Abstract
Background
Adherence to oral endocrine therapy in adjuvant breast cancer settings is a substantial clinical problem.
Methods
To provide current perspective on adherence to oral endocrine therapies, a comprehensive literature review was conducted.
Results
In adjuvant trials, endocrine therapy adherence is relatively high with greater adherence for aromatase inhibitors compared to tamoxifen. In contrast, adherence to adjuvant therapy in clinical practice is relatively poor, with only about 50% of women successfully completing five years therapy. Importantly, good adherence (> 80% use), has been associated with lower recurrence risk. Endocrine therapy adherence in primary breast cancer prevention trials parallels that seen in adjuvant trials. Factors associated with non-adherence include low recurrence risk perception, side effects, age extremes, medication cost, suboptimal patient-physician communication, and lack of social support. Few prospective studies have evaluated interventions designed to improve adherence. Interventions currently proposed reflect inferences from clinical trial procedures where clinical contacts are commonly greater than in usual practice settings.
Conclusions
For optimal breast cancer outcome, adherence to endocrine therapy must improve. While general recommendations likely to improve adherence can be made based on clinical trial results and preliminary prospective trial findings, research specifically targeting this issue is needed to establish effective intervention strategies.
Adherence to oral endocrine therapy for adjuvant breast cancer treatment and for breast cancer prevention are substantial problems for clinicians and healthcare systems (1, 2, 3). In the adjuvant setting, adherence to endocrine therapy now takes on greater importance given reports that adjuvant tamoxifen of greater than five years duration is associated with lower recurrence risk (4, 5).
Adherence is defined as a composite of compliance (how well physician’s orders are followed) and persistence (how long an individual continues on prescribed therapy) (6). Currently no gold standard method exists for adherence measurement. Adherence can be estimated from prescription and medical claims and pharmacy databases, medical record review, hospital databases, pill counts, patient self-reports, prospective studies, and, rarely, pharmacologic assessments of drug concentrations. Methodological concerns were raised by a study of 242 patients where correlations of only 0.2 to 0.4 was seen comparing endocrine therapy adherence estimates from self-report, physician rating, refill records, and anastrozole concentrations (7). Other studies found breast cancer patients overestimated adherence to tamoxifen based on prescription checks (8) or microelectronic monitoring (9). In this regard, in a report comparing non-adherence to adjuvant anastrozole using three separate databases in the same population, estimates of non-adherence varied from 32% to 50% (10); however, subjects in these databases had variable medical insurance coverage which may partially explain adherence differences. Despite these concerns, consistent general conclusions have emerged from studies using various methods of adherence assessment.
Adjuvant endocrine therapy adherence in clinical trials and clinical practice
Clinical adjuvant endocrine therapy trials, where adherence is commonly closely monitored, did not suggest a major adherence problem. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) B-14 trial in breast cancer patients receiving adjuvant tamoxifen or placebo, discontinuation rates were 23% in both groups at 60 months median follow-up (11). In the NSABP B-24 adjuvant intraductal breast cancer trial, 60 month discontinuation rates for placebo were 30% compared to 33% for tamoxifen (12).
The seminal reports by Partridge and colleagues (1, 2) brought attention to the issue of poor adjuvant tamoxifen adherence in clinical practices. In a retrospective analysis of prescription claims (Medicaid and Pharmaceutical Assistance to Aged and Disabled [PAAD]) databases from the years 1990 to 1996, adherence to adjuvant tamoxifen was 83% after one year, 68% after two years, 61% after three years, and only 50% after four years. Other studies also found that more than half of breast cancer patients discontinue endocrine therapy prior to completion of a recommended five-year treatment (13, 14). For aromatase inhibitors, the commonly experienced arthralgias raise particular adherence concerns (15, 16, 17). However, in several adjuvant clinical trials, adherence to aromatase inhibitors was closely comparable, or even superior to tamoxifen. In the Arimidex, Tamoxifen, Alone and Combined (ATAC) trial after five years, 2.1% of the anastrozole-treated patients and 14.3% of the tamoxifen-treated patients had discontinued use due to adverse events (18). Similar adherence was seen in both treatment groups in adjuvant trials comparing the aromatase inhibitor, exemestane, to tamoxifen (14% discontinued therapy in both arms) (19) and the aromatase inhibitor, letrozole, to placebo (only 10% discontinued therapy in both arms) (20).
Recent systematic reviews on adherence and/or persistence to adjuvant endocrine therapy in clinical practice settings identified 29 reports. Adherence in tamoxifen users ranged from 41% to 88%. While adherence in aromatase inhibitor users ranged from 50% to 91% (21). These findings were extended by Huiart and colleagues (22) who conducted meta-regression analyses to provide summary estimates of non-persistence in 17 trials. For tamoxifen, 5-year nonpersistence was 47.2% (95% CI 41.1%–53.5%) compared to 31.0% (95% CI 25.9–37.5%) for aromatase inhibitors (Table 1).
Table 1.
Systematic Reviews of Adherence to Adjuvant Endocrine Therapy
| Tamoxifen | Aromatase inhibitor | |
|---|---|---|
| Adherence (range)1,2 | 41% to 88% | 52% to 91% |
| Therapy discontinuation (range)1 | 15% to 20% within year 1 | 5% to 25% within 2 years |
| 5 year therapy discontinuation from meta-regression analysis2 | 47.2% (95% CI, 41.1% to 53.5%)0 | 31.0% (95% CI, 25.9 % to 37.5%) |
Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Research Treat 2012; 134 (2): 459–78.
Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: a summarizing the data for clinicians. Breast Cancer Res Treat 2013 Feb 3 [Epub ahead of print].
The findings are somewhat mixed considering aromatase inhibitor adherence in clinical practices (23, 24); however, in the United Kingdom (UK) general practice database, the one year discontinuation rate for adjuvant aromatase inhibitor use was 5% compared to about 10% for tamoxifen in women > 49 years old and 20% for tamoxifen in women < 40 years old (25). Similarly, in the Disease Analyses database (IMS Health, Germany), among 16,865 breast cancer patients, 3 year discontinuation rates were 52% for tamoxifen, 47% for anastrozole and 44% for letrozole (26). A randomized adjuvant adherence trial found shorter time to treatment discontinuation for exemestane, compared to letrozole (HR 1.5, 95% CI 1.1–2.1) (27).
