Abstract
Borrelia burgdorferi, the causative agent of Lyme disease, alternates between the microenvironments of the tick vector, Ixodes scapularis, and a mammalian host. The environmental conditions the spirochete encounters during its infectious cycle are suspected to differ greatly in many aspects, including available nutrients, temperature, and pH. Here we identify alterations in the membrane protein profile, as determined by immunoblotting and two-dimensional nonequilibrium pH gradient gel electrophoresis (2D-NEPHGE), that occur in virulent B. burgdorferi B31 as the pH of the medium is altered. Initial comparisons of cultures incubated at pHs 6.0, 7.0, and 8.0 yielded alterations in the expression of seven membrane proteins as determined by probing with hyperimmune rabbit serum. Six of these membrane proteins (54, 45, 44, 43, 35, and 24 kDa) were either present in increased amounts in or solely expressed by cultures incubated at pHs 6.0 and 7.0. The 24-kDa protein that decreased in expression at pH 8.0 was identified as outer surface protein C (OspC). In addition, a 42-kDa membrane protein increased in amount in cultures incubated at pH 8.0. Similar changes were observed with serum from a mouse infected by tick bite, with the recognition of two additional bands (48 and 46 kDa) unique to pHs 6.0 and 7.0. When membrane fractions were analyzed by 2D-NEPHGE, at least 37 changes in the membrane protein profile between cells incubated at pHs 6.0, 7.0, and 8.0 were observed by immunoblotting and silver staining. Environmental cues such as pH may prove important in the regulation of virulence determinants and factors necessary for the adaptation of B. burgdorferi to the tick or mammalian microcosm.
Like many other bacterial pathogens (2, 6, 9, 11, 16, 20, 25–27, 29, 30, 32, 39, 40, 46, 49, 50) Borrelia burgdorferi, the causative agent of Lyme disease, has been shown to alter transcription and protein expression in response to changes in the environment. Schwan et al. (37) and Stevenson et al. (43) observed that a subset of membrane proteins, OspC and the OspE- and -F-related proteins (Erps), are temperature regulated. Additionally, OspC and OspA are differentially expressed within the tick vector and the mammalian host, and recent evidence has led to the conclusion that some other factor(s) besides temperature may play a role in the regulation of OspC expression (13, 15, 37). In the past few years there have been several reports of differential expression in B. burgdorferi with respect to in vivo versus in vitro cultivation (12, 22, 44, 48). This evidence suggests that several environmental factors may be involved in the ability of B. burgdorferi to adapt to the different environments of the tick vector and mammalian host.
Considering the complex life cycle of B. burgdorferi and its ability to infect a wide variety of hosts (41), this response to environmental cues is not surprising. The bacterium is transmitted by ticks of the genus Ixodes; the spirochetes are concentrated and reside in the tick midgut, which is alkaline (3, 33), in close association with the epithelial lining. When the tick feeds, the bacterium migrates to the salivary glands and saliva, which has a reported pH of 9.5 (7), and transmission occurs via salivation into the feeding lesion (4, 19, 31, 34, 36, 51). In the case of the human host, the first sign of infection is the spreading, red rash called erythema migrans at the initial tick bite (5). Erythema migrans is observed in approximately 60% of infected individuals (42) and is typical of an acute inflammatory response, with recruitment of neutrophils and macrophage to the site (17, 18). The mammalian environment offers an initial pH of 7.4, but during the onset of a localized inflammatory response tissues often undergo a drop in pH (tissue acidosis) (1, 21, 35, 47). The microenvironments encountered by B. burgdorferi during the transmission cycle can differ greatly in temperature, nutrients, and pH. In this study we examine the in vitro protein expression of B. burgdorferi at pH 6.0 to pH 8.0 and identify over 37 proteins that are regulated by the environmental pH.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Low-passage (<5 passages) infectious B. burgdorferi B31 (8) was grown to mid-log phase (5 × 107 cells per ml) under an atmosphere of 5% CO2 at 35°C in BSK-H medium (Sigma Chemical Co., Saint Louis, Mo.). The cells were then concentrated by centrifugation (8,000 × g; 10 min; 24°C) and resuspension in BSK-H. The spirochetes were then inoculated at a final concentration of 107 per ml into BSK-H buffered with 25 mM HEPES and adjusted to pH 6.0, 7.0, or 8.0 with the addition of either HCl or NaOH. Cells grown at pHs 7.0 and 8.0 were incubated for 2 days, while cells grown at pH 6.0 were incubated for 4 days due to an increase in the doubling time. The cells were harvested by centrifugation (8,000 × g; 10 min; 4°C) when they reached 5 × 107 per ml. The pH of the spent medium was then checked, with no observable change from the original pH. Virulent strains were previously tested in Syrian hamsters as described elsewhere (23).
Isolation and quantitation of protein samples.
Once the bacterial cells grew to the desired density, they were harvested by centrifugation (8,000 × g; 10 min; 4°C). The cell pellets were gently rinsed with cold 50 mM NaCl in 20 mM HEPES, pH 7.6 (HEPES buffer), centrifuged a second time, and suspended in HEPES buffer. The cell suspensions were lysed by two passes through a French pressure cell (16,000 lb/in2; SLM-Aminco, Rochester, N.Y.), and cell debris and insoluble material were removed by centrifugation (10,000 × g; 10 min; 4°C). Total membranes were separated from the soluble protein fraction by ultracentrifugation (100,000 × g; 1 h, 4°C). The membranes were rinsed once in HEPES buffer to remove residual soluble proteins, pelleted again by ultracentrifugation, and resuspended with the aid of a glass tissue homogenizer (Kontes Glass Co., Vineland, N.J.) in 250 μl of HEPES buffer. Aliquots of cell lysates and rinsed membranes were stored at −20°C. Protein concentrations were determined by a modified Lowry protein assay (24) with bovine serum albumin as a standard.
