Summary:
Current breast augmentation options face limitations and potential associated complications. Implant-based augmentation introduces risks such as capsular contracture and malpositioning, whereas fat grafting poses issues such as induration and infections, necessitating revisions. Tissue engineering, integrating 3-dimensional (3D) printing and biomaterials science, aims to overcome these challenges. However, the clinical translation of these advancements remains challenging, with many approaches falling short in demonstrating the necessary volume regeneration. A 28-year-old yoga instructor with a disinterest in traditional options sought an alternative solution. Custom-made biocompatible thermoplastic copolyester implants were proposed, approved, and implemented. Our approach utilized artificial intelligence, magnetic resonance imaging, computer-aided design, and lattice structure engineering for customizing the implant design. Three-dimensional printing and plasma technology surface treatment created implants of 300 and 315 cm3 volumes, weighting around 33 g with biomimetic properties. Implants were placed in the subglandular plane; an 8-month follow-up revealed well-maintained implants without complications, except for a conservatively managed hematoma, and excellent cosmetic outcomes. Magnetic resonance imaging analysis revealed revascularization and new tissue formation within the implant, demonstrating tissue integration without complications. The study addresses biomechanical issues and foreign body reactions that cause capsular contracture in breast augmentation and proposes a novel 3D-printed implant with ultralight weight, tissue integrative porous structure, and biomimetic environments for scaffold-guided tissue regeneration. In conclusion, the presented solution shows promise in overcoming current breast augmentation limitations, demonstrating safety, biocompatibility, and patient satisfaction. Further adoption and long-term studies with larger cohorts are needed to validate its clinical effectiveness and feasibility.
Takeaways
Question: Can tissue engineering bring forth a clinically applicable substitute to tackle the fundamental issues tied to the complications of the silicone implants currently used in breast augmentation?
Findings: Through the optimization of biomaterial selection, scaffold architecture mechanics and biological microenvironment, 300-cm3 thermoplastic copolyester porous implants weighing 33 g successfully regenerated the patient’s autologous tissue. This was achieved through a single-stage, cell-free procedure, as evidenced by the fat digital subtraction function in contrast-enhanced magnetic resonance imaging, with no clinical or radiological signs of capsule formation or other complications.
Meaning: Scaffold-assisted breast augmentation emerges as a promising innovation in future personalized plastic surgery, seamlessly replacing like with like.
INTRODUCTION
Breast augmentation causes biomechanical implant-breast tissue interactions, causing tissue deformation and stretching, depicted by a nonlinear stress–strain curve. This process, especially prominent with higher implant volumes, thin soft-tissue coverage, and changes in breast structures, is predisposed to complications such as “bottoming-out,” “double bubble,” tissue atrophy, and visible implant edges.1,2
Clinically significant capsular contracture is reported in up to 45% of cases after silicone implant placement, predominantly within the first-year postsurgery necessitating implant exchange.3 Notably, a study highlighted an inverse correlation between polyurethane porous coating on silicone implants and contracture occurrence, suggesting the exploration of nontoxic, nonbiodegradable alternatives with similar properties.3
