 | ||
| Never | 306 (37) | 206 (38) | |
| Current | 69 (8) | 50 (9) | |
| Former | 452 (55) | 283 (53) | |
| Alcohol consumption | .81 | ||
| None | 475(57) | 299 (56) | |
| 1–4 standard units/week | 182 (22) | 129 (24) | |
| >4–7 standard units/week | 69 (8) | 49 (9) | |
| >7 standard units/week | 101 (12) | 58 (11) |
Diet
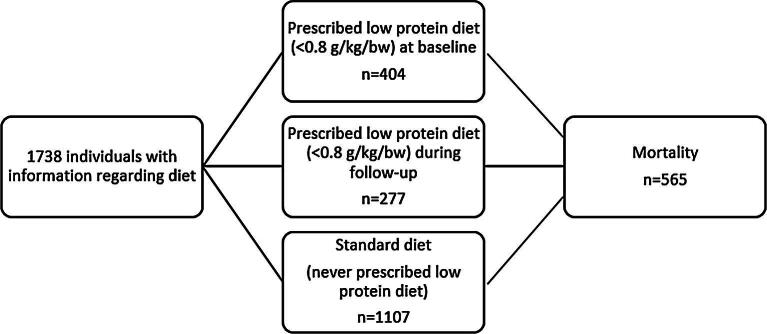
Among the 1738 patients, 631 patients (36%) were prescribed a LPD at some point during the follow-up (Fig. 1). Of these patients, 363 were prescribed a LPD with ≤0.6 g/kg bodyweight/day, of which 38 patients were prescribed a very-low-protein diet (0.3–0.4 g/kg). In total, 404 (23%) had a LPD at the first visit when included in the study.
Figure 1:
Description of the exposure, prescribed diet.
Decline in nutritional status
During follow-up, 268 (20%) patients declined in the 7-point SGA by ≥2 points and 342 (26%) died before they reached the SGA endpoint. The crude incidence rate over 4 years per 100 person-years was 7.7 (IQR 6.7–8.9) in those with a standard diet and 10.9 (IQR 9.2–13.2) in individuals prescribed a LPD (≤0.8 g/kg) (Table 2). In the group prescribed ≤0.6 g/kg, the incidence rate was 9.8 (IQR 7.6–12.8) (Table 2). The adjusted odds ratio (OR) for a decrease in the 7-pooint SGA was 1.11 (IQR 0.74–1.66) in those prescribed a LPD ≤0.8 g/kg compared with a standard diet. There was a statistically significant interaction (P < .05) between the risk of nutritional status decline and LPD treatment for age and comorbidity, while the interaction term was not consistent for LPD 0.8 g/kg or ≤0.6 g/kg for sex and diabetes (Supplementary Table S3). The results indicated a higher risk of a SGA decrease in those >75 years of age and a higher comorbidity score, but the individual subgroups did not reach statistical significance.
Table 2:
Risk of decrease in SGA in older adults prescribed a LPD according to prescription and measured protein intake over the follow-up period.
| Prescribed diet (n = 1319) | Number of events/person-years | Incidence rate over 4 years per 100 person-years (IQR) | Unadjusted OR (95% CI) | Adjusted ORa (95% CI) |
|---|---|---|---|---|
| Standard diet (>0.8 g protein/kg) (n = 1004) | 196/2529 | 7.7 (6.7–8.9) | Ref | Ref |
| LPD ≤0.8 g/kg (n = 483) | 117/1065 | 10.9 (9.2–13.2) | 1.43 (1.11–1.86) | 1.11 (0.74–1.66) |
| LPD ≤0.6 g/kg (n = 288) | 56/569 | 9.8 (7.6– 12.8) | 1.65 (1.23–2.22) | 1.31 (0.85–2.01) |
| Measured diet (n = 528) | Incidence rate per 100 person-years (IQR) | Unadjusted RR (95% CI) | Adjusted RRb (95% CI) | |
| Standard diet, adherent (n = 227)b | 30/453 | 6.6 (4.6–9.5) | ref | ref |
| LPD ≤0.8 g/kg, adherent (n = 194)b | 37/420 | 8.8 (6.4–12.1) | 1.77 (0.85–3.72) | 1.67 (0.75–3.77) |
| LPD ≤0.8 g/kg, non-adherent (n = 219)b | 47/505 | 9.3 (7.0–12.4) | 1.37 (0.70–2.66) | 0.95 (0.50–1.78) |
| Standard diet, spontaneously low protein intake (n = 139)b | 27/266 | 10.1 (7.0–14.8) | Ref | Ref |
| LPD ≤0.8 g/kg, adherent (n = 164)b | 33/374 | 8.8 (6.3–12.4) | 0.82 (0.40–1.67) | 0.77 (0.30–2.01)c |
Ref; reference; RR: relative risk.
