Abstract
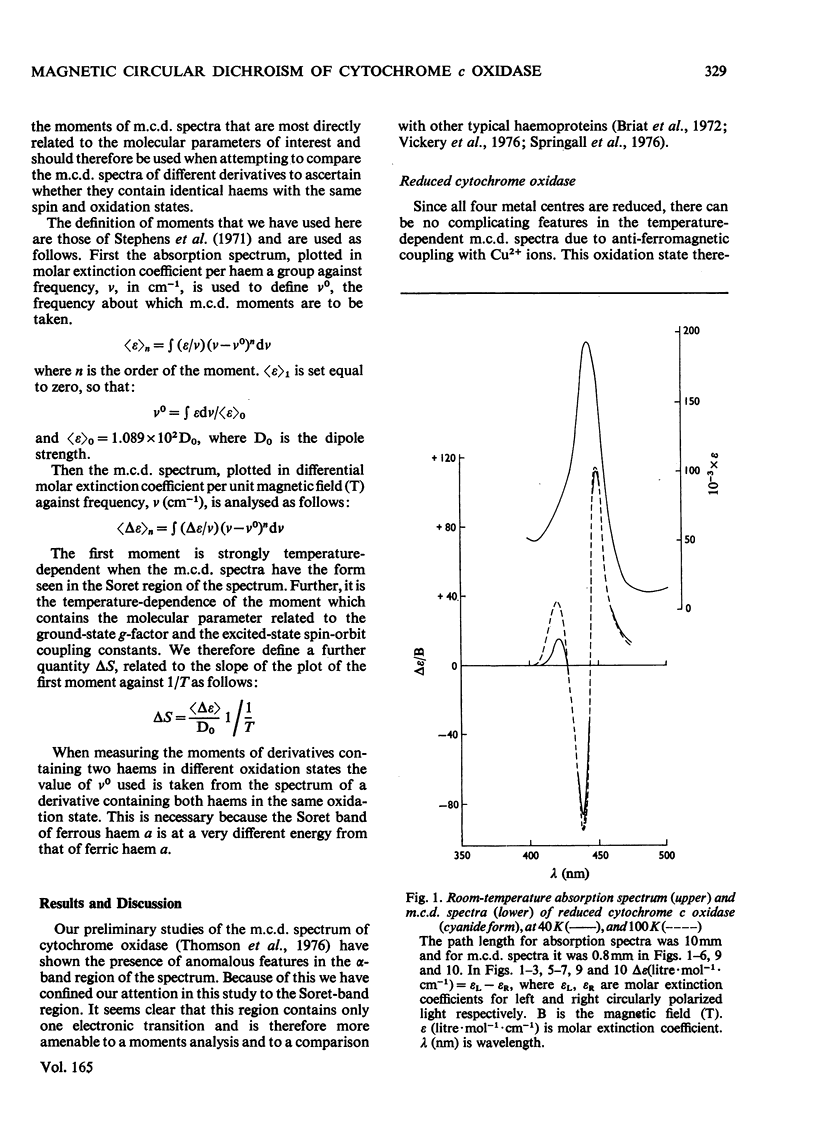
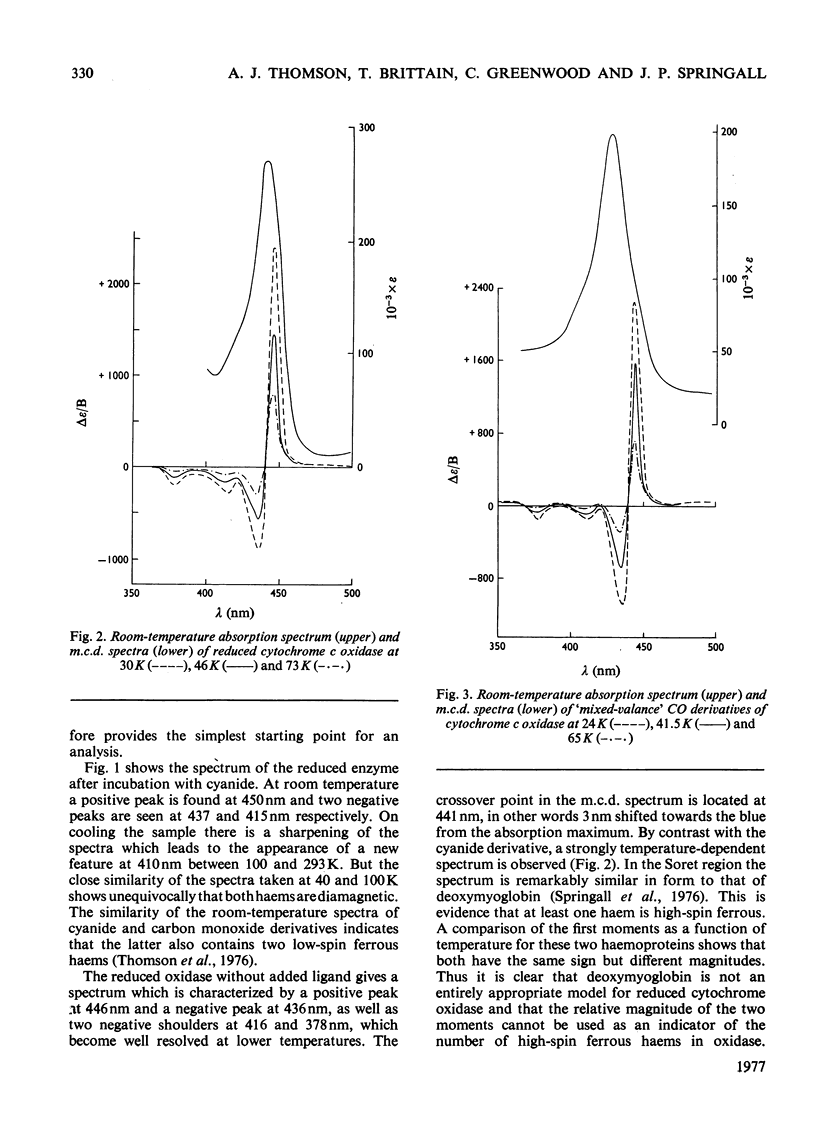
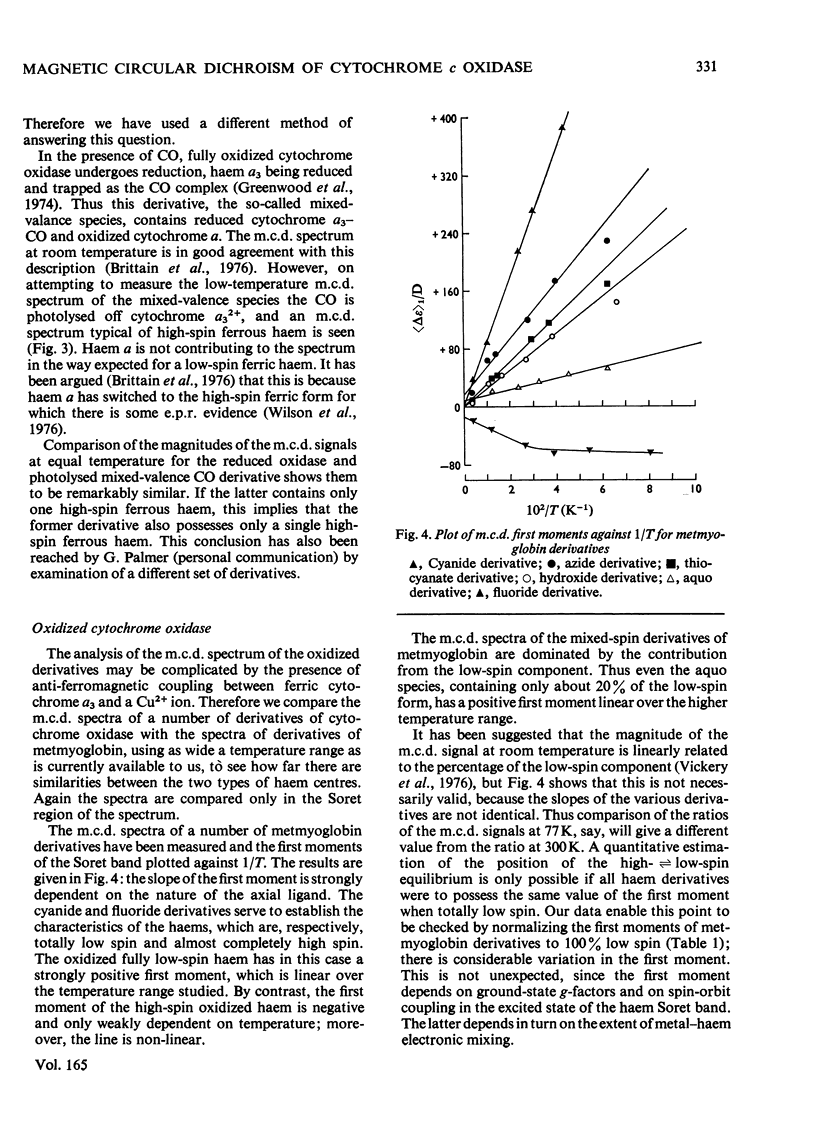
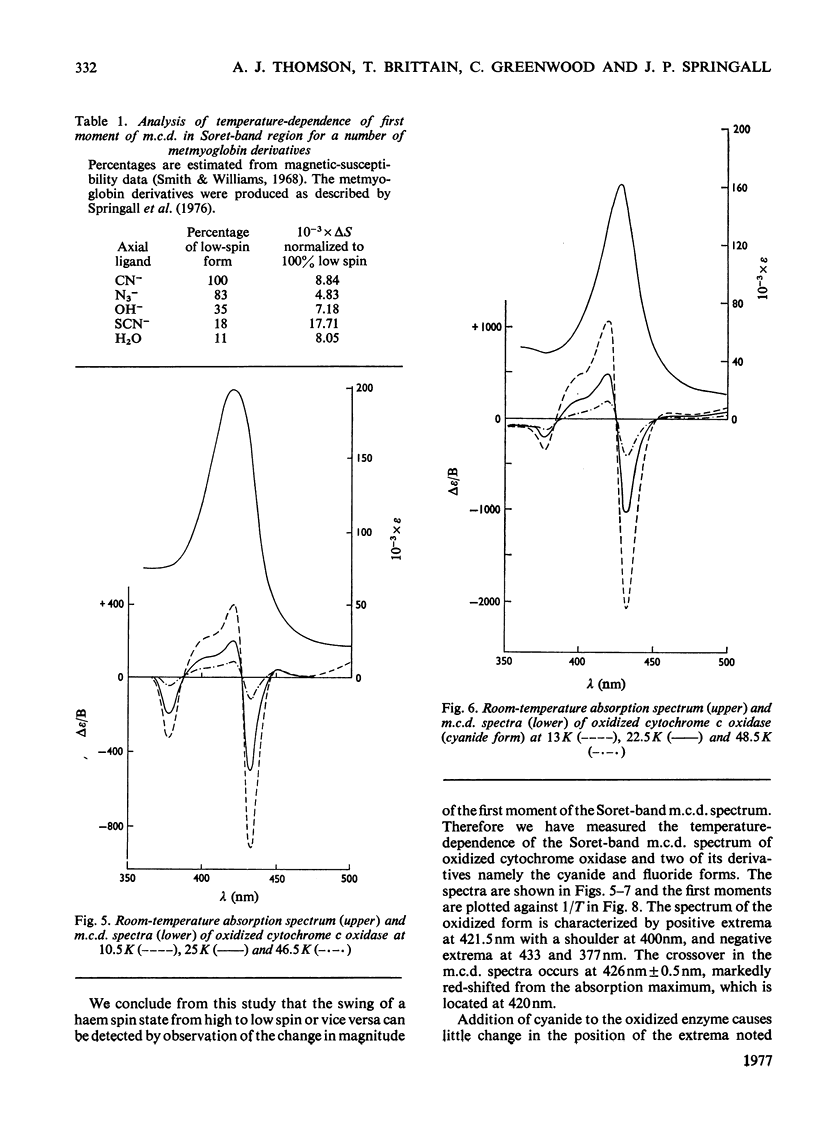
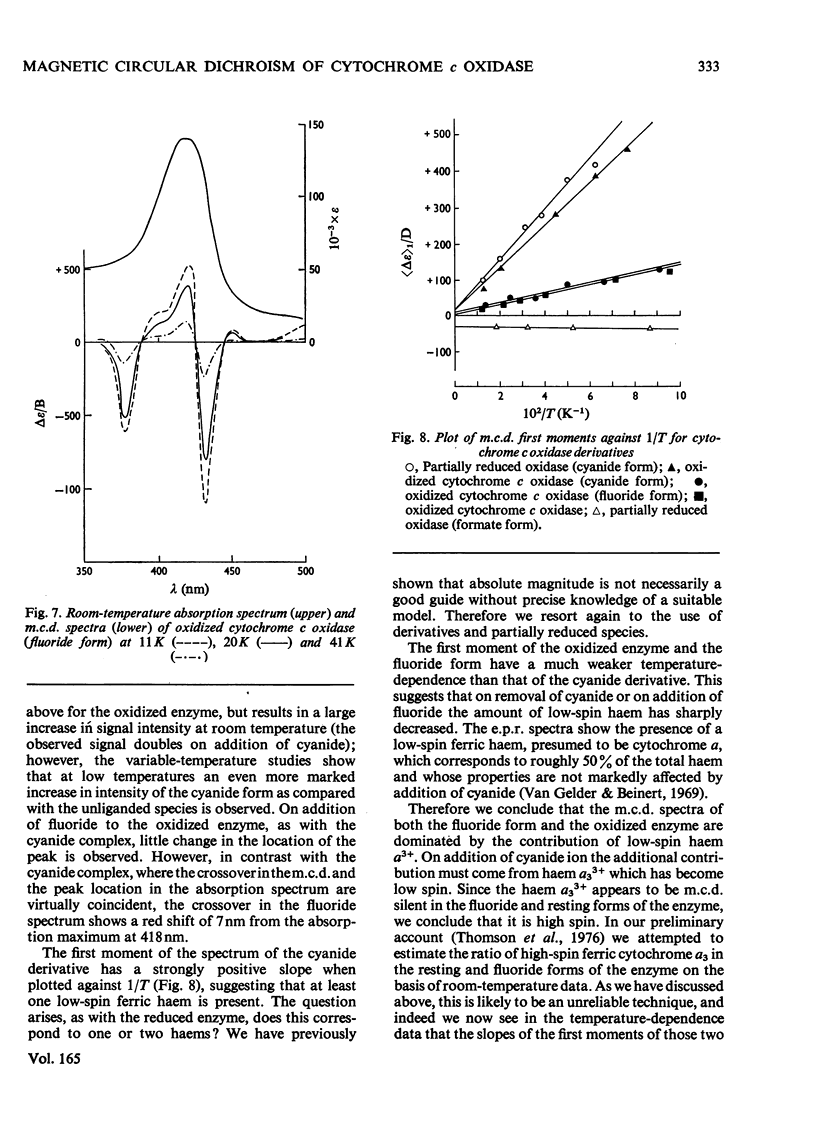
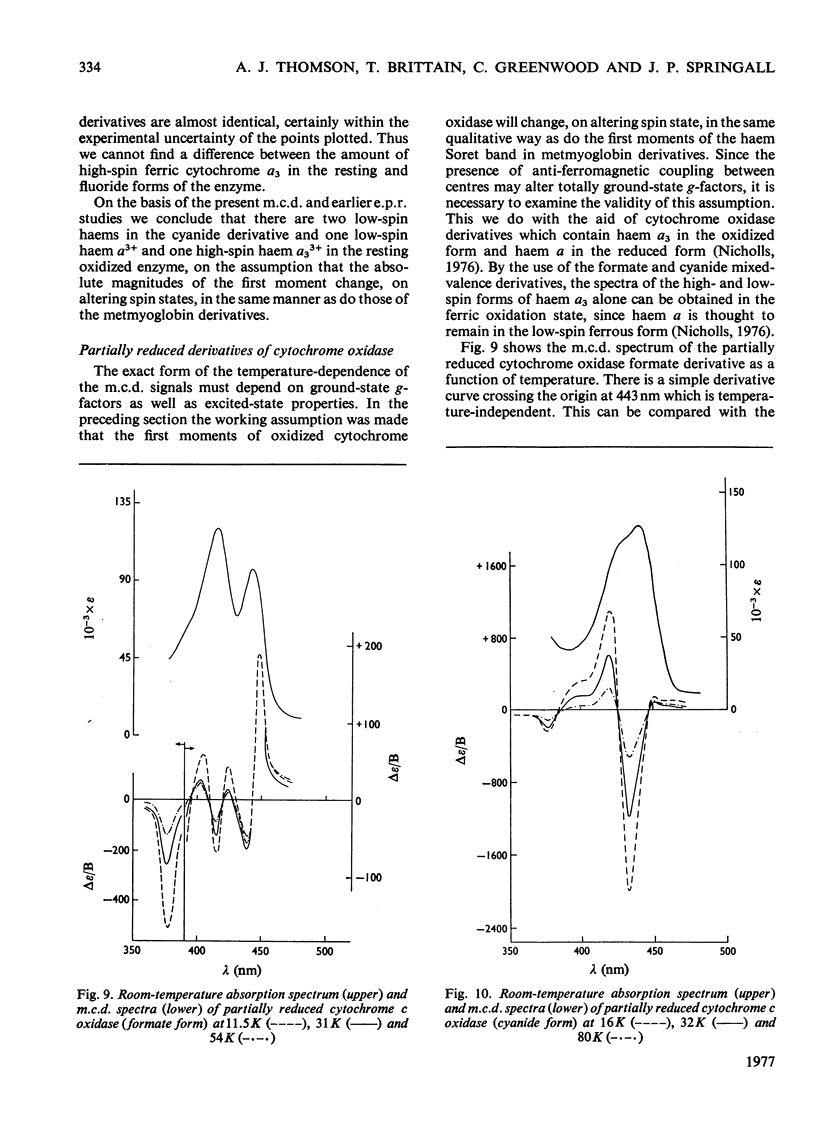
A detailed study of the effect of temperature on the m.c.d. (magnetic circular dichroism) spectra of cytochrome c oxidase and some of its derivatives was undertaken to characterize the spin states of haem a and a3. The fully reduced enzyme contains haem a32+ in its high-spin form and haem a2+ in the low-spin state. This conclusion is reached by comparing the spectrum with that of the mixed-valence CO derivatives and its photolysis product. The cyanide derivative of the fully reduced enzyme contains both haem a and a3 in the low-spin ferrous form. The m.c.d. spectra of the fully oxidized derivatives are consistent with the presence of one low-spin ferric haem group, assigned to a, which remains unaltered in the presence of ligands. Haem a3 is high spin in the resting enzyme