Abstract
Glyoxalase I (EC 4.4.1.5) was purified about 10000-fold from pig erythrocytes in a yield of approx. 20%. The purification included affinity chromatography on S-hexylglutathione coupled to Sepharose 4B. The purified enzyme normally contained two catalytically active components which were resolved by polyacrylamide-gel electrophoresis. After treatment with reduced glutathione only one component was found. The two components were also demonstrable after isoelectric focusing or DEAE-cellulose chromatography and could also in these cases be fused into one species by preincubation with reduced glutathione. It is proposed that the most acidic form of glyoxalase I is a mixed disulphide with glutathione. Except for these interconvertible forms, the purified enzyme was homogeneous, as judged by disc electrophoresis and sodium dodecyl sulphate/polyacrylamidegel electrophoresis. The molecule is a dimer (48000 daltons), composed of apparently identical subunits (24000 daltons). The isoelectric point was 4.8 at 4°C. The amino acid composition was consistent with the low isoelectric point. The enzyme contained about two thiol groups per enzyme molecule. EDTA inactivated the enzyme and bivalent metal ions could restore fully or partially the catalytic activity; Mg2+ and Mn2+ gave highest activity. It is proposed that a major biological function of glyoxalase I is the detoxification of methylglyoxal formed by enterobacteria in the alimentary canal.
Full text
PDF


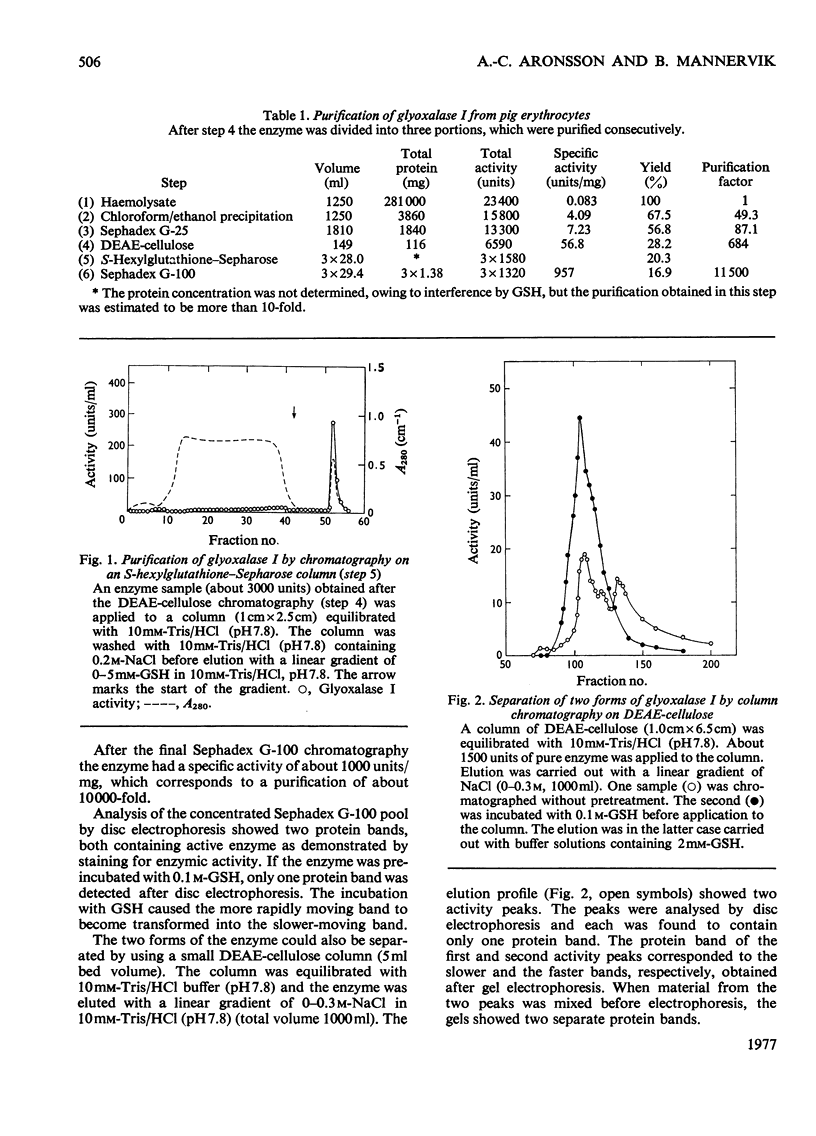
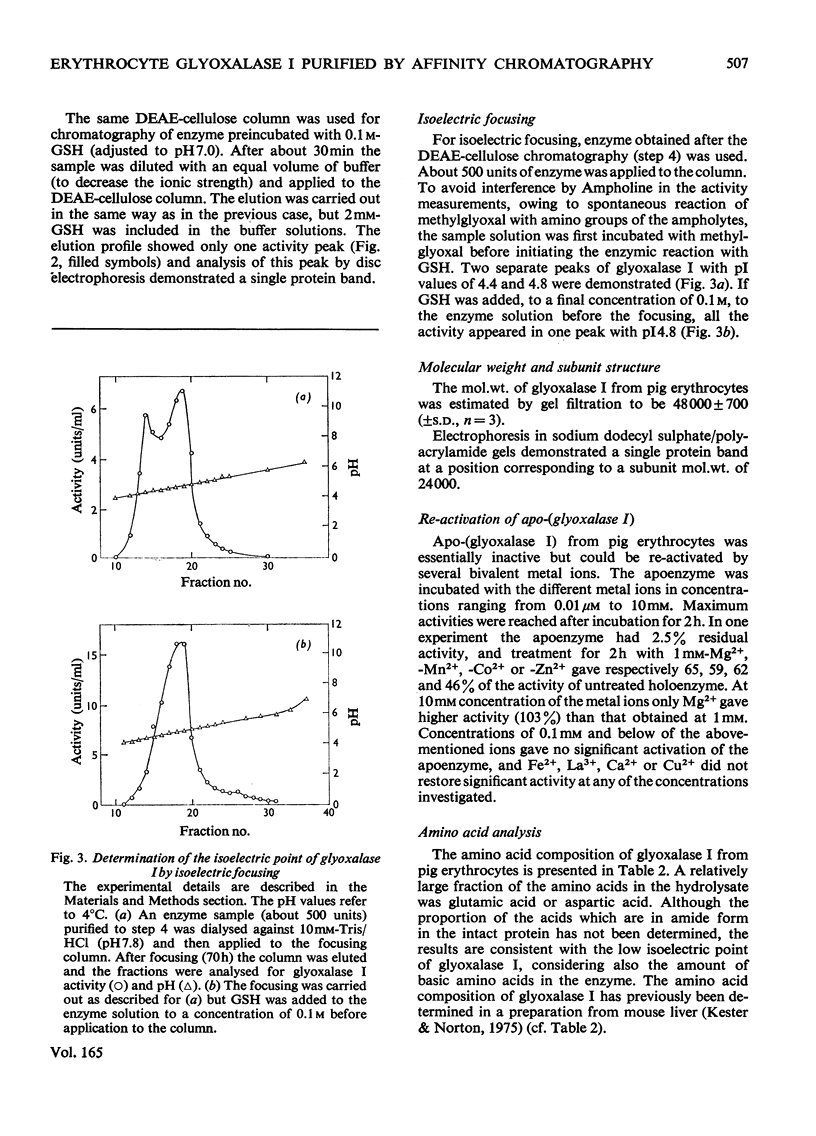


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Davis K. A., Williams G. R. Glyoxalase I, a lyase or an oxidoreductive isomerase? Can J Biochem. 1969 May;47(5):553–556. doi: 10.1139/o69-086. [DOI] [PubMed] [Google Scholar]
- Kester M. V., Norton S. J. The isolation and characterization of mouse liver glyoxalase I. Biochim Biophys Acta. 1975 May 23;391(1):212–221. doi: 10.1016/0005-2744(75)90168-0. [DOI] [PubMed] [Google Scholar]
