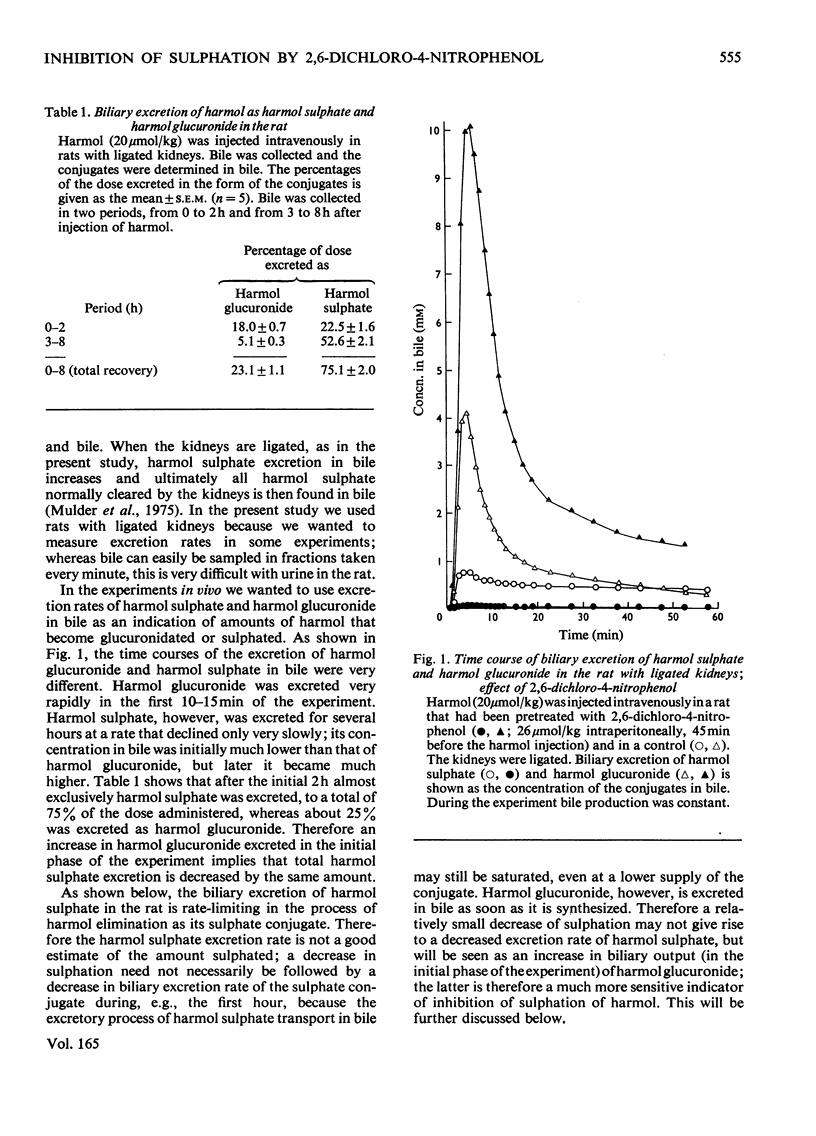
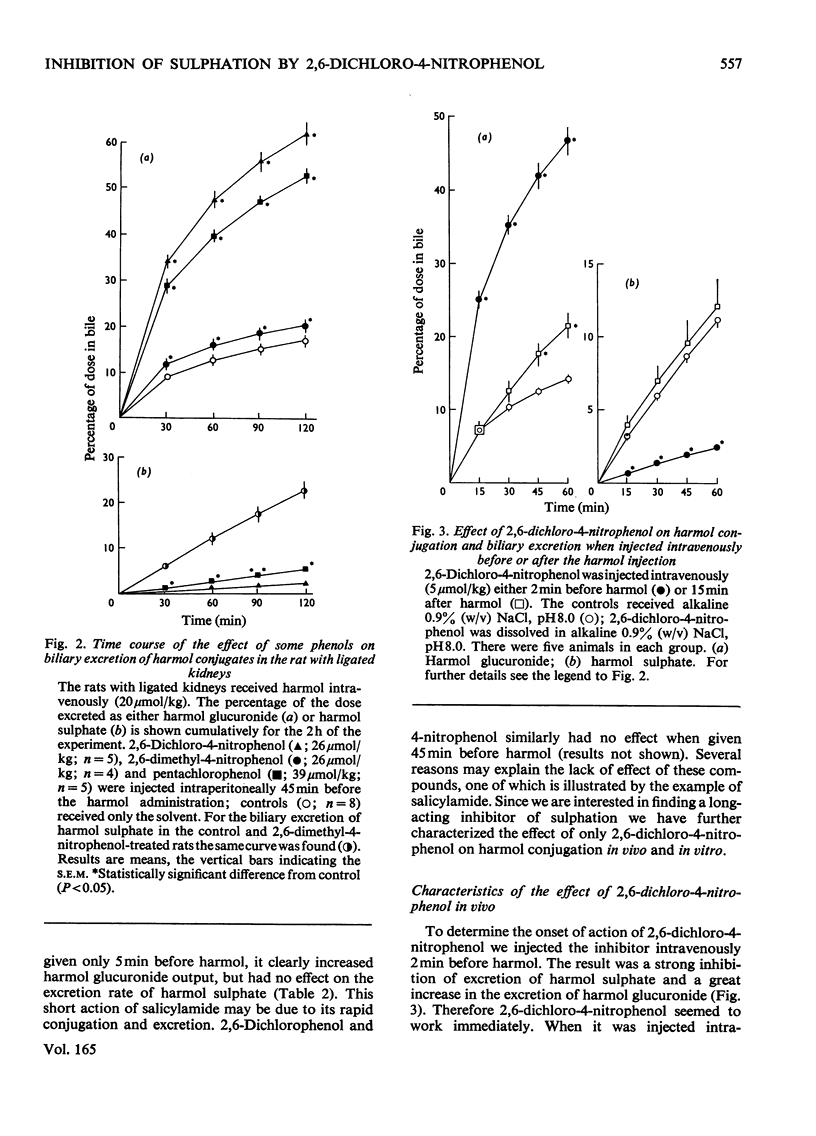
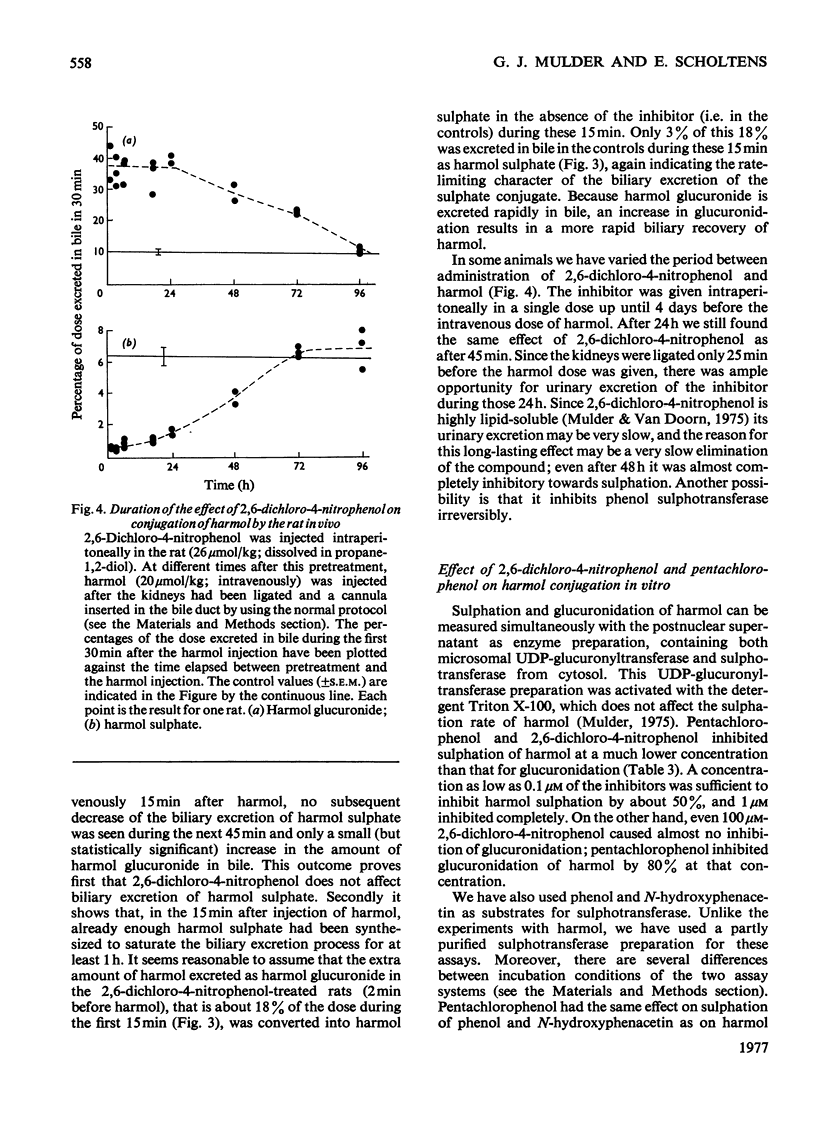
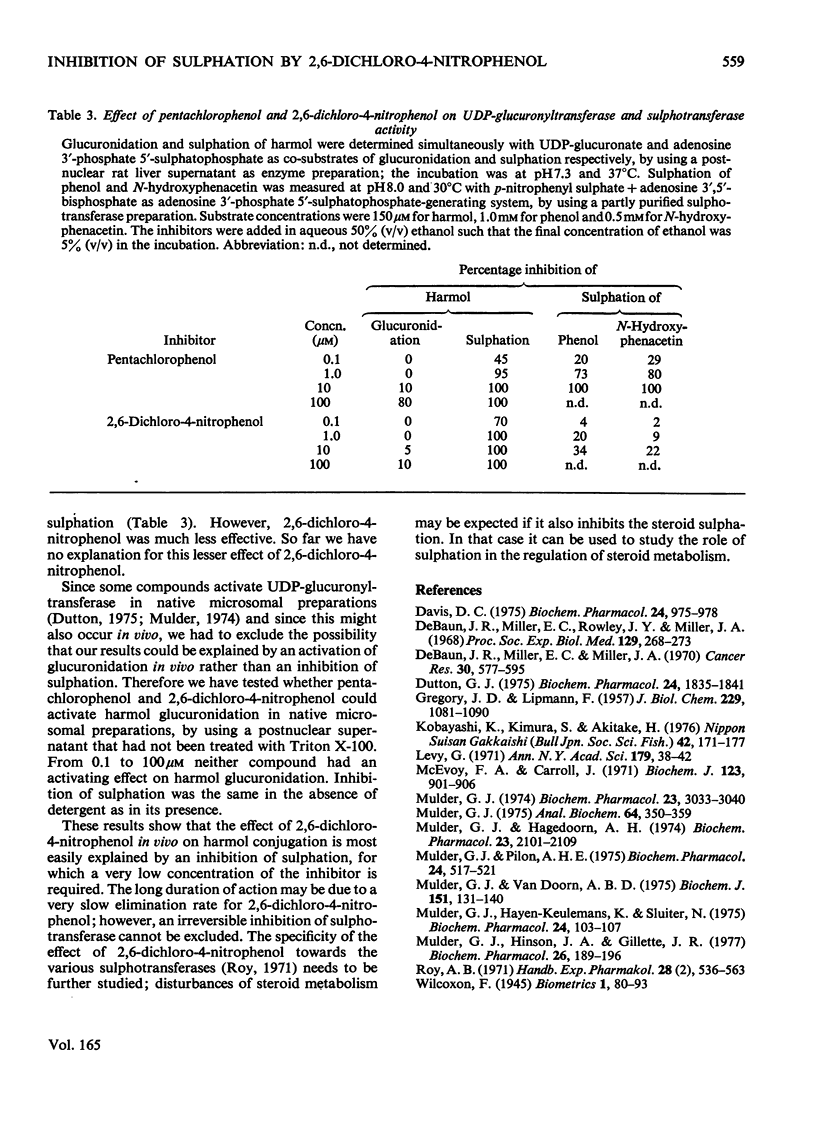
Abstract
Microsomal UDP-glucuronyltransferase and cytosolic sulphotransferase share many substrates, such as phenols and hydroxamic acids. In a search for a selective inhibitor of sulphation, several phenolic compounds were tested. 2,6-Dichloro-4-nitrophenol is introduced as a selective inhibitor of sulphation in vivo, having no effect on UDP-glucuronyltransferase activity. As substrate for both conjugating enzymes the phenolic drug harmol (7-hydroxy-1-methyl-9H-pyrido[3,4-b]indole) was used. In the rat in vivo 2,6-dichloro-4-nitrophenol caused almost complete inhibition of harmol sulphation after a single intraperitoneal injection (26μmol/kg) for 48h; the percentage of harmol sulphated decreased from 75% in controls to 5% in the treated rats. The percentage of harmol glucuronidated increased from 25 to 95%. Pentachlorophenol was equally effective but also highly toxic. Salicylamide had only a very-short-lasting inhibitory effect on sulphation. In vitro, 2,6-dichloro-4-nitrophenol inhibited sulphation of harmol by a rat liver postmitochondrial