Oral belzutifan, a novel HIF-2ɑ inhibitor, was associated with volume reduction of a giant retinal hemangioblastoma with extrascleral extension associated with von Hippel–Lindau. The medication was well tolerated with minimal systemic side effects. This treatment may be considered in patients with complex retinal hemangioblastoma that is unamendable to standard approaches.
Key words: hemangioblastoma, ocular oncology, retina, systemic disease, therapeutics, von Hippel–Lindau
Abstract
Purpose:
To describe the clinical response and safety profile of the novel HIF-2ɑ inhibitor belzutifan in treating a giant retinal hemangioblastoma with extrascleral extension associated with von Hippel–Lindau syndrome.
Methods:
A 71-year-old woman with Von Hippel–Lindau syndrome presented with a giant retinal hemangioblastoma with extrascleral extension in her only remaining eye. She had no light perception in the right eye and intraocular pressure was 48. She requested enucleation because of chronic pain, but because of concern for significant bleeding given the size of the neoplasm, a trial of belzutifan was initiated.
Results:
Within 3 months of treatment initiation, the patient reported an 80% reduction in pain. Magnetic resonance imaging showed 30% reduction in longest tumor diameter. Dose adjustments were guided by serum hemoglobin levels, allowing the patient to remain on the medication for over a year with continued tumor regression on MRI and avoid enucleation.
Conclusion:
Retinal hemangioblastoma with extrascleral extension is exceedingly rare and its treatment is complex, often requiring enucleation or external beam radiotherapy. This report demonstrates the use of belzutifan to safely and successfully reduce ocular tumor burden of complicated retinal hemangioblastoma with extrascleral extension, ultimately decreasing the need for enucleation.
Von Hippel–Lindau syndrome (VHL) is characterized by the development of multiple neoplasm primarily of the retina, CNS, kidney, and pancreas. Von Hippel–Lindau syndrome has an incidence of ∼1/36,0001 and retinal hemangioblastomas (RHB) are often the earliest manifestations of the disease.2 Current treatments for RHB include laser photocoagulation, radiotherapy, and enucleation.3 In August 2021, the FDA approved the HIF-2ɑ inhibitor belzutifan as a treatment of VHL-associated tumors. We report the first use of belzutifan to treat VHL-related complex RHB with extrascleral extension in a patient without other concurrent VHL tumors.
Case Report
A 71-year-old woman with history of VHL since the age of 16 presented to ocular oncology clinic for her first examination in 6 years. She had a history of numerous VHL-related tumors, including cerebellar hemangioblastoma, thoracic meningioma, and giant RHB in the left eye treated with complex enucleation in 2006.
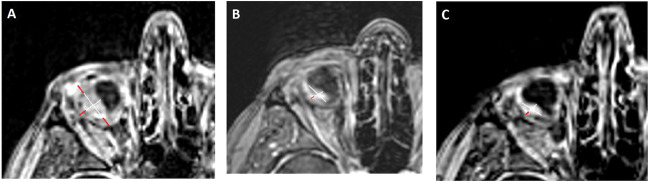
On presentation, she reported 4/10 pain and irritation caused by large RHB with extrascleral extension in the right eye (Figure 1A). She had no light perception in the right eye and intraocular pressure was 48. B-scan revealed an 8.6-mm thick intraocular component superotemporally with an extrascleral component up to 9 mm (Figure 1B), whereas MRI showed a tumor sized 2.0 cm × 1.2 cm (Figure 2A). The patient requested enucleation because of pain, but given the tumor size and extraocular component, surgery was not recommended because of the significant risk of complications. Conservative treatment with prednisolone acetate and atropine was recommended for comfort, but was minimally effective. At this point, a trial of 120 mg belzutifan daily was initiated by neuro-oncology to decrease tumor size. Three months later, the patient reported 80% reduction in pain and could tolerate wearing a scleral lens for cosmesis for the first time. Around the same time, the tumor's extrascleral component also appeared less vascularized (Figure 1C). MRI demonstrated 30% tumor regression in the long dimension from 2.0 cm × 1.2 cm 3 months before (Figure 2A) to 1.4 cm × 0.8 cm (Figure 2B), consistent with a “partial response” as per the Response Evaluation Criteria in Solid Tumors version 1.1.4 Belzutifan dose was reduced to 80 mg because of a decline in hemoglobin from a baseline of 15.3 to 11.5. Four months after therapy initiation, the patient reported continued improvement in pain and B scan showed a 31% decrease in intraocular mass thickness and 60% reduction in transscleral component (Figure 1D). Intraocular pressure improved to 21 and hemoglobin improved to 11.8. The patient was able to tolerate belzutifan at 80 mg daily and after 1 year of therapy, her hemoglobin was stable at 12.6 with improved energy levels and eye pain. Magnetic resonance imaging at this time showed continued tumor regression of nearly 50% to 1.1 cm × 0.5 cm (Figure 2C).