Dynamic inverse probability weighted analysis. Probability of treatment weights and stabilized censoring weights included baseline sex, country, Charlson comorbidity index, diabetes, ischaemic heart disease, peripheral arterial disease, heart failure, cancer, education, marital status, smoking, alcohol intake, time-updated age, eGFR, BMI, haemoglobin, albumin, urea, potassium, phosphate, systolic and diastolic BP and time (natural cubic spline with 3 knots). The outcome model further included age at inclusion, sex, country, comorbidity (diabetes, ischaemic heart disease, stroke, heart failure, peripheral arterial disease), eGFR at inclusion, SGA, smoking, BMI, haemoglobin and albumin at inclusion and time-varying kidney replacement therapy.
Time-varying exposure resulting in a patient that may count in several categories.
Country and level of education dropped due to failure of convergence.
Among the subgroup with measured protein intake there were 528 individuals from three countries. The SGA decreased by >2 points with an incidence of 6.6 per 100 person-years [95% confidence interval (CI) 4.6–9.5] in those with ‘standard diet—adherent’ as compared with patients with a spontaneously low protein intake [incidence 10.1 per 100 person-years (95% CI 7.0–14.8)]. As compared with patients with ‘standard diet—adherent’, the adjusted OR was 1.67 (95% CI 0.75–3.77) for a LPD ≤0.8 g/kg adherent and 0.95 (95% CI 0.50–1.78) for LPD ≤0.8 g/kg non-adherent. Comparison with patients with a spontaneously low protein intake, the OR was 0.77 (95% CI 0.30–2.01) for patients with LPD ≤0.8 g/kg adherent. The cumulative incidence of SGA decrease for patients with a spontaneously low protein intake versus standard diet adherent is illustrated in Fig. 2. As compared with a standard diet adherent, a spontaneously low protein intake was associated with a SGA decrease in the unadjusted analyses [hazard ratio (HR) 1.81 (95% CI 1.03–3.2)], but not in analyses adjusted for age, sex, eGFR and country [HR 1.54 (95% CI 0.82–2.87)] (data not shown).
Figure 2:
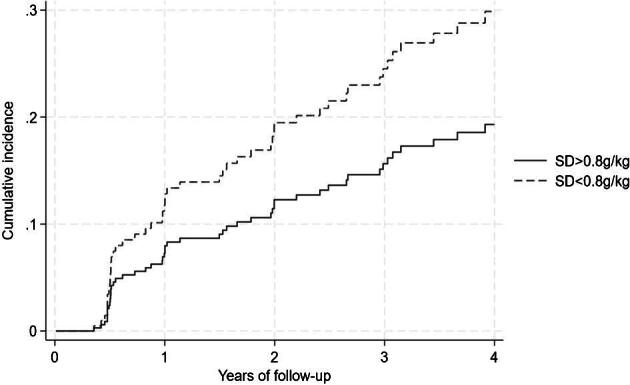
Cumulative incidence for decrease in the 7-point SGA in patients with spontaneously low protein intake (prescribed standard diet, but low protein intake <0.8 g/kg/day) and a standard diet with standard intake of protein (>0.8 g/kg/day).
