Abstract
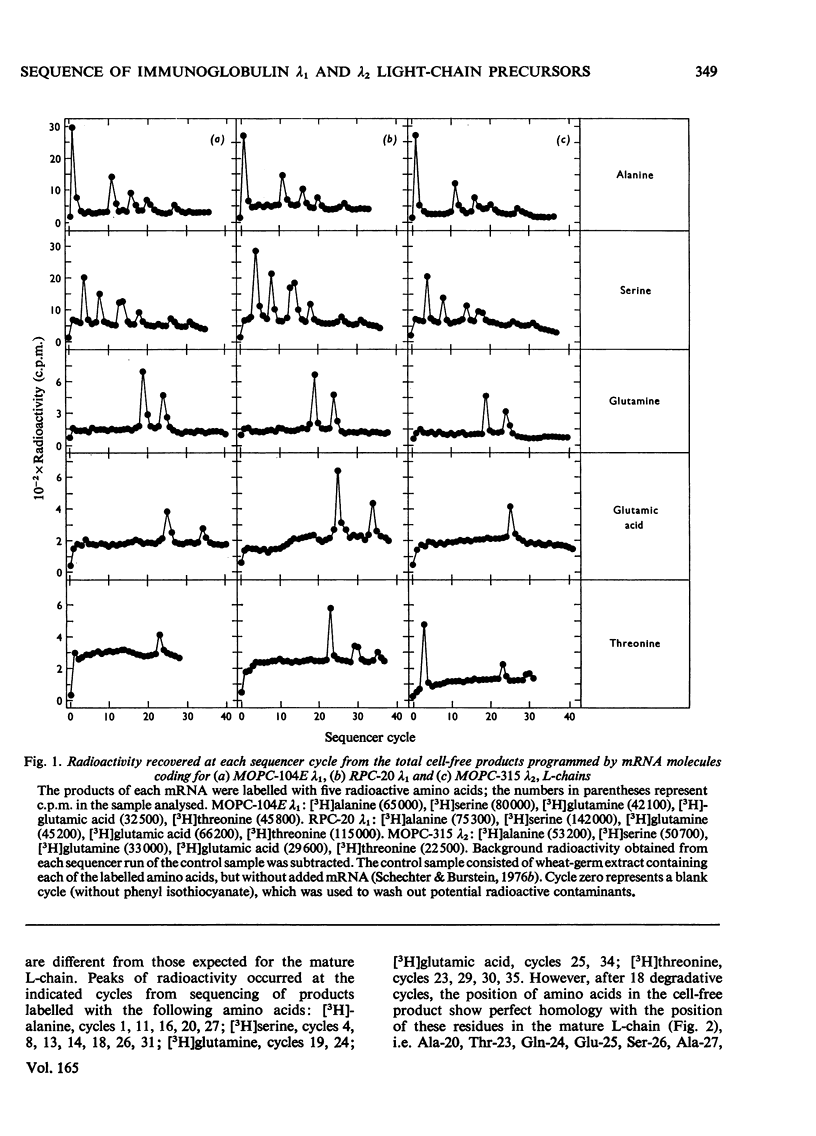
The mRNA molecules coding for three mouse immunoglobulin λ-type light (L) chains (MOPC-104E λ1, RPC-20 λ1, MOPC-315 λ2) programme the cell-free synthesis of precursors larger than the mature proteins. Radioactive amino acid-sequence analyses of each of the three precursors labelled with [3H]alanine, [3H]serine, [3H]glutamine, [3H]glutamic acid and [3H]threonine showed that an extra piece, at least 18 residues long, is linked to the N-terminus of the mature L-chains. The N-terminal extra-peptide segment may be 19 residues long, since analyses of precursors labelled with [35S]methionine indicated an additional N-terminal methionine residue which was recovered in low yields. Presumably this is the initiator methionine, which is known to be short lived in eukaryotes. The mature forms of MOPC-104E, RPC-20 and MOPC-315 λ L-chains are blocked at the N-termini by pyrrolid-2-one-5-carboxylic acid (pyroglutamic acid). Sequence analyses of precursors labelled with [3H]glutamine and [3H]glutamic acid showed incorporation only of glutamine in a position that matches with the position of pyrrolid-2-one-5-carboxylic acid in the mature forms of all three precursors, and incorporation of glutamic acid in other positions. The data showed the absence of glutamine–glutamic acid interconversion, since the radioactive peaks obtained from either 3H-labelled amino acid were discrete, and free from cross-contamination. These results prove that glutamine is the precursor amino acid of pyrrolid-2-one-5-carboxylic acid at the N-termini of the mature MOPC-104E λ1, RPC-20 λ1 and MOPC-315 λ2 L-chains. Thus the formation of pyrrolid-2-one-5-carboxylic acid by cyclization of glutamine is a post-translational event which occurs after, or concomitant with, cleavage of the extra piece from the precursor to yield the mature L-chain. The variable (V) regions (110 amino acid residues) of mouse λ L-chains are quite similar: when compared with that of MOPC-104E λ1 chain, the V-region of RPC-20 λ1 chain differs in one residue, and the V-region of MOPC-315 λ2 chain differs in 11 residues. The partial sequence data show that the N-terminal extra pieces of the two λ1 L-chain precursors have, so far, identical partial sequences; the extra piece of the λ2 L-chain precursor differs from these in at least three out of 19 positions.