PATIENTS AND METHODS
Patient Characteristics
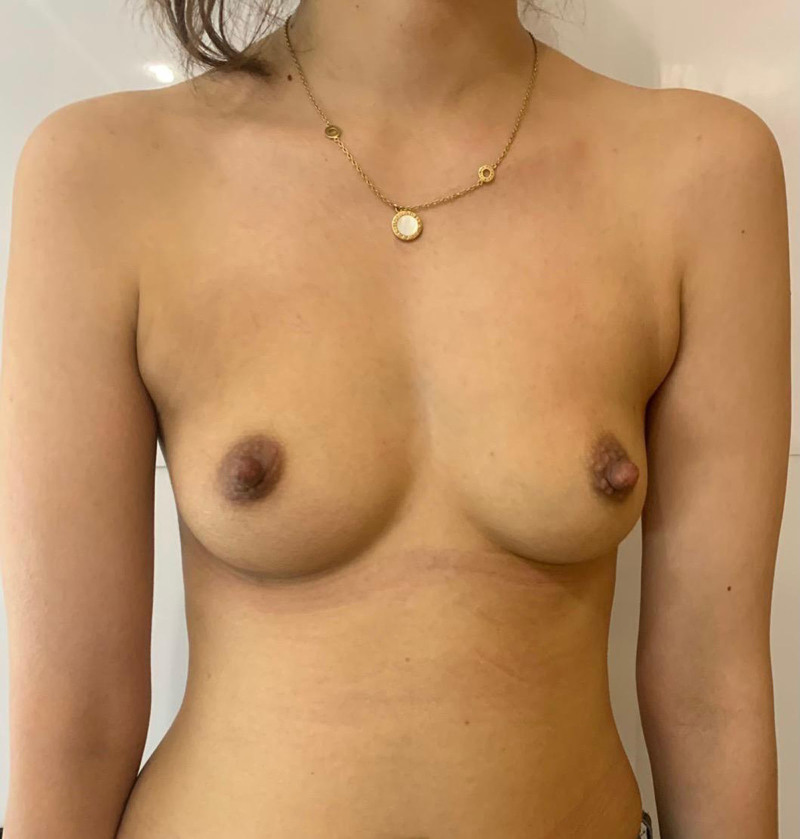
A 28-year-old woman, gravida 1, para 1, 167 cm in height, and 52 kg in weight, a yoga instructor, sought assistance for breast enhancement and correction of a slight asymmetry. Upon examination and discussion of available options, the patient expressed a disinterest in silicone implants, especially subpectoral or in a dual-plane manner, due to concerns about her thin physique and active lifestyle. The patient’s low body mass index, which corresponded to the 15th percentile, ruled out the use of autologous fat grafting. No preexisting breast conditions or medical comorbidities were reported (Fig. 1). Given this scenario, a personalized solution was needed. In response, we proposed, discussed, and obtained her approval for custom-made, lightweight, and biocompatible thermoplastic copolyester (TPC) implants.
Fig. 1.
Preoperative photograph of the patient.
Ethical Approval and Informed Consent
The study adhered to the ethical principles of the Declaration of Helsinki. Written informed consent was obtained from the patient for the procedure of breast augmentation using custom-made TPC implants, radiological evaluation at three months postoperatively, and for scientific publication.
Designing Customized Implants
The design process integrated the artificial intelligence software Crisalix (Crisalix S.A., Lausanne, Switzerland) and magnetic resonance imaging (MRI) to refine anatomical implant characterization. Proposed implant volumes (300 and 315 cc) were used to generate a computer-aided design file for the implant frame using SolidWorks (Dassault Systèmes, SolidWorks Corporation, Boston, MA).
The computer-aided design file was transferred to nTopology (nTopology, Inc, New York, NY) for lattice structure design, aiming for lattice with mechanical properties similar to breast tissue. Standard tessellation language files for the implants were produced after optimization and testing.
Fabrication of 3D-printed Implants
Standard tessellation language files were sent to a custom-made medical device manufacturer (Alive Biotechnology Solutions UK, London, England) for 3D printing and surface activation using plasma technology per ISO13485 (Medical Device Manufacturing Quality System). Medical-grade biostable TPC passed ISO10993-relevant bio-compatibility tests (Alive Biotechnology Solutions UK) were used. Details of the chemical formulation, lattice structure, printing technology, and postprinting surface functionalization are confidential to the manufacturer. Implants were sterilized in an autoclave according to the manufacturer’s instructions. All processes adhered to the final document of the International Medical Device Regulators Forum 2020 concerning personalized medical devices.4
Surgical Procedures
Under general anesthesia, 200 mL of fat tissue was liposuctioned by superwet technique via power suction and divided into 2 sterilized jars. Implants were immersed in separate jars after discarding the oil layer, following manufacturer’s instructions, to initiate surface functionalization. An inframammary incision, subglandular dissection, and radial scoring for breast fat contact were performed. Implants were extracted from the lipoaspirate and inserted in the respective pockets after irrigation. Hemostasis was ensured, and wound closure was finalized without suction drains. (See Video [online], which displays custom-made TPC breast implant design, manufacturing, implantation, and clinical result 8 months postoperatively.)
Video 1. displays custom-made TPC breast implant design, manufacturing, implantation, and clinical result 8 months postoperatively.
