Abstract
The adaptive immune system exhibits exquisite specificity and memory and is involved in virtually every process in the human body. Redirecting adaptive immune cells, in particular T cells, to desired targets has the potential to lead to the creation of powerful cell-based therapies for a wide range of maladies. While conventional effector T cells (Teff) would be targeted towards cells to be eliminated, such as cancer cells, immunosuppressive regulatory T cells (Treg) would be directed towards tissues to be protected, such as transplanted organs. Chimeric antigen receptors (CAR) are designer molecules comprising an extracellular recognition domain and an intracellular signaling domain that drives full T cell activation directly downstream of target binding. Here, we describe procedures to generate and evaluate human CAR CD4+ helper T cells, CD8+ cytotoxic T cells, and CD4+FOXP3+ regulatory T cells.
Keywords: T cell, regulatory T cell, chimeric antigen receptor, synthetic immunology, immune assay
1. Introduction
Our adaptive immune system has evolved to specifically recognize a virtually infinite variety of molecules (antigens). This allows it to distinguish pathogens (non-self) and cancerous cells (altered self) from our own tissues (self) and symbiotic microbiota (extended self). T cells are at the heart of this recognition system, with each T cell bearing a T cell receptor (TCR) specific for a unique peptide epitope presented on a major histocompatibility complex (MHC) molecule. Cytotoxic CD8+ T cells are restricted to MHC class I molecules, whereas helper CD4+ T cells are restricted to MHC class II molecules [1]. It is thus highly desirable to identify, isolate, and expand antigen-specific T cells for therapy. Yet, these are vanishingly rare. Moreover, disease epitopes are often unknown, and it remains extremely challenging to predict TCR-epitope pairs based on amino acid sequence alone [2].
Synthetic biology can be used to impart a desired specificity to T cells, partly circumventing the hurdles indicated above and greatly expanding what targets can be pursued using T cell-based therapies. Chimeric antigen receptors (CARs) are synthetic immune receptors consisting of an extracellular antibody-based antigen-binding domain, known as a single-chain fragment variable (scFv), and an intracellular signaling domain. By combining T cell signal 1 (TCR recognition, typically CD3zeta) and signal 2 (co-stimulation, typically CD28) in its endomain, second-generation CARs elicit potent T cell activation directly downstream of antigen recognition [3].
CAR T cells have changed the landscape of cancer therapy, inducing high rates of remission in previously incurable cancers. Currently, there are five CAR T cell therapies for cancer approved by the U.S. Food and Drug Administration (FDA). Nevertheless, most of these are CAR T cells specific for CD19, a B cell-derived leukemia marker, and all of these are for liquid tumors. Solid tumors remain refractory to CAR T cell therapy and the search for effective targets is object of intense investigation [4]. Still, the success of CD19 CAR T cell therapies has prompted researchers to look beyond cancer, creating CAR T cells that target other undesirable cells for destruction, such as senescent cells [5] and activated fibroblasts during acute cardiac injury [6]. Moreover, it has kindled interest in engineering other immune cells with CARs, including natural killer (NK) cells [7], macrophages [8], and regulatory T cells (Tregs) [9]. A subset of CD4+ T cells dedicated to suppressing immune responses, CD4+FOXP3+ Tregs are essential to maintain immune tolerance and homeostasis. Manipulating Treg specificity with CAR technology offers the opportunity to modulate the immune system with antigen specificity in organ transplant rejection, autoimmunity, and inflammation [10]. Hence, CARs can be used both to redirect CD4+ helper T cells and CD8+ cytotoxic T cells to increase immunity in the context of cancer, and to redirect CD4+FOXP3+ Tregs to decrease immunity in the context of organ transplant rejection and autoimmune disease (Figure 1A).
Figure 1. Redirecting T cells using chimeric antigen receptors.