In summary, a substantial problem regarding adherence and persistence to adjuvant endocrine therapy remains in clinical practice. Somewhat surprisingly, adherence to aromatase inhibitors has been similar or superior to adherence to tamoxifen in several settings.
Adjuvant endocrine therapy adherence and clinical outcome
Evidence that adherence to adjuvant endocrine therapy could influence clinical outcomes came from a series of randomized adjuvant breast cancer trials evaluating duration of tamoxifen use. As summarized In Early Breast Cancer Trialist Cooperative Group (EBCTCG) analyses, compared to no therapy/placebo, with tamoxifen for one year, reduction was 27% for 2 years, reduction was 33%; and for 5 years, reduction was 47%, P trend < 0.00001(28).
Adherence to adjuvant tamoxifen therapy and breast cancer outcome has been examined in several cohort studies. In a U.S. cohort of 1,837 older women with early-stage breast cancer, those who used tamoxifen less than one year had substantially higher breast cancer mortality than those who used the drug for five or more years (HR 6.26, 95% CI 3.10–12.64) (29). Similar findings were reported from a Scottish cohort of 2,080 early-stage breast cancer patients. In that study, tamoxifen adherence < 80% was associated with increased mortality (HR 1.100, 95% CI 1.001–1.21) (30).
In the managed care Kaiser Permanente Northern California population, among 8,769 breast cancer patients, 2,761 (31%) discontinued therapy within 6 months of diagnosis (based on automated pharmacy records); of those who continued, 1,684 (28%) were non-adherent (possession ratios <80%; defined as days with index prescription supplies/total days of follow-up). The survival at 10 years was 80.7% and 73.6% for those who continued therapy compared to those who discontinued therapy, respectively (P < 0.001) (31). Of those who continued therapy, survival was 81.7% in those adherent to therapy, compared to 73.6% in those non-adherent. In a similar study in Kaiser Permanente Southern California, although breast cancer recurrence was lowest in women with greater adherence (possession ratios >80%), the rates were not markedly different from women with less regular use (32). In a retrospective cohort study of 3,361 Scottish breast cancer patients, low adherence of < 80% to adjuvant tamoxifen in aromatase inhibitor was associated with poor survival (HR 1.20 95% CI 1.03–1.40, p = 0.019) (33).
In a prospective cohort of 417 localized breast cancer patients in Sweden, non-adherence at one year was associated with increased early breast cancer events (HR 2.97,95% CI 1.08–8.15) (34). In a study with 857 low-income women with early breast cancer, more recurrences and cancer-deaths were observed in women non-adherent to endocrine therapy, but the results were not statistically significant (35). Similarly, in a study of 690 women International Breast Cancer Study Group trials 13–39 and 14–93, those with ≥ 4 years SERM use had longer disease-free survival compared to those with < 4 year use (71% vs. 64%, HR 1.31, 95% CI 0.86–1.98, p = 0.20) (36) (Table 2). In a small study of 116 men with breast cancer overall survival was greater in those adherent to tamoxifen adjuvant therapy (37).
Table 2.
Studies Relating Duration of and/or Adherence to Adjuvant Endocrine Therapy to Breast Cancer Outcome
| Lead Author | Study | Findings |
|---|---|---|
| EBCTCG – Early Breast Cancer Trialist Collaborative Group 2001 | Overview analyses of randomized clinical trials evaluating duration of tamoxifen use | Tamoxifen duration 1 year, recurrence reduced 27%; tamoxifen duration 2 years, recurrence reduced 33%; tamoxifen duration 5 years, recurrence reduced 47%; P=trend < 0.00001 |
| Yood 2008 | Cohort of 1,837 US early stage breast cancer patients ≥ 65 years old | Adjuvant tamoxifen < 1 year vs. ≥ 5 years with higher breast cancer mortality (HR 6.26, 95% CI: 3.10–12.64) |
| McCowan 2008 | Resospective cohort of 2080 Scotish early stage breast cancer patients | Adherence to tamoxifen < 80% associated with poorer survival (HR 1.10, 95% CI: 1.001–1.21) |
| Hershman 2010 | Northern California Kaiser Permanente cohort of 8769 women with early stage, hormone-sensitive breast cancer and endocrine therapy adherence (drug availability) | 31% discontinued therapy, 10 years survival was 73.6% 69% continued therapy, 10 year survival was 80.7%; P<0.001 |
| Xu 2012 | Cohort of 116 men with early stage, hormone sensitive breast cancer and hormone therapy adherence | For those adherent, 10 year survival was 79.6%; For those non-adherent, 10 year survival was 50.5%, P=0.008 |
| Markula 2012 | Prospective cohort of 417 patients with early stage breast cancer Sweden and adherence (self-report) to adjuvant endocrine therapy | Non-adherence at the 1-year visit associated with increased early breast cancer events HR 2.97, 95% CI 1.08–8.15 |
| Haque 2012 | Southern California Kaiser Permanente cohort of 22,850 women with early stage breast cancer and endocrine therapy adherence (drug availability) | Women with high adherence had greater recurrence risk reduction (e.g., HR=0.42, 95% CI: 0.36–0.47 for tamoxifen) compared to those with less adherence (HR=0.46, 95% CI: 0.41–0.52 for tamoxifen) but the difference was not statistically significant. |
| Pagani 2013 | International Breast Cancer Study Group trials 13–93 and 14–93 with 690 women with early stage breast cancer or SERM’s | Women with ≥ 4 years of SERM had longer 10-year disease-free survival (71%) compared to < 4 years use (64%), p value = 0.20 |
Thus, lack of adherence and persistence to prescribed endocrine adjuvant therapy represents a barrier to achieving favorable outcomes for breast cancer patients. The magnitude of the benefit of being adherent to adjuvant endocrine therapy is comparable to that seen with the addition of adjuvant chemotherapy.
