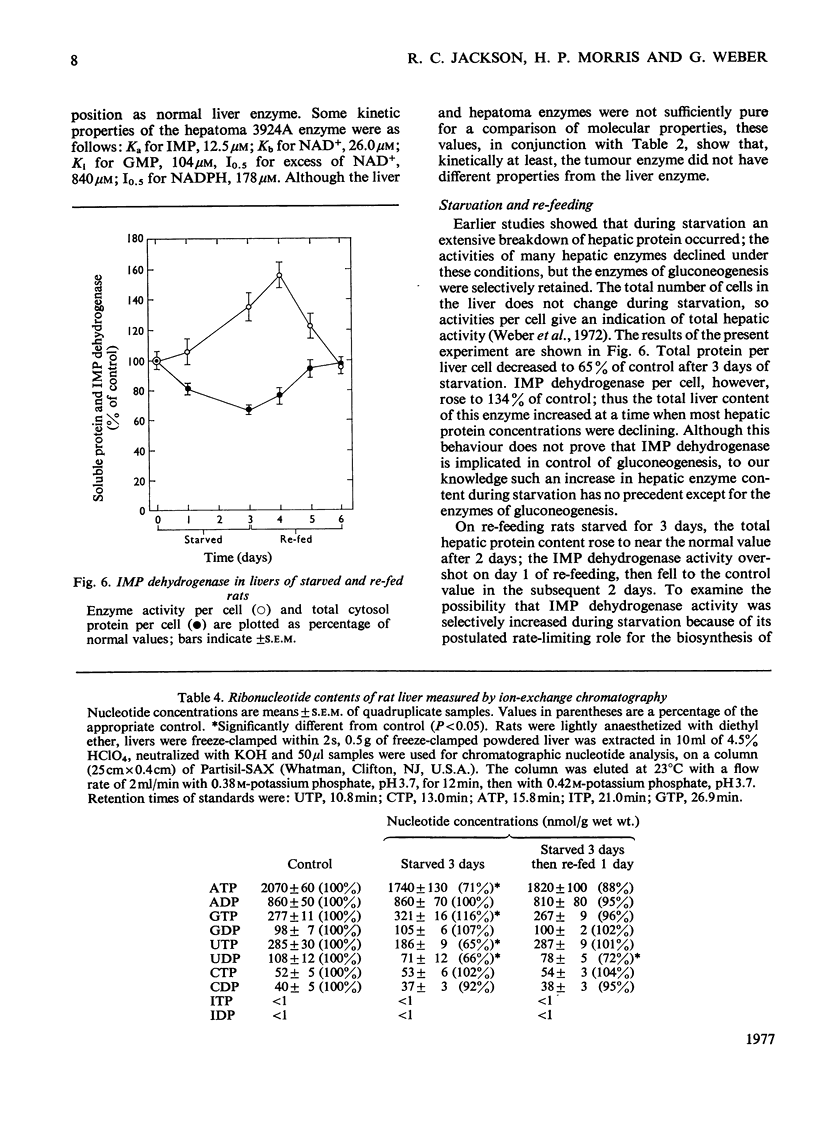
Abstract
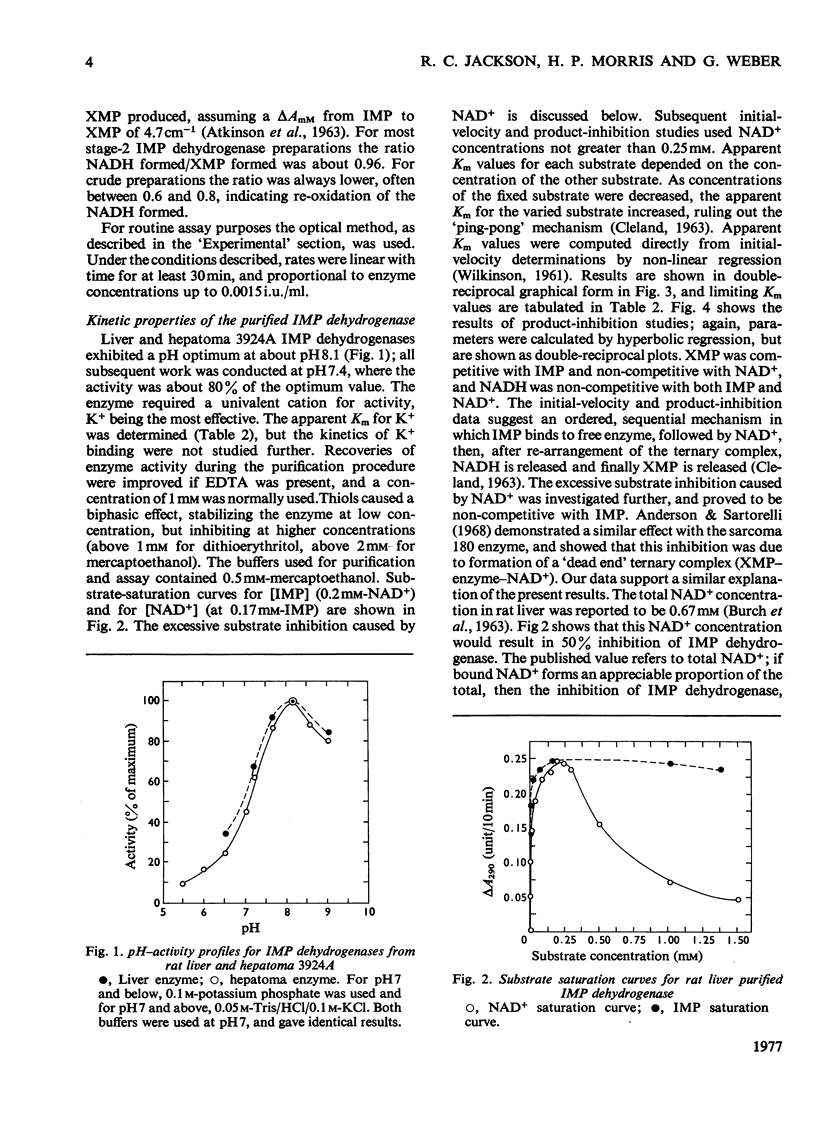
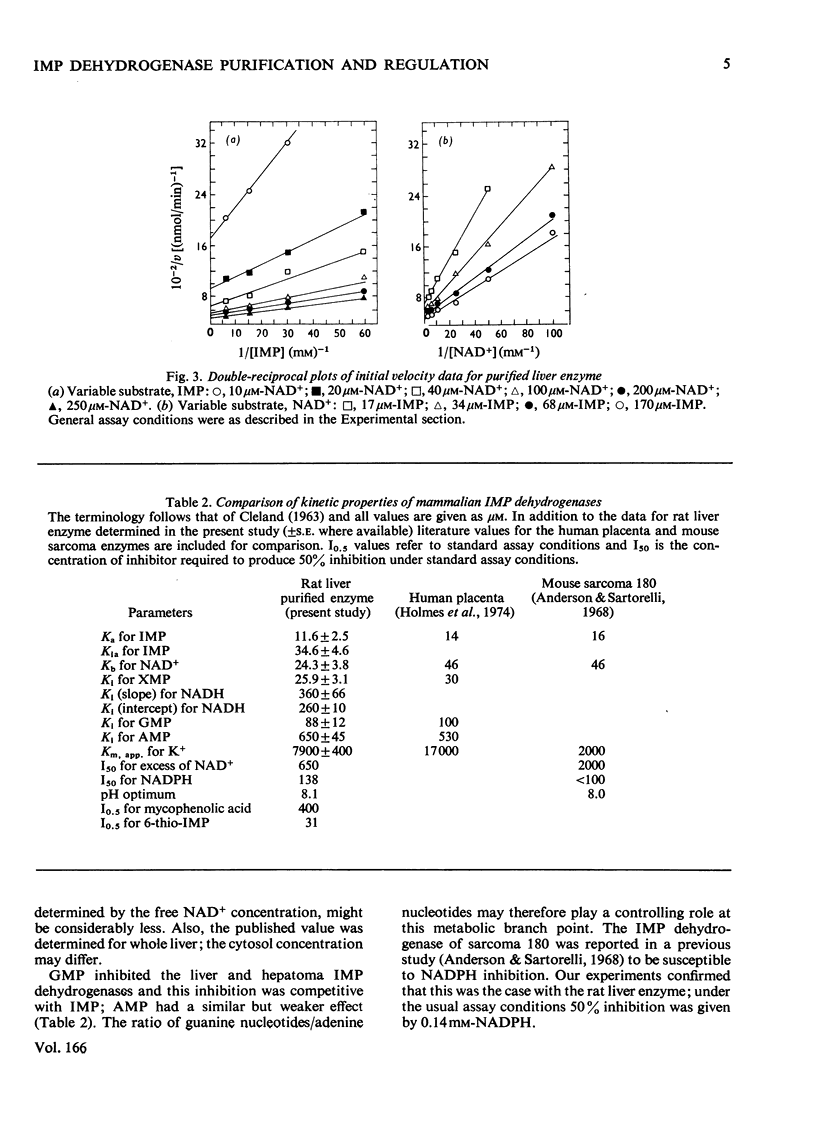
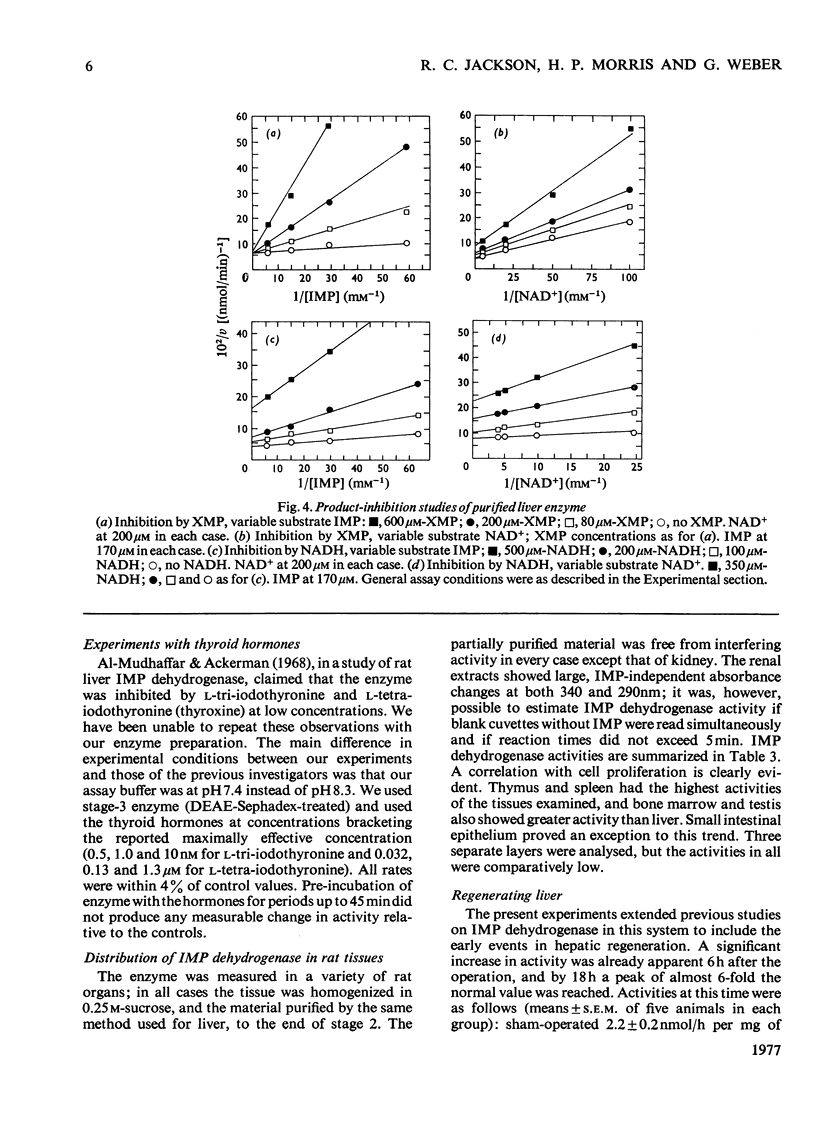
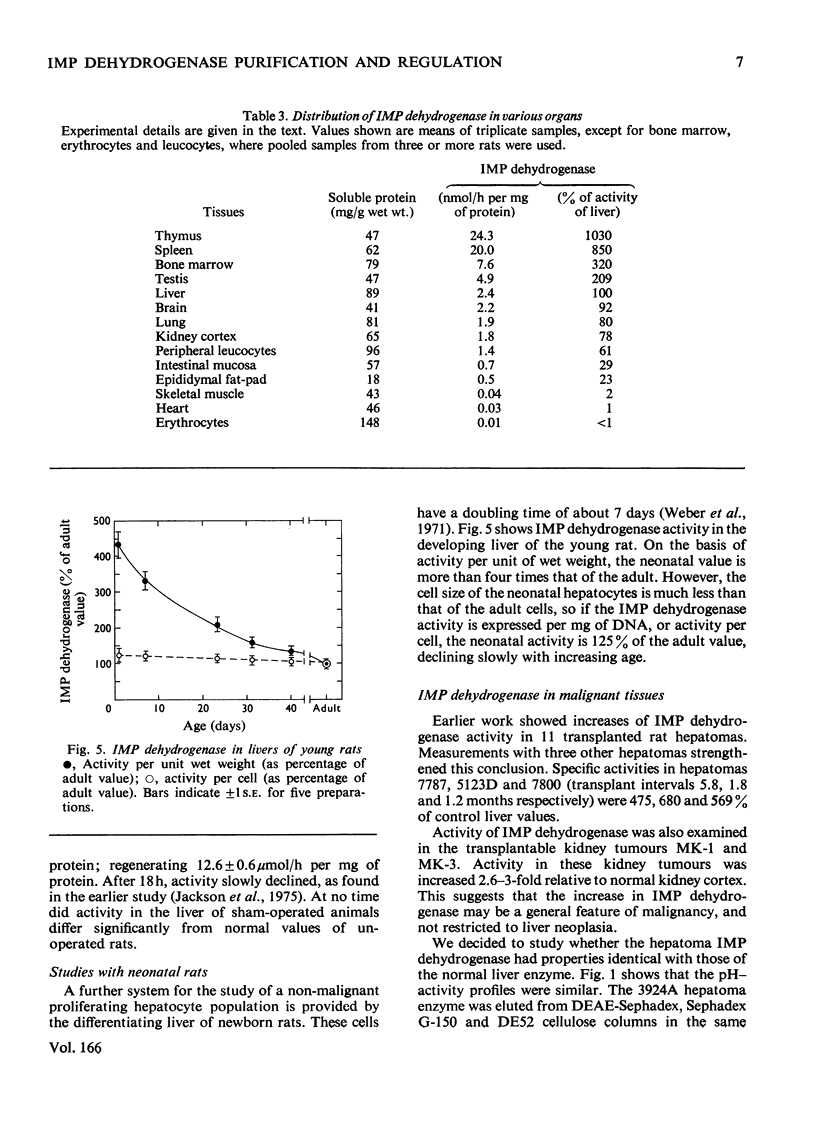
IMP dehydrogenase (EC 1.2.1.14) was purified 180-fold from rat liver and from the transplantable rat hepatoma 3924A. The enzymes from the two sources were apparently identical; they exhibited hyperbolic saturation kinetics and an ordered, sequential mechanism, and were subject to inhibition by a number of purine nucleotides. Km values for the substrates, IMP and NAD+, were 12 and 24 micrometer respectively. IMP dehydrogenase activity in a spectrum of rat hepatomas was increased, relative to normal liver, by 2.5--13-fold; these increases correlated with tumour growth rate. Activity in two rat kidney tumours was increased 3-fold relative to that in normal renal cortex; control of activity of this enzyme is apparently altered in neoplastic cells. After partial hepatectomy, IMP dehydrogenase activity began to rise 6 h after operation, reaching a peak of 580% of normal activity by 18 h. Activity in neonatal liver, however, was only slightly higher than that in the adult. Organ-distribution studies showed highest enzyme activities in spleen and thymus. In livers of rats starved for 3 days, where all enzymes, except those involved in gluconeogenesis, showed