Abstract
Mycoplasma fermentans incognitus has been isolated from human tissue in patients both with and without AIDS who died of systemic infection. M. fermentans incognitus and other strains of M. fermentans have been associated with rheumatoid arthritis. While cell extracts of M. fermentans incognitus can induce changes in murine and human cells of the monocytic lineage, little is known about interactions of viable organisms with such cells. Because of the central role of macrophages in chronic inflammation, we examined the effects of M. fermentans incognitus on surface markers and functions of THP-1 cells, a well-characterized human monocytic cell line. This cell line has been used extensively in studies of macrophage differentiation, especially following exposure to phorbol esters. Changes in cell morphology, phagocytosis, rate of cell division, and selected surface markers were evaluated in cultures of THP-1 cells exposed to phorbol myristate acetate (PMA), M. fermentans incognitus, or both. As reported by other investigators, PMA induced THP-1 cells to differentiate into cells resembling tissue macrophages. M. fermentans incognitus only minimally affected changes induced by PMA, slightly increasing the percentage of cells positive for FCγRI and major histocompatibility complex (MHC) class II antigens. M. fermentans incognitus alone induced an incomplete arrest in the cell cycle at G0 phase, increased phagocytic ability, and enhanced expression of FCγRI, CR3, CR4, and MHC class II antigens.
Macrophages serve a wide range of functions in host defense and inflammation, including antigen presentation, cytokine secretion, phagocytosis, and microbial and tumor cell killing (2, 4). After differentiation, their functional repertoire can be altered, and it is largely determined by the surrounding tissue microenvironment (the surrounding cells and their secreted products). Phorbol esters, such as phorbol 12-myristate 13-acetate (PMA), are capable of inducing complete differentiation of monocytes into tissue macrophages (4, 5). PMA-induced differentiation occurs through activation of protein kinase C (PKC) and resembles diacylglycerol-induced differentiation, which occurs naturally (14, 19). Phenotypically, macrophages induced by PMA resemble resident tissue macrophages in having reduced expression of FCγRI and major histocompatibility complex (MHC) class II antigens and increased expression of the complement receptors CR3 (CD18-CD11b) and CR4 (CD18-CD11c) (3, 21).
In contrast, inflammatory macrophages express greater amounts of FCγRI and MHC class II antigens and have enhanced phagocytic ability (2). Enhanced expression of FCγRI correlates with antibody-dependent cell cytotoxicity. Activation of FCγRI also leads to activation of phospholipase A2 and the proinflammatory arachidonic acid cascade (2). Enhanced MHC class II expression correlates with effective antigen-presenting capacity (2, 8, 27).
Various Mycoplasma fermentans cell components have immunomodulatory effects on monocytes, resulting in secretion of proinflammatory cytokines (tumor necrosis factor [TNF], interleukin 1 [IL-1], and IL-6), alterations in expression of MHC class II antigens, arrest of cell division, and induction of differentiation (8–10, 12, 22, 27). However, the functional capabilities of M. fermentans-differentiated macrophages have not been described. Our objective was to determine the effect of M. fermentans incognitus infection on the differentiation and functional capacity of THP-1 cells, a monocytoid leukemic cell line, by assessing changes in cell cycle, cell morphology, phagocytic ability, and expression of MHC class II antigens, FCγRI, and complement receptors CR3 and CR4.
We examined the effect of M. fermentans incognitus on THP-1 cells that were either resting or differentiating in response to PMA. M. fermentans incognitus did not induce complete differentiation in resting THP-1 cells, but exposure did increase the phagocytic ability and enhance expressions of FCγRI, CR3, CR4, and MHC class II antigens. Furthermore, M. fermentans incognitus altered the phenotypes of cells induced to differentiate by PMA so that the cells resembled inflammatory macrophages rather than resident tissue macrophages.
MATERIALS AND METHODS
Mycoplasmas.
M. fermentans incognitus was used for all experiments. This culture was the seventh passage of a stock culture obtained from J. G. Tully, National Institutes of Health. To ensure identical inocula for all experiments, a large-volume culture was grown to late log phase in SP4 medium, and 5-ml aliquots were frozen at −80°C. The frozen inoculum contained 108 CFU per ml of broth. In preliminary experiments, we found that M. fermentans continued to grow in our THP-1 cell culture system and that the number of final CFU varied until this system was saturated. After saturation, there was little change in the number of CFU during the period in which these experiments were conducted. We therefore could not test differing doses, and to reduce variability, we saturated our cultures by using inocula that contained the highest number of CFU (108 CFU/ml of RPMI 1640 medium) that did not kill THP-1 cells over a 1-week period. Prior to inoculation into THP-1 cell cultures, the mycoplasmas were washed three times in sterile, endotoxin- and preservative-free 0.9% saline and resuspended in sterile, antibiotic- and endotoxin-free RPMI medium containing 10% fetal calf serum and 25 mM HEPES. For the duration of each experiment, all THP-1 cultures infected with M. fermentans incognitus received only one inoculum. For each experiment, the concentration of viable organisms in the prepared inoculum was verified by culture.
