Abstract
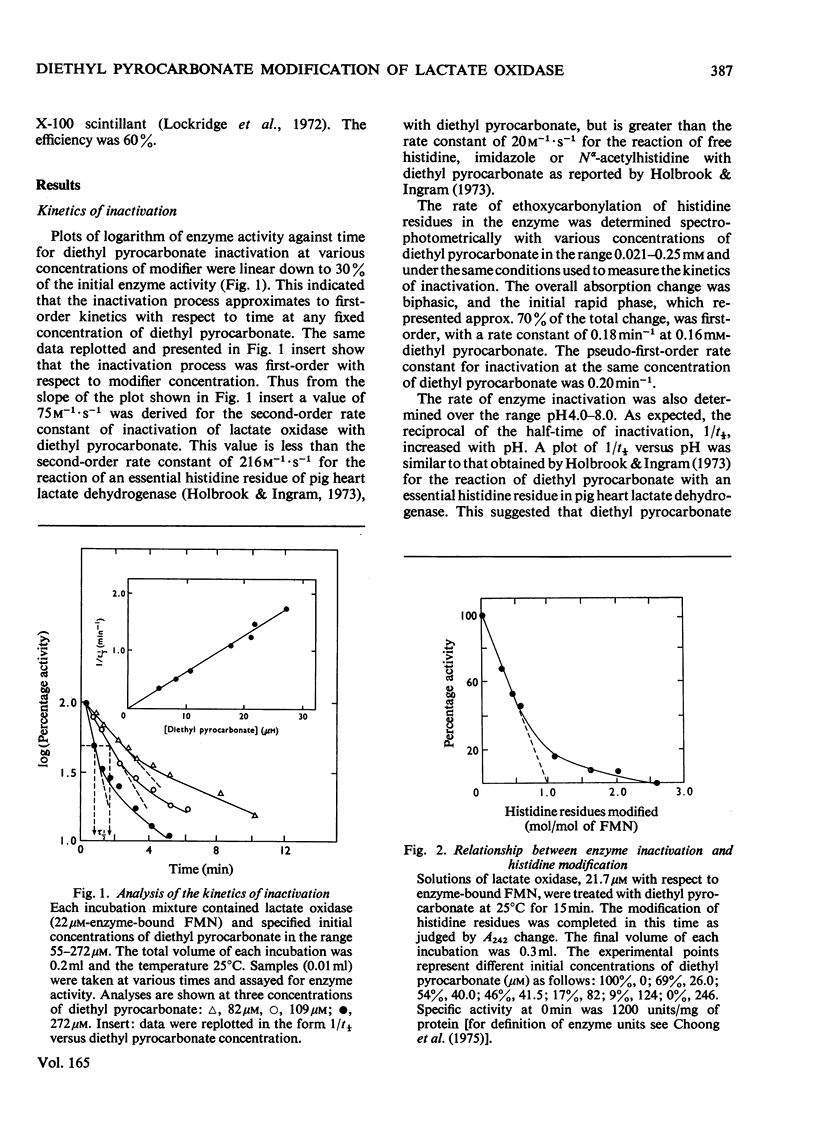
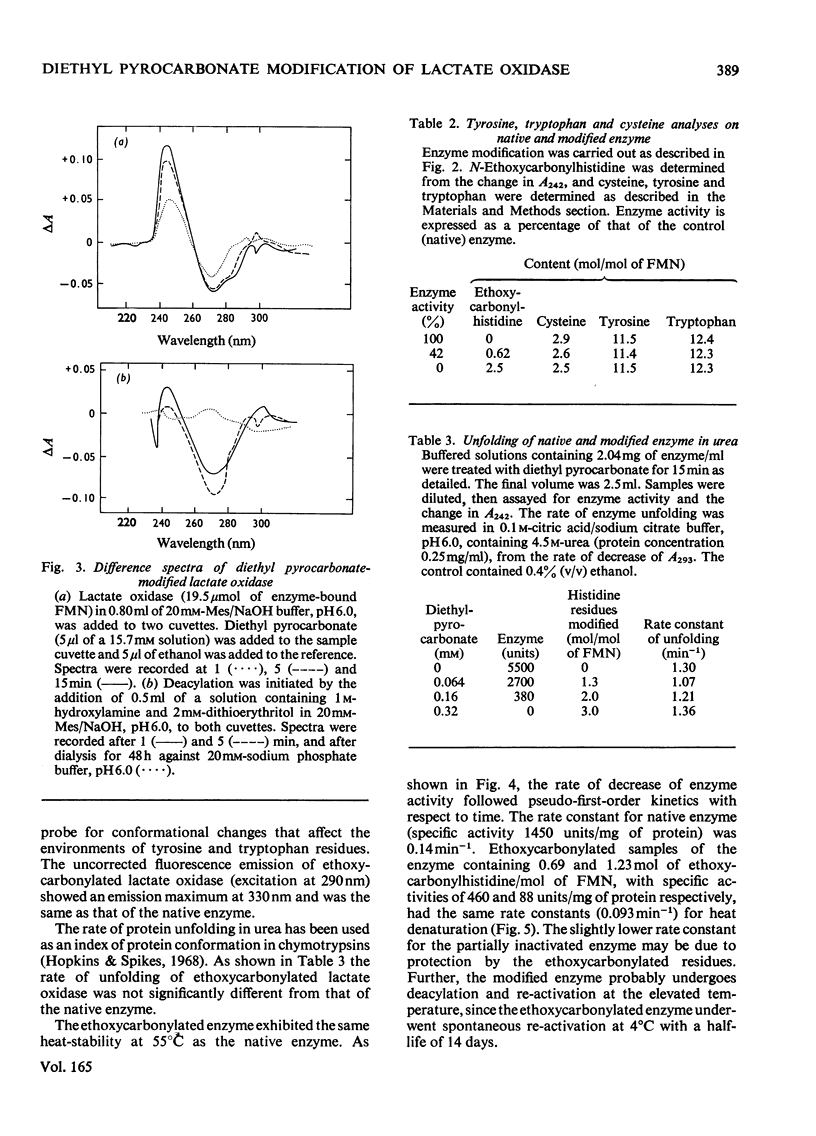
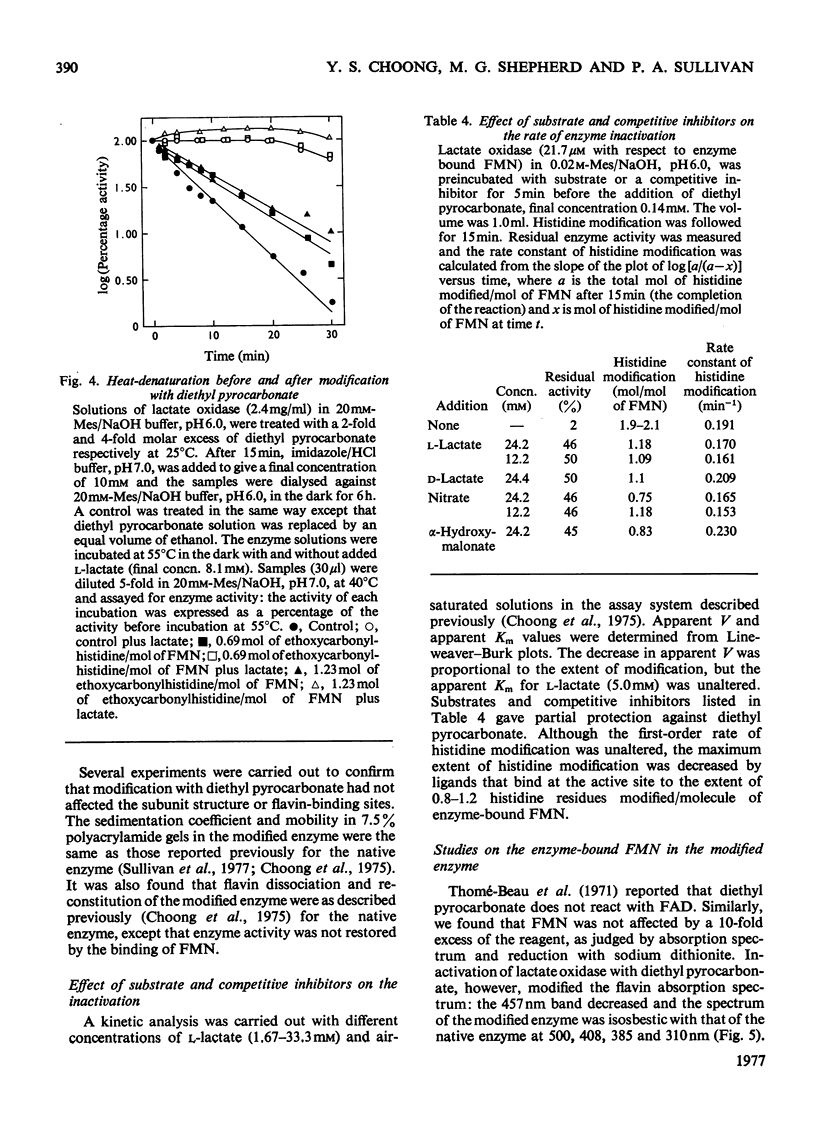
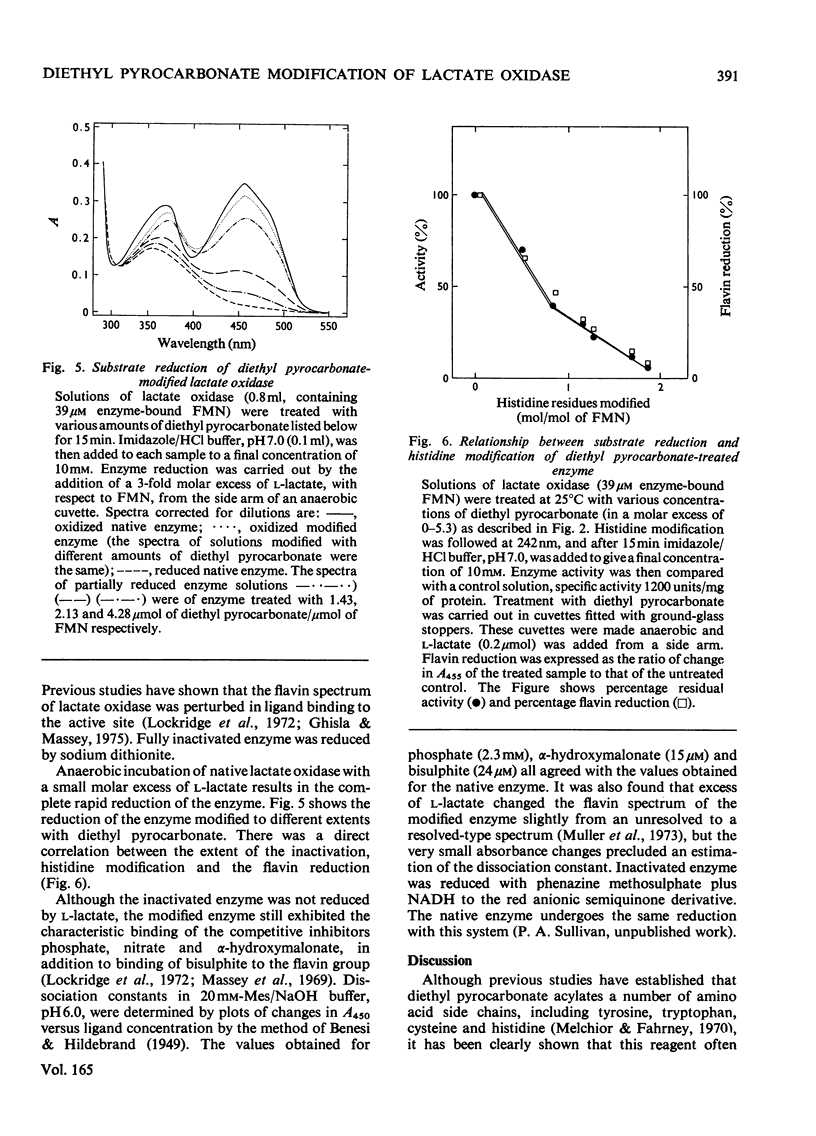
1. Diethyl pyrocarbonate inactivated l-lactate oxidase from Mycobacterium smegmatis. 2. Two histidine residues underwent ethoxycarbonylation when the enzyme was treated with sufficient reagent to abolish more than 90% of the enzyme activity, but analyses of the inactivation showed that the modification of one histidine residue was sufficient to cause the loss of enzyme activity. The rates of enzyme inactivation and histidine modification were the same. 3. Substrate and competitive inhibitors decreased the maximum extent of inactivation to a 50% loss of enzyme activity and modification was decreased from 1.9 to 0.75–1.2 histidine residues modified/molecule of FMN. 4. Treatment of the enzyme with diethyl [14C]pyrocarbonate (labelled in the carbonyl groups) confirmed that only histidine residues were modified under the conditions used and that deacylation of the ethoxycarbonylhistidine residues by hydroxylamine was concomitant with the removal of the 14C label and the re-activation of the enzyme. 5. No evidence was found for modification of tryptophan, tyrosine or cysteine residues, and no difference was detected between the conformation and subunit structure of the modified and native enzyme. 6. Modification of the enzyme with diethyl pyrocarbonate did not alter the following properties: the binding of competitive inhibitors, bisulphite and substrate or the chemical reduction of the flavin group to the semiquinone or fully reduced states. The normal reduction of the flavin by lactate was, however, abolished.