decreased activity IMP dehydrogenase activity was increased; this change was accompanied by a rise in hepatic GTP concentrations. It is concluded that IMP dehydrogenase is a key enzyme in the regulation of GTP production, and thus involved in regulation of nucleic acid biosynthesis. The increased activity of IMP dehydrogenase in liver of starved rats may be related to the requirements for GTP for gluconeogenesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS R., BENTLEY M. Biosynthesis of nucleic acid purines. II. Role of hypoxanthine and xanthine compounds. Arch Biochem Biophys. 1955 Sep;58(1):109–118. doi: 10.1016/0003-9861(55)90098-9. [DOI] [PubMed] [Google Scholar]
- ATKINSON M. R., MORTON R. K., MURRAY A. W. INHIBITION OF INOSINE 5'-PHOSPHATE DEHYDROGENASE FROM EHRLICH ASCITES-TUMOUR CELLS BY 6-THIONINOSINE 5'-PHOSPHATE. Biochem J. 1963 Oct;89:167–172. doi: 10.1042/bj0890167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. H., Sartorelli A. C. Inhibition of inosinic acid dehydrogenase by 6-chloropurine nucleotide. Biochem Pharmacol. 1969 Oct;18(10):2735–2745. [PubMed] [Google Scholar]
- Anderson J. H., Sartorelli A. C. Inosinic acid dehydrogenase of sarcoma 180 cells. J Biol Chem. 1968 Sep 25;243(18):4762–4768. [PubMed] [Google Scholar]
