Abstract
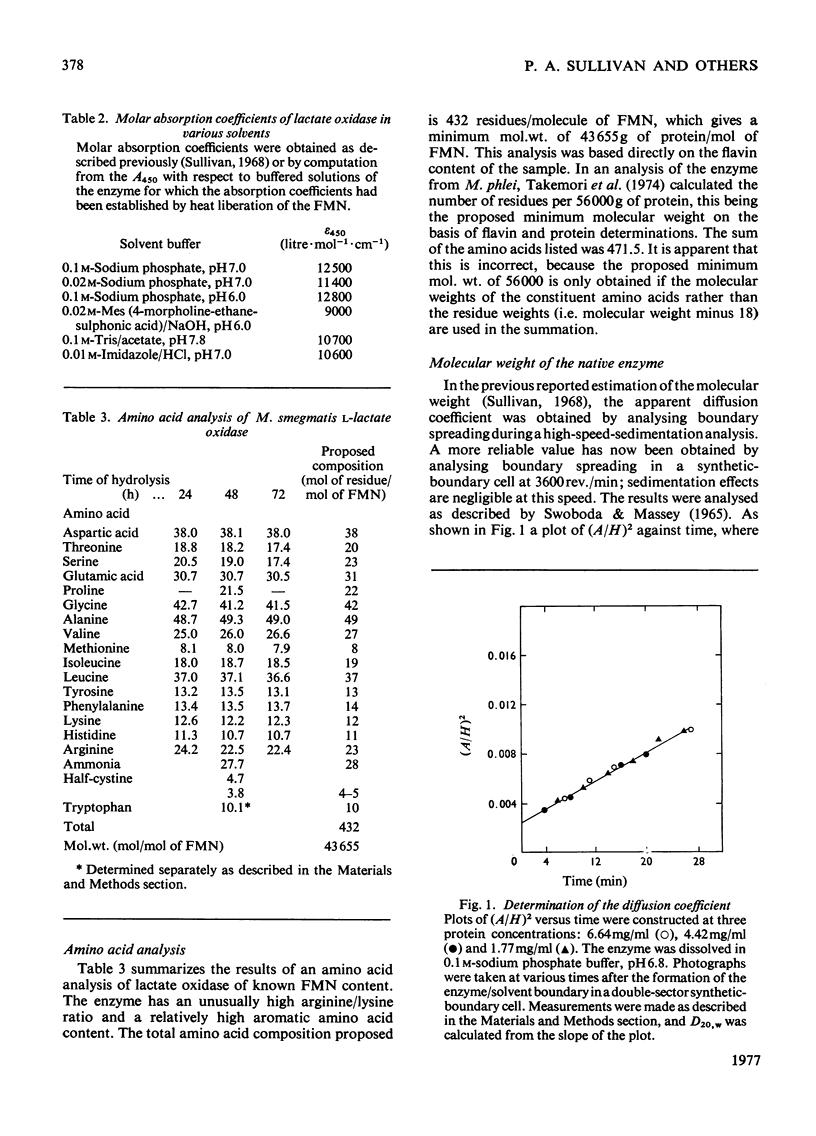
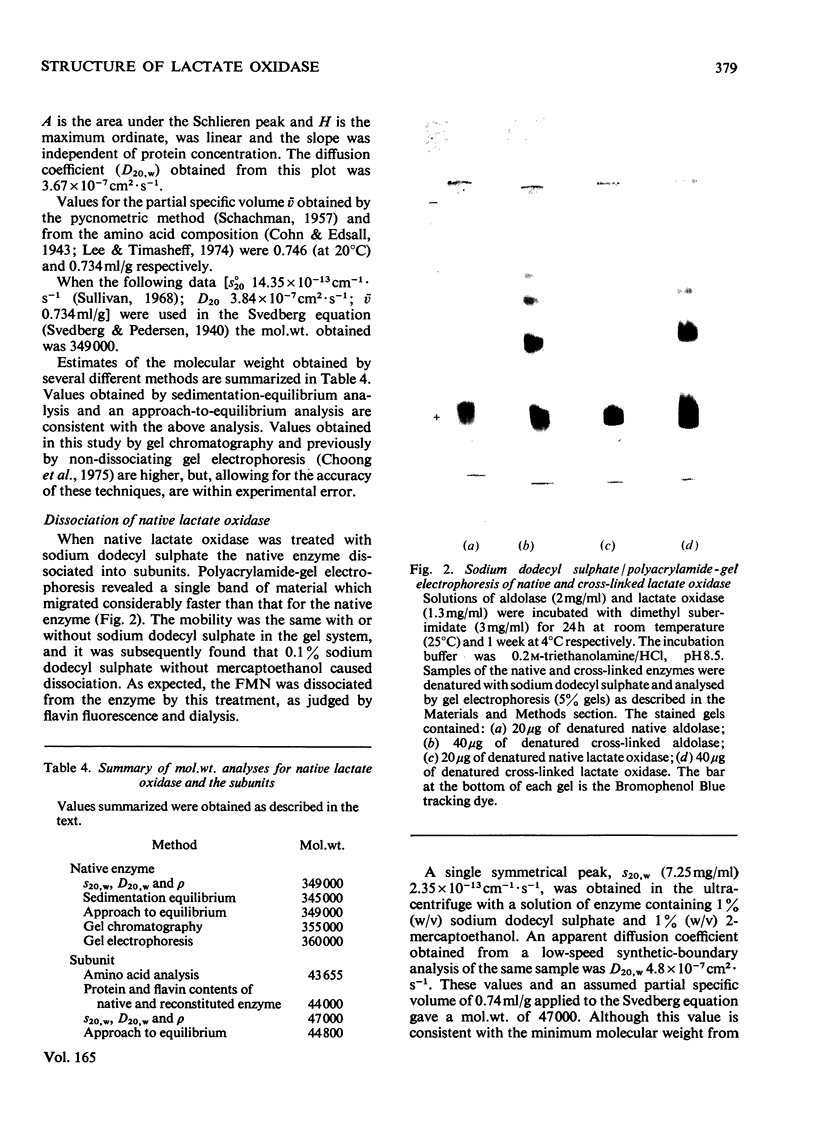
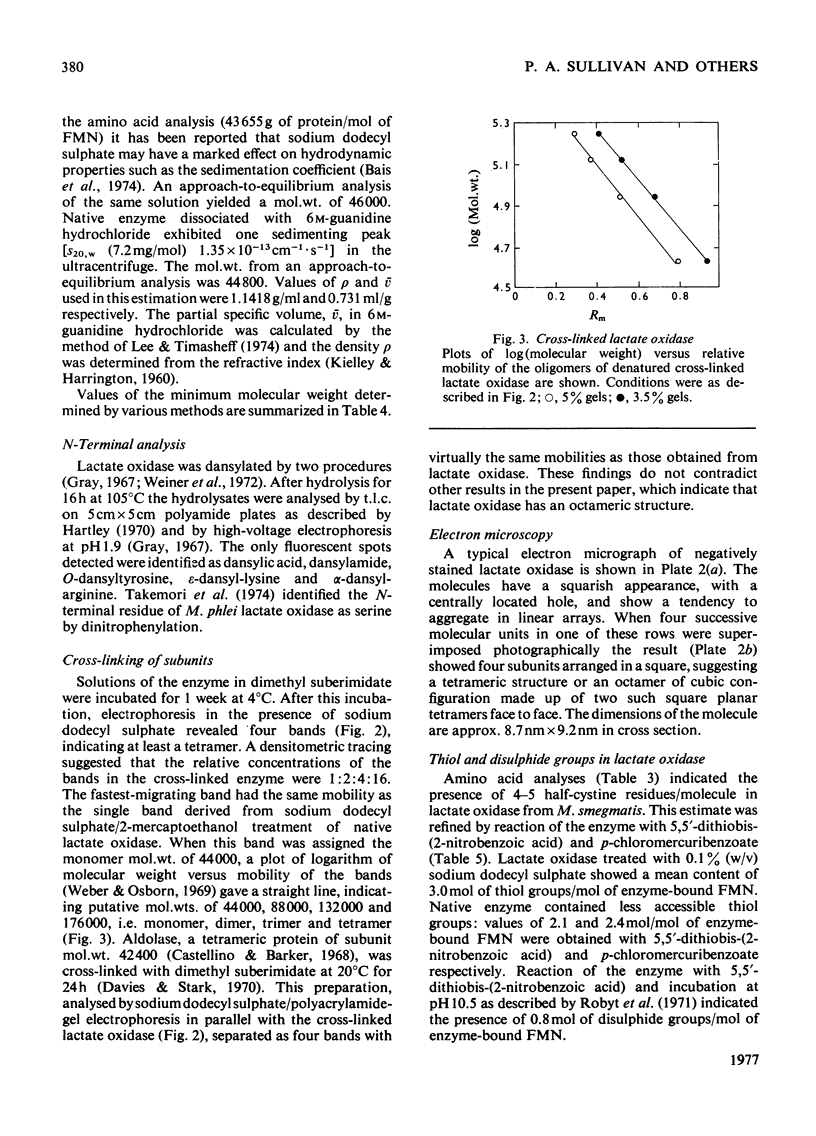
1. An improved purification was developed for L-lactate oxidase from Mycobacterium smegmatis. 2. The mol.wt. of the native enzyme by a sedimentation-equilibrium analysis was 345 000, and other ultracentrifuge methods gave values in the range 345 000-350 000. 3. An amino acid analysis, determinations of protein and flavin, a sedimentation-velocity analysis and an approach to equilibrium analysis gave values for the subunit mol.wt. in the range 43 500-47 000. 4. It was concluded that L-lactate oxidase contains eight subunits of mol.wt. 43 500. 5. Cross-linking of the subunits with dimethyl suberimidate and electron-microscopy studies were consistent with an octameric structure.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais R., Greenwell P., Wallace J. C., Keech D. B. Influence of sodium dodecyl sulphate on the sedimentation velocity of proteins. FEBS Lett. 1974 Apr 15;41(1):53–57. doi: 10.1016/0014-5793(74)80952-x. [DOI] [PubMed] [Google Scholar]
- Castellino F. J., Barker R. Examination of the dissociation of multichain proteins in guanidine hydrochloride by membrane osmometry. Biochemistry. 1968 Jun;7(6):2207–2217. doi: 10.1021/bi00846a025. [DOI] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Choong Y. S., Shepherd M. G., Sullivan P. A. Preparation of the lactate oxidase apoenzyme and studies on the binding of flavin mononucleotide to the apoenzyme. Biochem J. 1975 Jan;145(1):37–45. doi: 10.1042/bj1450037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies G. E., Stark G. R. Use of dimethyl suberimidate, a cross-linking reagent, in studying the subunit structure of oligomeric proteins. Proc Natl Acad Sci U S A. 1970 Jul;66(3):651–656. doi: 10.1073/pnas.66.