supernatant completely at 1μm, whereas even at 100μm it had no effect on glucuronidation of harmol. It is concluded that 2,6-dichloro-4-nitrophenol is a selective inhibitor of sulphation and, further, that its long duration of action makes it suitable for studies on the regulatory role of sulphation in some biological processes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis D. C. Radioisotopic assay for rat liver sulfotransferase activity. Biochem Pharmacol. 1975 May 1;24(9):975–978. doi: 10.1016/0006-2952(75)90430-x. [DOI] [PubMed] [Google Scholar]
- DeBaun J. R., Miller E. C., Miller J. A. N-hydroxy-2-acetylaminofluorene sulfotransferase: its probable role in carcinogenesis and in protein-(methion-S-yl) binding in rat liver. Cancer Res. 1970 Mar;30(3):577–595. [PubMed] [Google Scholar]
- DeBaun J. R., Rowley J. Y., Miller E. C., Miller J. A. Sulfotransferase activation of N-hydroxy-2-acetylaminofluorene in rodent livers susceptible and resistant to this carcinogen. Proc Soc Exp Biol Med. 1968 Oct;129(1):268–273. doi: 10.3181/00379727-129-33301. [DOI] [PubMed] [Google Scholar]
- Dutton G. J. Commentary: Control of UDP-glucuronyltransferase activity. Biochem Pharmacol. 1975 Oct 15;24(20):1835–1841. doi: 10.1016/0006-2952(75)90399-8. [DOI] [PubMed] [Google Scholar]