THP-1 cell cultures.
Mycoplasma-free THP-1 cells obtained from the American Type Culture Collection (Manassas, Va.) were maintained at 37°C, 5% CO2, and 95% relative humidity (RH) in antibiotic- and endotoxin-free RPMI medium containing 10% fetal calf serum and 25 mM HEPES. Both cell extracts and cell supernatants were repeatedly screened by PCR for mycoplasmal contamination. DNA was extracted from the samples by proteinase K digestion (23). The primers used detected a 16S ribosomal gene sequence that is characteristically present in the class Mollicutes. The primers used were (from the 5′ to 3′ sequence) P1, AGAGTTGATCCTGGCTCAGGA-3′ (23), and MGSO, TCGACCATCTGTCACTCTGTTAACCTC (13), which yielded a 1,042-bp product. For amplification of DNA, the PCR assay was performed in 50 μl of reaction mixture containing 50 mM Tris-HCL, 50 mM NaCl, 2 mM MgCl2, a 0.5 μM concentration of each primer, a 200 mM concentration of each deoxynucleoside triphosphate, and 2.5 U of Taq DNA polymerase (Promega, Madison, Wis.). This assay consistently detects mycoplasmal DNA extracted from cultures containing 100 CFU/μl. DNA from mycoplasmal cultures was extracted by proteinase K digestion. Each THP-1 cell sample had a corresponding positive control containing 10 copies of either Mycoplasma pulmonis or M. fermentans DNA. The thermal profile involved one 5-min delay cycle at 95°C followed by 40 cycles of denaturation at 94°C for 1 min, primer annealing at 55°C for 1 min, and primer extension at 72°C for 1 min. Twenty-microliter aliquots of the amplified product were analyzed on a 1.5% agarose gel. DNA was stained with ethidium bromide.
PMA treatments.
A 10−7 M concentration of PMA (Sigma, St. Louis, Mo.) in RPMI medium was used to differentiate THP-1 cells. The differentiation-inducing dose of 10−7 M PMA for THP-1 cells was determined in preliminary dose-response experiments (data not shown). The criteria for differentiation of THP-1 cells were cell adherence, changes in cell morphology, and changes in the cell surface marker expression profile (integrin, FCγRI, CD4, and MHC class II antigen) that is associated with the macrophage phenotype. A 10−2 M stock solution of PMA was prepared by dissolving PMA in sterile dimethylsulfoxide (Sigma). The stock solution was stored frozen at −20°C. Immediately prior to use, the PMA stock solution was diluted in RPMI medium to 10−7 M concentration.
Cell surface markers.
Nonadherent cells were detached by gentle scraping with a sterile rubber policeman. The cells were counted in a hemocytometer, and viability was assessed by trypan blue exclusion. THP-1 cells were examined by flow cytometry for changes in CR3 and CR4, FCγRI, and MHC class II antigen expression. The following monoclonal antibodies were used to detect changes in cell surface marker expression: for CR3, we used phycoerythrin (PE)-labeled anti-CD11b (IV MO47; Pharmingen, San Diego, Calif.); for CR4, we used PE-labeled anti-CD11c (IV NO12; Pharmingen), fluorescein isothiocyauate (FITC)-labeled anti-CD64 (10.1; Pharmingen) and FITC-labeled anti-HLA-DR, -DP, and -DQ (TU39; Pharmingen). Both FITC- and PE-labeled mouse immunoglobulin G1 (MOPC-21; Pharmingen) were included as isotype controls. Approximately 0.5 × 106 to 1 × 106 cells were prepared and stained with each antibody according to the manufacturer’s protocol. Briefly, the cells were pelleted by centrifugation at 400 × g, washed with sterile phosphate-buffered saline containing 0.1% sodium azide, and then stained with the appropriate monoclonal antibody. The cells were analyzed for forward and side scatter characteristics and for one-color fluorescence with a FACScan (Becton Dickinson, San Jose, Calif.). SPHERO 3.0-μm Rainbow Fluorescent Particles (Pharmingen) were used in each experiment to calibrate fluorescence intensity. The results were analyzed with PC Lysis software (Becton Dickinson); only samples that were at least 1% positive for each marker were analyzed for mean fluorescence.
Cell cycle analysis.