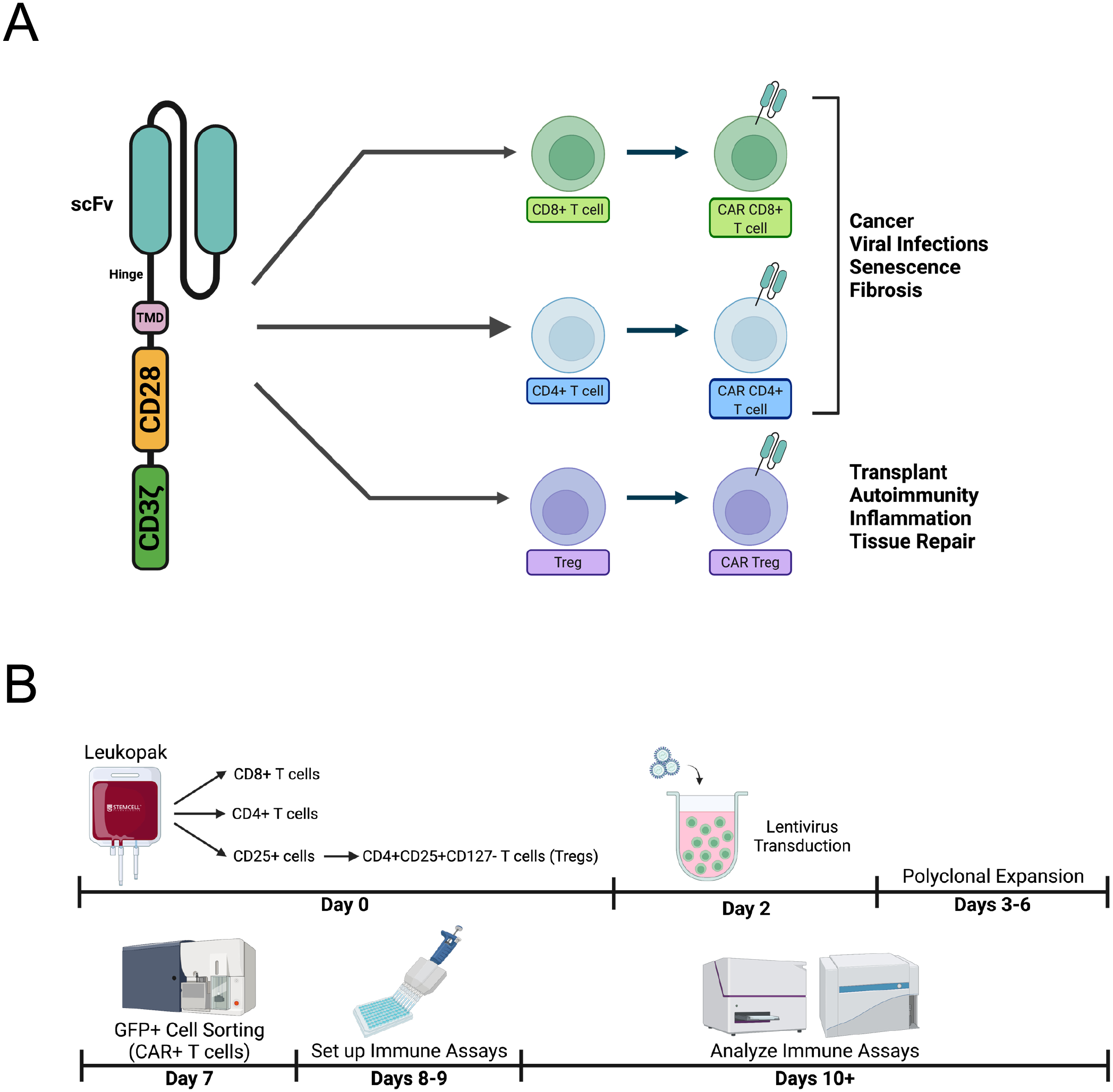
A. A chimeric antigen receptor (CAR) comprises an antigen recognition domain, typically a single chain fragment variable (scFv). This domain is connected to an intracellular signaling domain, typically a tandem CD28-CD3zeta domain, by a hinge and a transmembrane domain. Introducing a CAR in CD4+ T cells and/or CD8+ T cells can be used to combat cancer, viral infections, senescence, and fibrosis, whereas introducing a CAR in regulatory T cells (Tregs) can be used to prevent transplant rejection, treat autoimmunity and excessive inflammation, as well as aid tissue repair. B. The workflow of generating human CAR T cells involves purifying and activating T cell subsets from a human peripheral blood product, e.g. a leukopak (Day 0), transducing activated T cells with CAR lentivirus (Day 2), polyclonally expanding transduced cells (Days 3–6), sorting CAR+ T cells (e.g. by sorting cells expressing a CAR reporter gene, such as GFP) (Day 7), setting up immune assays, namely cytoxicity or suppression (Days 8–9), and finally analyzing such immune assays (Day 10 and beyond).
Altogether, much ongoing research is dedicated to creating and testing new CAR targets and CAR designs. CARs can be used to target T cells to specific tissues and disease states independently of endogenous antigens recognized by resident lymphocytes, which have often revealed difficult to identify, especially in humans. More broadly, the CAR platform has the power to help dissect how specificity, affinity, and signaling modulate the function of different T cells subsets in tolerance and immunity, ultimately informing the design and development of new engineered immune cell-based therapies for a variety of disorders. Given the increasing and broadening importance of engineering CAR T cell subsets, we provide detailed protocols to create and test human CAR CD4+ helper T cells, CD8+ cytotoxic T cells, and CD4+FOXP3+ regulatory T cells.