Sera used for immunoblots.
Hyperimmune rabbit antiserum raised against live, low-passage B. burgdorferi B31 (hyperimmune serum) was produced as previously described (10). Antiserum from a white-footed mouse, Peromyscus leucopus, infected with B31 by the bite of an infected I. scapularis nymph (tick bite immune serum) was also raised for these studies. Rabbit polyclonal immune serum raised against OspC (anti-OspC) was produced as previously described (38).
Electrophoresis and immunoblotting.
Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with an SE600 gel apparatus (Hoefer Scientific, San Francisco, Calif.). Twenty-five to 35 μg of protein was applied to each lane. Two-dimensional nonequilibrium pH gradient gel electrophoresis (2D-NEPHGE), using a Hoefer SE600 gel apparatus, was performed as described by O’Farrell (28) with the following modifications: (i) 60 μg of B. burgdorferi B31 TM protein was solubilized in NEPHGE sample buffer (9 M urea, 4% Nonidet P-40, 2% β-mercaptoethanol, 2% ampholytes in distilled H2O) for 2 h at 24°C, (ii) insoluble debris was removed by ultracentrifugation (100,000 × g; 1 h; 24°C), and (iii) the samples were loaded onto 10- to 13-cm-diameter tube gels. The tube gels were focused for a total of 2,400 to 2,500 V · h. Preblended pH 3.5 to 9.5 ampholytes were purchased from Pharmacia Biotech (Piscataway, N.J.). Proteins were visualized by staining with the Silver Stain Plus kit (Bio-Rad Laboratories, Hercules, Calif.) or prepared for immunoblotting. Molecular mass standards were purchased from Bio-Rad Laboratories.
For immunoblotting, the proteins were electrophoretically transferred to nitrocellulose (0.45 μM Trans-Blot Transfer Medium; Bio-Rad Laboratories) as described by Towbin et al. (45) with a Bio-Rad Trans Blot cell (100 mA; 12 h; 4°C). After transfer, the proteins were visualized with Ponceau red (0.1% Ponceau red dye in 1.0% acetic acid) and the standards were marked. The nitrocellulose membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (150 mM NaCl in 10 mM Tris-HCl, pH 8.0) with the addition of 0.1% Tween 20 (TBS-T20) (3 h; 24°C), and immune serum diluted either 1:500, 1:1,000, or 1:10,000 in TBS-T20 (primary antibody) was applied to the blot (1 h; 24°C). The blot was washed twice in 100 to 200 ml of TBS-T20 for 10 min to remove residual primary antibody. Secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse antibody) (Sigma Chemical Co.) was diluted 1:5,000 in TBS-T20 and applied to the blot (45 min; 24°C), followed by three washes with 100 to 200 ml of TBS-T20. Reactive bands were visualized with the enhanced chemiluminescence kit (Amersham, Arlington Heights, Ill.) in accordance with the manufacturer’s specifications. The relative molecular masses of protein bands or spots were estimated by a two-variable statistic linear regression with molecular mass standards purchased from Bio-Rad Laboratories. Integrated density values were measured by an AlphaImager 2000 digital imaging system (Alpha Innotech Corporation, San Leandro, Calif.).
RESULTS
Comparison of membrane protein profiles by silver staining and immunoblotting of B. burgdorferi B31 grown at pHs 6.0, 7.0, and 8.0.
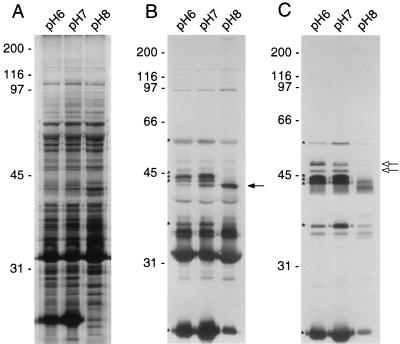
A silver stain of membrane proteins from B. burgdorferi grown at varying pH revealed few alterations in the protein profile (Fig. 1A). Most noticeable was the decrease in the amount of a 25-kDa protein as the pH of the medium grew more alkaline. To identify immunogenic protein variations due to the influence of environmental pH, membrane preparations from B. burgdorferi B31 were separated by SDS-PAGE, transferred to nitrocellulose, and probed with a 1:10,000 dilution of hyperimmune serum (Fig. 1B). Initially, we were able to identify seven immunogenic bands with the hyperimmune serum that clearly increased or decreased as the pH varied. Three of these proteins (relative molecular masses, 54, 35, and 24 kDa) were present in greater amounts in membrane samples incubated in BSK-H at pHs 6.0 and 7.0 than at pH 8.0, and three immunoreactive proteins (relative molecular masses, 45, 44, and 43 kDa) were unique to samples incubated at pHs 6.0 and 7.0 (Fig. 1B). The 54-kDa protein band displayed the greatest intensity in samples incubated at pH 6.0. The hyperimmune serum detected a protein band at 42 kDa that increased under more alkaline conditions (Fig. 1B).
FIG. 1.
Silver stain (A) and comparable immunoblots probed with rabbit hyperimmune serum (B) or mouse serum infected by the bite of a tick (C) of total membrane proteins from cultures incubated at pHs 6.0, 7.0, and 8.0. The asterisks indicate similar-sized proteins recognized by both sera. The solid arrow marks the presence of an alkaline-induced protein detected by the hyperimmune serum. The open arrows mark immunoreactive proteins recognized by the tick bite immune serum. Molecular mass standards in kilodaltons are indicated to the left of each panel.