Adherent cells were detached by a rubber policeman and were counted in a hemacytometer, and viability was assessed by trypan blue exclusion. The cells were washed once with sterile phosphate-buffered saline and lysed, and the cell DNA was stained with ethidium bromide as previously described (20). Using a FACScan (Becton Dickinson, Mountain View, Calif.), the nuclear DNA content was determined by simultaneous flow cytometric measurements of ethidium bromide fluorescence and the side scatter intensity of cell nuclei. The percentages of cells in G0-G1 phase, S phase, and G2 phase were analyzed by MODFIT for Windows (Verity Software House, Topsham, Maine).
Phagocytosis assays.
Monolayers of THP-1 cells were washed twice with sterile RPMI medium and then incubated with 0.2% 0.7-μm-diameter sterile latex-coated polystyrene beads (Sigma) at 37°C, 5% CO2, and 95% RH. At 2, 4, 6, and 8 h of incubation, at least 200 cells from each sample were counted. Cells that contained at least five beads were considered positive for phagocytosis.
Experimental design.
THP-1 cells were cultured in multiple-well tissue culture plates at a concentration of 106/ml and incubated at 37°C, 5% CO2, and 95% RH. The cell cultures were divided into four treatment groups: control (untreated), M. fermentans incognitus only, PMA only, and M. fermentans incognitus combined with PMA. Each treatment group had at least three to five replicates per experiment. The cells in each group were examined for changes in cell morphology, cell cycle, phagocytic ability, and expression of CR3 and CR4 receptors (CD11b and CD11c), FCγRI (CD64), and MHC class II antigen (HLA-DR, -DP, and -DQ). All assays were done at 24, 48, and 72 h poststimulation. In order to avoid complications from nutrient depletion, the RPMI medium in all treatment groups was changed daily. Cells treated with PMA received RPMI medium containing freshly prepared 10−7 M PMA. MFI-infected cultures were inoculated once; therefore, subsequent medium changes were done with RPMI and/or RPMI containing PMA.
Statistical analysis.
Analyses were performed with the Statview SE computer program (ABACUS Concepts, Berkeley, Calif.). Parametric data were analyzed by two-way and one-way analysis of variance followed by Fisher’s test for multiple-means comparison.
RESULTS
Cell adherence and morphology.
Control THP-1 cells maintained a round shape and did not clump or adhere to the culture plate surface. More than 90% of M. fermentans incognitus-infected and non-M. fermentans incognitus-infected THP-1 cells treated with PMA aggregated, became flat and amoeboid, and adhered to the culture plate surface. There were no obvious differences in cell shape or percentage of adhered cells between these two groups. Cells that received only M. fermentans incognitus displayed marked clumping; only 5 to 10% of these cells displayed a flat, amoeboid shape. Approximately 40 to 50% of these cells adhered to the culture plate surface.
Phagocytosis assay.
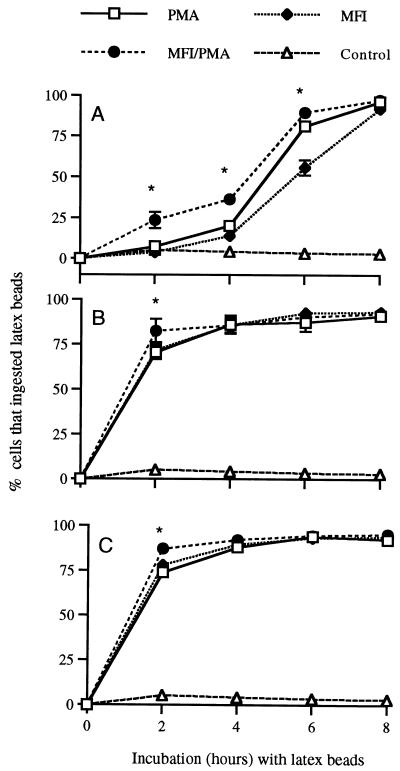
The ability of THP-1 cells to phagocytize latex beads is summarized in Fig. 1. Cells exposed to M. fermentans incognitus PMA, or both displayed significantly greater phagocytosis than control cells (P ≤ 0.0001). Regardless of the stimulus, maximum phagocytosis occurred by 4 h of incubation for cells that had been in culture for either 48 or 72 h. In contrast, cells in culture for only 24 h required exposure to latex beads for 6 to 8 h for maximum phagocytosis. Among cells that had been cultured for 24 h, those that received both PMA and M. fermentans incognitus exhibited greater phagocytosis than cells that received either agent alone (P < 0.0001). This was not seen in cells that had been cultured for either 48 or 72 h, except at 2 h of incubation.
FIG. 1.