2. Materials
2.1. T cell isolation
1/10 Leukopak (STEMCELL Technologies #200-0092)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
RPMI 1640 medium, no glutamine (Gibco #11875093)
Fetal Bovine Serum (FBS) (Gibco #26140079)
Penicilin-Streptomycin solution (Gibco #15140122)
GlutaMAX (Gibco #35050061)
Sodium pyruvate (Gibco #11360070)
Non-essential amino acids (NEAA) solution (Gibco #11140050)
1M HEPES (Gibco #15630080)
Ammonium chloride solution (STEMCELL Technologies #07850)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
0.5M EDTA, pH 8.0 (Gibco #15575020)
EasySep magnet (STEMCELL Technologies #18000)
EasySep Human CD8+ T cell Enrichment Kit (STEMCELL Technologies #19053)
EasySep Human CD4+ T cell Enrichment Kit (STEMCELL Technologies #19052)
Easy 50 EasySep magnet (STEMCELL Technologies #18002)
EasySep Human Pan-CD25 Positive Selection and Depletion Kit (STEMCELL Technologies #17861)
Anti-human CD4 FITC (clone SK3, Biolegend #344604)
Anti-human CD25 APC (clone BC96, Biolegend #302610)
Anti-human CD127 PE (clone hIL-7R-M21, BD Biosciences #557938)
Anti-human CD8 PerCP (clone SK1, Biolegend #344707)
Flow cytometer (e.g. Beckman CytoFLEX)
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
2.2. Lentivirus production
HEK293T cells (ATCC #CRL-3216)
Jurkat cells (ATCC #TIB-152)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
RPMI10 complete medium (see section 3.1)
DMEM, high glucose, no glutamine medium (Gibco #11960044)
Fetal Bovine Serum (FBS) (Gibco #26140079)
GlutaMAX (Gibco #35050061)
Sodium pyruvate (Gibco #11360070)
Non-essential amino acids (NEAA) solution (Gibco #11140050)
Trypsin-EDTA (0.25%) (Gibco #25200056)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
Chloroquine diphosphate (Fisher Scientific #AAJ6445914)
Polyethylenimine (PEI), linear (Sigma-Aldrich #919012-100MG)
Nuclease-free water (New England Biolabs #B1500L)
Opti-MEM reduced serum medium (Gibco #31985070)
Packaging DNA plasmid (e.g. psPAX2, Addgene #12260)
Envelope DNA plasmid (e.g. VSV-G, Addgene #8454)
Transfer DNA plasmid (e.g. CD19CAR-2A-GFP, Addgene #135991)
ViralBoost (ALSTEM #VB100)
Flow cytometer (e.g. Beckman CytoFLEX)
2.3. T cell transduction
T cells isolated and activated in section 3.1
Lentivirus produced and titrated in section 3.2
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
RPMI10 complete medium (see section 3.1)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
Flow cytometer (e.g. Beckman CytoFLEX)
2.4. CAR+ T cell sorting
T cells transduced in section 3.3
RPMI10 complete medium (see section 3.1)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
FACS sorter (e.g. BD FACS Aria II Cell Sorter)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
2.5. Immune assays
2.5.1. Activation
CAR+ T cells sorted in section 3.4
K562 cells (ATCC #CCL-243)
CAR target-expressing K562 cells (e.g. CD19-K562)
Cesium-137 irradiator
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
RPMI10 complete medium (see section 3.1)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
Anti-human CD4 PE/Cy7 (clone SK3, Biolegend #344612)
Anti-human CD8 PerCP (clone SK1, Biolegend #344708)
Ghost BV510 viability dye (TONBO #13-0870-T100)
Anti-human CD71 PE (clone SK1, Biolegend #334106)
Anti-human CD25 APC (clone BC96, Biolegend #302610)
Flow Cytometer (e.g. Beckman CytoFLEX)
Human IFN-γ Quantikine ELISA kit (R&D Systems #DIF50C)
Human IL-10 Quantikine ELISA kit (R&D Systems #D1000B)
Microplate reader (e.g. Molecular Devices Spectramax)
2.5.2. Cytotoxicity
CAR+ T cells sorted in section 3.4
K562 cells (ATCC #CCL-243)