A complementary immunoblot was probed with a 1:1,000 dilution of serum from a mouse infected by tick bite (Fig. 1C). The tick bite immune serum reacted with the same six proteins that were detected by the hyperimmune serum in samples incubated at pHs 6.0 and 7.0 (Fig. 1B and C) but failed to react with the 42-kDa immunogenic protein that was more abundant in samples incubated at pH 8.0 (Fig. 1B and C). Two additional immunoreactive proteins (48 and 46 kDa), which were unique to cells incubated at pHs 6.0 and 7.0, were recognized by the tick bite immune serum and not by the hyperimmune serum (Fig. 1C).
Differences in immunoreactivity between the rabbit hyperimmune serum and the tick bite immune serum were evident (Fig. 1), reflecting differences in how the organism was presented to the host. The rabbit hyperimmune serum was raised against a large number of B. burgdorferi organisms grown in vitro and recognizes many antigens, including OspA, which is not expressed by the bacterium during transmission from the tick vector to the mammalian host (14, 37). Conversely, the immunogenic profile observed by immunoblotting and probing with tick bite immune serum reflects the antigens the host is actually exposed to during the course of a natural transmission and infection cycle.
An immunoblot of membrane samples from cultures incubated at pHs 6.0, 7.0, and 8.0 was probed with anti-OspC polyclonal antiserum (Fig. 2). OspC was present in large amounts at pHs 6.0 and 7.0 but decreased at pH 8.0. Comparisons of the bands shown in Fig. 2 by densitometer indicated that the density of OspC at pH 7.0 was 10-fold greater than that of cultures incubated at pH 8.0 (data not shown). OspC was observed to be regulated at the transcriptional level, where transcript of ospC was detected at pH 7.0 but not at pH 8.0 (data not shown).
FIG. 2.
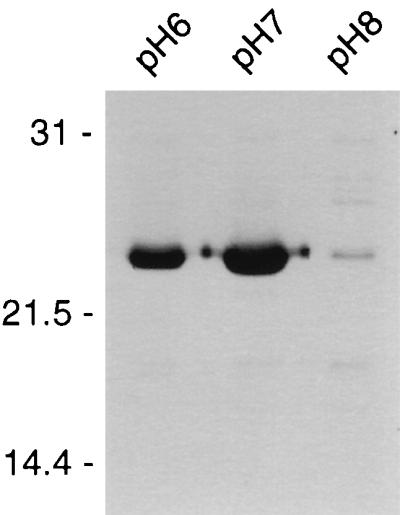
Immunoblot of total membrane samples from cultures incubated at pH 6.0, pH 7.0, and pH 8.0 probed with polyclonal serum raised against OspC. Molecular mass standards in kilodaltons are indicated on the left.
Comparison of membrane samples from cells grown at pHs 6.0, 7.0, and 8.0 by 2D-NEPHGE and immunoblotting.
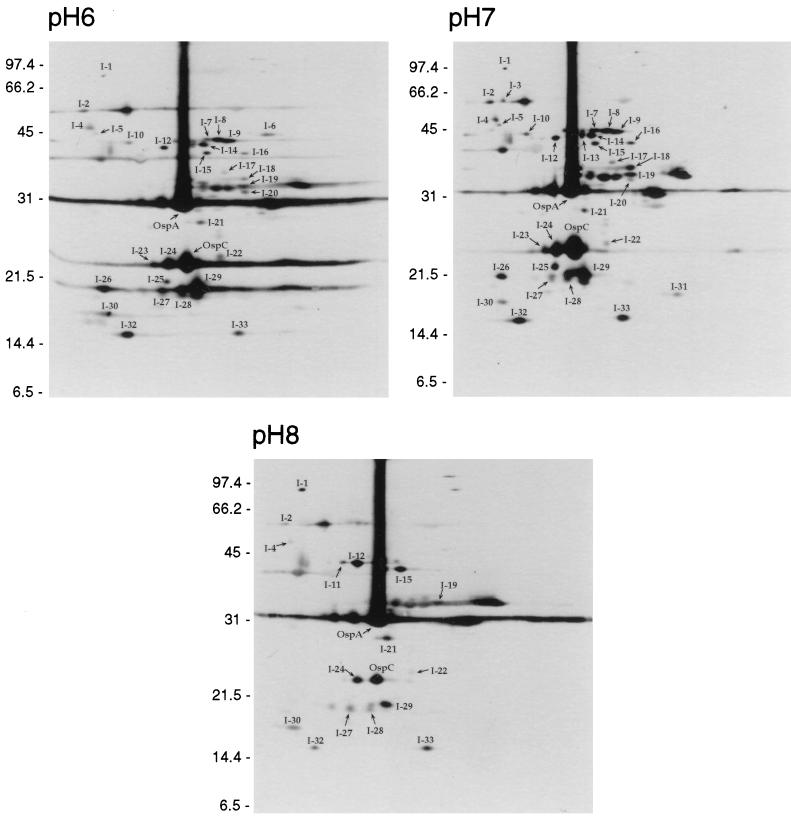
To determine if any additional immunogenic protein changes could be observed, membrane proteins from low-passage B. burgdorferi B31 cultures incubated at different pHs were analyzed by 2D-NEPHGE and immunoblotting. These experiments were performed twice, independently of one another. When the immunoblots were probed with the hyperimmune serum, at least 34 alterations in the 2D-NEPHGE protein profile were detected (Fig. 3). As the pH of the growth medium was decreased from 7.0 to 6.0, many proteins were either less abundant or undetectable. At pH 6.0 the intensities of protein spots I-4, I-5, I-7, I-10, I-12, I-16, I-17, I-18, I-19, and I-25 were noticeably decreased, and protein spots I-13 and I-31 were no longer detectable by immunoblotting (Fig. 3). Incubation at pH 6.0 displayed an increase in the amounts of protein spots I-20, I-26, I-27, and I-30 compared to incubation at pH 7.0, with the appearance of spot I-6 solely at pH 6.0 (Fig. 3).