Mean percentage ± standard deviation (n = 5) of THP-1 cells that ingested latex beads after 24 (A), 48 (B), and 72 (C) h of exposure to PMA and/or M. fermentans incognitus (MFI). The values labeled with an asterisk represent treatments that were significantly different at that time point (P < 0.0001).
Cell cycle studies.
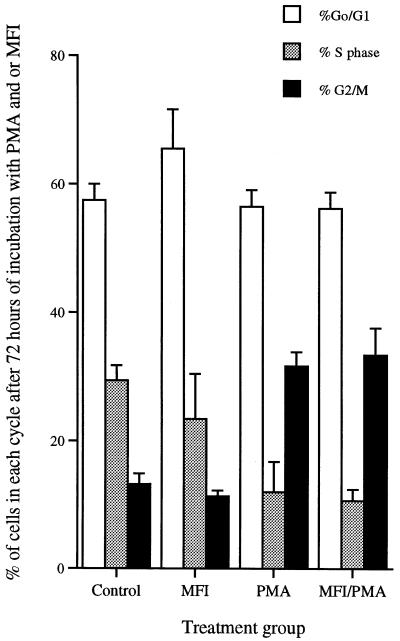
All cultures initially contained 2 × 106 THP-1 cells. Following 24 h in culture, there were no differences in numbers of cells between groups. By 48 h, cultures containing PMA had significant decreases in cell numbers (P < 0.0001), although the remaining cells were able to exclude trypan blue (Table 1). Cultures containing both M. fermentans incognitus and PMA had significantly more cells (P < 0.0001) than those containing PMA alone. After 24 h in culture, there were no differences in the percentages of cells in G0-G1 phase in any treatment group (data not shown). Trends were the same following 48 and 72 h in culture; only the data for 72-h cultures are presented in Fig. 2. PMA significantly decreased the percentage of cells in S phase (P < 0.0001) and increased the percentage in G2 phase (P < 0.0001). M. fermentans incognitus alone induced small, but statistically significant (P < 0.0001), decreases in the percentage of cells in S phase and corresponding increases in the percentage of cells in G0-G1 phase (P < 0.02). M. fermentans incognitus did not significantly alter the proportion of cells in various phases of the growth cycle following exposure to PMA.
TABLE 1.
Number of cells in culture after incubation with PMA and/or M. fermentans incognitus (MFI)a
| Incubation time (h) | No. of cells (106)
|
|||
|---|---|---|---|---|
| Control (n = 6) | PMA (n = 6) | MFI (n = 6) | MFI-PMA(n = 6) | |
| 24 | 1.84 ± 0.29b | 1.42 ± 0.37b | 1.92 ± 0.14b | 1.69 ± 0.44b |
| 48 | 1.81 ± 0.51c | 0.73 ± 0.17d | 1.86 ± 0.29c | 1.46 ± 0.43c |
| 72 | 2.0 ± 0.33e | 0.69 ± 0.25g | 1.60 ± 0.38ef | 1.29 ± 0.41f |
All cultures initially contained 2 × 106 viable cells. Greater than 90% of cells in any culture were able to exclude trypan blue. Groups within the same row that share the superscript letters b to g are not statistically different at P < 0.05.
FIG. 2.
Mean percentage ± standard deviation of cells in different phases of the cell cycle (n = 6) as determined by ethidium bromide staining and fluorescence-activated cell sorter analysis. The data are the averages of two separate experiments. MFI, M. fermentans incognitus.
Changes in cell surface marker expression.
