ABSTRACT
Enteric duplication cysts and reversed intestinal rotation (RIR) are rare congenital anomalies, with their coexistence being exceptionally uncommon. We report a 4-year-old girl who presented with chronic anemia and intermittent abdominal symptoms since infancy. Detailed workup for medical causes of anemia was inconclusive. Computed tomography of the abdomen revealed intestinal malrotation with a grossly dilated small bowel loop. Intraoperative findings revealed a long duodenojejunal tubular duplication with a separate mesentery (Type 1a) and RIR. The patient underwent a Ladd’s procedure, resection of the duplication cyst, and end-to-end anastomosis. This case underscores the anatomical rarity, varied clinical presentation, and challenges in making an accurate and timely diagnosis in such a case.
KEYWORDS: Enteric duplication cyst, reverse intestinal rotation, malrotation, anemia
INTRODUCTION
Intestinal malrotation involves the partial-to-complete failure of the midgut to undergo a 270° counterclockwise rotation around the superior mesenteric vessels during fetal development.[1] Reversed intestinal rotation (RIR) is the rarest form of midgut rotational anomalies.[2] Enteric duplication cysts (EDCs) are rare congenital anomalies, with approximately half occurring in the small bowel.[3] The coexistence of intestinal malrotation and EDCs in the same patient is exceptionally rare.
We present a case involving a female child who presented at age 4 with varied symptoms associated with malrotation and an intestinal duplication cyst. To our knowledge, this is the first reported case of a type 1a duodenojejunal (DJ) duplication cyst associated with RIR. This case underscores the anatomical rarity, varied presentation, and challenges in making an accurate and timely diagnosis.
CASE REPORT
A 4-year-old girl presented to hematology clinic with severe anemia requiring multiple blood transfusions and parenteral iron therapy since infancy. She also had a history of recurrent episodes of low-grade fever, loose stools, upper abdominal distension after feeds, and nonbilious vomiting, since 3 months of age. Clinically, she exhibited severe pallor, stunted growth, hemolytic facies, and pedal edema. There was no icterus, lymphadenopathy, or hepatosplenomegaly. Despite extensive work-up and symptomatic treatment at an outside hospital, no definitive diagnosis had been reached. The mother also revealed that serial antenatal scans had demonstrated a fixed dilated bowel loop. However, this finding was not followed up in the postnatal period.
Hemogram revealed a hemoglobin level of 5.1 g/dL. Peripheral blood film and bone marrow aspiration indicated an anisopoikilocytic blood picture and cellular marrow with erythroid preponderance. Further laboratory workup revealed severe deficiency of serum iron and 25-Hydroxy vitamin D levels. Serum ferritin, vitamin B12, hemoglobin electrophoresis, serum hepcidin, TORCH panel, Parvovirus B19 serology, thyroid profile, and high-performance liquid chromatography were all reported within the normal range. Targeted next-generation sequencing to diagnose hereditary hemolytic anemia did not reveal any mutation and multiplex ligation-dependent probe amplification for alpha-thalassemia and sequencing for beta thalassemia showed no deletions/duplications. Stool for occult blood was positive on 3 occasions, while the test for reducing substances was negative. Extensive evaluation by the pediatric gastroenterology team, including tests for celiac disease and malabsorption syndromes, also did not yield a definitive diagnosis.
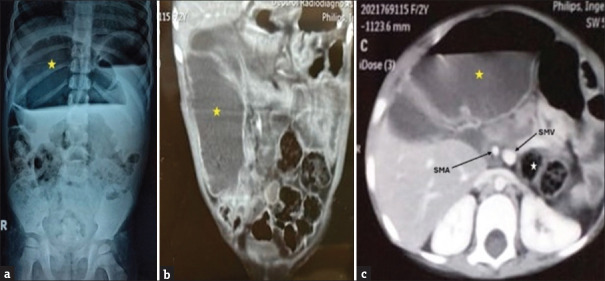
A plain abdominal X-ray showed a grossly dilated bowel loop, occupying almost 50% of abdomen [Figure 1a]. A computed tomography (CT) angioenterography of abdomen revealed a grossly dilated small bowel loop of diameter 5.2 cm [Figure 1b]. The superior mesenteric artery (SMA) and superior mesenteric vein (SMV) relation were reversed with the cecum and transverse colon lying posterior to the small bowel, suggestive of reversed intestinal rotational anomaly with proximal small bowel obstruction [Figure 1c].
Figure 1.