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appella E. Amino acid sequences of two mouse immunoglobulin lambda chains. Proc Natl Acad Sci U S A. 1971 Mar;68(3):590–594. doi: 10.1073/pnas.68.3.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmayer H. Initiation of protein synthesis in intact cells and in isolated chloroplasts of Acetabularia mediterranea. Biochim Biophys Acta. 1970;209(2):584–586. doi: 10.1016/0005-2787(70)90759-8. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Kantour F., Schechter I. Partial amino-acid sequence of the precursor of an immunoglobulin light chain containing NH2-terminal pyroglutamic acid. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2604–2608. doi: 10.1073/pnas.73.8.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid-sequence variability at the N-terminal extra piece of mouse immunoglobulin light-chain precursors of the same and different subgroups. Biochem J. 1976 Jul 1;157(1):145–151. doi: 10.1042/bj1570145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari I. M., Weigert M. Mouse lambda-chain sequences. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2112–2116. doi: 10.1073/pnas.70.7.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugan E. S., Bradshaw R. A., Simms E. S., Eisen H. N. Amino acid sequence of the light chain of a mouse myeloma protein (MOPC-315). Biochemistry. 1973 Dec 18;12(26):5400–5416. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Green M., Graves P. N., Zehavi-Willner T., McInnes J., Pestka S. Cell-free translation of immunoglobulin messenger RNA from MOPC-315 plasmacytoma and MOPC-315 NR, a variant synthesizing only light chain. Proc Natl Acad Sci U S A. 1975 Jan;72(1):224–228. doi: 10.1073/pnas.72.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Packman S. Quantitation of constant and variable region genes for mouse immunoglobulin lambda chains. Biochemistry. 1976 Jun 29;15(13):2780–2785. doi: 10.1021/bi00658a012. [DOI] [PubMed] [Google Scholar]
- Honjo T., Packman S., Swan D., Nau M., Leder P. Organization of immunoglobulin genes: reiteration frequency of the mouse kappa chain constant region gene. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3659–3663. doi: 10.1073/pnas.71.9.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honjo T., Swan D., Packman S., Polsky F., Leder P. Purification and translation of an immunoglobulin lambda chain messenger RNA from mouse myeloma. Biochemistry. 1976 Jun 29;15(13):2775–2779. doi: 10.1021/bi00658a011. [DOI] [PubMed] [Google Scholar]