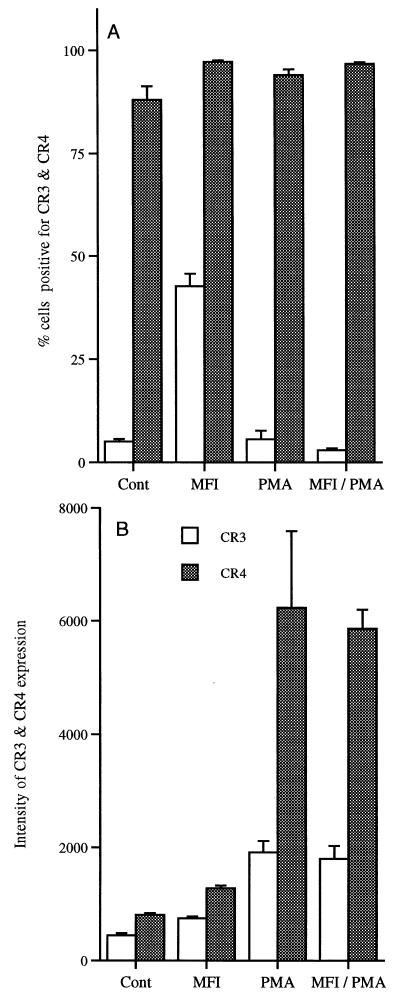
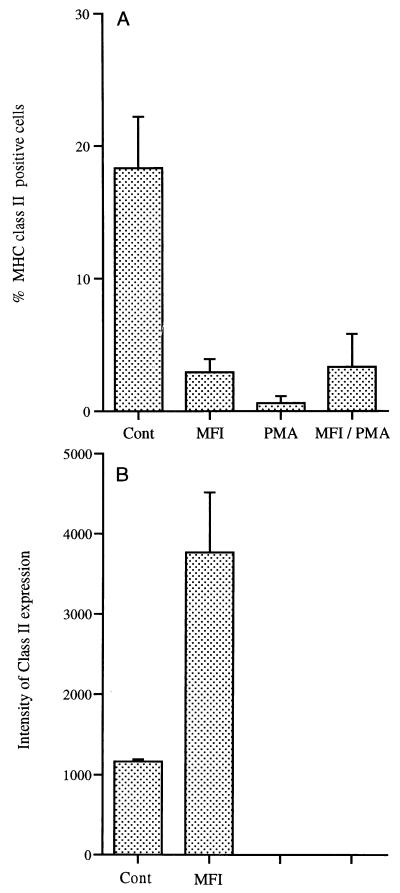
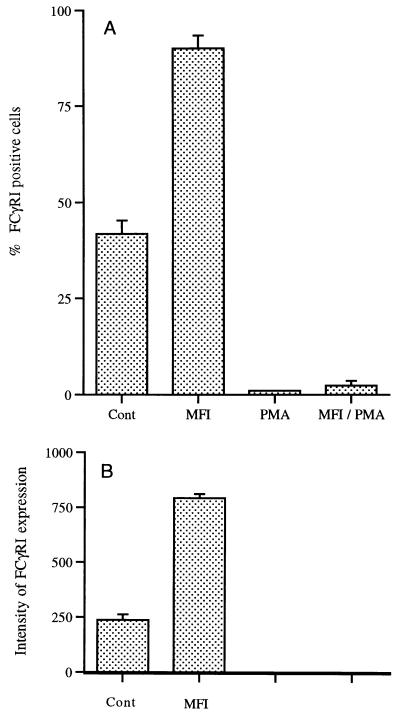
Data regarding the expression of cell surface markers are expressed as percentages because of the drop in cell numbers in cultures exposed to PMA. Only data from the 72-h time point are shown in Fig. 3 to 5. In cultures containing M. fermentans incognitus only, there was a slight increase in the percentage of cells expressing CR4 and more than an eightfold increase in cells expressing CR3 (Fig. 3A) (P < 0.001). While PMA did not significantly increase the percentage of cells expressing either CR3 or CR4, it significantly increased the expression of both markers on positive cells (Fig. 3B) (P < 0.0001). As shown in Fig. 4A, M. fermentans incognitus significantly increased the percentage of cells expressing FCγRI (P < 0.001) and PMA significantly decreased (P < 0.001) the percentage of cells expressing this marker. There was some suggestion that M. fermentans incognitus and PMA were antagonistic inasmuch as there was a small, but significant, difference in the percentage of cells expressing this marker in cultures containing PMA alone and those containing both PMA and M. fermentans incognitus (P < 0.001). Due to the autofluoresence of PMA-treated cultures, changes in the intensity of FCγRI could not be assessed; however, M. fermentans incognitus increased the expression of this marker on positive cells (Fig. 4B). Figure 5A shows that exposure to either PMA or M. fermentans incognitus significantly decreased (P < 0.0001) the percentage of cells positive for MHC class II antigens. Again, due to autofluorescence, intensity of expression could not be evaluated in cultures treated with PMA, but M. fermentans incognitus increased the intensity of expression of the marker (P < 0.0001).
FIG. 3.
Mean percentage ± standard deviation of cells positive for CR3 or CR4 (n = 5) (A) and intensity of expression for positive cells as measured in mean fluorescence units (average ± standard deviation) (B). MFI, M. fermentans incognitus.
FIG. 5.
Mean percentage ± standard deviation of cells positive for MHC class II expression (n = 5) (A) and intensity of expression for positive cells in control and M. fermentans incognitus (MFI)-treated groups as measured in mean fluorescence units (average ± standard deviation). Only two of five samples in the PMA-treated group were positive for MHC class II expression. Intensity of expression was not determined in PMA and MFI-PMA groups because of autofluorescence.
FIG. 4.