Adjuvant endocrine therapy adherence and the oncologist
Emerging data suggests that a substantial proportion of women who qualify for adjuvant endocrine therapy are not receiving this intervention. In a population of 13,753 early stage hormone-receptor positive breast cancer patients in the managed care Kaiser Permanente Northern California group, studied within one area year of diagnosis, 30% of women did not initiate endocrine adjuvant therapy defined as having < 2 prescriptions for tamoxifen or aromatase inhibitor filled within the first year after the cancer diagnosis (38). In the Kaiser Permanente Southern California population of breast cancer survivors, nearly 24% (3,237/13,412) of patients with estrogen receptor positive disease did not use endocrine therapy (or had discontinued treatment within six months) despite having pharmacy coverage (32). This finding stimulated that organization to implement a medication adherence tool in the electronic medical records to potentially improve adherence. In the Women’s Health Initiative cohort, in 3,588 patients with hormone receptor positive, early-stage invasive breast cancer evaluated within five years of diagnosis by survey questionnaire, while adjuvant endocrine therapy use was reported by 83%,17% reported no use. In their response, women cited “lack of physician recommendation” as the most common reason for non-use. (39). Finally, 743 patients, identified from SEER registries, eligible for adjuvant endocrine therapy were surveyed four years after diagnosis, surprisingly 10.8% never initiated therapy, and 15.1% started therapy but discontinued before four years (40) (Table 3). While detailed information on the characteristics of those not initiating adjuvant endocrine therapy are not currently available, further exploration of this issue is warranted.
Table 3.
Adjuvant Hormone Therapy Use for Hormone Receptor Positive Postmenopausal Women with Early Stage Breast Cancer
| In Women’s Health Initiative Cohort1 | Kaiser Permanente Southern California2 | In SEER Population by Survey3 | Kaiser Permanente Northern California4 |
|---|---|---|---|
| 3,588 surveyed 2009–2010 | 22,850 in years 1996–2006 | 743 surveyed in years 2005–2007 | 13,753 studied in year 1996–2007 |
| Use AI 33%, SERM 31%, mix 36% | Use: SERM 38%, 19% AI, mix 16% | Use: Endocrine 75% | Not examined |
| 17% none | 24% none | 10.8% none | 30% none |
| 33% of users became non-adherent | 21% users became non-adherent | 15.1% uses became non-adherent by year 4 | Not examined |
Livaudais J, LaCroix A, Chlebowski RT, et al. Use of and adherence to adjuvant hormonal therapy for breast cancer in the Women’s Health Initiative. Cancer Epidemiol Biomark Prev 2013;22(3):365–73.
Haque R, Ahmed SA, Fisher A, Avila CC, Shi J, Guo A, Craig Cheetham T, Schottinger JE. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012 Dec; 1(3):318–27.
Frease CR, Pini TM, Li y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat 2013;138:931–939.
Livaudais JC, Hershman DL, Habel L, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat 2012; 131(2):607–617.
Data is sparse regarding the communication between oncologists and breast cancer patients on their therapeutic plan, as it is difficult to conduct linguistic communication studies. However, one study videotaped the initial breast cancer adjuvant therapy discussion in a series of 28 early stage patients and found the issue of adherence to be poorly addressed. Much of the discussions on endocrine therapy focused on side effects and trial findings rather than on the importance of adherence (41).
Finally, a recent study found substantial discordance in adherence to adjuvant endocrine therapy when comparing results among prescription refill information, patient self-report, and oncologists’ estimates. The oncologists estimated their patient’s adherence at over 94% which was less than estimated by telephone questionnaire self-report (P=0.003), or by the pharmacy database where only 67% of women > 65 years old were identified as having drug available (P=0.0001) (42).
Adjuvant endocrine therapy adherence in long-duration clinical trials
Interest in adherence to long-term adjuvant endocrine therapy regimen was enhanced by the recent report from the worldwide Adjuvant Tamoxifen Longer Against Shorter (ATLAS) adjuvant trial where continued tamoxifen use for longer than five years reduced breast cancer recurrence (P= 0.002) and overall mortality (P= 0.01) (4). Based on self-report, five year adherence was an excellent 84% for continued tamoxifen users. In contrast, the Investigation on the Duration of Extended Adjuvant Letrozole treatment (IDEAL) trial entered 1,250 early breast cancer patients comparing 2.5 years to 5 years of extended letrozole use after 5 years of adjuvant endocrine therapy found overall non-adherence was 18.4% at 2.5 years (43). It is not clear whether this apparent difference between long term continued tamoxifen and continued aromatase inhibitor use represents real differences in tolerability, or are the result of the limited data on the aromatase inhibitors currently available. In any event, more information is needed regarding persistence to long-term aromatase inhibitor adjuvant use.
Endocrine therapy adherence in breast cancer prevention trials
Available evidence suggests that adherence to endocrine therapy in primary breast cancer prevention trial participants is similar to that seen in the adjuvant setting. In the NSABP P-1 prevention trial, discontinuation rates after 54.6 months mean follow-up were 23.7% on tamoxifen, and 19.7% on placebo (11). Discontinuation rates were somewhat higher in the International Breast Intervention Study-1(IBIS-1) where, in a primary prevention setting, the 50 month median follow-up discontinuation rate for tamoxifen was 36% compared to 26% for placebo (44). In the longer intervention duration Royal Marsden Hospital trial comparing tamoxifen to placebo, therapy was prematurely discontinued at a median of 70 months in 46% of tamoxifen and 36% of placebo participants, respectively (45). In the NSABP STAR prevention trial, 5-year adherence was 70.8% for tamoxifen and 73.9% for raloxifene (p < 0.001) (46).
The aromatase inhibitor, exemestane, has been compared to placebo for primary breast cancer prevention in the Mammary Prevention (MAP).3 trial. After median 35 months follow-up, a 65%, statistically significant, relative reduction in invasive breast cancer incidence was seen for exemestane (47). During the study, exemestane was discontinued because of “intolerable side effects” by 15.4% of participants but surprisingly, 10.8% of placebo participants discontinued study pills for the same reason. With only a net 5.3% difference, a major influence of factors other than drug side effects likely influenced the adherence results seen. A similar result was seen in the MA.17 adjuvant trial, where about 20% of breast cancer patients in the placebo group reported climactic symptoms (48). These results point to the importance of placebo controls to generate the most reliable tolerability information.