CAR target-expressing K562 cells (e.g. CD19-K562)
RPMI10 complete medium (see section 3.1)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
CyQUANT LDH Cytotoxicity Assay (ThermoFisher #C20300)
Microplate reader (e.g. Molecular Devices Spectramax)
2.5.3. Suppression
CAR+ Treg cells sorted in section 3.4
CAR target-expressing K562 cells (e.g. CD19-K562)
Cesium-137 irradiator
Unmodified CD4+ T cells from section 3.1
Unmodified CD8+ T cells from section 3.1
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
RPMI10 complete medium (see section 3.1)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
CellTrace Violet Proliferation Kit (ThermoFisher #C34571)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
Anti-human CD4 PE/Cy7 (clone SK3, Biolegend #344612)
Anti-human CD8 PerCP (clone SK1, Biolegend #344708)
Flow Cytometer (e.g. Beckman CytoFLEX)
2.5.4. Expansion
CAR+ T cells sorted in section 3.4
CAR target-expressing K562 cells (e.g. CD19-K562)
Cesium-137 irradiator
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
RPMI10 complete medium (see section 3.1)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
Anti-human CD4 PE/Cy7 (clone SK3, Biolegend #344612)
Anti-human CD8 PerCP (clone SK1, Biolegend #344708)
Ghost BV510 viability dye (TONBO #13-0870-T100)
Precision Count Beads (Biolegend #424902)
Flow Cytometer (e.g. Beckman CytoFLEX)
2.5.5. Exhaustion
CAR+ T cells sorted in section 3.4
CAR target-expressing K562 cells (e.g. CD19-K562)
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
RPMI10 complete medium (see section 3.1)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
Anti-human CD4 Alexa Fluor 700 (clone SK3, Biolegend #344622)
Anti-human CD8 APC/Cy7 (clone SK1, Biolegend #344714)
Anti-human PD-1 PerCP (clone EH12.2H7, Biolegend #329938)
Ghost BV510 viability dye (TONBO #13-0870-T100)
Flow Cytometer (e.g. Beckman CytoFLEX)
2.5.6. Stability
CAR+ Treg cells sorted in section 3.4
CAR target-expressing K562 cells (e.g. CD19-K562)
Cesium-137 irradiator
Human CD3/28 T Cell Expansion and Activation Dynabeads (Gibco #11131D)
RPMI10 complete medium (see section 3.1)
Recombinant human interleukin-2 (rhIL-2) (Peprotech #200-02)
DynaMag-15 magnet (ThermoFisher #12301D)
Trypan Blue solution (Sigma #T8154-100ML)
Cell counter (TC20 Automated Cell Counter, Bio-Rad #1450102)
Cell Counting Slides (Bio-Rad #1450016)
Ca2+ and Mg2+ free Dulbecco’s Phosphate Buffered Saline (DPBS) (Gibco #14190144)
eBioscience Foxp3 transcription factor staining buffer set (ThermoFisher #00-5523-00)
Anti-human CD4 PE/Cy7 (clone SK3, Biolegend #344612)
Anti-human CD25 APC (clone BC96, Biolegend #302610)
Ghost BV510 viability dye (TONBO #13-0870-T100)
Anti-human FOXP3 eFluor 450 (clone PCH101, ThermoFisher #48-4776-42)
Anti-human HELIOS PE (clone 22F6, Biolegend #137216)
Anti-human CTLA-4 PerCP-e710 (clone 14D3, ThermoFisher #46-1529-42)
Flow Cytometer (e.g. Beckman CytoFLEX)
3. Methods
3.1. T cell isolation
Prior to generating chimeric antigen receptor (CAR) T cells via viral transduction of human T cells and testing them in immune assays, T cells must first be isolated from peripheral blood mononuclear cells (PBMCs) obtained from a leukopak (Figure 1B). Subsequently, these PBMCs are magnetically enrichment with various kits to isolate different T cell subsets, such as CD4+ (Figure 2A) and CD8+ T cells (Figure 2B). For the isolation of regulatory T cells (Tregs), their rarity (ca. 1% of PBMCs or 5% of CD4+ T cells) requires a magnetic enrichment step to obtain a cell population enriched in CD25+ cells, followed by a fluorescence-assisted cell sorting (FACS) step to isolate CD4+CD25+CD127− Tregs with over 95% purity (Figure 2C).