Mean percentage ± standard deviation of cells positive for FcγRI (n = 5) (A) and intensity of expression for positive cells in control and M. fermentans incognitus (MFI)-treated groups as measured in mean fluorescence units (average ± standard deviation) (B). Only one of five samples in the PMA-treated group was positive for FcγRI expression. Intensity of expression was not determined in PMA and MFI-PMA groups because of autofluorescence.
DISCUSSION
THP-1 is a well-characterized human monocytic leukemic cell line; the cells resemble monocytes with respect to several criteria (4, 5) and can be differentiated into macrophage-like cells by treatment with PMA (4, 5). PMA-induced differentiation is associated with alterations in cell morphology, cell adherence, and expression of several genes (4). The changes in cell morphology, adherence, phagocytosis, and cell surface receptors noted in the these studies have been reported previously by Auwerx et al. (4, 5). Furthermore, PMA is known to induce cell cycle arrest in both G0-G1 and G2 phases through activation of PKC (14). We also found significant decreases in the number of cells in culture, which suggests that cell death may also be occurring. PMA induces apoptosis in other cell lines (7, 18), which may be related to PKC activation (7, 18). M. fermentans incognitus infection of PMA-treated cells did not greatly alter the effects of PMA on THP-1 cells, although phagocytosis and expression of both FCγRI and MHC class II antigens were significantly increased. Further studies would be required to determine if these differences are biologically relevant in terms of macrophage function.
M. fermentans incognitus also induces changes in the THP-1 phenotype, but the final phenotype following M. fermentans incognitus exposure differs significantly from that induced by PMA. Cells exposed to MFI do not display amoeboid characteristics (data not shown), do not undergo complete arrest in cell division (Fig. 2), and have significant increases, instead of decreases, in the percentage of cells positive for FCγRI (Fig. 4). In addition, the magnitude of cell loss in cultures exposed to M. fermentans incognitus is much less than that in cultures exposed to PMA. However, increased phagocytosis, increased expression of CR3 and CR4, and a decrease in the percentage of cells expressing MHC class II molecules are similar in cells exposed to M. fermentans incognitus and those exposed to PMA. This suggests that THP-1 cells are activated by both M. fermentans incognitus and PMA and that the two agents activate different, but overlapping, cellular mechanisms.
Phorbol esters, such as PMA, are thought to regulate gene expression principally through the activation of PKC, a family of structurally related kinases with potentially unique activation requirements and substrate specificities (14, 19). Specific PKC isoenzyme activities correlate with specific biological functions. For example activation of PKC-α, and -δ correlates with myeloid cell differentiation (14), which encompasses cell cycle arrest. Bacterial phospholipase can also activate PKC isoforms and other membrane phospholipids, some of which can modulate PKC’s effects (19). Mycoplasmas, including M. fermentans incognitus, are known to possess membrane-bound phospholipase C (26), which is capable of producing diacylglycerol and inositol 1,4,5-triphosphate (IP3). Both agents modulate PKC activity, diacylglycerol through its direct activation, and indirectly through IP3-mediated Ca2+ mobilization (19). IP3 can modulate the state of activation in Ca2+-dependent PKC isoenzymes (19). It is probable that the overlapping effects of M. fermentans incognitus and PMA on THP-1 cells occur through PKC modulation.
Changes in FCγRI, CR3, and CR4 expression are dependent on cell cycle arrest but not on PKC activation (5). For instance, PKC inhibitors do not alter PMA-induced changes in expression of FCγRI, CR3, and CR4 (5). Our data suggest that M. fermentans incognitus alters expression of FCγRI, CR3, and CR4 without inducing complete cell cycle arrest. Between MFI-treated and control cultures, there is no significant decrease of cells in S phase and no increase in cells in the G2-M phase, both of which occur in PMA-treated cultures. Thus, for at least 72 h, the cell cycle is not arrested in cultures treated with M. fermentans incognitus only. Integrin expression (CR3 and CR4) on phagocytes can be regulated through TNF (3). A likely explanation is that M. fermentans incognitus induces THP-1 cells to produce cytokines that alter the expression of these markers. M. fermentans cell extracts can induce monocytes and macrophages to produce TNF-α and IL-1 (9, 17, 22). Cytokine receptors modulate intracellular signaling through the JAK family of kinases (11), which in turn stimulates gene transcription. Both FcγRI and integrin molecules are involved in inflammatory processes. Engagement of FcγRI potentiates the inflammatory process through antibody-dependent cell cytotoxicity and activation of phospholipase A and production of arachidonic acid (2). The expression of integrin molecules (CR3 and CR4) is necessary for cell-to-cell adhesion and leukocyte trafficking to sites of inflammation (3). Thus, M. fermentans incognitus can increase the proinflammatory capacities of these cells.