MRI Analysis
Implant assessment at 3 months postoperatively using MRI involved a device with a minimum 1.5 T magnetic field strength and a dedicated coil. Pulse sequences for T1-weighted and T2-weighted imaging were incorporated. Water and fat saturation signals were selectively imaged using distinct pulse sequences. Intravenous paramagnetic contrast was recommended for enhanced implant evaluation and identification of inflammatory processes linked to implant complications.5
RESULTS
Clinical Outcomes
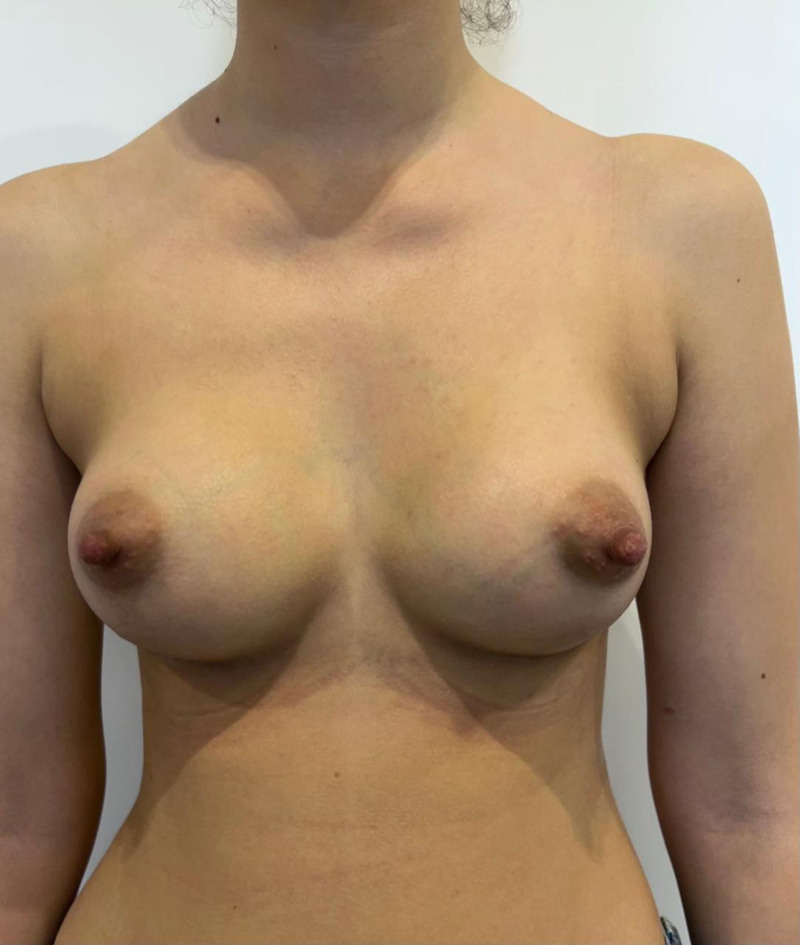
The implants were well maintained during the 8-month follow-up period. No complications, such as wound dehiscence, infection, or soft tissue vascular compromise, were observed, only bilateral breast hematoma, conservatively managed with close monitoring. The cosmetic outcome was highly satisfactory for the patient, displaying no discernible breast asymmetry or implant malposition (Fig. 2; see Video [online]).
Fig. 2.
Postoperative result.
MRI-based Analysis
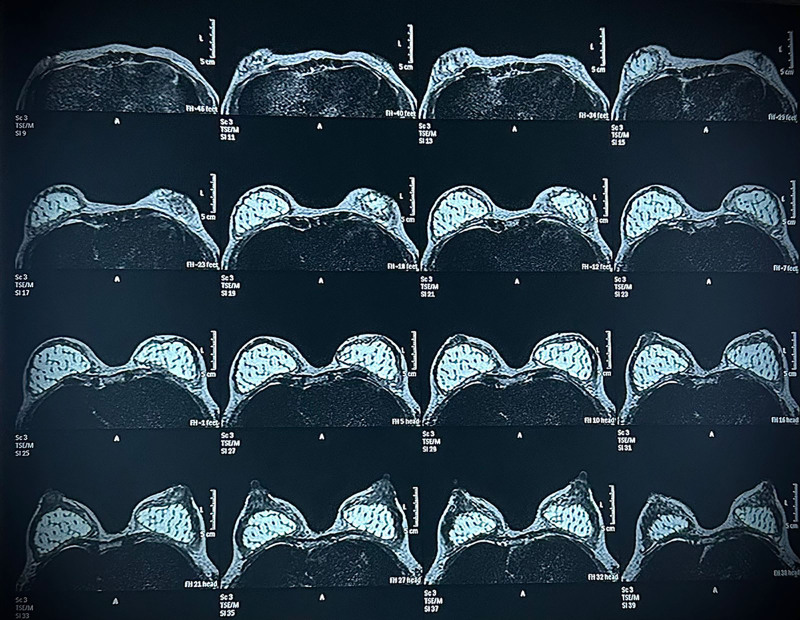
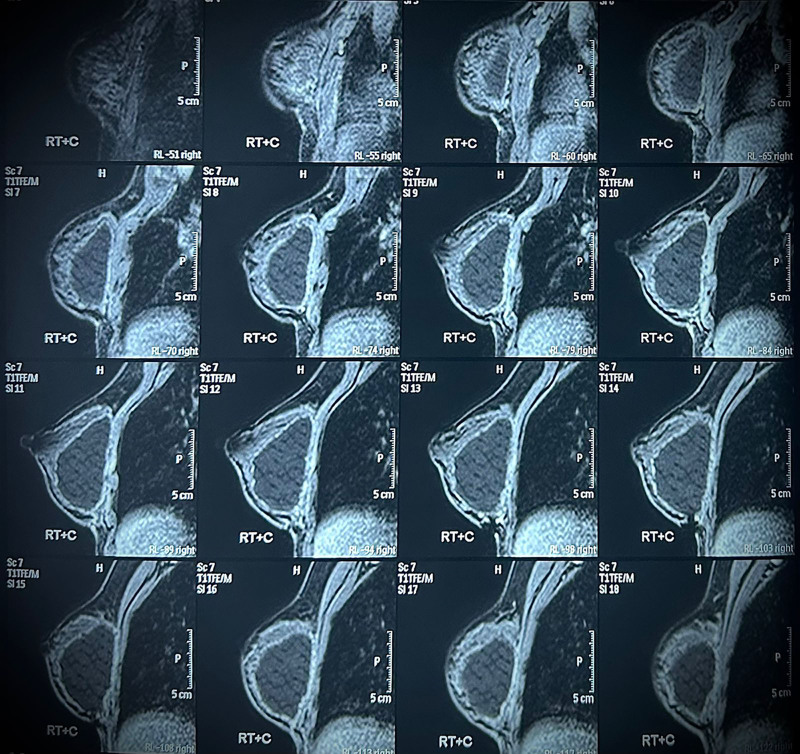
MRI analysis revealed evidence of revascularization and tissue regeneration within the implant. No signs of implant-associated complications were detected, showcasing tissue integration without encapsulation (Figs. 3–4).
Fig. 3.
MRI of breast axial planes using the short-Tau inversion recovery sequence, showing bilateral augmentation of both breasts by subglandular porous implants. Soft-tissue regeneration within the pores of the implant is evidenced.
Fig. 4.
Postcontrast sagittal T1-weighted fat suppression images of the right breast, showing the porous structure of the implant and demonstrating the suppressed signal of the contents after digital fat subtraction, thus indicating fat regeneration within the pores of the implant.