(a) Erect abdominal X-ray showing a grossly dilated bowel loop occupying almost 50% of the abdomen (yellow star) (b) CT angioenterography of abdomen (coronal section) showing a grossly dilated small bowel loop of diameter 5.2 cm (yellow star) (c) CT angioenterography of the abdomen (axial section) showing reversal of SMA and SMV relationship, with cecum and transverse colon (white star) lying posterior to the small bowel (yellow star), suggestive of reversed intestinal rotation
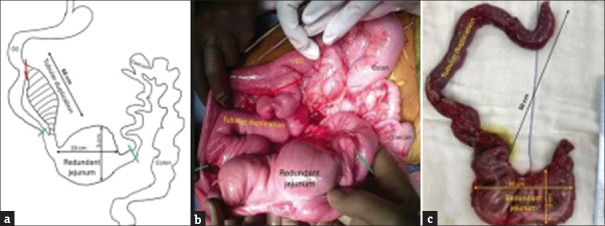
Exploratory laparotomy was performed after the optimization of hemoglobin and serum albumin levels. Intraoperatively, malrotation was confirmed with the DJ flexure and clumped small bowel loops lying on the right side of the abdomen with large bowel lying on the left and posterior to the small bowel. Thick Ladd’s bands were extending from the cecum to the lateral parietal wall, compressing the duodenum. A long tubular duplication cyst was found with a separate muscular coat and mesentery, extending from the third portion of the duodenum distally for approximately 50 cm, terminating into a dilated redundant portion of the jejunum, approximately 20 cm in length and 5 cm in diameter [Figure 2a-c].
Figure 2.
(a) Schematic diagram, (b) intra-operative photograph, and (c) resected specimen, showing a long tubular duplication with a separate muscular coat and mesentery, extending from the third portion of duodenum distally for approximately 50 cm, terminating into a dilated redundant portion of the jejunum, approximately 20 cm in length and 5 cm in diameter. Resection was performed along the red and green lines, followed by closure of the duodenum (along the red line) and end-to-end anastomoses (between green lines) in 2 layers. Also note the cecum and transverse colon lying posterior and to the left of duodenojejunal loops, suggestive of reversed intestinal rotation
The patient underwent a Ladd’s procedure (without appendectomy), resection of the DJ duplication cyst with the redundant portion of the jejunum, and end-to-end duodenojejunal anastomosis. The residual small bowel after resection was approximately 110 cm with an intact ileocecal valve. The patient tolerated the procedure well.
The patient made an uneventful recovery. Feeds were gradually increased postoperatively until full enteral feeds were reached. Histopathology confirmed an EDC without any evidence of ectopic gastric mucosa, pancreatic rest, dysplasia, or malignancy. At 18-month follow-up, the patient is asymptomatic and thriving well with good weight gain. The hemoglobin and serum albumin levels are within the normal range.
DISCUSSION
EDCs are rare congenital anomalies that can be cystic (80%) or tubular (20%), affecting any part of the gastrointestinal tract.[4] Based on their relation to the mesentery, EDCs are classified into two types: parallel (Type I) and intramesenteric (Type II), with further subdivisions based on the mesentery and muscular coat characteristics.[5] Type I is further subclassified into Ia, Ib, and Ic. Type Ia has a separated mesentery, Ib shares a common mesentery with the neighboring bowel, and Ic shares a common muscular coat with the adjacent bowel. Type II is also subdivided into IIa and IIb. In Type IIa, the duplication is distinct from the normal bowel, while in Type IIb, they share a common muscular coat. This classification has surgical implications for managing intestinal duplications, as is evident in our case. Our patient had a type Ia anatomy with a long, tubular dudenojejunal duplication with a separate mesentery. As it did not share the blood supply or muscular coat with the adjacent bowel, we were able to resect it completely without resecting the neighboring bowel [Figure 2a-c].
Reversed rotation occurs as an anomaly during the second stage of midgut development. In this phase, the caudal portion of the midgut reenters the abdomen first, causing the duodenum to rotate in a clockwise direction rather than the typical counterclockwise manner.[6] Consequently, the duodenum passes in front of the SMA in reversed rotation, while the colon, instead of passing anteriorly, courses posterior to the SMA. Normal colonic rotation is rarely observed in such cases. In 1968, Amir-Jahed[7] categorized reversed rotation into four types: (1) Type I, right-sided prearterial cecum; (2) Type II, left-sided prearterial cecum; (3) Type III, right-sided retro-arterial cecum; and (4) Type IV, a left-sided retroarterial cecum. As per this classification, our case fits into type IV, as the cecum was retroarterial and on left side.
The combination of intestinal malrotation and an EDC is extremely rare, with only a few case reports describing this association.[8] Our patient’s rare pathology included a Type 1a DJ duplication cyst, large redundant jejunal loop, short small bowel length, and RIR. In this case, malrotation could be a possible cause of early embryological vascular insult leading to subsequent impairment of midgut growth. Furthermore, long-term extrinsic compression of the jejunum from thick peritoneal bands in fetal life could have led to luminal narrowing and pronounced redundancy of the proximal jejunal segment.