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Ogata H., Massey V., Schonbrunn A., Abeles R. H., Walsh C. T. Kinetic studies on the inactivation of L-lactate oxidase by [the acetylenic suicide substrate] 2-hydroxy-3-butynoate. Biochemistry. 1976 May 4;15(9):1791–1797. doi: 10.1021/bi00654a002. [DOI] [PubMed] [Google Scholar]
- Hartley B. S. Strategy and tactics in protein chemistry. Biochem J. 1970 Oct;119(5):805–822. doi: 10.1042/bj1190805f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., HARRINGTON W. F. A model for the myosin molecule. Biochim Biophys Acta. 1960 Jul 15;41:401–421. doi: 10.1016/0006-3002(60)90037-8. [DOI] [PubMed] [Google Scholar]
- Kato N., Omori Y., Tani Y., Ogata K. Alcohol oxidases of Kloeckera sp. and Hansenula polymorpha. Catalytic properties and subunit structures. Eur J Biochem. 1976 May 1;64(2):341–350. doi: 10.1111/j.1432-1033.1976.tb10307.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The calculation of partial specific volumes of proteins in guanidine hydrochloride. Arch Biochem Biophys. 1974 Nov;165(1):268–273. doi: 10.1016/0003-9861(74)90164-7. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Massey V., Sullivan P. A. Mechanism of action of the flavoenzyme lactate oxidase. J Biol Chem. 1972 Dec 25;247(24):8097–8106. [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Robyt J. F., Ackerman R. J., Chittenden C. G. Reaction of protein disulfide groups with Ellman's reagent: a case study of the number of sulfhydryl and disulfide groups in Aspergillus oryzae -amylase, papain, and lysozyme. Arch Biochem Biophys. 