and the fluoride derivatives, and low spin in the cyanide form. The partially reduced formate and cyanide derivatives have temperature-dependent m.c.d. spectra due to the presence of high- and low-spin haem a33+ respectively. Haem a is low-spin ferrous in both. A comparison of the magnitude of the temperature-dependence of haem a33+ in the fully oxidized and partially reduced forms shows a marked difference which is tentatively ascribed to the presence of anti-ferromagnetic coupling in the fully oxidized form of the enzyme, and to its absence from the partially reduced derivatives, owing to the reduction of both Cu2+ ions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasa R., Albracht P. J., Falk K. E., Lanne B., Vänngard T. EPR signals from cytochrome c oxidase. Biochim Biophys Acta. 1976 Feb 13;422(2):260–272. doi: 10.1016/0005-2744(76)90137-6. [DOI] [PubMed] [Google Scholar]
- Brittain T., Springall J., Greenwood C., Thomson A. J. Low-temperature studies on mixed-valence cytochrome oxidase by using magnetic circular dichroism. Biochem J. 1976 Dec 1;159(3):811–813. doi: 10.1042/bj1590811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dervartanian D. V., Lee I. Y., Slater E. C., van Gelder B. F. Effects of ATP, antimycin and cyanide on the EPR spectra of cytochromes in phosphorylating submitochondrial particles. Biochim Biophys Acta. 1974 May 22;347(2):321–327. doi: 10.1016/0005-2728(74)90056-5. [DOI] [PubMed] [Google Scholar]
- GIBSON Q. H., PALMER G., WHARTON D. C. THE BINDING OF CARBON MONOXIDE BY CYTOCHROME C OXIDASE AND THE RATIO OF THE CYTOCHROMES A AND A3. J Biol Chem. 1965 Feb;240:915–920. [PubMed] [Google Scholar]
- Greenwood C., Wilson M. T., Brunori M. Studies on partially reduced mammalian cytochrome oxidase. Reactions with carbon monoxide and oxygen. Biochem J. 1974 Feb;137(2):205–215. doi: 10.1042/bj1370205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauzerall D. Multiple excitations in photosynthetic systems. Biophys J. 1976 Jan;16(1):87–91. doi: 10.1016/S0006-3495(76)85665-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls P., Petersen L. C. Haem-haem interactions in cytochrome aa3 during the anaerobic-aerobic transition. Biochim Biophys Acta. 1974 Sep 20;357(3):462–467. doi: 10.1016/0005-2728(74)90038-3. [DOI] [PubMed] [Google Scholar]
- Nicholls P. The effect of formate on cytochrome aa3 and on electron transport in the intact respiratory chain. Biochim Biophys Acta. 1976 Apr 9;430(1):13–29. doi: 10.1016/0005-2728(76)90218-8. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Williams R. J. Analysis of the visible spectra of some sperm-whale ferrimyoglobin derivatives. Biochem J. 1968 Nov;110(2):297–301. doi: 10.1042/bj1100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springall J., Stillman M. J., Thomson A. J. Low temperature magnetic circular dichroism spectra of met- and myoglobin derivatives. Biochim Biophys Acta. 1976 Dec 22;453(2):494–501. doi: 10.1016/0005-2795(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Thomson A. J., Brittain T., Greenwood C., Springall J. Determination of the heme spin states in cytochrome c oxidase using magnetic circular dichroism. FEBS Lett. 1976 Aug 1;67(1):94–98. doi: 10.1016/0014-5793(76)80877-0. [DOI] [PubMed] [Google Scholar]
- Tiesjema R. H., Hardy G. P., van Gelder B. F. Biochemical and biophysical studies on cytochrome c oxidase. XVIII. Potentiometric titrations of cytochrome c oxidase followed by circular dichroism. Biochim Biophys Acta. 1974 Jul 25;357(1):24–33. doi: 10.1016/0005-2728(74)90108-x. [DOI] [PubMed] [Google Scholar]
- Tsudzuki T., Okunuki K. Studies on cytochrome a. XIX. On the subunit structure and spin states of cytochrome oxidase from beef heart muscle. J Biochem. 1971 May;69(5):909–922. doi: 10.1093/oxfordjournals.jbchem.a129542. [DOI] [PubMed] [Google Scholar]
- Van Gelder B. F., Beinert H. Studies of the heme components of cytochrome c oxidase by EPR spectroscopy. Biochim Biophys Acta. 1969 Sep 16;189(1):1–24. doi: 10.1016/0005-2728(69)90219-9. [DOI] [PubMed] [Google Scholar]
- Vickery L., Nozawa T., Sauer K. Magnetic circular dichroism studies of low-spin cytochromes. Temperature dependence and effects of axial coordination on the spectra of cytochrome c and cytochrome b5. J Am Chem Soc. 1976 Jan 21;98(2):351–357. doi: 10.1021/ja00418a006. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Erecińska M., Owen C. S. Some properties of the redox components of cytochrome c oxidase and their interactions. Arch Biochem Biophys. 1976 Jul;175(1):160–172. doi: 10.1016/0003-9861(76)90495-1. [DOI] [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. I. Absolute and difference absorption spectra. J Biol Chem. 1960 Mar;235:845–852. [PubMed] [Google Scholar]
- YONETANI T. Studies on cytochrome oxidase. III. Improved preparation and some properties. J Biol Chem. 1961 Jun;236:1680–1688. [PubMed] [Google Scholar]
- van Buuren K. J., Nicholis P., van Gelder B. F. Biochemical and biophysical studies on cytochrome aa 3 . VI. Reaction of cyanide with oxidized and reduced enzyme. Biochim Biophys Acta. 1972 Feb 28;256(2):258–276. doi: 10.1016/0005-2728(72)90057-6. [DOI] [PubMed] [Google Scholar]