Fig. 1.
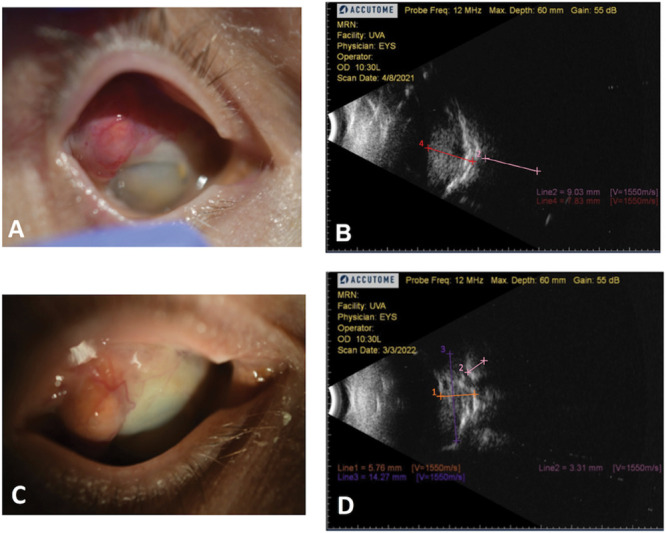
Retinal hemangioblastoma at initial presentation versus 4 months of belzutifan therapy. A. Gross visualization of RHB in the right eye during initial presentation. Note vascularized transscleral component. B. Initial RHB B scan that showed transscleral component up to 9.03 mm (pink line 2). White arrows indicate sclera. C. Gross visualization of RHB in the right eye after 4 months of belzutifan therapy. D. Follow-up B scan after belzutifan therapy that showed transscleral component up to 3.31 mm (pink line 2). White arrows indicate sclera.
Fig. 2.
Progression of RHB on MRI. A. MRI before initiation of belzutifan that showed tumor size of 2.0 cm × 1.2 cm. Outer red diameter lines represent transscleral component. B. MRI after 4 months of belzutifan therapy that showed tumor regression to 1.4 cm × 0.8 cm. C. MRI after 1 year of belzutifan therapy with evidence of significant tumor regression to 1.1 cm × 0.5 cm.
Discussion
We report the first known use of belzutifan for treatment of ocular retinal hemangioblastoma in a patient with VHL without concurrent VHL-related tumors. Belzutifan is a novel HIF-2α inhibitor approved for patients with VHL who require therapy for renal cell carcinoma, central nervous system hemangioblastomas, or pancreatic neuroendocrine tumors. Few studies have documented belzutifan's efficacy outside of a phase 1 and 2 trial, and have focused on belzutifan's use for renal cell carcinoma therapy.5,6 All patients in the phase 2 trial with associated RHBs experienced tumor regression, indicating belzutifan is a promising treatment with a more tolerable side effect profile compared with the standard of care. However, further study is required to better understand belzutifan's role in treating other VHL-disease-associated tumors apart from renal cell carcinoma, such as RHB. Current treatment options for large RHBs and hemangioblastomas affecting the optic nerve head are very limited and not efficacious. External beam radiotherapy, plaque brachytherapy, or selective surgical excision can lead to significant local complications, including retinal detachment, exudation, and ultimate loss of vision.3 The RHB with transscleral and extrascleral extension that we report here is exceedingly rare, but aggressive retinal angiomas with extraocular spread have been reported in the past,7 and often require enucleation, which in itself can be complicated by significant bleeding.8 The ability to control RHB with an orally administered medication can be vision saving and life changing for patients with VHL.