In a prevention study, adherence was related to outcome in the Women’s Health Initiative (WHI) trial of estrogen alone. In this study, when 10,739 postmenopausal women with prior hysterectomy were randomized to conjugated equine estrogen alone or placebo, surprisingly, a statistically significant, lower breast cancer incidence was seen in the estrogen alone group in intent-to-treat analyses (HR 0.77, 95% CI 0.62–0.95) (49). However, in sensitivity analyses, censoring participants with less than 80% adherence to the pill taking regimen, an even stronger association between estrogen alone use and lower breast cancer incidence was seen (HR 0.68, 95% CI 0.49–0.95).
Factors associated with non-adherence to endocrine therapy in breast cancer prevention trials
Factors predictive of tamoxifen chemoprevention non-adherence were examined in the P-1 breast cancer prevention trial. Current smokers and heavy alcohol users had lower tamoxifen adherence while obesity and lower physical activity were unrelated to adherence (50). Similar findings were seen in 100 participants in the IBIS-1 study where women with smoking history also were less likely to persist with their randomized drug (51). In addition, in the IBIS-1 trial, use of additional prescribed medication was an important factor in predicting successful completion of therapy (P = 0.04) (51). The latter findings suggest that women already using other prescription medications may represent a potentially favorable population, and thus, be more likely to accept and adhere to endocrine chemoprevention regimens. Lack of influence of obesity and low physical activity on adherence suggests factors other than an unhealthy lifestyle are related to medication discontinuation.
Endocrine therapy for prevention in clinical practice
Currently, use of the two drugs approved for chemoprevention in the US (tamoxifen and raloxifene) continues to be low (52), and, for this reason, information on adherence in clinical practice settings is not available. However, a review of a clinical experience from the Partners HealthCare System identified 2,938 women with breast lesions with atypia. Women who received no chemoprevention had 10 year breast cancer incidence of 21.3% compared to 7.5% (p<0.001) in women who did receive chemoprevention (53).
Factors associated with non-adherence to adjuvant endocrine therapy
Factors associated with non-adherence to adjuvant hormonal therapy include lack of physician recommendation (32), patient perception of low risk for recurrence (54), adverse effects of therapy (55, 56, 57), age extremes: older age (23, 58), and younger age (23, 59), medication costs (60, 61, 62), low social economic status (63), sub-optimal patient-physician communication (64), higher co-morbidity (23, 59, 62), cigarette smoking (50, 51) and lack of social support (65) (Table 4). Similar factors were associated with adherence in a low-income population in California (66). Findings regarding adherence by race/ethnicity have produced mixed results (23, 38, 67).
Table 4.
Correlates Associated with Discontinuing Endocrine Therapy or Non-Adherence
| Reason | Study |
|---|---|
| Side effects | Demissie 2001, Kahn 2007, Lash 2006, Cluze 2012 |
| Higher co-morbidity | Hershman 2010, Hadji 2013, Sedjo 2011 |
| Financial considerations or low SES | Kimmeck 2001, Neugut 2011, Liu 2013, Riley 2011 |
| Very young or older age | Hershman 2010, Owusu 2008, Land 2011 |
| Lack of physician recommendation | Davidson 2007 |
| Perception of low risk of recurrence | Fink 2004 |
| Lack of social support | Cluze 2013, Land 2011 |
| Follow-up care with general practitioner vs. oncologist | Murphy 2012 |
| African American race/ethnicity | Hershman 2010 |
| Cigarette smoking | Land 2011 |
| Presence of anxiety/depression linked to better adherence | KyverNitankis 2013, Hadji 2013 |
| Alcohol use | Land 2011 |
Many oncologists likely consider endocrine therapy side effects to be a major factor influencing therapy adherence. However, the available evidence identifies a less straight forward relationship. In breast cancer patients in the Commonly used Medications and Breast Cancer outcomes (COMBO) study, among 538 participants, 18.2% discontinued use before completing 5 years of therapy, while 25% of discontinued after < 1 years use (68). As in several prior reports, women who discontinued therapy were more likely to have been tamoxifen (43.9%) compared to aromatase inhibitor users (22.4%). Of interest, the only adverse effect significantly associated with discontinuation of both aromatase inhibitor and tamoxifen was headaches, an adverse event not commonly associated with these therapies. Such findings suggest, that while control of adverse effects is an important clinical consideration, adverse effects of endocrine therapy use may not play a major role in determining adherence and persistence to adjuvant endocrine therapy.
Factors adversely influencing adherence, perhaps in unexpected ways, are anxiety and depression. Following a breast cancer diagnosis, anxiety and depression decreases from about 50% in year one to about 15% in year five (69), a reciprocal to endocrine therapy adherence over the same period (1, 3, 70) (Figure 1). Supporting the concept that greater patient anxiety correlates with better adjuvant hormone therapy adherence are findings from the prospective COMPAS study where breast cancer patients with higher anxiety levels had better adherence to adjuvant endocrine therapy (P= 0.028) (71). In an extremely large breast cancer population from IMS HEALTH, Germany with 17,512 patients, depression (p < 0.002) was also associated with decreased risk of treatment discontinuation (26). As anxiety and depression can be linked in a cancer population, unraveling the relative contribution of these two factors on adjuvant endocrine therapy adherence requires further study. In this regard, despite early concerns, evidence from the NSABP placebo-controlled clinical prevention trial found depression was not increased by tamoxifen use (72, 73).
Figure 1.