Figure 2. Purifying T cell subsets from human peripheral blood.
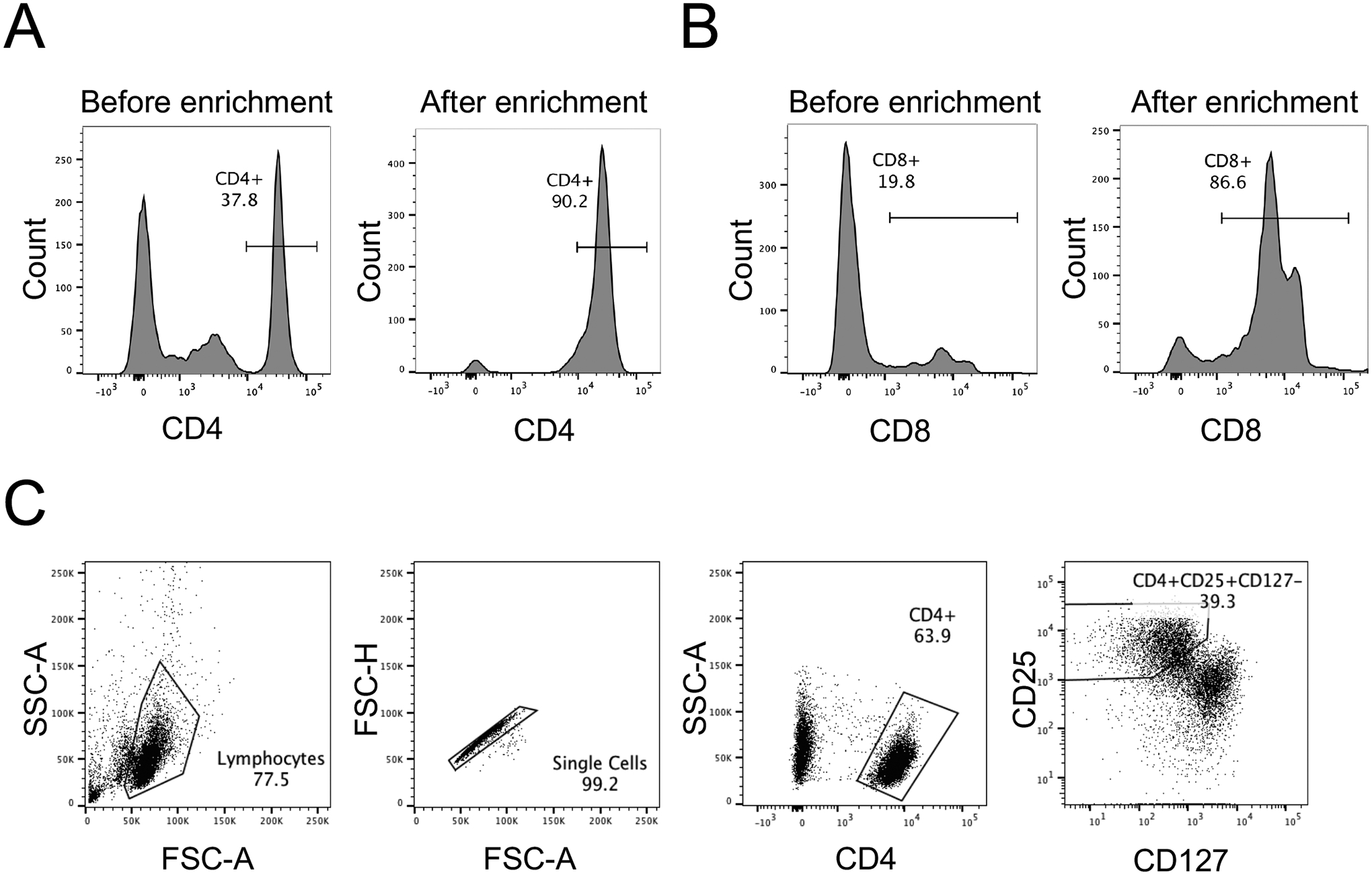
A. Purity of human CD4+ T cells after magnetic negative selection enrichment from a leukopak, as assessed by flow cytometry with anti-human CD4 PE/Cy7. B. Purity of human CD8+ T cells after magnetic negative selection enrichment from a leukopak, as assessed by flow cytometry with anti-human CD8 PerCP. C. Fluorescence-assisted cell sorting (FACS) gating strategy to purify human CD4+CD25+CD127− Treg cells after magnetic positive selection enrichment of CD25+ cells from a leukopak, followed by staining with anti-human CD4 FITC, anti-human CD25 APC, and anti-human CD127 PE.
3.1.1. Leukopak processing
Dilute the leukopak in an equivalent volume of DPBS + 2% FBS. Mix by pipetting up and down slowly.
Count cells by diluting them first 1:100 with DPBS (10 μl of cell suspension + 90 μl of DPBS, and then 10 μl of cells diluted 1:10 + 90 μl of DPBS) and then 1:1 with Trypan Blue solution (10 μl of cells diluted 1:100 + 10ul Trypan Blue). If using an automated cell counter, make sure to adjust cell count to this 200-fold cell dilution by multiplying the result by 100, as most cell counters assume a 1:1 dilution.
Centrifuge the cells at 300 g for 10 minutes at room temperature (RT) with the brake on.
Aspirate the supernatant, leaving some volume behind to avoid disturbing the cell pellet.
Resuspend the cell pellet, add 2 mL of DPBS + 2% FBS, and pipet up and down.
Add 8 mL of ammonium chloride solution to the cell suspension (4:1 ratio). Pipet up and down.
Incubate on ice for 15 minutes.
Centrifuge at 500 g for 10 minutes at RT with the brake on.
Aspirate the supernatant.
Wash by adding 30 mL DPBS + 2% FBS.
Centrifuge at 150 g for 10 minutes at RT with the brake off.
Aspirate the supernatant and resuspend the cell pellet in 30 mL DPBS + 2% FBS.
Count cells by diluting them first 1:100 with DPBS (10 μl of cell suspension + 90 μl of DPBS, and then 10 μl of cells diluted 1:10 + 90 μl of DPBS) and then 1:1 with Trypan Blue solution (10 μl of cells diluted 1:100 + 10ul Trypan Blue). If using an automated cell counter, make sure to adjust cell count to this 200-fold cell dilution by multiplying the result by 100, as most cell counters assume a 1:1 dilution.
3.1.2. CD4+ T cell enrichment (Negative Selection)
Collect 15×106 PBMCs and centrifuge at 500 g for 5 minutes at RT. The starting cell number will depend on your specific needs.
Resuspend the cells in Cell Separation Buffer (DPBS + 10 mM EDTA + 2% FBS) at 50×106 cells/ml.
Follow EasySep Human CD4+ T cell Enrichment Kit protocol.
Count cells in a 1:1 ratio with Trypan Blue.
Assess CD4+ T cell purity using flow cytometry (Figure 2A).
3.1.3. CD8+ T cell enrichment (Negative Selection)
Collect 50×106 PBMCs and centrifuge at 500 g for 5 minutes at RT. The starting cell number will depend on your specific needs.
Resuspend the cells in Cell Separation Buffer (DPBS + 10 mM EDTA + 2% FBS) at 50×106 cells/ml.
Follow EasySep Human CD8+ T cell Enrichment Kit protocol.
Count cells in a 1:1 ratio with Trypan Blue.
Assess CD8+ T cell purity using flow cytometry (Figure 2B).
3.1.4. CD25+ Cell enrichment (Positive Selection)
-
1.
Collect 109 PBMCs and centrifuge at 500 g for 5 minutes at RT. The starting cell number will depend on your specific needs.
Resuspend the cells in Cell Separation Buffer (DPBS + 10 mM EDTA + 2% FBS) at 108 cells/mL
Follow EasySep Human Pan-CD25 Positive Selection and Depletion Kit protocol.