DISCUSSION
Recently, tissue engineering enabled a novel approach, integrating 3D printing technology with biomaterial science for scaffold-guided tissue regeneration. This involves careful modulation of biomaterials, architecture, surface chemistry, and mechanical properties. Despite recent promising research, challenges remain in clinical translation. Various published approaches to adipose tissue engineering are defective in demonstrating the necessary volume regeneration for clinically feasible breast augmentation.6,7
Thermoplastic elastomers have well-proven safety and biocompatibility and utilization in vascular tissue engineering and synthetic vascular grafts.8 Lattice structures are promising in engineering tissue scaffolds, with controllable mechanics and tunable functions.9 Plasma surface modification is a powerful tool to increase the bioactivity and biocompatibility of medical implants by increasing the hydrophilicity of the surface and functional coating deposition.10
The newly proposed 3D-printed implant addresses concerns of breast augmentation through its ultralight weight design (33 g), significantly lighter than the 300-cm3 silicone implant (approximately 350 g), reducing stress on the soft-tissue envelope and lowering the risk of malposition by soft-tissue attenuation.1,2 Its engineered porous structure, with full interconnection between pores, creates a persistent porous implant–tissue interface, promoting tissue integration instead of encapsulation.3,6,7 Functioning as a scaffold, it provides a biomimetic environment, supporting vascularization, tissue regeneration, and sequential remodeling.6,7 Manufactured without a gel component, it eliminates the risk of rupture.