MHC class II expression is typically an expression of macrophage activation and can be induced by exposure to gamma interferon or macrophage-activating factor (2, 8). There is conflicting evidence regarding the effects of M. fermentans incognitus on expression of MHC class II antigens. Killed M. fermentans incognitus induces MHC class II expression in both human and murine monocytic cells (27). However, others have found that lipid extracts from M. fermentans D15-86 suppresses gamma interferon-induced expression of MHC class II antigens on monocytic cells (8). Our data show that viable M. fermentans incognitus decreases the percentage of cells that are positive for MHC class II expression but that intensity of expression increases per positive cell, suggesting M. fermentans incognitus exposure induces THP-1 cells to differentiate into more than one subpopulation.
M. fermentans has been implicated in human disease since it was isolated from the joints of patients with leukemia or rheumatoid arthritis (24, 25). More recently, the incognitus strain of the organism has been identified in the tissues of patients both with and without AIDS and is thought to induce both respiratory and systemic disease in some individuals (6, 15, 16). Our data, along with those of others, indicate that M. fermentans incognitus induces changes in cells of the monocytic lineage that may contribute to disease pathogenesis through increased phagocytosis (1), alterations in local cytokine networks (9, 12, 17, 22, 28), and altered presentation of antigens (8, 27). Alterations in cytokine networks, along with alterations in antigen presentation, may lead to changes in T-helper-cell activity and could lead to autoimmune mechanisms.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health grant RO1AI33164. L. Reyes was supported by a fellowship from the National Institutes of Health (T32RR07001).
REFERENCES
- 1.Abe T, Ohno M, Sato T, Murakami M, Kajiki M, Kodara R. “Differentiation induction” culture of human leukemic myeloid cells stimulates high production of macrophage differentiation inducing factor. Cytotechnology. 1991;5:S75–S93. [PubMed] [Google Scholar]
- 2.Adams D O, Hamilton T A. The cell biology of macrophage activation. Annu Rev Immunol. 1984;2:283–318. doi: 10.1146/annurev.iy.02.040184.001435. [DOI] [PubMed] [Google Scholar]
- 3.Arnaout M A. Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 4.Auwerx J. The human leukemia cell line, THP-1: a multifaceted model for the study of monocyte-macrophage differentiation. Experientia. 1991;47:22–31. doi: 10.1007/BF02041244. [DOI] [PubMed] [Google Scholar]
- 5.Auwerx J, Staels B, Van Vaeck F, Ceuppens J L. Changes in IgG Fc receptor expression induced by phorbol 12-myristate 13-acetate treatment of THP-1 monocyte leukemia cells. Leukoc Res. 1992;16:317–327. doi: 10.1016/0145-2126(92)90070-n. [DOI] [PubMed] [Google Scholar]
- 6.Beecham H J, III, Lo S C, Lewis D E, Comer S W, Riley K J, Oldfield E C., III Letter. Lancet. 1991;338:1014–1015. doi: 10.1016/0140-6736(91)91874-t. [DOI] [PubMed] [Google Scholar]
- 7.De Vente J, Kiley S, Garris T, Bryant W, Hooker J, Posekany K, Parker P, Cook P, Fletcher D, Ways D K. Phorbol ester treatment of U937 cells with altered protein kinase C content and distribution induces cell death rather than differentiation. Cell Growth Differ. 1995;6:371–382. [PubMed] [Google Scholar]
- 8.Frisch M, Gradehandt G, Mulradt P F. Mycoplasma fermentans-derived lipid inhibits class II major histocompatibility complex expression without mediation by interleukin-6, interleukin-10, tumor necrosis factor, transforming growth factor-beta, type 1 interferon, prostaglandins or nitric oxide. Eur J Immunol. 1996;26:1050–1057. doi: 10.1002/eji.1830260514. [DOI] [PubMed] [Google Scholar]
- 9.Gallily R, Salman M, Tarshis M, Rottem S. Mycoplasma fermentans (incognitus strain) induces TNF-alpha and IL-1 production by human monocytes and murine macrophages. Immunol Lett. 1992;34:27–30. doi: 10.1016/0165-2478(92)90023-h. [DOI] [PubMed] [Google Scholar]
- 10.Hall R E, Agarwal S, Kestler D P, Cobb J A, Goldstein K M, Chang N. cDNA and genomic cloning and expression of the p48 monocytic differentiation/activation factor, a Mycoplasma fermentans gene product. Biochem J. 1996;319:919–927. doi: 10.1042/bj3190919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karnitz L M, Abraham R T. Cytokine receptor signaling mechanisms. Curr Opin Immunol. 1995;7:320–326. doi: 10.1016/0952-7915(95)80105-7. [DOI] [PubMed] [Google Scholar]