The decision to use belzutifan in a monocular patient with a long-standing giant RHB and extraocular extension was an attempt to forestall the need for enucleation or make enucleation less complex. Although the extraocular extension may represent a pyogenic granuloma related to the phthisical state of the eye, there is a high likelihood that it is an extrascleral component of the intraocular lesion based on the clinical appearance of the vascular lesion that is continuous with the intraocular hemangioma on ultrasonography and MRI. The response to treatment with clinical decrease in size, associated discomfort, and vascularity further support this assessment. Our patient demonstrated a 30% reduction in tumor size within 3 months of belzutifan initiation with significant decrease in ocular pain. Tumor size reduction continued further to 50% over the first year of treatment. The patient developed a moderate anemia that responded to dose reduction from 120 mg to 80 mg with continued observed effect of the therapy on the tumor. Belzutifan treatment was not associated with increased exudation or inflammatory reactions observed with other therapies and even contributed to deceased tumor vascularity. The time to response and side effect profile reported here align well with reported phase 2 data.
Our findings suggest that belzutifan can be considered as a treatment modality for difficult-to-treat ocular hemangioblastomas in VHL, including large tumors, those associated with extensive exudative detachment, and those involving the optic nerve. The benefits of therapy are oral formulation with acceptable safety profile, lack of tissue destruction, and no increased exudation, which may make belzutifan a better option compared with traditionally used treatments such as radiation.
Future studies on smaller tumors are needed to assess treatment impact on vision. In addition, long-term effects of this medication or its cessation will need to be assessed and we will closely follow this patient because durability of effect and need for lifelong treatment are also unclear.
Conclusions
Belzutifan, a novel HIF-2ɑ inhibitor, was used for a giant RHB with extrascleral extension associated with VHL, demonstrating significant tumor regression and symptom improvement. Notably, the use of belzutifan was associated with an acceptable side effect profile and not associated with a significant local exudation reaction, unlike traditional treatments for RHB. Our case suggests this novel agent can be used in the management of complex RHB associated with VHL.
Footnotes
Y. (Eugene) Shildkrot: Former member of Castle Bioscience advisory board, Former employee of Roche/Genentech. The remaining authors have no financial/conflicting interests to disclose.
C. C. Cotton, A. S. Chandrabhatla, and P. H. Andrews have contributed equally.
Contributor Information
Caroline C. Cotton, Email: cc4hsv@virginia.edu.
Anirudha S. Chandrabhatla, Email: ac2fp@virginia.edu.
Patrick H. Andrews, Email: pha6ve@virginia.edu.
Benjamin W. Purrow, Email: bwp5g@uvahealth.org.
References
- 1.Ganapathy A, Diaz EJ, Coleman JT, Mackey KA. Tumor syndromes: neurosurgical evaluation and management. Neurosurg Clin N Am 2022;33:91–104. [DOI] [PubMed] [Google Scholar]
- 2.Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med 1990;77:1151–1163. [DOI] [PubMed] [Google Scholar]
- 3.Wiley HE, Krivosic V, Gaudric A, et al. Management of retinal hemangioblastoma in von Hippel-Lindau disease. Retina 2019;39:2254–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nat Med 2021;27:802–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for renal cell carcinoma in von Hippel–Lindau disease. N Engl J Med 2021;385:2036–2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumari N, Das S, Bhaduri A, Gandhi A. Retinal hemangioblastoma with extraocular extension: report of three cases. Ocul Oncol Pathol 2021;7:177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menon SG, Ramachandran GM, Jacob M, et al. Aggressive-fungating retinal hemangioblastoma. J Cancer Res Ther 2021;17:279–281. [DOI] [PubMed] [Google Scholar]