- Atkinson M. R., Morton R. K., Murray A. W. Inhibition of adenylosuccinate synthetase and adenylosuccinate lyase from Ehrlich ascites-tumour cells by 6-thioinosine 5'-phosphate. Biochem J. 1964 Aug;92(2):398–404. doi: 10.1042/bj0920398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURCH H. B., LOWRY O. H., VONDIPPE P. THE STABILITY OF TRIPHOSPHOPYRIDINE NUCLEOTIDE AND ITS REDUCED FORM IN RAT LIVER. J Biol Chem. 1963 Aug;238:2838–2842. [PubMed] [Google Scholar]
- Buzzee D. H., Levin A. P. Demonstration of an effector site for the enzyme inosine 5'-phosphate dehydrogenase. Biochem Biophys Res Commun. 1968 Mar 27;30(6):673–677. doi: 10.1016/0006-291x(68)90565-2. [DOI] [PubMed] [Google Scholar]
- CLELAND W. W. The kinetics of enzyme-catalyzed reactions with two or more substrates or products. I. Nomenclature and rate equations. Biochim Biophys Acta. 1963 Jan 8;67:104–137. doi: 10.1016/0006-3002(63)91800-6. [DOI] [PubMed] [Google Scholar]
- DE LAMIRANDE G., ALLARD C., CANTERO A. Purine-metabolizing enzymes in normal rat liver and Novikoff hepatoma. Cancer Res. 1958 Sep;18(8 Pt 1):952–958. [PubMed] [Google Scholar]
- Ferdinandus J. A., Morris H. P., Weber G. Behavior of opposing pathways of thymidine utilization in differentiating, regenerating, and neoplastic liver. Cancer Res. 1971 May;31(5):550–556. [PubMed] [Google Scholar]