FIG. 3.
Immunoblots of total membrane samples from cultures incubated at pH 6.0, pH 7.0, and pH 8.0 separated by 2D-NEPHGE and probed with hyperimmune serum. The acidic ends are to the left. Alterations in the protein profiles are indicated by the prefix “I−” (for immunoblot) with a corresponding number designation for easy comparison between blots. The locations of OspA and OspC are indicated as reference marks. Molecular mass standards in kilodaltons are indicated on the left of each panel.
When B. burgdorferi B31 was incubated at pH 8.0, the alterations in the 2D-NEPHGE immunogenic protein profile were much more striking. Several protein spots were undetectable. Protein spots I-3, I-5, I-7, I-8, I-9, I-10, I-16, I-17, I-18, I-20, I-23, I-25, I-26, and I-31, all present in samples at pH 7.0, were undetectable in samples at pH 8.0 (Fig. 3). In addition, numerous spots appeared to decrease in amount, including I-2, I-19, I-22, I-24, I-28, I-29, I-32, I-33, and OspC. At pH 8.0 spots I-1, I-12, I-15, and I-21 increased, with spot I-11 being specific for pH 8.0 (Fig. 3).
Integrated density values and relative molecular masses of a representative subset of reactive proteins from the immunoblots shown in Fig. 3 were measured and are displayed in Table 1. OspA was present in relatively equal amounts, while other reactive proteins, such as OspC, were present in varying amounts as the environmental pH was changed.
TABLE 1.
Integrated density values (IDV) for immunoreactive proteins identified by 2D-NEPHGE immunoblotting
| Protein | Molecular massa | IDVb
|
||
|---|---|---|---|---|
| pH 6.0 | pH 7.0 | pH 8.0 | ||
| OspA | 31.4 | 25,056 | 27,666 | 22,968 |
| OspC | 25.4 | 15,180 | 25,990 | 1,150 |
| I-1 | 97.1 | 451 | 656 | 820 |
| I-2 | 60.2 | 1,260 | 1,530 | 990 |
| I-4 | 50.2 | 1,700 | 1,870 | 595 |
| I-7 | 44.9 | 5,166 | 7,056 | 0 |
| I-8 | 44.7 | 4,800 | 6,300 | 0 |
| I-9 | 44.0 | 7,696 | 6,660 | 0 |
| I-10 | 44.0 | 810 | 1,080 | 0 |
| I-11 | 43.2 | 0 | 680 | 1,115 |
| I-12 | 43.2 | 903 | 1,806 | 4,386 |
| I-13 | 43.8 | 2,856 | 3,780 | 0 |
| I-14 | 43.8 | 2,736 | 3,312 | 0 |
| I-15 | 40.8 | 285 | 684 | 2,166 |
| I-17 | 36.2 | 528 | 1144 | 0 |
| I-18 | 35.1 | 2,847 | 5,183 | 0 |
| I-19 | 33.6 | 6,586 | 8,277 | 6,586 |
| I-20 | 32.9 | 780 | 360 | 0 |
| I-21 | 28.0 | 1,892 | 2,150 | 3,440 |
| I-22 | 26.3 | 2,268 | 1,998 | 378 |
| I-23 | 25.4 | 4,806 | 4,628 | 0 |
| I-24 | 25.4 | 9,167 | 10,680 | 3,837 |
| I-25 | 23.0 | 2,964 | 3,876 | 0 |
| I-26 | 21.7 | 5,289 | 4,128 | 0 |
| I-27 | 21.4 | 7,030 | 2,565 | 2,565 |
| I-28 | 21.4 | 10,700 | 7,811 | 2,461 |
| I-29 | 21.4 | 27,416 | 25,024 | 8,464 |
| I-30 | 18.4 | 3,950 | 2,054 | 2,528 |
| I-32 | 16.3 | 5,402 | 5,550 | 1,702 |
| I-33 | 17.0 | 3,225 | 5,805 | 4,902 |
Molecular mass in kilodaltons compared to molecular mass standards (Fig. 3).
IDV = Σ(each pixel value − background).
Comparison of membrane samples from cells grown at pHs 6.0, 7.0, and 8.0 by 2D-NEPHGE and silver staining.