- Krebs H. A., Perkins J. R. The physiological role of liver alcohol dehydrogenase. Biochem J. 1970 Jul;118(4):635–644. doi: 10.1042/bj1180635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kömpf J., Bissbort S., Gussmann S., Ritter H. Polymorphism of red cell glyoxalase I (EI: 4.4.1.5); a new genetic marker in man. Investigation of 169 mother-child combinations. Humangenetik. 1975;27(2):141–143. doi: 10.1007/BF00273329. [DOI] [PubMed] [Google Scholar]
- LINDSKOG S. Purification and properties of bovine erythrocyte carbonic anhydrase. Biochim Biophys Acta. 1960 Apr 8;39:218–226. doi: 10.1016/0006-3002(60)90156-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mannervik B., Lindström L., Bártfai T. Partial purification and characterization of glyoxalase I from porcine erythrocytes. Eur J Biochem. 1972 Sep 18;29(2):276–281. doi: 10.1111/j.1432-1033.1972.tb01985.x. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Marmstål E., Ekwall K., Górna-Hall B. Inactivation of glyoxalase I from porcine erythrocytes and yeast by amino-group reagents. Eur J Biochem. 1975 May 6;53(2):327–333. doi: 10.1111/j.1432-1033.1975.tb04072.x. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Penke B., Ferenczi R., Kovács K. A new acid hydrolysis method for determining tryptophan in peptides and proteins. Anal Biochem. 1974 Jul;60(1):45–50. doi: 10.1016/0003-2697(74)90129-8. [DOI] [PubMed] [Google Scholar]
- RACKER E. The mechanism of action of glyoxalase. J Biol Chem. 1951 Jun;190(2):685–696. [PubMed] [Google Scholar]
- Tsai P. K., Gracy R. W. Isolation and characterization of crystalline methylglyoxal synthetase from Proteus vulgaris. J Biol Chem. 1976 Jan 25;251(2):364–367. [PubMed] [Google Scholar]
- Uotila L., Koivusalo M. Purification and properties of glyoxalase I from sheep liver. Eur J Biochem. 1975 Apr 1;52(3):493–503. doi: 10.1111/j.1432-1033.1975.tb04019.x. [DOI] [PubMed] [Google Scholar]
- Vince R., Daluge S., Wadd W. B. Studies on the inhibition of glyoxalase I by S-substituted glutathiones. J Med Chem. 1971 May;14(5):402–404. doi: 10.1021/jm00287a006. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]