- Holmes E. W., Pehlke D. M., Kelley W. N. Human IMP dehydrogenase. Kinetics and regulatory properties. Biochim Biophys Acta. 1974 Oct 17;364(2):209–217. doi: 10.1016/0005-2744(74)90006-0. [DOI] [PubMed] [Google Scholar]
- Ishii K., Shiio I. Regulation of purine ribonucleotide synthesis by end product inhibition. I. Effect of purine nucleotides on inosine-5'-phosphate dehydrogenase, xanthosine-5'-phosphate aminase and adenylosuccinate lyase of Bacillus subtilis. J Biochem. 1968 May;63(5):661–669. doi: 10.1093/oxfordjournals.jbchem.a128826. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Morris H. P., Weber G. Increased adenylosuccinase activity in hepatomas and kidney tumors. Life Sci. 1976 May 15;18(10):1043–1048. doi: 10.1016/0024-3205(76)90136-3. [DOI] [PubMed] [Google Scholar]
- Jackson R. C., Weber G., Morris H. P. IMP dehydrogenase, an enzyme linked with proliferation and malignancy. Nature. 1975 Jul 24;256(5515):331–333. doi: 10.1038/256331a0. [DOI] [PubMed] [Google Scholar]
- LAGERKVIST U. Biosynthesis of guanosine 5'-phosphate. I. Xanthosine 5'-phosphate as an intermediate. J Biol Chem. 1958 Jul;233(1):138–142. [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- Nijkamp H. J. Regulatory role of adenine nucleotides in the biosynthesis of guanosine 5'-monophosphate. J Bacteriol. 1969 Nov;100(2):585–593. doi: 10.1128/jb.100.2.585-593.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehlke D. M., McDonald J. A., Holmes E. W., Kelley W. N. Inosinic acid dehydrogenase activity in the Lesch-Nyhan syndrome. J Clin Invest. 