Mortality
During follow-up, 565 (32.5%) patients died. The mortality rate (crude incidence rate per 100 person-years) was 11.2 (95% CI 10.1–12.4) in those with a standard diet, 12.5 (95% CI 10.8–14.4) in those prescribed a LPD ≤0.8 g/kg and 12.3 (95% CI 10.1–14.9) in those prescribed a LPD ≤0.6 g/kg (Table 3). As compared with a standard diet, the adjusted OR for all-cause mortality was 1.15 (95% CI 0.86–1.55) for those prescribed a LPD ≤0.8 g/kg and 1.01 (95% CI 0.73–1.40) for a LPD ≤0.6 g/kg (Table 3). There was a statistically significant interaction (P < .05) between age, comorbidity and 7-pooint SGA, suggesting a higher risk for patients >75 years of age treated with LPD, a higher Charlson comorbidity score (>6 points) or lower nutritional status (7-point SGA <6). However, none of the risk estimates for the individual subgroups reached statistical significance (Supplementary Table S3).
Table 3:
Mortality risk in older adults prescribed a LPD, according to prescription and measured protein intake over the follow-up period.
| All-cause mortality | ||||
|---|---|---|---|---|
| Prescribed diet (n = 1738) | Number of events/person-years | Crude incidence rate per 100 person-years (IQR) | Unadjusted OR | Adjusted ORa |
| Standard diet (n = 1329b) | 368/3298 | 11.2 (10.1–12.4) | Ref | Ref |
| LPD ≤0.8 g/kg (n = 631b) | 191/1529 | 12.5 (10.8–14.4) | 1.10 (0.93–1.31) | 1.15 (0.86–1.55) |
| LPD ≤0.6 g/kg (n = 363b) | 99/808 | 12.3 (10.1–14.9) | 1.06 (0.85–1.31) | 1.01 (0.73–1.40) |
| Restricted analysis according to measured protein intake (n = 778)b | All-cause mortality | |||
| Standard diet, adherent (n = 280) | 47/593 | 7.9 (5.9–10.6) | Ref. | Ref. |
| LPD ≤0.8 g/kg, adherent (n = 229) | 68/669 | 12.4 (9.8–15.7) | 1.49 (1.03–2.16) | 0.81 (0.46–1.43) |
| LPD ≤0.8 g/kg, non-adherent (n = 280) | 77/697 | 11.0 (8.8–13.8) | 1.33 (0.92–1.92) | 0.97 (0.60–1.58) |
| Standard diet, spontaneously low protein intake (n = 169) | 41/361 | 11.4 (8.4–15.4) | Ref. | Ref. |
| LPD ≤0.8 g/kg, adherent (n = 180) | 56/464 | 12.1 (9.3–15.7) | 1.04 (0.71–1.53) | 1.23 (0.68–2.20) |
Ref: reference.
Dynamic inverse probability of treatment weights and stabilized censoring weights included baseline sex, country, Charlson comorbidity index, diabetes, ischaemic heart disease, peripheral arterial disease, heart failure, cancer, education, marital status, smoking, alcohol intake, time-updated age, eGFR, SGA, BMI, haemoglobin, albumin, urea, potassium, phosphate, systolic and diastolic BP and time (natural cubic spline with 3 knots). The outcome marginal structural model further included age at inclusion, sex, country, comorbidity (diabetes, ischaemic heart disease, stroke, heart failure, peripheral arterial disease), eGFR at inclusion, SGA, smoking, BMI, haemoglobin and albumin at inclusion and time-varying kidney replacement therapy.
Time-varying exposure resulting in a patient that may count in several categories.
In the restricted analysis according to measured protein intake (n = 778), the adjusted OR was 0.81 (95% CI 0.46–1.43) in patients with a LPD ≤0.8 g/kg adherent and 0.97 (95% CI 0.60–1.58) for patients with a LPD ≤0.8 g/kg non-adherent. The adjusted mortality rates were similar in those with a spontaneously low protein intake and a LPD ≤0.8 g/kg adherent. Sensitivity analyses where missing data were imputed and according to measured protein intake per actual bodyweight demonstrated similar results as the main analyses (Supplementary Table S4).