Count CD25+ cells in a 1:1 ratio with Trypan Blue.
3.1.5. CD4+CD25+CD127− Treg sorting (FACS)
-
1.
Centrifuge CD25+ cells obtained in section 3.1.4 at 500 g for 5 minutes at RT.
Resuspend the cells in 200 μL of DPBS.
Add the following antibodies: anti-human CD4 FITC, anti-human CD25 APC, anti-human CD127 PE at 1 μL of antibody per 106 cells. Make sure to use the antibody clones indicated in the materials section (2.1), especially αCD25 (clone BC96), as the CD25+ cell fraction will have some of the CD25 epitopes blocked by the antibodies used in the EasySep Human Pan-CD25 Positive Selection and Depletion Kit.
Incubate at 4°C for 30 minutes in the dark.
Wash by adding 10 mL of DPBS, then centrifuge at 500 g for 5 minutes at RT.
Resuspend cells at 15×106 cells/ml in DPBS.
Filter cell suspension through a 40 μm filter cap into a FACS tube and put on ice.
Prepare collection tubes for sorting by adding 3 mL RPMI10 complete medium (Note 4.1) per 15 mL conical tube and put on ice. Collection tube type and complete medium volume will depend on the specific cell sorting instrument used.
Sort CD4+CD25+CD127− cells using fluorescence assisted cell sorting (FACS) (Figure 2C).
Count the cells post-sort in a 1:1 ratio with Trypan Blue.
3.1.6. T cell activation
After T cell subset purification and counting, activate T cells in a 24-well plate with Human T-Activator CD3/28 for T cell Expansion and Activation Dynabeads and recombinant human IL-2 (rhIL-2) in RPMI10 complete medium.
Add 25 μL of anti-CD3/CD28 dynabeads for every 106 T cells obtained for a 1:1 ratio of dynabeads to T cells.
- Add recombinant human IL-2:
- Add 1000 IU/ml of rhIL-2 to Treg cells
- Add 100 IU/ml of rhIL-2 to CD4+ T cells
- Add 300 IU/ml of rhIL-2 to CD8+ T cells
Plate T cells at 5×105 cells per well of a 24-well plate in 1 ml RPMI10 complete medium and rhIL-2 and put in a 37°C 5% CO2 tissue culture incubator (Note 4.2, Figure 3).
Figure 3. Activation of purified human T cell subsets.
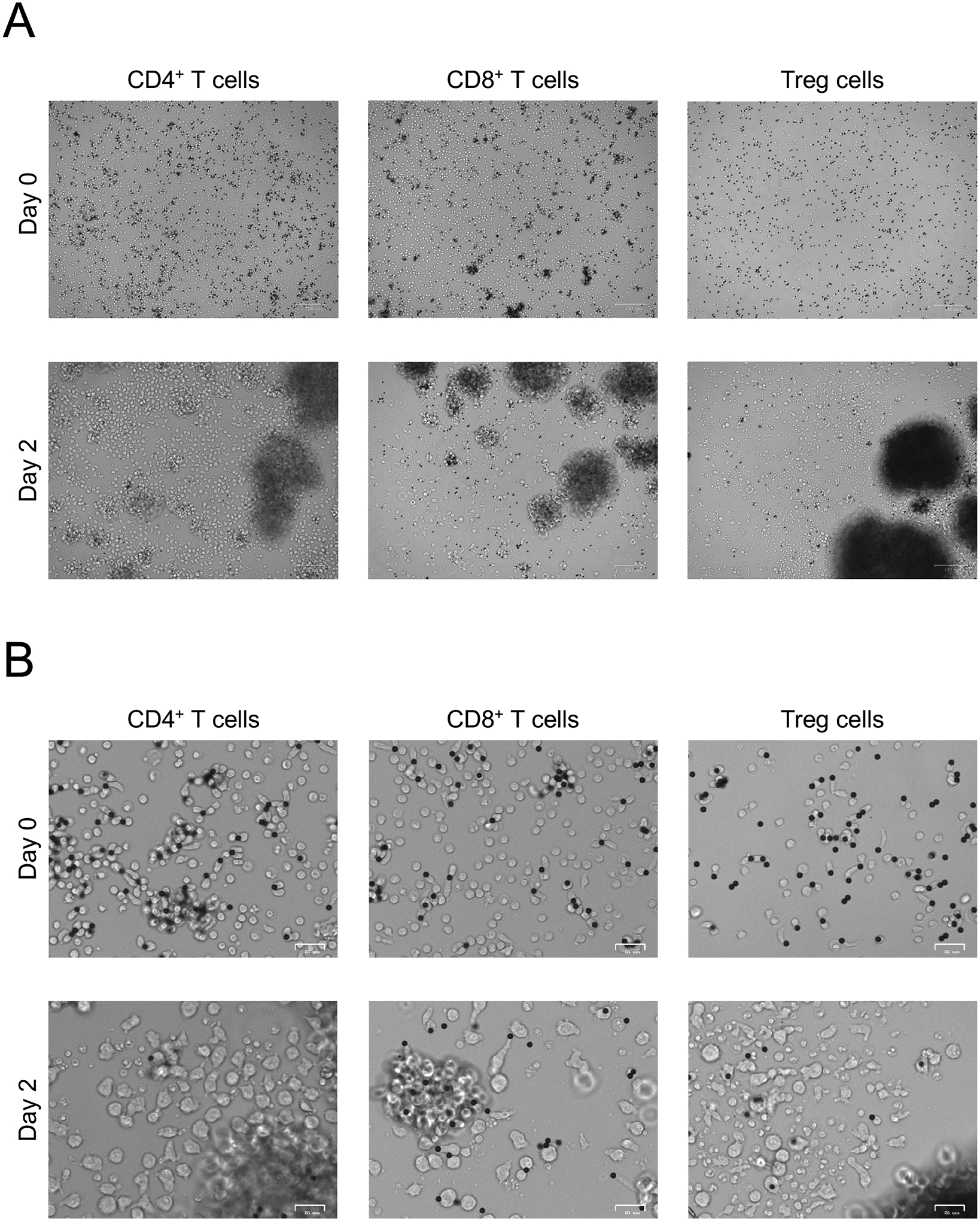
A. Anti-CD3/CD28 bead-mediated activation (1:1 bead to cell ratio) of human CD4+ T cells, CD8+ T cells, and Treg cells for 48h. Images at 5x magnification. B. Anti-CD3/CD28 bead-mediated activation of human CD4+ T cells, CD8+ T cells, and Treg cells for 48h. Images at 20x magnification.