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averill B. A., Schonbrunn A., Abeles R. H. Studies on the mechanism of Mycobacterium smegmatis L-lactate oxidase. 5-Deazaflavin mononucleotide as a coenzyme analogue. J Biol Chem. 1975 Feb 25;250(4):1603–1605. [PubMed] [Google Scholar]
- Bredderman P. J. Tryptophan analysis of proteins in 6M guanidine hydrochloride: modification for more general application. Anal Biochem. 1974 Sep;61(1):298–301. doi: 10.1016/0003-2697(74)90360-1. [DOI] [PubMed] [Google Scholar]
- Burstein Y., Walsh K. A., Neurath H. Evidence of an essential histidine residue in thermolysin. Biochemistry. 1974 Jan 1;13(1):205–210. doi: 10.1021/bi00698a030. [DOI] [PubMed] [Google Scholar]
- Choong Y. S., Shepherd M. G., Sullivan P. A. Preparation of the lactate oxidase apoenzyme and studies on the binding of flavin mononucleotide to the apoenzyme. Biochem J. 1975 Jan;145(1):37–45. doi: 10.1042/bj1450037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dann L. G., Britton H. G. The reaction of diethyl pyrocarbonate with pyruvate kinase. Biochem J. 1974 Feb;137(2):405–407. doi: 10.1042/bj1370405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fisher J., Spencer R., Walsh C. Enzyme-catalyzed redox reactions with the flavin analogues 5-deazariboflavin, 5-deazariboflavin 5'-phosphte, and 5-deazariboflavin 5'-diphosphate, 5' leads to 5'-adenosine ester. Biochemistry. 1976 Mar 9;15(5):1054–1064. doi: 10.1021/bi00650a016. [DOI] [PubMed] [Google Scholar]
- Ghisla S., Massey V. Mechanism of inactivation of the flavoenzyme lactate oxidase by oxalate. J Biol Chem. 1975 Jan 25;250(2):577–584. [PubMed] [Google Scholar]