1972 Jun;51(6):1398–1404. doi: 10.1172/JCI106935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquie J. Regulation of the biosynthesis of purine nucleotides in Schizosaccharomyces pombe. II. Kinetic studies of IMP dehydrogenase. Biochim Biophys Acta. 1969;185(2):310–315. doi: 10.1016/0005-2744(69)90424-0. [DOI] [PubMed] [Google Scholar]
- Prajda N., Katunuma N., Morris H. P., Weber G. Imbalance of purine metabolism in hepatomas of different growth rates as expressed in behavior of glutamine-phosphoribosylpyrophosphate amidotransferase (amidophosphoribosyltransferase, EC 2.4.2.14). Cancer Res. 1975 Nov;35(11 Pt 1):3061–3068. [PubMed] [Google Scholar]
- Prajda N., Morris H. P., Weber G. Imbalance of purine metabolism in hepatomas of different growth rates as expressed in behavior of xanthine oxidase (EC 1.2.3.2). Cancer Res. 1976 Dec;36(12):4639–4646. [PubMed] [Google Scholar]
- REID E., LEWIN I. Adenosine deaminase, nucleoside phorphorylase and xanthine oxidase in liver tumours. Br J Cancer. 1957 Sep;11(3):494–498. doi: 10.1038/bjc.1957.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKOOG W. A., BECK W. S. Studies on the fibrinogen, dextran and phytohemagglutinin methods of isolating leukocytes. Blood. 1956 May;11(5):436–454. [PubMed] [Google Scholar]