3.2. Lentivirus production
Second generation lentivirus production employs transfection of human embryonic kidney 293T (HEK293T) cells with DNA plasmids encoding lentiviral packaging proteins, lentiviral envelope proteins, and the construct of interest, in this case a CAR. HEK293T cells are ideal to produce lentivirus as they are easily maintained in culture and transfected with very high efficiencies. Once the plasmid DNA is taken up by the cells, the HEK293T cellular machinery produces lentivirus particles containing the CAR gene and releases them into the culture medium (Figure 4A). The medium containing the lentivirus particles is then titrated in Jurkat cells and stored in aliquots at - 80°C for subsequent transduction of primary human T cells.
Figure 4. Lentivirus production and titration.
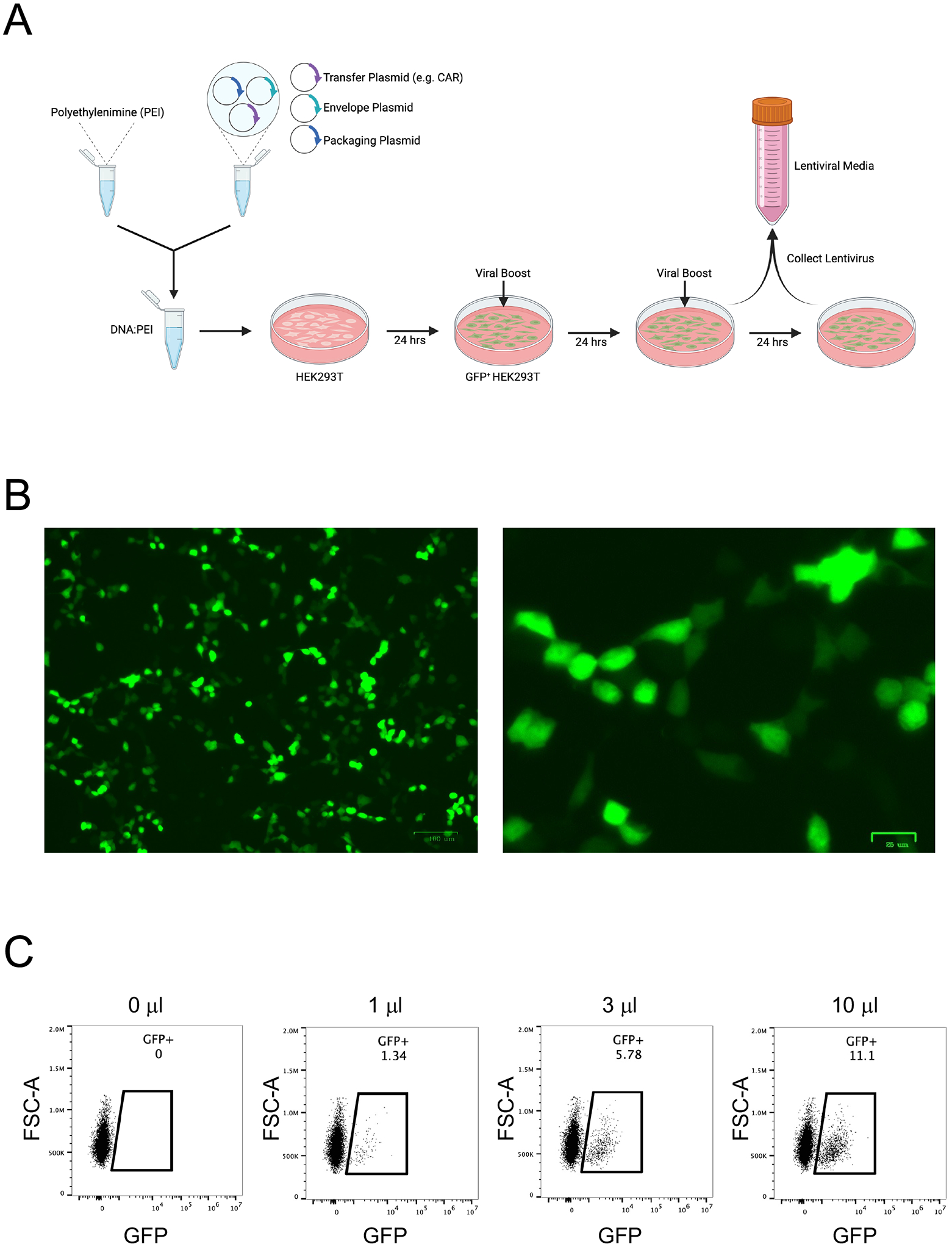
A. The workflow for lentivirus production involves transfecting chloroquine-treated HEK293T cells with transfer plasmid (CAR), envelope plasmid, and packaging plasmid using polyethylenimine (PEI). One day later, the medium is replaced with fresh medium containing ViralBoost. On the third and fourth days, lentivirus-containing medium is collected into conical tubes and stored at 4°C. B. GFP expression by HEK293T cells 18h after transfection with CD19CAR-2A-GFP accompanied by envelope plasmid and packaging plasmid, imaged at 5X (left) and 20X (right) magnification. C. Titration of CD19CAR-2A-GFP lentivirus particle-containing medium in Jurkat cells.
Day 0
Count HEK293T cells with Trypan Blue and seed at a density of 3.8 × 106 cells per 10 cm dish in DMEM10 complete medium. Cells should be expanded ahead of time and then split into the desired number of plates. The timeline of transfection is as follows:
Prepare stock solutions of chloroquine diphosphate and polyethylenimine (PEI) for transfection.
Dissolve 0.129g of chloroquine diphosphate salt in 10 mL of sterile nuclease-free water to prepare a 25mM chloroquine stock solution.
Sterilize the 25mM chloroquine stock solution using a 10 mL syringe and 0.22 μm filter. Aliquot and store at −20°C as needed.
Dissolve 0.1g of PEI in 90 mL of ultrapure water (via Milli-Q Water Purification System) in a 150 mL autoclaved beaker and stir using a magnetic stir bar/stir plate to prepare a 1 mg/ml PEI stock solution.
Adjust pH to 7.0 by progressively adding small volumes of either HCl (to decrease pH) or NaOH (to increase pH) while measuring the pH using a pH meter.