In terms of study limitations, we need a larger number of cases and a longer follow-up period to assess the long-term safety, like biofilm formation and managing complications that may necessitate explantation with bloody dissection due to tissue integration.
CONCLUSIONS
The presented solution is the first successful translation of scaffold technology for breast augmentation in a real clinical scenario; it represents a promising tool for personalized plastic surgery, after demonstrating safety and clinical adaptability, along with patient satisfaction with the shape and the implant feel.
DISCLOSURES
The author is the sole founder, owner, and chairman of Alive Biotechnology Solutions UK (London, England), the developer of the utilized implant and its raw material. The technology has been exclusively licensed to Auxetica BioMed (London, England), of which the author is also the sole founder, owner, and chairman.
ACKNOWLEDGMENTS
The author shows his deepest gratitude to Dr. Soheir Omar, Senior Chemist, and Eng. Mohamed Nagy, Senior Design Engineer at Alive Biotechnology Solutions UK, London, England, for generously sharing their invaluable insights with him. Additionally, the author thanks Prof. Naglaa Abd El Razek, Professor of Radiology at the Faculty of Medicine, Cairo University, Egypt, whose expertise assisted this work. The author also shows his greatest gratitude to Prof. Wael Ayad, Professor of Plastic Surgery, Al Azhar University, Cairo, Egypt, for being his role model in surgery and life. The author also expresses his sincere appreciation to his early bird believers in him, Debbie Battat, Caroline Gwilliam, Prof. Alberto Rancati Chief of Oncoplastic Surgery of the Institute Oncologico Henry Moore, at Buenos Aires Argentina, and Director of Plastic Surgery for Latin America at the CFS-UCSD (Center for the Future of Surgery, at University of California, San Diego, CA), Prof. Maurizio Nava Professor of Plastic Surgery at Milan University School of Medicine,Italy, Prof. Per Heden, Professor of Plastic Surgery at the Karolinska University, Stockholm, Sweden.
Footnotes
Published online 13 December 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
The author confirms that the data supporting the findings of this study are available within the article and its supplementary materials except for data containing information related to the know-how that could compromise potential intellectual property rights.
REFERENCES
- 1.Medor MC, Bouhadana G, Churchill IF, et al. How big is too big? Exploring the relationship between breast implant volume and postoperative complication rates in primary breast augmentations. Plast Reconstr Surg Glob Open. 2023;11:e4843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janzekovic J, Hunt J, Peltz T, et al. Biomechanical principles of breast implants and current state of research in soft tissue engineering for cosmetic breast augmentation. Aesthetic Plast Surg. 2022;46:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Castel N, Soon-Sutton T, Deptula P, et al. Polyurethane-coated breast implants revisited: a 30-year follow-up. Arch Plast Surg. 2015;42:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IMDRF Personalized Medical Devices (PMD) Working Group. Personalized medical devices—regulatory pathways. 2020. Available at https://www.imdrf.org/sites/default/files/docs/imdrf/final/technical/imdrf-tech-200318-pmd-rp-n58.pdf. Accessed March 30, 2023. [Google Scholar]
- 5.Mund DF, Farria DM, Gorczyca DP, et al. MR imaging of the breast in patients with silicone-gel implants: spectrum of findings. AJR Am J Roentgenol. 1993;161:773–778. [DOI] [PubMed] [Google Scholar]
- 6.Cheng M, Heald A, Wagels M, et al. Scaffold-guide breast tissue engineering: the future of breast implants. Aust J Plast Surg. 2023;6:1–3. [Google Scholar]
- 7.Donnely E, Griffin M, Butler PE. Breast reconstruction with a tissue engineering and regenerative medicine approach (systematic review). Ann Biomed Eng. 2020;48:9–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fathi-Karkan S, Banimohamad-Shotorbani B, Saghati S, et al. A critical review of fibrous polyurethane-based vascular tissue engineering scaffolds. J Biol Eng. 2022;16:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao B, Xia RF, Li W, et al. 3D-printed Ti6Al4V scaffolds with graded triply periodic minimal surface structure for bone tissue engineering. J Mater Eng Perform. 2021;30:4993–5004. [Google Scholar]
- 10.Liu P, Wang G, Ruan Q, et al. Plasma-activated interfaces for biomedical engineering. Bioact Mater. 2021;6:2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]