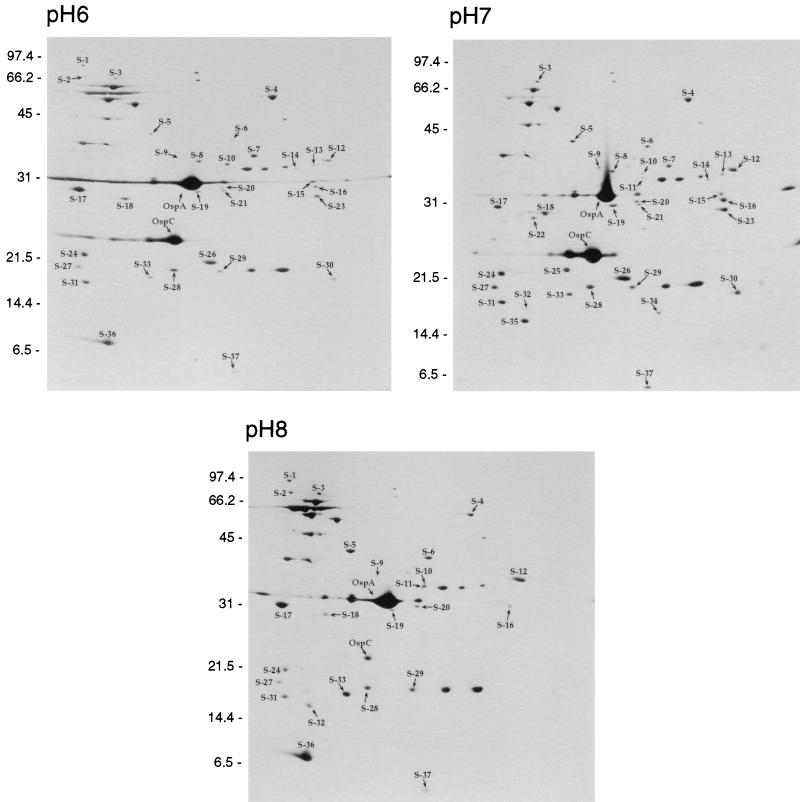
Membrane protein samples from B. burgdorferi B31 incubated at pHs 6.0, 7.0, and 8.0 were also subjected to 2D-NEPHGE and stained with silver (Fig. 4). These experiments were performed three times, independently of one another. Comparison of silver-stained 2D-NEPHGE protein spots at pHs 6.0 and 7.0 (Fig. 4) revealed several alterations not detected by immunoblotting. Membrane protein spots S-1, S-2, and S-36 were present in pH 6.0 samples but were not detected when the cells were incubated at pH 7.0. We observed an increase of spots S-4, S-10, and S-17 in samples incubated at pH 6.0. Proteins that were less abundant at pH 6.0 included S-5, S-6, S-9, S-11, S-12, S-13, S-16, S-18, S-19, S-20, S-21, S-23, S-26, S-27, S-28, S-29, S-30, S-31, S-33, and S-37. OspC, although still present, decreased when the cells were incubated at pH 6.0.
FIG. 4.
Silver stains of total membrane samples from cultures incubated at pH 6.0, pH 7.0, and pH 8.0 separated by 2D-NEPHGE. The acidic ends are to the left. Alterations in the protein profiles are indicated by the prefix “S−” (for silver stain) with a corresponding number designation for easy comparison between panels. The locations of OspA and OspC are indicated as reference marks. Molecular mass standards in kilodaltons are indicated on the left of each panel.
Again, B. burgdorferi incubated in medium at pH 8.0 displayed a 2D-NEPHGE profile significantly different from that of cells incubated at pH 7.0 (Fig. 4). Many protein spots present at pH 7.0 (as well as 6.0) were undetectable in cells incubated at pH 8.0. These included spots S-7, S-8, S-13, S-15, S-21, S-23, S-26, and S-30. Protein spots S-9, S-14, S-16, S-18, S-19, S-24, S-27, S-28, S-31, S-37, and OspC decreased in amount as the pH was increased from 7.0 to 8.0. Conversely, spots S-3, S-4, S-5, S-6, S-10, S-11, S-12, S-17, and S-33 increased when the cells were incubated at pH 8.0. In addition, spot S-32 was observed exclusively in samples incubated at pH 8.0. Spots S-1, S-2, and S-36 were present when the cells were incubated at pHs 6.0 and 8.0 but not at pH 7.0 (Fig. 4). Lastly, protein spots S-22, S-25, S-34, and S-35 were observed in the samples only when incubated at pH 7.0 and not in samples incubated at pHs 6.0 and 8.0.
The integrated density values and relative molecular masses of a subset of silver-stained proteins (Table 2) allowed better assessment of the observed alterations. Again, the density values for OspA are equal while other protein density values vary in response to changes in the environmental pH.
TABLE 2.
Integrated density values (IDV) for proteins separated by 2D-NEPHGE and stained with silver
| Protein | Molecular massa | IDVb
|
||
|---|---|---|---|---|
| pH 6.0 | pH 7.0 | pH 8.0 | ||
| OspA | 31.0 | 19,240 | 19,240 | 19,832 |
| OspC | 24.0 | 12,118 | 16,102 | 1,328 |
| S-1 | 93.8 | 27 | 0 | 162 |
| S-2 | 76.3 | 17 | 0 | 119 |
| S-3 | 73.5 | 308 | 308 | 836 |
| S-4 | 59.5 | 1,628 | 1,490 | 880 |
| S-5 | 42.3 | 132 | 660 | 1,628 |
| S-6 | 40.4 | 18 | 108 | 378 |
| S-7 | 36.0 | 688 | 989 | 0 |
| S-8 | 34.9 | 252 | 450 | 0 |
| S-9 | 35.4 | 36 | 72 | 36 |
| S-10 | 34.4 | 360 | 72 | 192 |
| S-11 | 33.7 | 0 | 270 | 900 |
| S-12 | 35.1 | 528 | 1,584 | 2,068 |
| S-13 | 34.4 | 148 | 592 | 0 |
| S-15 | 31.0 | 989 | 1,333 | 0 |
| S-16 | 30.2 | 407 | 1,332 | 370 |
| S-17 | 29.1 | 4,387 | 1,177 | 3,745 |
| S-18 | 28.1 | 3,600 | 5,100 | 1,950 |
| S-19 | 29.1 | 306 | 594 | 324 |
| S-23 | 28.8 | 860 | 1,462 | 0 |
| S-24 | 22.3 | 1,496 | 1,452 | 792 |
| S-26 | 22.1 | 7,446 | 8,030 | 0 |
| S-27 | 21.4 | 1,200 | 2,304 | 1,008 |
| S-28 | 21.0 | 4,800 | 6,225 | 5,025 |
| S-29 | 21.4 | 532 | 912 | 722 |
| S-30 | 20.3 | 732 | 1,708 | 0 |
| S-31 | 18.8 | 1,736 | 4,154 | 1,922 |
| S-33 | 20.1 | 1,098 | 2,501 | 4,819 |
| S-36 | 7.1 | 4,960 | 0 | 5,394 |
| S-37 | 4.2 | 228 | 456 | 190 |
Molecular mass in kilodaltons compared to molecular mass standards (Fig. 4).