- Ghisla S., Ogata H., Massey V., Schonbrunn A., Abeles R. H., Walsh C. T. Kinetic studies on the inactivation of L-lactate oxidase by [the acetylenic suicide substrate] 2-hydroxy-3-butynoate. Biochemistry. 1976 May 4;15(9):1791–1797. doi: 10.1021/bi00654a002. [DOI] [PubMed] [Google Scholar]
- Hegyi G., Premecz G., Sain B., Mühlrád A. Selective carbethoxylation of the histidine residues of actin by diethylpyrocarbonate. Eur J Biochem. 1974 May 2;44(1):7–12. doi: 10.1111/j.1432-1033.1974.tb03452.x. [DOI] [PubMed] [Google Scholar]
- Hellerman L., Coffey D. S. Studies on crystalline D-amino acid oxidase. V. Characterization of borohydride-reduced enzyme-subtrate intermediate. Synthesis of epsilon-N-(1-carboxyethyl)-L-lysine. J Biol Chem. 1967 Feb 25;242(4):582–589. [PubMed] [Google Scholar]
- Hersh L. B., Jorns M. S. Use of 5-deazaFAD to study hydrogen transfer in the D-amino acid oxidase reaction. J Biol Chem. 1975 Nov 25;250(22):8728–8734. [PubMed] [Google Scholar]
- Holbrook J. J., Ingram V. A. Ionic properties of an essential histidine residue in pig heart lactate dehydrogenase. Biochem J. 1973 Apr;131(4):729–738. doi: 10.1042/bj1310729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins T. R., Spikes J. D. Denaturation of proteins in 8M urea as monitored by tryptophan fluorescence: trypsin, trypsinogen and some derivatives. Biochem Biophys Res Commun. 1968 Mar 12;30(5):540–545. doi: 10.1016/0006-291x(68)90086-7. [DOI] [PubMed] [Google Scholar]
- Huc C., Olomucki A., Lê-Thi-Lan, Dang-Ba-Pho, Nguyen-Van-Thoai Essential histidyl residues of octopine dehydrogenase. Eur J Biochem. 1971 Jul 29;21(2):161–169. doi: 10.1111/j.1432-1033.1971.tb01452.x. [DOI] [PubMed] [Google Scholar]