DISCUSSION
In this large European cohort with older CKD adults, we did not find an increased risk of nutritional status decline or mortality in patients prescribed a LPD as compared with a standard diet. However, the results suggested that there may be differences in safety depending on underlying patient characteristics. Although the individual subgroups did not reach statistical significance, there was an interaction between the risk for both mortality and nutritional status decline, indicating a higher risk with a LPD in patients >75 years of age and those with a higher comorbidity burden. Our results further suggested that patients with a spontaneously low protein intake had a higher risk of a decline in nutritional status compared with patients who adhered to a standard diet.
There are limited data on the long-term use of a LPD in elderly patients with CKD and its association with safety outcomes. Brunori et al. [24] studied mortality in a prospective, randomized controlled trial that included 112 patients >70 years of age. They compared individuals with a very-low-protein diet and patients on dialysis over a 48-month follow-up period. The study concluded that a very-low-protein diet was safe and postponed dialysis treatment by a median of 10.7 months. In a National Health and Nutrition Examination Survey study, a high protein intake was associated with mortality in people with an eGFR <60 ml/min/1.73 m2 and a mean age of 72 years, whereas lower levels of protein intake were not associated with death [25]. Hung et al. [26] performed a study in which 103 older adults with an eGFR <45 ml/min/1.73 m2 and a LPD or standard diet were followed up to 1 year, concluding that although BMI decreased progressively, muscle mass was preserved according to bioimpedance measurements. In our subgroup analyses we found that patients on a standard diet with a spontaneously low protein intake, which often is the result of a diminished appetite, had the highest rate of decline in nutritional status, although the analyses failed to reach statistical significance. These results align with other observations where a spontaneous reduction in protein intake without careful monitoring of energy intake has been reported as harmful [27].
Usually patients prescribed a LPD are carefully monitored by the nephrologist and dietitian, who also consider the total energy intake and assess clinical signs of PEW [10]. Even if the patients do not completely adhere to the prescribed diet, the nutritional counselling and monitoring itself might be beneficial. In a study by Perrez-Torres et al. [28], a nutritional education program during the pre-dialysis phase showed positive effects on nutritional status, decreased hospital admissions and increased survival in patients starting dialysis. According to KDOQI guidelines, it is important to consider in what context the LPD is initiated [1]. If the patients are metabolically instable or suffer from acute disease, a LPD should not be prescribed. In line with these recommendations, we observed important interactions for older versus younger age and higher versus lower comorbidity burden. For mortality, there was also an interaction for baseline SGA assessment. Although none of the point estimates of these subgroups reached the significance level, possibly due to the sample size, the results call for caution in treating patients >75 years of age, with a higher comorbidity burden and a lower SGA score with LPD.
The major strength of this study is the large population with incident advanced CKD from six countries with extensive, prospectively collected, repeated clinical data, making the results generalizable to the clinical practice of nephrology care in Europe. Furthermore, the patients in our study were included when their eGFR dropped below the pre-defined level of 20 ml/min/1.73 m2, thus minimizing the risk of survivor bias. The use of rich, prospectively collected information made it possible to analyse the data using marginal structural models with dynamic inverse probability weights. This type of analysis considers not only informative censoring, but also time-varying confounding. The decision to start a patient on a LPD is often influenced by current kidney function and interlinked uraemic symptoms, metabolic disturbances and nutritional status. Since these factors also influence future prognosis, time-varying confounding (or reverse causation) will be present unless it is considered in the analyses.
Another strength is our use of a repeated 7-point SGA to evaluate nutritional status. There are several methods to measure dietary adherence in CKD [1]. Subjective approaches such as food diaries and food frequency questionnaires are often used. In our study we used a combination of information of prescribed diet directly from the nephrologists’ questionnaire and calculations based on a repeated urea nitrogen levels from 24-hour urine collections, which are regarded as an objective, relatively unbiased method to assess protein intake. Since there is no international standard on which bodyweight should be used when prescribing protein level (real bodyweight regardless of BMI or adjusted body weight), we standardized our measured protein intake to a normal bodyweight with a BMI of 23 kg/m2. However, changing the estimations to real bodyweight did not meaningfully change the results.