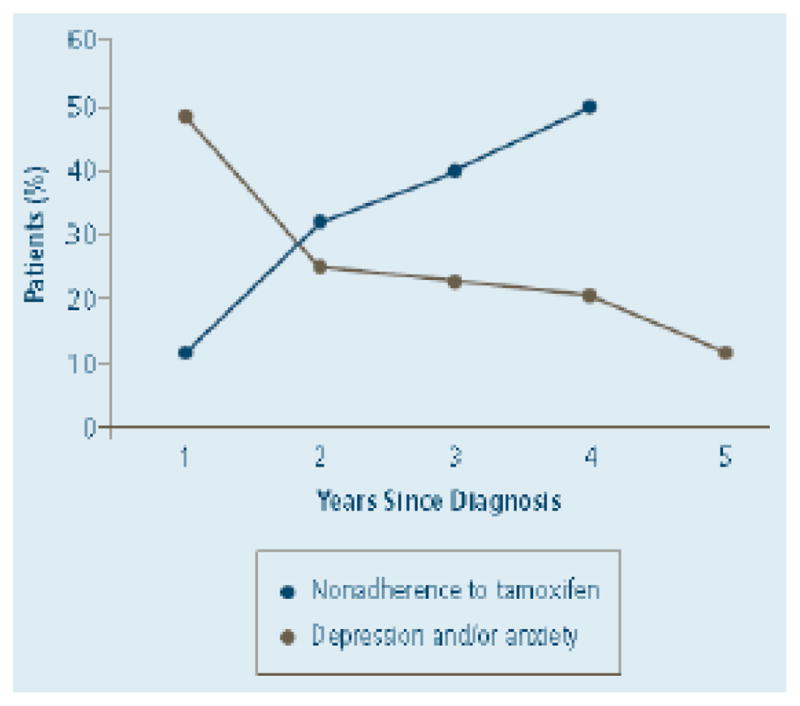
Non-adherence Rates for Adjuvant Tamoxifen Therapy in Clinical Practice and Incidence of Depression and/or Anxiety
Clinical trials to improve endocrine therapy adherence
Few prospective studies have evaluated interventions designed to improve adherence to endocrine adjuvant therapy. However, to guide future study designs, theoretical models of factors influencing adherence and persistence have been proposed (74).
While adherence to endocrine therapy in breast cancer adjuvant and prevention settings remains problematic, there are limitations to the currently available information. As reviewed (59, 75), only modest information about factors associated with continued hormone therapy use are known and, importantly, few of the factors identified are easily modifiable. In addition, current medical claims databases, commonly used in adherence analyses, contain limited information on healthcare practice patterns or patient characteristics needed to identify new potentially modifiable factors.
Despite the important influence of adjuvant endocrine therapy adherence on clinical outcome, there has only been one full scale, randomized intervention trial designed to improve adherence completed to date. The Patient’s Anastrozole Compliance to Therapy (PACT) program was a randomized, prospective, multicenter study designed to improve persistence and compliance to adjuvant endocrine therapy (76). In this trial, 4,844 patients were randomly assigned to standard therapy or standard therapy plus mailed educational materials (EM) including monthly reminders on persistence and additional letters and brochures. Questionnaires were completed before therapy was initiated, at 12 and 24 months and at treatment discontinuation. At one year, there was no difference in the primary endpoint of compliance (88.5% vs. 88.8 %, respectively, p = 0.81). Thus, provision of education materials did not increase adherence to adjuvant endocrine therapy (76).
A more promising result was seen in a smaller COMPAS study of 181 patients receiving adjuvant aromatase inhibitor therapy. The randomization was either to a control condition, a letter group where participants received 5 mailings in the first year and 3 in the second, and a telephone group where participants were contacted by a study nurse using a semi-structured interview technique at the same intervals as in the letter group (77). Adherence was determined as a composite of self-report using a standardized questionnaire plus medication possession ratios calculated from pharmacy prescription refill information. At 12 months, 48% in the control group, 63% in the telephone group, and 65% in the letter group were judged adherent. While the differences between the groups were not statistically significant, a post hoc analysis pooling both interventions versus control indicated a significant difference favoring intervention (p=0.039). These encouraging results provide a foundation for a future confirmation trial as either intervention would be feasible for implementation in clinical practice settings.
Focus on endocrine therapy patient education: “Optimization of expectations”
There is emerging evidence that a patient’s expectation regarding the benefits and drawbacks of a therapy can influence of adverse and persistence with therapy. A meta-analysis identified significant associations between cancer patient’s expectation of developing adverse effects and the actual adverse effect experience (78). When 597 early stage breast cancer patients prescribed tamoxifen were followed for two years, 17% discontinued tamoxifen use. Of these, women with neutral or negative beliefs about tamoxifen efficacy were significantly more likely to discontinue than those with more positive beliefs. Based on these and similar findings, several strategies to enhance endocrine therapy adherence are now focused on the development and testing of structured educational sessions implementing at the beginning of therapy with the goal of optimization of expectations. In another study, the balance between efficacy and side effects was assessed in women receiving adjuvant endocrine therapy with an Adaptive Conjoint Analysis (ACA) customized to each patient. Using such information, a benefit/drawback ratio was calculated and the 16% of women who valued the efficacy less than the adverse effects had substantially lower adherence (79). Based on such findings, an ongoing randomized, controlled trial is evaluating a three session program of cognitive, behavioral training designed to provide a realistic and balanced view of endocrine therapy (80).
Recommendations for improving adherence and clinical practice
Despite the paucity of full scale clinical trial evidence, there are strategies for implementation in current clinical practice which would likely have a favorable effect on endocrine therapy adherence resulting in more favorable clinical outcome.
Adherence to endocrine adjuvant therapy has been higher in clinical trials where patient contacts are commonly greater than in clinical practice settings, and where concerned attention is directed at encouraging the maintenance of adherence. Strategies to increase patient contacts which incorporate emerging technologies such as email reminder programs and use of cell phone apps (81, 82, 83, 84) shown to improve adherence in other disease settings, seem promising to evaluate in breast cancer trials. Strategies to increase contacts with patients in practice settings include use of automated telephone refill reminders and implementing medication adherence tools in electronic medical records. The concept that increased contacts with patients would increase endocrine therapy adherence is strengthened by the findings from the COMPAS trial where both additional mailings and telephone contacts seem to influence favorable adherence (77).
While a waiting results of ongoing clinical studies, one could reasonably conclude that attention to endocrine therapy patient education to optimize realistic patient expectations for adjuvant endocrine therapy, besides being good medical practice, also could improve therapy adherence. For infusional chemotherapy, in many practices, the benefits and risks of therapy, originally discussed by the oncologist, are reinforced in formal chemotherapy education sessions by a mid-level provider. A similar approach to improve adjuvant endocrine therapy patient education could be considered. Educational interventions should focus on increasing patient’s understanding of the benefits and risks of therapy including the relationship between therapy adherence and persistence and higher efficacy of the therapy in reducing cancer recurrence. Implementation of these recommendations is likely to favorably impact adherence in clinical practice at this time. More definitive evidence must come from future activity in the research arena.