- Lockridge O., Massey V., Sullivan P. A. Mechanism of action of the flavoenzyme lactate oxidase. J Biol Chem. 1972 Dec 25;247(24):8097–8106. [PubMed] [Google Scholar]
- Massey V., Ganther H. On the interpretation of the absorption spectra of flavoproteins with special reference to D-amino acid oxidase. Biochemistry. 1965 Jun;4(6):1161–1173. doi: 10.1021/bi00882a027. [DOI] [PubMed] [Google Scholar]
- Massey V., Müller F., Feldberg R., Schuman M., Sullivan P. A., Howell L. G., Mayhew S. G., Matthews R. G., Foust G. P. The reactivity of flavoproteins with sulfite. Possible relevance to the problem of oxygen reactivity. J Biol Chem. 1969 Aug 10;244(15):3999–4006. [PubMed] [Google Scholar]
- Melchior W. B., Jr, Fahrney D. Ethoxyformylation of proteins. Reaction of ethoxyformic anhydride with alpha-chymotrypsin, pepsin, and pancreatic ribonuclease at pH 4. Biochemistry. 1970 Jan 20;9(2):251–258. doi: 10.1021/bi00804a010. [DOI] [PubMed] [Google Scholar]
- Müller F., Mayhew S. G., Massey V. On the effect of temperature on the absorption spectra of free and protein-bound flavines. Biochemistry. 1973 Nov 6;12(23):4654–4662. doi: 10.1021/bi00747a017. [DOI] [PubMed] [Google Scholar]
- SIMPSON R. T., RIORDAN J. F., VALLEE B. L. FUNCTIONAL TYROSYL RESIDUES IN THE ACTIVE CENTER OF BOVINE PANCREATIC CARBOXYPEPTIDASE A. Biochemistry. 1963 May-Jun;2:616–622. doi: 10.1021/bi00903a039. [DOI] [PubMed] [Google Scholar]
- Schonbrunn A., Abeles R. H., Walsh C. T., Ghisla S., Ogata H., Massey V. The structure of the covalent flavin adduct formed between lactate oxidase and the suicide substrate 2-hydroxy-3-butynoate. Biochemistry. 1976 May 4;15(9):1798–1807. doi: 10.1021/bi00654a003. [DOI] [PubMed] [Google Scholar]