IDV = Σ(each pixel value − background).
DISCUSSION
When the pH of BSK-H was adjusted to 6.0, 7.0, and 8.0, we observed at least 37 alterations in the membrane protein profile, suggesting that pH may play a regulatory role in the expression of many of these membrane proteins. Initially, six of these changes were seen by immunoblotting with hyperimmune serum or serum derived from a tick-acquired infection, suggesting that the immunogens observed at pHs 6.0 and 7.0 (Fig. 1) are expressed during infection. The hyperimmune serum also reacted with a 42-kDa membrane protein that increased in amount as the pH of the medium was increased from 6.0 to 8.0 (Fig. 1). In addition, the tick bite immune serum recognized a 48- and a 46-kDa protein that went undetected when probed with hyperimmune serum. This also suggests that there may be numerous membrane proteins expressed at pHs 6.0 and 7.0 which may be differentially expressed during the infectious cycle and not recognized by the hyperimmune serum. These alterations in membrane proteins as the pH of the medium was changed led us to use a more powerful technique to characterize which proteins may be under pH regulation in B. burgdorferi. 2D-NEPHGE with immunoblotting and silver staining (Fig. 3 and 4) demonstrated additional changes in the membrane protein composition as a function of the changing extracellular pH.
The alterations in protein expression observed by 2D-NEPHGE between pHs 6.0 and 7.0 were few, yet comparisons between 2D-NEPHGE membrane protein profiles of cells incubated at pH 7.0 (or pH 6.0) and that of cells incubated at pH 8.0 exhibited more pronounced differences (Fig. 3 and 4). This seemed reasonable because, while within the tick vector, an alkaline environment (3, 7, 33), B. burgdorferi would presumably alter its gene expression for survival in the arthropod and decrease the expression of genes necessary for survival in the mammal. Comparing samples incubated at pHs 7.0 and 8.0, we detected the loss of at least 13 proteins by 2D-NEPHGE with immunoblotting (I-7, I-8, I-9, I-10, I-13, I-14, I-16, I-17, I-18, I-20, I-23, I-26, and I-31 [Fig. 3]) and 12 by silver staining (S-7, S-8, S-13, S-14, S-15, S-21, S-22, S-23, S-25, S-30, S-34, and S-35 [Fig. 4]). Many of the protein alterations visualized by immunoblotting correlate with those visualized by silver staining, but strangely, immunoreactive protein spots I-7, I-8, and I-9 observed at pHs 6.0 and 7.0 (Fig. 3) were not visible by silver staining (Fig. 4). This could be due to the sensitivity of immunoblotting or the stainability of those proteins with silver.
In comparing the results of the immunoblot in Fig. 1 to those of the 2D-NEPHGE immunoblot in Fig. 3, we observed that the 45-, 44-, and 43-kDa protein bands identified in Fig. 1 actually resolved into seven protein spots (I-6, I-7, I-8, I-9, I-10, I-13, and I-14) of similar molecular masses (Fig. 3 and Table 1). The 42-kDa band observed to increase as the in vitro pH became more alkaline (Fig. 1B) resolved into two protein spots (I-12 and I-15) when the samples were subjected to 2D-NEPHGE (Fig. 3 and Table 1). This illustrates the ability of 2D-NEPHGE to separate and distinguish immunogens of similar molecular masses.
We observed a large reduction in the amount of OspC in cells incubated under alkaline conditions (Fig. 2), and Northern blots comparing RNA from cells grown at pHs 7.0 and 8.0 suggest that OspC is regulated at the level of transcription as the pH varies. This supported the previous findings, which indicated that OspC was down-regulated in the midgut of unfed ticks (37). Interestingly, OspC was present by silver staining and immunoblotting at pH 8.0 even though transcript could not be detected. This could suggest that OspC has a long turnover time and what we observed at pH 8.0 was residual protein, or it could be a reflection of the nonclonal nature of the strain used in these experiments. Schwan et al. (37) indicated that temperature alone was unable to regulate OspC expression in unfed ticks. Quite possibly OspC may be under the coordinate regulation of temperature and pH. This could help explain why OspC was not expressed in the midgut of unfed, infected ticks even when the ticks were shifted from 24 to 35°C. Presumably the tick midgut pH would have remained alkaline, even at 35°C, and may have maintained a regulatory influence on ospC expression. Furthermore, one might expect the pH of the midgut lumen to initially decrease from alkaline to a more neutral pH as infected ticks feed, due to the influx of blood, allowing for the switch to OspC expression characteristic of B. burgdorferi in the midguts of infected ticks during feeding. Interestingly the Erps, which are regulated by temperature as well (43), showed no significant change in expression at pH 6.0, 7.0, or 8.0 (data not shown), indicating that not all proteins under temperature regulation are under pH regulation.
Several membrane proteins appeared to be up-regulated under alkaline growth conditions (Fig. 3, spots I-11, I-12, and I-15), and their expression may be important in the colonization of the tick vector. Note that the increase in the amounts of spots I-12 and I-15 can be better assessed by silver staining (Fig. 4, spots S-5 and S-6, respectively). Additional silver-stained membrane proteins that were up-regulated when the cells were exposed to an alkaline environment included spots S-1, S-2, S-3, S-17, S-32, and S-36 (Fig. 4). Oddly, spots S-1, S-2, and S-36 were also present in membrane samples from cells incubated at pH 6.0 (Fig. 4) yet were undetectable in membrane protein preparations from pH 7.0 cultures (Fig. 4). It is possible that these membrane proteins play a role in the survival of B. burgdorferi under pH stress, and their induction under such conditions warrants further investigation.