Acknowledgments
Funding/Support: Studies from the Women’s Health Initiative (WHI) program reported here were funded by the National Heart, Lung, and Blood Institute with additional support from the National Cancer Institute.
Footnotes
Disclosure: Dr. Chlebowski has received consulting fees from AstraZeneca, Novartis and Pfizer and lecture fees from Novartis. No other authors have conflicts.
Contributor Information
Jisang Kim, Email: jikim1120@gmail.com.
Reina Haque, Email: Reina.Haque@kp.org.
References
- 1.Partridge AH, Avorn J, Wang PS, et al. Adherence to therapy with oral antineoplastic agents. J Natl Cancer Inst. 2002;94:652–661. doi: 10.1093/jnci/94.9.652. [DOI] [PubMed] [Google Scholar]
- 2.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–6. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Geller ML. Adherence to endocrine therapy for breast cancer. Oncology. 2006;71:1–9. doi: 10.1159/000100444. [DOI] [PubMed] [Google Scholar]
- 4.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at five years after diagnosis of estrogen receptor-positive breast cancer: ATLAS, a randomized trial. Lancet. 2013;381(9869):805–16. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray RD, Rea DW, Handley K, et al. aTTom (adjuvant Tamoxifen – To offer more?): randomized trial of 10 versus 5 years of adjuvant tamoxifen among 6,934 women with estrogen receptor-positive (ER+) or ER untested breast cancer – Preliminary results. J Clin Oncol. 2008;26(155):513. [Google Scholar]
- 6.Dezii CM. Persistence with drug therapy: a practical approach using administrative claims data. Manag Care. 2001;10:42–45. [PubMed] [Google Scholar]
- 7.Oberguggenberger AS, Sztankay M, Beer B, et al. Adherence evaluation of endocrine treatment in breast cancer: methodological aspects. BMC Cancer. 2012;12:474. doi: 10.1186/1471-2407-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine thearpy in postmenopausal women with breast cancer. Annals of Oncol. 2009;20:431–436. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 9.Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: a comparison of patient self-report, pill count, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–97. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 10.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–62. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 11.Fisher B, Constantino JP, Wickerham L, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 1998;90:1371–30. 70. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Dignam J, Wolmark N, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomized controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 13.van Herk-Sukel MP, van de Poll-Franse LV, Voogd CA, et al. Half of breast cancer patients discontinue tamoxifen and any endocrine treatment before the end of the recommended treatment period of 5 years: a population-based analysis. Breast Cancer Res Treat. 2010;122:843–51. doi: 10.1007/s10549-009-0724-3. [DOI] [PubMed] [Google Scholar]
- 14.Nekhlyudov L, Li L, Ross-Degnan D, et al. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Research Treat. 2011;130:681–9. doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 15.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–3883. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 16.Chlebowski RT, Cuzick J, Amakye D, et al. Clinical perspectives on utility of aromatase inhibitors for the adjuvant treatment of breast cancer. The Breast. 2009;18 (Supplement 2):S1–S11. doi: 10.1016/S0960-9776(09)70002-5. [DOI] [PubMed] [Google Scholar]
- 17.Visvanathan K, Chlebowski RT, Hurley P, et al. American Society of Clinical Oncology clinical practice guideline update on the use of pharmacologic interventions including tamoxifen, raloxifene, and aromatase inhibition for breast cancer risk reduction. Clin Oncol. 2009;27:3235–58. doi: 10.1200/JCO.2008.20.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The ATAC Trialists Group. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of five years adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 19.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 20.Goss PE, Ingle JN, Martino S, et al. A randomized trial of letrozole in postmenopausal women after five years of tamoxifen therapy early-stage breast cancer. N Engl J Med. 2003;349:1793–802. doi: 10.1056/NEJMoa032312. [DOI] [PubMed] [Google Scholar]
- 21.Murphy CC, Bartholomew LK, Carpentier MY, et al. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Research Treat. 2012;134:459–78. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huiart L, Ferdynus C, Giorgi R. A meta-regression analysis of the available data on adherence to adjuvant hormonal therapy in breast cancer: a summarizing the data for clinicians. Breast Cancer Res Treat. 2013;138:325–8. doi: 10.1007/s10549-013-2422-4. [DOI] [PubMed] [Google Scholar]
- 23.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayres LR, Baldoni AD, Borges AP, Pereira LR. Adherence and discontinuation of oral hormonal therapy in patients with hormone receptor positive breast cancer. Int J Clin Pharm. 2013 doi: 10.1007/s11096-013-9833-5. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 25.Huiart L, Dell’Aniello S, Suissa S. Use of tamoxifen and aromatase inhibitors in a large population-based cohort of women with breast cancer. British Journal of Cancer. 2011;104:1558–1568. doi: 10.1038/bjc.2011.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hadji P, Ziller V, Kyvernitakis J, et al. Persistence in patients with breast cancer treated with tamoxifen or aromatase inhibitors: a retrospective database analysis. Breast Cancer Res Treat. 2013 doi: 10.1007/s10549-013-2417-1. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Henry NL, Azzouz F, Desta Z, et al. Predictors of aromatase inhibitor discontinuation as a result of treatment emergent symptoms in early stage breast cancer. J Clin Oncol. 2012;30:936–942. doi: 10.1200/JCO.2011.38.0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Early Breast Cance Trialist Collaborative Group. Tamoxifen for early breast cancer. Cochrane Database Syst Rev. 2001;(1):CD 000486. [Google Scholar]