1971 Nov;147(1):262–269. doi: 10.1016/0003-9861(71)90334-1. [DOI] [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Schonbrunn A., Abeles R. H., Walsh C. T., Ghisla S., Ogata H., Massey V. The structure of the covalent flavin adduct formed between lactate oxidase and the suicide substrate 2-hydroxy-3-butynoate. Biochemistry. 1976 May 4;15(9):1798–1807. doi: 10.1021/bi00654a003. [DOI] [PubMed] [Google Scholar]
- Spencer R. L., Wold F. A new convenient method for estimation of total cystine-cysteine in proteins. Anal Biochem. 1969 Oct 15;32(1):185–190. doi: 10.1016/0003-2697(69)90123-7. [DOI] [PubMed] [Google Scholar]
- Sullivan P. A. Crystallization and properties of L-lactate oxidase from Mycobacterium smegmatis. Biochem J. 1968 Nov;110(2):363–371. doi: 10.1042/bj1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori S., Nakazawa K., Nakai Y., Suzuki K., Katagiri M. A lactate oxygenase from Mycobacterium phlei. Improved purification and some properties of the enzyme. J Biol Chem. 1968 Jan 25;243(2):313–319. [PubMed] [Google Scholar]
- Takemori S., Tajima H., Kawahara F., Nakai Y., Katagiri M. A lactate oxygenase from Mycobacterium phlei. 3. Evidence for the subunit structure. Arch Biochem Biophys. 1974 Jan;160(1):289–303. doi: 10.1016/s0003-9861(74)80037-8. [DOI] [PubMed] [Google Scholar]
- Walsh C. T., Schonbrunn A., Lockridge O., Massey V., Abeles R. H. Inactivation of a flavoprotein, lactate oxidase, by an acetylenic substrate. J Biol Chem. 1972 Sep 25;247(18):6004–6006. [PubMed] [Google Scholar]
- Walsh C., Lockridge O., Massey V., Abeles R. Studies on the mechanism of action of the flavoenzyme lactate oxidase. Oxidation and elimination with beta-chlorolactate. J Biol Chem. 1973 Oct 25;248(20):7049–7054. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]