Our findings suggest that the in vitro expression of over 37 membrane proteins in B. burgdorferi B31 is regulated by the extracellular pH. A majority of the protein alterations we observed occur between pH 7.0 and pH 8.0, a difference comparable to the environment of the mammalian host (1, 21, 35, 47) versus that of the arthropod vector (3, 7, 33). It is likely that B. burgdorferi grown in BSK-H is receiving a mixed set of regulatory signals from the surroundings, and such regulatory studies performed in vitro may not truly mimic the conditions experienced by B. burgdorferi during the infectious cycle. However, performing these initial studies will allow future in vivo analysis. How the in vitro pH regulation we observed applies to the regulation of these membrane proteins in vivo is not clear. Understanding how these or other membrane proteins are regulated could prove to be important in understanding the pathogenesis of Lyme disease and how B. burgdorferi is transmitted between arthropod vector and mammalian host.
ACKNOWLEDGMENTS
We thank S. Porcella, P. Rosa, and L. Barker for comments on the manuscript; G. Hettrick and R. Evans for artwork and photography; and A. Golden for secretarial assistance.
REFERENCES
- 1.Ahlqvist J. A hypothesis on the pathogenesis of rheumatoid and other non-specific synovitides. IV A. The possible intermediate role of local hypoxia and metabolic alterations. Med Hypotheses. 1984;13:257–302. doi: 10.1016/0306-9877(84)90162-2. [DOI] [PubMed] [Google Scholar]
- 2.Ankenbauer R G, Nester E W. Sugar-mediated induction of Agrobacterium tumefaciens virulence genes; structural specificity and activities of monosaccharides. J Bacteriol. 1990;172:6442–6446. doi: 10.1128/jb.172.11.6442-6446.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balashov Y S. Bloodsucking ticks (Ixodoidea)—vectors of diseases of man and animals. Misc Publ Entomol Soc Am. 1972;8:161–376. [Google Scholar]
- 4.Benach J L, Coleman J L, Skinner R A, Bosler E M. Adult Ixodes dammini on rabbits: a hypothesis for the development and transmission of Borrelia burgdorferi. J Infect Dis. 1987;155:1300–1306. doi: 10.1093/infdis/155.6.1300. [DOI] [PubMed] [Google Scholar]
- 5.Berger B. Erythema chronicum migrans of Lyme disease. Arch Dermatol. 1984;120:1017–1021. [PubMed] [Google Scholar]
- 6.Binns A N, Thomashow M F. Cell biology of Agrobacterium infection and transformation in plants. Annu Rev Microbiol. 1988;42:575–606. [Google Scholar]
- 7.Bowman A S, Sauer J R, Zhu K, Dillwith J W. Biosynthesis of salivary prostaglandins in the lone star tick, Amblyomma americanum. Insect Biochem Mol Biol. 1995;25:735–741. doi: 10.1016/0965-1748(95)00013-l. [DOI] [PubMed] [Google Scholar]
- 8.Burgdorfer W, Barbour A G, Hayes S F, Benach J L, Grunwaldt E, Davis J P. Lyme disease—a tick-borne spirochetosis? Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. [DOI] [PubMed] [Google Scholar]
- 9.Cangelosi G A, Ankenbauer R G, Nester E W. Sugars induce the Agrobacterium virulence genes through a periplasmic binding protein and a transmembrane signal protein. Proc Natl Acad Sci USA. 1990;87:6708–6712. doi: 10.1073/pnas.87.17.6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll J A, Gherardini F C. Membrane protein variations associated with in vitro passage of Borrelia burgdorferi. Infect Immun. 1996;64:392–398. doi: 10.1128/iai.64.2.392-398.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty T, Leimeister-Wachter M, Domann E, Hartl M, Goebel W, Nichterlein T, Notermans S. Coordinate regulation of virulence genes in Lysteria monocytogenes requires the product of the prfA gene. J Bacteriol. 1992;174:568–574. doi: 10.1128/jb.174.2.568-574.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Champion C I, Blanco D R, Skare J T, Haake D A, Giladi M, Foley D, Miller J N, Lovett M A. A 9.0-kilobase-pair circular plasmid of Borrelia burgdorferi encodes an exported protein: evidence for expression only during infection. Infect Immun. 1994;62:2653–2661. doi: 10.1128/iai.62.7.2653-2661.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coleman J L, Gebbia J A, Piesman J, Degen J L, Bugge T H, Benach J L. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 14.de Silva A M, Telford III S R, Brunet L R, Barthold S W, Fikrig E. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med. 1996;183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Silva A M, Zeidner N S, Zhang Y, Dolan M C, Piesman J, Fikrig E. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun. 1999;67:30–35. doi: 10.1128/iai.67.1.30-35.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duray P H. Histopathology of clinical phases of human Lyme disease. Rheum Dis Clin N Am. 1989;15:691–710. [PubMed] [Google Scholar]
- 18.Duray P H, Steere A C. Clinical pathological correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79. doi: 10.1111/j.1749-6632.1988.tb31839.x. [DOI] [PubMed] [Google Scholar]
- 19.Gern L, Zhu Z, Aeschlimann A. Development of Borrelia burgdorferi in Ixodes ricinus females during blood feeding. Ann Parasitol Hum Comp. 1990;65:89–93. [Google Scholar]
- 20.Goldberg M B, Boyko S A, Calderwood S B. Transcriptional regulation by iron of a Vibrio cholerae virulence gene and homology of the gene to the Escherichia coli fur system. J Bacteriol. 1990;172:6863–6870. doi: 10.1128/jb.172.12.6863-6870.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habler C. Uber den K- und Ca- gehalt von eiter und exsudaten und seine beziehungen zum entzundungsschmerz. Klin Wochenschr. 1929;8:1569–1572. [Google Scholar]