- 29.Yood MU, Owusu C, Buist DS, et al. Mortality impact of less-than-standard therapy in older breast cancer patients. J Am Coll Surg. 2008;206:66–75. doi: 10.1016/j.jamcollsurg.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 30.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. British Journal of Cancer. 2008;99:1763–1768. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hershman DL, Shao T, Kushi LH, Buono D, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Research Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque R, Ahmed SA, Fisher A, et al. Effectiveness of aromatase inhibitors and tamoxifen in reducing subsequent breast cancer. Cancer Med. 2012 Dec;1(3):318–27. doi: 10.1002/cam4.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makubate B, Donnan PT, Dewar JA, et al. Cohort study of adherence to adjuvant endocrine therapy, breast cancer recurrence and mortality. Br J Cancer. 2013;108(7):1515–24. doi: 10.1038/bjc.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Markkula A, Hietala M, Henningson M, et al. Clinical profiles predict early non-adherence to adjuvant endocrine treatment in a prospective breast cancer cohort. Cancer Prev Res. 2012;5:735–745. doi: 10.1158/1940-6207.CAPR-11-0442. [DOI] [PubMed] [Google Scholar]
- 35.Weaver KE, Camacho F, Hwang W, et al. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol. 2013;36:181–7. doi: 10.1097/COC.0b013e3182436ec1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pagani O, Gelber S, Colleoni M, et al. Impact of SERM adherence on treatment effect: International Breast Cancer Study Group Trials 13–39 and 14–39. Breast Cancer Res Treat. 2013 doi: 10.1007/s10549-013-2757-x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S, Yang Y, Tao W, et al. Tamoxifen adherence and its relationship to mortality in 116 men with breast cancer. Breast Cancer Research Treat. 2012;13:495–502. doi: 10.1007/s10549-012-2286-z. [DOI] [PubMed] [Google Scholar]
- 38.Livaudais JC, Hershman DL, Habel L, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–617. doi: 10.1007/s10549-011-1762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Livaudais J, LaCroix A, Chlebowski RT, et al. Use of and adherence to adjuvant hormonal therapy for breast cancer in the Women’s Health Initiative. Cancer Epidemiol Biomark Prev. 2013;22(3):365–73. doi: 10.1158/1055-9965.EPI-12-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friese CR, Pini TM, Li Y, et al. Adjuvant endocrine therapy initiation and persistence in a diverse sample of patients with breast cancer. Breast Cancer Res Treat. 2013;138(3):931–9. doi: 10.1007/s10549-013-2499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson B, Vogel V, Wickerham L. Oncologist-patient discussion of adjuvant hormonal therapy in breast cancer: results of the linguistic study focusing on adherence and persistence to therapy. J Support Oncol. 2007;5:139–43. [PubMed] [Google Scholar]
- 42.Font R, Espinas JA, Gil-Gil M, et al. Prescription refill, patient self-report and physician report in assessing adherence to oral endocrine therapy in early breast cancer patients: a retrospective cohort study in Catalonia, Spain. Br J Cancer. 2012;107:1249–56. doi: 10.1038/bjc.2012.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontaine DB, Nortier JW, Liefers GJ, et al. High non-compliance in the use of letrozole after 2.5 years of extended adjuvant endocrine therapy. Results from the IDEAL randomized trial. Eur J Surg Oncol. 2012;3:107–7. doi: 10.1016/j.ejso.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Cuzick J, Forbes J, Edwards R, et al. First results from the International Breast Cancer Intervention Study (IBIS-1): a randomized prevention trial. Lancet. 2002;360:817–24. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 45.Powles TJ, Ashley S, Tidy A, et al. Twenty-year follow-up of the Royal Marsden randomized, double-blinded tamoxifen breast cancer prevention trial. J Natl Cancer Inst. 2007;99:283–90. doi: 10.1093/jnci/djk050. [DOI] [PubMed] [Google Scholar]
- 46.Land SR, Wickerham DL, Constantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or tamoxifen for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–51. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 47.Goss PE, Ingle JN, Ales-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;365:2381–91. doi: 10.1056/NEJMoa1103507. [DOI] [PubMed] [Google Scholar]
- 48.Whelan TJ, Goss PE, Ingle JN, et al. Assessment of quality of life in MA. 17: a randomized, placebo-controlled trial of letrozole after 5 years of tamoxifen in postmenopausal women. J Clin Oncol. 2005;23:6931–6940. doi: 10.1200/JCO.2005.11.181. [DOI] [PubMed] [Google Scholar]
- 49.Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine estrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncology. 2012;13:476–86. doi: 10.1016/S1470-2045(12)70075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Land SR, Cronin WM, Wickerham DL, et al. Cigarette smoking, fitness, and obesity as predictors of chemoprevention adherence among women in the National Surgical Adjuvant Breast and Bowel Program (NSABP) Breast Cancer Prevention Trial. Cancer Prev Res (Phila) 2011;4:1393–1400. doi: 10.1158/1940-6207.CAPR-11-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maurice A, Howell A, Evans DG, et al. Predicting compliance in a breast cancer prevention trial. The Breast Journal. 2006;12:446–450. doi: 10.1111/j.1075-122X.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- 52.Waters EA, McNeel TS, Stevens WM, et al. Use of tamoxifen and Raloxifene for breast cancer chemoprevention in 2010. Breast Cancer Res Treat. 2012;134(2):875–80. doi: 10.1007/s10549-012-2089-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136(3):627–33. doi: 10.1007/s10549-012-2318-8. [DOI] [PubMed] [Google Scholar]
- 54.Fink AK, Gurwitz J, Rakowski W, et al. Patient beliefs and tamoxifen discontinuing in older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2004;22:3309–15. doi: 10.1200/JCO.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 55.Demissie S, Silliman RA, Lash TL. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation older women. Journal of Clin Oncol. 2001;19:322–8. doi: 10.1200/JCO.2001.19.2.322. [DOI] [PubMed] [Google Scholar]
- 56.Kahn KL, Shneider EC, Malin JL, et al. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Medical Care. 2007;45:41–9. doi: 10.1097/01.mlr.0000257193.10760.7f. [DOI] [PubMed] [Google Scholar]
- 57.Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Research Treat. 2006;99:215–20. doi: 10.1007/s10549-006-9193-0. [DOI] [PubMed] [Google Scholar]