- Steenkamp D. J., Schabort J. C., Holzapfel C. W., Ferreira N. P. The role of essential histidines in the mechanism of catalysis of the flavoenzyme, beta-cyclopiazonate oxidocyclase. Biochim Biophys Acta. 1974 Jul 17;358(1):126–143. doi: 10.1016/0005-2744(74)90265-4. [DOI] [PubMed] [Google Scholar]
- Sullivan P. A., Soon C. Y., Schreurs W. J., Cutfield J. F., Shepherd M. G. The structure of L-lactate oxidase from Mycobacterium smegmatis. Biochem J. 1977 Aug 1;165(2):375–383. doi: 10.1042/bj1650375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomé-Beau F., Lê-Thi-Lan, Olomucki A., van Thoai N. Essential histidyl residues in arginine oxygenase (decarbosylating). Comparison with amino acid oxidases. Eur J Biochem. 1971 Mar 11;19(2):270–275. doi: 10.1111/j.1432-1033.1971.tb01314.x. [DOI] [PubMed] [Google Scholar]
- Walsh C. T., Krodel E., Massey V., Abeles R. H. Studies on the elimination reaction of D-amino acid oxidase with -amino- -chlorobutyrate. Further evidence for abstraction of substrate -hydrogen as a proton. J Biol Chem. 1973 Mar 25;248(6):1946–1955. [PubMed] [Google Scholar]
- Walsh C. T., Schonbrunn A., Abeles R. H. Studies on the mechanism of action of D-amino acid oxidase. Evidence for removal of substrate -hydrogen as a proton. J Biol Chem. 1971 Nov 25;246(22):6855–6866. [PubMed] [Google Scholar]
- Walsh C., Lockridge O., Massey V., Abeles R. Studies on the mechanism of action of the flavoenzyme lactate oxidase. Oxidation and elimination with beta-chlorolactate. J Biol Chem. 1973 Oct 25;248(20):7049–7054. [PubMed] [Google Scholar]