- Saccoccia P. A., Jr, Miech R. P. Inosinic acid dehydrogenase in mammalian tissues. Mol Pharmacol. 1969 Jan;5(1):26–29. [PubMed] [Google Scholar]
- Sweeney M. J., Hoffman D. H., Esterman M. A. Metabolism and biochemistry of mycophenolic acid. Cancer Res. 1972 Sep;32(9):1803–1809. [PubMed] [Google Scholar]
- WALFORD R. L., PETERSON E. T., DOYLE P. Leukocyte antibodies in human sera and in immune rabbit sera. Blood. 1957 Nov;12(11):953–971. [PubMed] [Google Scholar]
- WEBER G., CANTERO A. Studies on hormonal factors influencing hepatic glucose-6-phosphatase. Endocrinology. 1957 Dec;61(6):701–712. doi: 10.1210/endo-61-6-701. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber G., Ferdinandus J. A., Queener S. F., Dunaway G. A., Jr, Trahan L. J. Metabolic imbalance in carbohydrate, pyrimidine and ornithine utilization. Adv Enzyme Regul. 1972;10:39–62. doi: 10.1016/0065-2571(72)90005-2. [DOI] [PubMed] [Google Scholar]
- Weber G., Queener S. F., Ferdinandus J. A. Control of gene expression in carbohydrate, pyrimidine and DNA metabolism. Adv Enzyme Regul. 1970;9:63–95. doi: 10.1016/s0065-2571(71)80038-9. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Mudhaffar S., Ackerman C. J. Inhibition of inosine monophosphate-dehydrogenase by thyroid hormones in vitro. Endocrinology. 1968 May;82(5):912–918. doi: 10.1210/endo-82-5-912. [DOI] [PubMed] [Google Scholar]