Our study has also various limitations that should be addressed. As in all observational studies, we cannot confer causality, and although we have used all available data for adjustment in our models, there may still be residual confounding present. Another limitation is that the time points for collecting information on prescribed diet were fixed and the actual start of changes in a prescribed diet for the individual patient may have occurred between two study visits. However, in our subgroup analyses with measured protein intake, this should have been accounted for at least to some extent since measurements were repeated. Furthermore, we examined the protein intake and not the source of the proteins. Recent data suggest that protein from red meat and fish may have different effects on the progression of CKD [29]. Furthermore, renal care and the routine of nutritional management may differ between participating countries. By tradition, Italy, Sweden, and The Netherlands use a LPD to treat CKD patients, whereas Poland, Germany and the UK seldom do. When prescribing a LPD, the recommendation is to increase the intake of fat and carbohydrates to maintain the energy balance. However, the procedures for nutritional counselling and evaluating the energy and nutrient intake in the clinical setting may differ between the participating countries and are not standardized in the study protocol.
In conclusion, this study suggests that a traditional LPD prescribed and monitored according to routine clinical practice in Europe is a safe treatment for older adults with CKD. In people with a higher risk profile, such as the very old and those with a large comorbidity burden, the benefits of a protein-restricted diet should be carefully weighed against the potential risks of accelerated nutritional status decline. Since our study further suggests that a spontaneously low protein intake is associated with higher risk of nutritional status decline compared with adherence to a standard diet, our results further underscore the importance of monitoring the nutritional status and diet over time regardless of the dietary regimen.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all the patients and health professionals participating in the EQUAL study.
Contributor Information
Karin Windahl, Division of Renal Medicine, Department of Clinical Intervention and Technology, Karolinska Institutet, Stockholm, Sweden; Division of Clinical Nutrition and Dietetics, Department of Orthopedics, Danderyds Hospital, Stockholm, Sweden.
Nicholas C Chesnaye, ERA Registry, Amsterdam UMC location University of Amsterdam, Medical Informatics, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, The Netherlands.
Gerd Faxén Irving, Division of Clinical Geriatrics, Department of Neurobiology, Care Science and Society, Karolinska Institutet, Stockholm, Sweden.
Peter Stenvinkel, Division of Renal Medicine, Department of Clinical Intervention and Technology, Karolinska Institutet, Stockholm, Sweden.
Tora Almquist, Division of Nephrology, Department of Clinical Sciences, Danderyds Hospital, Stockholm, Sweden.
Maarit Korkeila Lidén, Division of Renal Medicine, Department of Clinical Intervention and Technology, Karolinska Institutet, Stockholm, Sweden.
Christiane Drechsler, Department of Medicine, Division of Nephrology, University Hospital of Würzburg, Würzburg, Germany.
Maciej Szymczak, Department of Nephrology and Transplantation Medicine, Wroclaw Medical University, Wroclaw, Poland.
Magdalena Krajewska, Department of Nephrology and Transplantation Medicine, Wroclaw Medical University, Wroclaw, Poland.
Esther de Rooij, Department of Clinical Epidemiology, Leiden University Medical Centre, Leiden, The Netherlands.
Claudia Torino, 4CNR-IFC, Clinical Epidemiology and Physiopathology of Renal Diseases and Hypertension, Reggio Calabria, Italy.
Gaetana Porto, G.O.M., Bianchi Melacrino Morelli, Reggio Calabria, Italy.
Fergus J Caskey, Department of Renal Medicine, North Bristol NHS Trust, Bristol, UK; Population Health Sciences, University of Bristol, Bristol, UK.
Christoph Wanner, Department of Clinical Research and Epidemiology, Comprehensive Heart Failure Center, University Hospital of Würzburg, Würzburg, Germany.
Kitty J Jager, ERA Registry, Amsterdam UMC location University of Amsterdam, Medical Informatics, Amsterdam, The Netherlands; Amsterdam Public Health Research Institute, Quality of Care, Amsterdam, The Netherlands.
Friedo W Dekker, Department of Clinical Epidemiology, Leiden University Medical Centre, Leiden, The Netherlands.