EDCs can be prenatally diagnosed through antenatal ultrasonography.[9] In this case, antenatal scans indicated a dilated bowel segment, but the finding was ignored postnatally, leading to delayed diagnosis. The clinical presentation of duplication cysts is influenced by their location, size, and mucosal pattern. In our case, intermittent postprandial upper abdominal pain and distension were likely due to the significant distension of the redundant jejunal loop after feeding. In addition, duodenal compression by Ladd’s bands can potentially explain the nonbilious vomiting seen in our patient.[10] Duplication cysts may also contribute to pain and intestinal obstruction by compressing the adjacent bowel segments.[11]
Chronic malnutrition, indicated by low weight for age, hypoalbuminemia, severe iron deficiency anemia, and vitamin D deficiency, can all be attributed to stasis and bacterial overgrowth within the duplication cyst and redundant jejunal segment.[12] Small intestinal bacterial overgrowth (SIBO) impedes nutrient absorption, resulting in weight loss and malnutrition.[12] Symptoms such as mild fever and diarrhea can also be explained by SIBO-associated enteritis. Despite the histopathological examination ruling out ectopic gastric mucosa in this case, the positive stool occult blood test could also have been due to SIBO-associated severe enteritis.[13]
The optimal treatment for EDCs involves complete resection of the duplication cyst with or without the adjacent bowel.[14] However, based on the proposed vascular classification, resecting the duplication without the adjacent bowel is advisable if it possesses an independent vascular supply, like in this case.[5]
CONCLUSION
The combination of intestinal malrotation and an EDC is extremely rare and can present with diverse life-threatening symptoms. Timely and accurate diagnosis with appropriate surgical intervention is crucial to avoid unnecessary investigations and patient distress. Awareness of this unique morphological combination aids in selecting optimal surgical procedures.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the legal guardian has given his consent for images and other clinical information to be reported in the journal. The guardian understands that names and initials will not be published and due efforts will be made to conceal the identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.McVay M, Kokoska ER, Jackson RJ, Smith SD. The changing spectrum of intestinal malrotation: diagnosis and management. Am J Surg. 2007:712–719. doi: 10.1016/j.amjsurg.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 2.Lileyman A, Levy RD, Sillar R. Reversed intestinal rotation: report of two cases and review of the published reports. ANZ J Surg. 2006;76:947–9. doi: 10.1111/j.1445-2197.2006.03893.x. [DOI] [PubMed] [Google Scholar]
- 3.Patiño Mayer J, Bettolli M. Alimentary tract duplications in newborns and children: diagnostic aspects and the role of laparoscopic treatment. World J Gastroenterol. 2014;20:14263–71. doi: 10.3748/wjg.v20.i39.14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.D’Agostino V, Castaldo A, Catelli A, Pesce I, Genovese S, Coppola L, et al. An ileal duplication cyst case report: From diagnosis to treatment. Radiol Case Rep. 2021;16:1597–1602. doi: 10.1016/j.radcr.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li L, Jin-Zhe Z, Yan-Xia W. Vascular Classification for Small Intestinal Duplications: Experience With 80 Cases. Journal of pediatric surgery. 1998;33:1243–45. doi: 10.1016/s0022-3468(98)90159-2. [DOI] [PubMed] [Google Scholar]
- 6.Fukuhara M, Yamaguchi Y, Izaki T. Intestinal reversed rotation in neonates: A case report and review of the literature. Journal of Pediatric Surgery Case Reports. 2023;97:102708. [Google Scholar]
- 7.Amir-Jahed A. K. Classification of reversed intestinal rotation. Surgery. 1968;64:1071–4. [PubMed] [Google Scholar]
- 8.Azzam A, Abdulkarim AN, Shehata AEM, Mahran I, Arafa A, Arafat A, et al. A report of two infant cases operated for jejunal duplication cyst associated with malrotation and volvulus. Int J Surg Case Rep. 2020;67:227–230. doi: 10.1016/j.ijscr.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, PicóAliaga S, Ibañez Pradas V. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging. 2018;9:1097–1106. doi: 10.1007/s13244-018-0660-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirbilek S, Karaman A, Gürünlüoğlu K, Akin M, Taş E, Aksoy RT, et al. Delayed gastric emptying in gastroesophageal reflux disease: the role of malrotation. Pediatr Surg Int. 2005;21:423–7. doi: 10.1007/s00383-005-1460-3. [DOI] [PubMed] [Google Scholar]
- 11.Iyer CP, Mahour GH. Duplications of the alimentary tract in infants and children. J Pediatr Surg. 1995;30:1267–70. doi: 10.1016/0022-3468(95)90482-4. [DOI] [PubMed] [Google Scholar]
- 12.Dukowicz AC, Lacy BE, Levine GM. Small Intestinal Bacterial Overgrowth. Gastroenterol Hepatol. 2007;3:112–122. [PMC free article] [PubMed] [Google Scholar]
- 13.David L, Babin A, Picos A, Dumitrascu DL. Small intestinal bacterial overgrowth is associated with intestinal inflammation in the irritable bowel syndrome. Clujul Medical. 2014;87:163–65. doi: 10.15386/cjmed-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bishop HC, Koop CE. Surgical management of duplications of the alimentary tract. Am J Surg. 1964;107:434–42. doi: 10.1016/0002-9610(64)90210-7. [DOI] [PubMed] [Google Scholar]