- Housman D., Jacobs-Lorena M., Rajbhandary U. L., Lodish H. F. Initiation of haemoglobin synthesis by methionyl-tRNA. Nature. 1970 Aug 29;227(5261):913–918. doi: 10.1038/227913a0. [DOI] [PubMed] [Google Scholar]
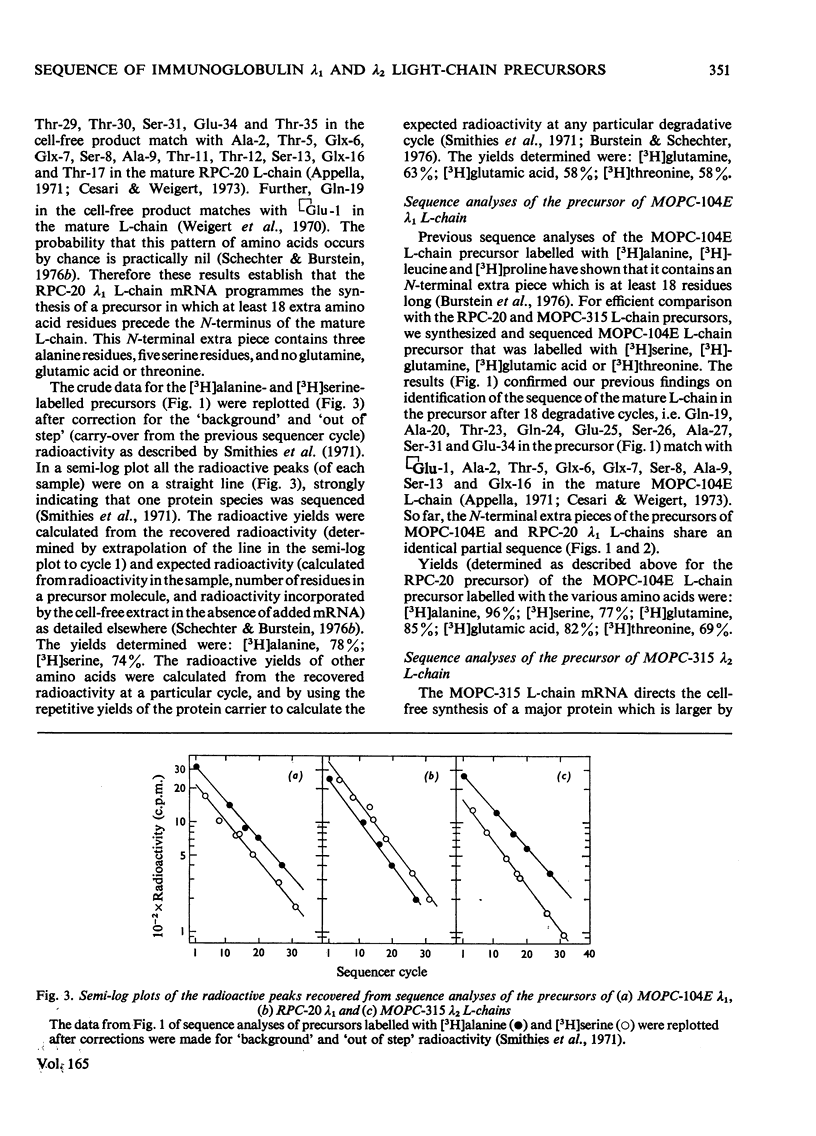
- Jackson R., Hunter T. Role of methionine in the initiation of haemoglobin synthesis. Nature. 1970 Aug 15;227(5259):672–676. doi: 10.1038/227672a0. [DOI] [PubMed] [Google Scholar]
- Leder P., Honjo T., Packman S., Swan D., Nau M., Norman B. The organization and diversity of immunoglobulin genes. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5109–5115. doi: 10.1073/pnas.71.12.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- MESSER M., OTTESEN M. ISOLATION AND PROPERTIES OF GLUTAMINE CYCLOTRANSFERASE OF DRIED PAPAYA LATEX. Biochim Biophys Acta. 1964 Nov 22;92:409–411. doi: 10.1016/0926-6569(64)90204-4. [DOI] [PubMed] [Google Scholar]
- McKean D., Potter M., Hood L. Mouse immunoglobulin chains. Pattern of sequence variation among kappa chains with limited sequence differences. Biochemistry. 1973 Feb;12(4):760–771. doi: 10.1021/bi00728a028. [DOI] [PubMed] [Google Scholar]
- Potter M. Immunoglobulin-producing tumors and myeloma proteins of mice. Physiol Rev. 1972 Jul;52(3):631–719. doi: 10.1152/physrev.1972.52.3.631. [DOI] [PubMed] [Google Scholar]
- Prasad C., Peterkofsky A. Initiation by methionine of mouse immunoglobulin light chain containing NH-2terminal pyroglutamic acid. J Biol Chem. 1975 Jan 10;250(1):171–174. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Aggregates of partially purified mRNA coding for immunoglobulin light-chain. Biochem Biophys Res Commun. 1974 Apr 8;57(3):857–864. doi: 10.1016/0006-291x(74)90625-1. [DOI] [PubMed] [Google Scholar]
- Schechter I. Biologically and chemically pure mRNA coding for a mouse immunoglobulin L-chain prepared with the aid of antibodies and immobilized oligothymidine. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2256–2260. doi: 10.1073/pnas.70.8.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Identification of N-terminal methionine in the precursor of immunoglobulin light chain. Initiation of translation of messenger ribonucleic acid in plants and animals. Biochem J. 1976 Mar 1;153(3):543–550. doi: 10.1042/bj1530543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Marked hydrophobicity of the NH2-terminal extra piece of immunoglobulin light-chain precursors: possible physiological functions of the extra piece. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3273–3277. doi: 10.1073/pnas.73.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Partial sequence of the precursors of immunoglobulin light-chains of different subgroups: evidence that the immunoglobulin variable-region gene is larger than hitherto known. Biochem Biophys Res Commun. 1976 Jan 26;68(2):489–496. doi: 10.1016/0006-291x(76)91172-4. [DOI] [PubMed] [Google Scholar]
- Schechter I. Further characterization of the mRNA coding for immunoglobulin light-chain. Biochem Biophys Res Commun. 1975 Nov 3;67(1):228–235. doi: 10.1016/0006-291x(75)90306-x. [DOI] [PubMed] [Google Scholar]
- Schechter I. Region of immunoglobulin light-chain mRNA transcribed into complementary DNA by RNA-dependent DNA polymerase of avian myeloblastosis virus. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2511–2514. doi: 10.1073/pnas.72.7.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Use of antibodies for the isolation of biologically pure messenger ribonucleic acid from fully functional eukaryotic cells. Biochemistry. 1974 Apr 23;13(9):1875–1885. doi: 10.1021/bi00706a016. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Cory S., Adams J. M. Translation of immunoglobulin mRNAs in a wheat germ cell-free system. Mol Biol Rep. 1974 Mar;1(6):355–363. doi: 10.1007/BF00309570. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Marcker K. A. N-formylmethionyl transfer RNA in mitochondria from yeast and rat liver. J Mol Biol. 1968 Dec 14;38(2):241–243. doi: 10.1016/0022-2836(68)90409-9. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Stott D. I., Munro A. J. The formation of pyrrolid-2-one-5-carboxylic acid at the N-terminus of immunoglobulin G heavy chain. Biochem J. 1972 Aug;128(5):1221–1227. doi: 10.1042/bj1281221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twardzik D. R., Peterkofsky A. Glutamic acid as a precursor to N-terminal pyroglutamic acid in mouse plasmacytoma protein (protein synthesis-initiation-immunoglobulins-pyrrolidone carboxylic acid). Proc Natl Acad Sci U S A. 1972 Jan;69(1):274–277. doi: 10.1073/pnas.69.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]