- 22.Indest K J, Ramamoorthy R, Sole M, Gilmore R D, Johnson B J B, Phillip M T. Cell-density-dependent expression of Borrelia burgdorferi lipoprotein in vitro. Infect Immun. 1997;65:1165–1171. doi: 10.1128/iai.65.4.1165-1171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson R C, Marek N, Kodner C. Infection of Syrian hamsters with Lyme disease spirochetes. J Clin Microbiol. 1984;20:1099–1101. doi: 10.1128/jcm.20.6.1099-1101.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 25.Mekalanos J J. Environmental signals controlling expression of virulence determinants in bacteria. J Bacteriol. 1992;174:1–7. doi: 10.1128/jb.174.1.1-7.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J F, Mekalanos J J, Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989;243:916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- 27.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson K, Mekalanos J J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pettersson J, Nordfelth R, Dubinina E, Bergman T, Gustafsson M, Magnusson K E, Wolf-Watz H. Modulation of virulence factor expression by pathogen target cell contact. Science. 1996;273:1231–1233. doi: 10.1126/science.273.5279.1231. [DOI] [PubMed] [Google Scholar]
- 31.Piesman J, Maupin G O, Campos E G, Happ C M. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J Infect Dis. 1991;163:895–897. doi: 10.1093/infdis/163.4.895. [DOI] [PubMed] [Google Scholar]
- 32.Pratt L A, Silhavy T J. The response regulator SprE controls the stability of RpoS. Proc Natl Acad Sci USA. 1996;93:2488–2492. doi: 10.1073/pnas.93.6.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro J M C. The midgut hemolysin of Ixodes dammini (Acari: Ixodidae) J Parasitol. 1988;74:532–537. [PubMed] [Google Scholar]
- 34.Ribeiro J M C, Mather T N, Piesman J, Spielman A. Dissemination and salivary delivery of Lyme disease spirochetes in vector ticks (Acari: Ixodidae) J Med Entomol. 1987;24:201–205. doi: 10.1093/jmedent/24.2.201. [DOI] [PubMed] [Google Scholar]
- 35.Sawyer R G, Spengler M D, Adams R B, Pruett T L. The peritoneal environment during infection: the effect of monomicrobial and polymicrobial bacteria on pO2 and pH. Ann Surg. 1991;213:253–260. doi: 10.1097/00000658-199103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwan T G. Ticks and Borrelia: model systems for investigating pathogen-arthropod interactions. Infect Agents Dis. 1996;5:167–181. [PubMed] [Google Scholar]
- 37.Schwan T G, Piesman J, Golde W T, Dolan M C, Rosa P A. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc Natl Acad Sci USA. 1995;92:2909–2913. doi: 10.1073/pnas.92.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwan T G, Schrumpf M E, Karstens R H, Clover J R, Wong J, Daugherty M, Struthers M, Rosa P A. Distribution and molecular analysis of Lyme disease spirochetes, Borrelia burgdorferi, isolated from ticks throughout California. J Clin Microbiol. 1993;31:3096–3108. doi: 10.1128/jcm.31.12.3096-3108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimamura T, Watanabe S, Sasaki S. Enhancement of enterotoxin production by carbon dioxide in Vibrio cholerae. Infect Immun. 1985;49:455–456. doi: 10.1128/iai.49.2.455-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soncini F C, Groisman E A. Two-component regulatory systems can interact to process multiple environmental signals. J Bacteriol. 1996;178:6796–6801. doi: 10.1128/jb.178.23.6796-6801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steere A C. Lyme disease. N Engl J Med. 1989;321:586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- 42.Steere A C, Craft J E, Hutchinson G J, Newman J H, Rahn D W, Sigal L H, Spieler P N, Stenn K S, Malawista S E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99:76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson B, Schwan T G, Rosa P A. Temperature-related differential expression of antigens in the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun. 1995;63:4535–4539. doi: 10.1128/iai.63.11.4535-4539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suk K, Das S, Sun W, Jwang B, Barthold S W, Flavell R A, Fikrig E. Borrelia burgdorferi genes selectively expressed in the infected host. Proc Natl Acad Sci USA. 1995;92:4269–4273. doi: 10.1073/pnas.92.10.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Via L E, Curcic R, Mudd M H, Dhandayuthapani S, Ulmer R J, Deretic V. Elements of signal transduction in Mycobacterium tuberculosis: in vitro phosphorylation and in vivo expression of the response regulator MtrA. J Bacteriol. 1996;178:3314–3321. doi: 10.1128/jb.178.11.3314-3321.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Ardenne M, Kruger W. Local tissue hyperacidification and lysosomes. Front Biol. 1979;48:161–194. [PubMed] [Google Scholar]
- 48.Wallich R, Brenner C, Kramer M D, Simon M M. Molecular cloning and immunological characterization of a novel linear-plasmid-encoded gene, pG, of Borrelia burgdorferi expressed in vivo. Infect Immun. 1995;63:3327–3335. doi: 10.1128/iai.63.9.3327-3335.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winans S C. Transcriptional induction of an Agrobacterium regulatory gene at tandem promoters by plant-released phenolic compounds, phosphate starvation, and acidic growth media. J Bacteriol. 1990;172:2433–2438. doi: 10.1128/jb.172.5.2433-2438.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Q L, Kong D, Lam K, Husson R N. A mycobacterial extracytoplasmic function sigma factor involved in survival following stress. J Bacteriol. 1997;179:2922–2929. doi: 10.1128/jb.179.9.2922-2929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zung J L, Lewengrub S, Rudzinska M A, Spielman A, Telford S R, Piesman J. Fine structural evidence for the penetration of the Lyme disease spirochete Borrelia burgdorferi through the gut and salivary tissues of Ixodes dammini. Can J Zool. 1989;67:1737–1748. [Google Scholar]