- GREGORY J. D., LIPMANN F. The transfer of sulfate among phenolic compounds with 3',5'-diphosphoadenosine as coenzyme. J Biol Chem. 1957 Dec;229(2):1081–1090. [PubMed] [Google Scholar]
- Levy G. Drug biotransformation interactions in man: nonnarcotic analgesics. Ann N Y Acad Sci. 1971 Jul 6;179:32–42. doi: 10.1111/j.1749-6632.1971.tb46889.x. [DOI] [PubMed] [Google Scholar]
- McEvoy F. A., Carroll J. Purification from rat liver of an enzyme that catalyses the sulphurylation of phenols. Biochem J. 1971 Aug;123(5):901–906. doi: 10.1042/bj1230901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder G. J. A method for comparison of the properties of UDP glucuronyltransferase from rat liver, with a joint substrate. Anal Biochem. 1975 Apr;64(2):350–359. doi: 10.1016/0003-2697(75)90442-x. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Hagedoorn A. H. UDP glucuronyltransferase and phenolsulfotransferase in vivo and in vitro. Conjugation of harmol and harmalol. Biochem Pharmacol. 1974 Aug;23(15):2101–2109. doi: 10.1016/0006-2952(74)90575-9. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Hayen-Keulemans K., Sluiter N. E. UDP glucuronyltransferase and phenolsulfotransferase from rat liver in vivo and in vitro. Characterization of conjugation and biliary excretion of harmol in vivo and in the perfused liver. Biochem Pharmacol. 1975 Jan 1;24(1):103–107. doi: 10.1016/0006-2952(75)90321-4. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Hinson J. A., Gillette J. R. Generation of reactive metabolites of N-hydroxy-phenacetin by glucoronidation and sulfation. Biochem Pharmacol. 1977 Feb 1;26(3):189–196. doi: 10.1016/0006-2952(77)90301-x. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., Pilon A. H. UDP glucuronyltransferase and phenolsulfotransferase from rat liver in vivo and in vitro. III. The effect of phenolphthalein and its sulfate and glucuronide conjugate on conjugation and biliary excretion of harmol. Biochem Pharmacol. 1975 Feb 15;24(4):517–521. doi: 10.1016/0006-2952(75)90138-0. [DOI] [PubMed] [Google Scholar]
- Mulder G. J., van Doorn A. B. A rapid NAD+-linked assay for microsomal uridine diphosphate glucuronyltransferase of rat liver and some observations on substrate specificity of the enzyme. Biochem J. 1975 Oct;151(1):131–140. doi: 10.1042/bj1510131. [DOI] [PMC free article] [PubMed] [Google Scholar]