- 58.Owusu C, Buist DSM, Field TS, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–55. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 59.Hadji P. Improving compliance and persistence to adjuvant tamoxifen and aromatase inhibitor therapy. Crit Rev Oncol Hematol. 2010;7:156–66. doi: 10.1016/j.critrevonc.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 60.Riley GF, Warren JL, Harlan LC, et al. Endocrine therapy use among elderly hormone receptor-positive breast cancer patients enrolled in Medicare part D. Medicare Medicaid Res Rev. 2011 Dec 13; doi: 10.5600/mmrr.001.04.a04. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29:2534–42. doi: 10.1200/JCO.2010.33.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sedjo RL, Devine S. Predictors of non-adherence to aromatase inhibitors among commercially insured women with breast cancer. Breast Cancer Res Treat. 2011;125:191–200. doi: 10.1007/s10549-010-0952-6. [DOI] [PubMed] [Google Scholar]
- 63.Kimmick G, Anderson G, Camacho F, et al. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27:3445–51. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pellegrini I, Sarradon-Eck A, Sooussan PB, et al. Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patient’s point of view. Psychooncology. 2010;19:472–9. doi: 10.1002/pon.1593. [DOI] [PubMed] [Google Scholar]
- 65.Cluze C, Rey D, Huiart L, et al. Adjuvant endocrine therapy with tamoxifen in young women with breast cancer: determinants of interruptions vary over time. Ann Oncol. 2012;23:882–890. doi: 10.1093/annonc/mdr330. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Malin JL, Diamart AL, et al. Adherence to adjuvant hormone therapy in low-income women with breast cancer: the role of provider-patient communication. Breast Cancer Res Treat. 2013;137:829–36. doi: 10.1007/s10549-012-2387-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bhatta SS, Hou N, Moton ZN, et al. Factors associated with compliance to adjuvant hormone therapy in Black and White women with breast cancer. Springerplus. 2013;2:356. doi: 10.1186/2193-1801-2-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aiello Bowles EJ, Boudreau DM, Chubak J, et al. Patient-reported discontinuation of endocrine therapy and related adverse effects among women with early-stage breast cancer. J Oncol Pract. 2012;8(6):e149–57. doi: 10.1200/JOP.2012.000543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burgess C, Cornelius V, Love S, et al. Depression and anxiety in women with early breast cancer: five year observational cohort study. BMJ. 2005;330:702. doi: 10.1136/bmj.38343.670868.D3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59:56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 71.Kyvernitakis I, Ziller V, Hars O, et al. Prevalence of menopausal symptoms and their influence on adherence in women with breast cancer: results of the COMPAS study. Climacteric. 2013 doi: 10.3109/13697137.2013.819327. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 72.Land SR, Wieand S, Day R, et al. Methodological Issues in the Analysis of Quality of Life Data in Clinical Trials: Illustrations from the National Surgical Adjuvant Breast and Bowel Project (NSABP) Breast Cancer Prevention Trial. Statistical Methods for Quality of Life Studies. 2002:71–85. [Google Scholar]
- 73.Day R, Ganz PA, Costantino JP. Tamoxifen and depression: more evidence from the National Surgical Adjuvant Breast and Bowel Project’s Breast Cancer Prevention (P-1) Randomized Study. J Natl Cancer Inst. 2001;93(21):1615–23. doi: 10.1093/jnci/93.21.1615. [DOI] [PubMed] [Google Scholar]
- 74.Moore S. Adherence to oral therapies for cancer: Barriers and models for change? J Adv Pract Oncol. 2010;1:155–164. [Google Scholar]
- 75.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res. 2011;4:1360–5. doi: 10.1158/1940-6207.CAPR-11-0380. [DOI] [PubMed] [Google Scholar]
- 76.Hadji P, Blettner M, Harbeck N, et al. The Patient’s Anastrozole Compliance to Therapy (PACT) program: a randomized, in practice study on the impact of a standardized information program on persistence and compliance to adjuvant endocrine therapy in postmenopausal women with early stage breast cancer. Annals Oncol. 2013;24:1505–12. doi: 10.1093/annonc/mds653. [DOI] [PubMed] [Google Scholar]
- 77.Ziller V, Kyvernitakis I, Knoll D, et al. Influence of a patient information program on adherence and persistence with an aromatase inhibitor in breast cancer treatment – the COMPAS study. BMC Cancer. 2013;13:407. doi: 10.1186/1471-2407-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sohl SJ, Schnur JB, Montgomery GH. A meta—analysis of the relationship between response expectancies and cancer treatment-related side effects. J Pain Symptom Manage. 2009;38:775–784. doi: 10.1016/j.jpainsymman.2009.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wouters H, Maatman GA, Van Dijk L, et al. Trade-off preferences regarding adjuvant endocrine therapy among women with estrogen receptor-positive breast cancer. Ann Oncol. 2013;24(9):2324–9. doi: 10.1093/annonc/mdt195. [DOI] [PubMed] [Google Scholar]
- 80.Von Blackenburg P, Schuricht F, Albert US, et al. Optimizing expectations to prevent side effects and enhance quality of life in breast cancer patients undergoing endocrine therapy: study protocol of a randomized controlled trial. BMC Cancer. 2013;13:426. doi: 10.1186/1471-2407-13-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cicolini G, Simonetti V, Comparcini D, et al. Efficacy of a nurse-led email reminder program for cardiovascular prevention risk reduction in hypertensive patients: a randomized controlled trial. Int J Nurs Stud. 2013 doi: 10.1016/j.ijnurstu.2013.10.010. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 82.Becker S, Kribben A, Meister S, et al. User profiles of a smartphone application to support drug adherence – experience from the iNephro project. PLoS One. 2013;8(10):e78547. doi: 10.1371/journal.pone.0078547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stuurman-Bieze AG, Hiddink EG, van Boven JF, Vegter S. Proactive pharmaceutical care interventions improve patients’ adherence to lipid-lowering medication. Ann Pharmacother. 2013;47(11):1448–56. doi: 10.1177/1060028013501146. [DOI] [PubMed] [Google Scholar]
- 84.Carter MC, Burley VJ, Nykjaer C, Cade JE. Adherence to a smartphone application for weight loss compared to website and paper diary: pilot randomized controlled trial. J Med Internet Res. 2013;15(4):e32. doi: 10.2196/jmir.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]