Marie Evans, Division of Renal Medicine, Department of Clinical Intervention and Technology, Karolinska Institutet, Stockholm, Sweden.
the EQUAL study investigators:
Karin Windahl, Nicholas C Chesnaye, Gerd Faxén Irving, Peter Stenvinkel, Tora Almquist, Maarit Korkeila Lidén, Christiane Drechsler, Maciej Szymczak, Magdalena Krajewska, Esther de Rooij, Claudia Torino, Gaetana Porto, Fergus J Caskey, Christoph Wanner, Kitty J Jager, Friedo W Dekker, and Marie Evans
FUNDING
Funding was received from the European Renal Association–European Dialysis and Transplant Association, the Swedish Medical Association, the Stockholm County Council ALF, Njurfonden (Sweden), Center for Innovative Medicine, the Italian Society of Nephrology, the Dutch Kidney Foundation (SB 142), a Young Investigators grant in Germany and the National Institute for Health Research in the UK.
AUTHORS’ CONTRIBUTIONS
K.W. and M.E. were responsible for the study conception and design. M.E. was responsible for statistical analyses. K.W. wrote the first draft of manuscript. All authors were responsible for data acquisition, critical revision of the manuscript and final approval of the version for publication.
DATA AVAILABILITY STATEMENT
The data underlying this article are sensitive health data and cannot be shared publicly for privacy reasons. The data will be shared upon reasonable request to the corresponding author.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Ikizler TA, Burrowes JD, Byham-Gray LDet al. KDOQI clinical practice guideline for nutrition in CKD: 2020 update. Am J Kidney Dis 2020;76(3 Suppl 1):S1–107. 10.1053/j.ajkd.2020.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Ku E, Kopple JD, Johansen KLet al. Longitudinal weight change during CKD progression and its association with subsequent mortality. Am J Kidney Dis 2018;71:657–65. 10.1053/j.ajkd.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Windahl K, Faxén Irving G, Almquist Tet al. Prevalence and risk of protein-energy wasting assessed by subjective global assessment in older adults with advanced chronic kidney disease: results from the EQUAL study. J Ren Nutr 2018;28:165–74. 10.1053/j.jrn.2017.11.002 [DOI] [PubMed] [Google Scholar]
- 4.Chesnaye NC, Dekker FW, Evans Met al. Renal function decline in older men and women with advanced chronic kidney disease-results from the EQUAL study. Nephrol Dial Transplant 2021;36:1656–63. 10.1093/ndt/gfaa140.MO074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Windahl K, Irving GF, Almquist Tet al. Patient-reported measures and lifestyle are associated with deterioration in nutritional status in CKD stage 4-5: the EQUAL cohort study. J Ren Nutr 2022;32:161–9. [DOI] [PubMed] [Google Scholar]
- 6.Bowling CB, Muntner P. Epidemiology of chronic kidney disease among older adults: a focus on the oldest old. J Gerontol A Biol Sci Med Sci 2012;67:1379–86. 10.1093/gerona/gls173 [DOI] [PubMed] [Google Scholar]
- 7.Clyne N. Caring for older people with chronic kidney disease—primum non nocere. Nephrol Dial Transplant 2021;36:953–6. 10.1093/ndt/gfaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrero JJ, Thomas F, Nagy Ket al. Global prevalence of protein-energy wasting in kidney disease: a meta-analysis of contemporary observational studies from the International Society of Renal Nutrition and Metabolism. J Ren Nutr 2018;28:380–92. 10.1053/j.jrn.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 9.Volkert D, Beck AM, Cederholm Tet al. ESPEN guideline on clinical nutrition and hydration in geriatrics. Clin Nutr 2019;38:10–47. 10.1016/j.clnu.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 10.Eyre S, Faxén-Irving G, Attman POet al. A practical approach to low protein diets in Sweden- 45 years of clinical use. BMC Nephrol 2016;17:89. 10.1186/s12882-016-0295-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccoli GB, Di Iorio BR, Chatrenet Aet al. Dietary satisfaction and quality of life in chronic kidney disease patients on low-protein diets: a multicentre study with long-term outcome data (TOrino-Pisa study). Nephrol Dial Transplant 2020;35:790–802. [DOI] [PubMed] [Google Scholar]