- 12.Kostyal D A, Butler G H, Beezhold D H. A 48 kilodalton Mycoplasma fermentans membrane protein induces cytokine secretion by human monocytes. Infect Immun. 1994;62:3793–3800. doi: 10.1128/iai.62.9.3793-3800.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuppeveld F J M, Van Der Logt J T M, Angulo A F, Van Zoest M J, Quint W G V, Niesters H G M, Galama J M D, Melchers W J G. Genus-and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992;58:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livneh E, Fishman D D. Linking protein kinase C to cell-cycle control. Eur J Biochem. 1997;248:1–9. doi: 10.1111/j.1432-1033.1997.t01-4-00001.x. [DOI] [PubMed] [Google Scholar]
- 15.Lo S C, Hates M M, Wang R Y, Pierce P F, Kotani H, Shih J W. Newly discovered mycoplasma isolated from patients infected with HIV. Lancet. 1991;338:1415–1418. doi: 10.1016/0140-6736(91)92721-d. [DOI] [PubMed] [Google Scholar]
- 16.Lo S C, Wear D J, Green S L, Jones P G, Legier J F. Adult respiratory distress syndrome without systemic disease associated with infections due to Mycoplasma fermentans. Clin Infect Dis. 1993;17:S259–S263. doi: 10.1093/clinids/17.supplement_1.s259. [DOI] [PubMed] [Google Scholar]
- 17.Muhlradt P F, Quentmeier H, Schmitt E. Involvement of interleukin-1 (IL-1), IL-6, IL-2, and IL-4 in generation of cytolytic T cells from thymocytes stimulated by a Mycoplasma fermentans-derived product. Infect Immun. 1991;59:3962–3968. doi: 10.1128/iai.59.11.3962-3968.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munn D H, Beall A C, Song D, Wrenn R W, Throckmorton D C. Activation-induced apoptosis in human macrophages: developmental regulation of a novel cell death pathway by macrophage colony-stimulating factor and interferon gamma. J Exp Med. 1995;181:127–136. doi: 10.1084/jem.181.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- 20.Nusse M, Beisker W, Hoffman C, Tarnok A. Flow cytometric analysis of G1- and G2/M phase subpopulations in mammalian cell nuclei using side scatter and DNA content measurements. Cytometry. 1990;11:813–821. doi: 10.1002/cyto.990110707. [DOI] [PubMed] [Google Scholar]
- 21.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 22.Rawadi G, Roman-Roman S. Mycoplasma membrane lipoproteins induce proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infect Immun. 1996;64:637–643. doi: 10.1128/iai.64.2.637-643.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson J A, Vekris A, Bebear C, Stemke G W. Polymerase chain reaction using 16s rRNA gene sequences distinguishes the two biovars of Ureaplasma urealyticum. J Clin Investig. 1993;31:824–830. doi: 10.1128/jcm.31.4.824-830.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaeverbeke T, Gilroy C B, Bebear C, Dehais J, Taylor-Robinson D. Mycoplasma fermentans, but not M. penetrans, detected by PCR assays in synovium from patients with rheumatoid arthritis and other rheumatic disorders. J Clin Pathol. 1996;49:824–828. doi: 10.1136/jcp.49.10.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaeverbeke T, Renaudin H, Clerc M, Lequen L, Vernhes J P, De Barbeyrac B, Bannwarth B, Bebear C, Dehaus J. Systematic detection of mycoplasmas by culture and polymerase chain reaction (PCR) procedures in 209 synovial fluid samples. Br J Rheumatol. 1997;36:310–314. doi: 10.1093/rheumatology/36.3.310. [DOI] [PubMed] [Google Scholar]
- 26.Shibata K, Sasaki T, Watanabe T. AIDS-associated mycoplasmas possess phospholipase C in the membrane. Infect Immun. 1995;63:4174–4177. doi: 10.1128/iai.63.10.4174-4177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stuart P M, Egan R M, Woodward J G. Characterization of MHC induction by Mycoplasma fermentans (incognitus strain) Cell Immunol. 1993;152:261–270. doi: 10.1006/cimm.1993.1286. [DOI] [PubMed] [Google Scholar]
- 28.Sugama K, Kuwano K, Furukawa M, Himeno Y, Satoh T, Arai S. Mycoplasmas induce transcription and production of tumor necrosis factor in a monocytic cell line, THP-1, by a protein kinase C-independent pathway. Infect Immun. 1990;58:3564–3567. doi: 10.1128/iai.58.11.3564-3567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]