- 12.Rhee CM, Wang AY, Biruete Aet al. Nutritional and dietary management of chronic kidney disease under conservative and preservative kidney care without dialysis. J Ren Nutr 2023;33(6 Suppl):S56–66. 10.1053/j.jrn.2023.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carrero JJ, González-Ortiz A, Avesani CMet al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat Rev Nephrol 2020;16:525–42. 10.1038/s41581-020-0297-2 [DOI] [PubMed] [Google Scholar]
- 14.Bellizzi V, Cupisti A, Locatelli Fet al. Low-protein diets for chronic kidney disease patients: the Italian experience. BMC Nephrol 2016;17:77. 10.1186/s12882-016-0280-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee CM, Ahmadi SF, Kovesdy CPet al. Low-protein diet for conservative management of chronic kidney disease: a systematic review and meta-analysis of controlled trials. J Cachexia Sarcopenia Muscle 2018;9:235–45. 10.1002/jcsm.12264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira RA, Alvarenga MS, Avesani CMet al. Strategies designed to increase the motivation for and adherence to dietary recommendations in patients with chronic kidney disease. Nephrol Dial Transplant 2021;36:2173–81. 10.1093/ndt/gfaa177 [DOI] [PubMed] [Google Scholar]
- 17.Piccoli GB, Cederholm T, Avesani CMet al. Nutritional status and the risk of malnutrition in older adults with chronic kidney disease—implications for low protein intake and nutritional care: a critical review endorsed by ERN-ERA and ESPEN. Clin Nutr 2023;42:443–57. 10.1016/j.clnu.2023.01.018 [DOI] [PubMed] [Google Scholar]
- 18.Jager KJ, Ocak G, Drechsler Cet al. The EQUAL study: a European study in chronic kidney disease stage 4 patients. Nephrol Dial Transplant 2012;27:iii27–31. 10.1093/ndt/gfs277 [DOI] [PubMed] [Google Scholar]
- 19.Maroni BJ, Steinman TI, Mitch WE. A method for estimating nitrogen intake of patients with chronic renal failure. Kidney Int 1985;27:58–65. 10.1038/ki.1985.10 [DOI] [PubMed] [Google Scholar]
- 20.Visser R, Dekker FW, Boeschoten EWet al. Reliability of the 7-point subjective global assessment scale in assessing nutritional status of dialysis patients. Adv Perit Dial 1999;15:222–5. [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fewell Z, Hernán MA, Wolfe Fet al. Controlling for time-dependent confounding using marginal structural models. Stata J 2004;4:402–20. 10.1177/1536867X0400400403 [DOI] [Google Scholar]
- 23.Hernán MA, Lanoy E, Costagliola Det al. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol 2006;98:237–42. 10.1111/j.1742-7843.2006.pto_329.x [DOI] [PubMed] [Google Scholar]
- 24.Brunori G. Treatment of chronic kidney disease in the elderly: diet or conservative management. J Nephrol 2012;25(Suppl 19):S28–31. 10.5301/jn.5000143 [DOI] [PubMed] [Google Scholar]
- 25.Narasaki Y, Okuda Y, Moore LWet al. Dietary protein intake, kidney function, and survival in a nationally representative cohort. Am J Clin Nutr 2021;114:303–13. 10.1093/ajcn/nqab011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hung KY, Chiou TT, Wu CHet al. Effects of diet intervention on body composition in the elderly with chronic kidney disease. Int J Med Sci 2017;14:735–40. 10.7150/ijms.19816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol 2013;24:337–51. 10.1681/ASN.2012010047 [DOI] [PubMed] [Google Scholar]
- 28.Pérez-Torres A, González García ME, Ossorio-González Met al. The effect of nutritional interventions on long-term patient survival in advanced chronic kidney disease. Nutrients 2021;13:621. 10.3390/nu13020621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai L, Massy ZA, Stenvinkel Pet al. The association between TMAO, CMPF, and clinical outcomes in advanced chronic kidney disease: results from the European QUALity (EQUAL) Study. Am J Clin Nutr 2022;116:1842–51. 10.1093/ajcn/nqac278 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are sensitive health data and cannot be shared publicly for privacy reasons. The data will be shared upon reasonable request to the corresponding author.