Sterilize the 1 mg/ml PEI pH 7.0 stock solution through a 0.22 μm filter using a 10 mL syringe. Aliquot and store at −80°C as needed.
Test chloroquine and PEI solutions on 6 × 105 of HEK293T cells in a 6-well with 1 μg of DNA to ensure they result in sufficient transfection efficiency prior to aliquoting/freezing the solutions. The ideal PEI:DNA ratio will be PEI batch dependent. In our hands, a 2:1 PEI:DNA mass ratio yielded the best results.
Day 1
Aspirate exhausted medium.
Add 10 mL fresh DMEM10 complete medium with 25 μM chloroquine (10 μL of 25mM chloroquine stock solution in 10 ml medium).
Incubate the plates at 37°C 10% CO2 in tissue culture incubator for 3–5 hours.
Thaw DNA plasmids and PEI stock solution aliquots on ice.
Calculate the volume needed of each DNA plasmid using a table as below. Fill out concentration and, subsequently, volume to add according to the specific plasmid DNA concentrations of the maxiprep DNA batches in use.
Dilute the required volumes of the three DNA plasmids needed per construct (total of 20 μg of DNA) in 500 μl Opti-MEM in a 1.5 ml microcentrifuge tube.
Dilute 40 μl of 1 mg/ml PEI in 500 μl Opti-MEM in a separate 1.5 ml microcentrifuge tube (for a 2:1 PEI:DNA ratio).
Close both tubes and gently flick to mix contents.
Briefly spin down both tubes to ensure contents are in the bottom of the tube.
Slowly add the diluted PEI to the diluted DNA tube dropwise.
Gently flick the combined tube to mix contents. Do not vortex to prevent shearing of the plasmid DNA.
Incubate the tube for 15 minutes at RT.
Retrieve the 10 cm plate with HEK293T cells to be transfected from the incubator.
Add PEI:DNA complexes dropwise to the HEK293T cells, covering the plate.
Place the transfected HEK293T cell plate back in the tissue culture incubator.
Day 2
24 hours after transfection, use the ZOE fluorescence cell imager to confirm transfection prior to changing medium. In this specific example, HEK293T will be GFP+ if successfully transfected (Figure 4B).
Aspirate exhausted medium and add 10 mL DMEM10 complete medium (Note 4.3) + 20 μL ViralBoost to maximize viral production.
Place the plate back in the tissue culture incubator.
Day 3
Collect lentivirus-containing medium from plate into a 50 mL conical tube. 48h post-transfection is the peak of lentivirus production.
Add 10 mL DMEM10 complete medium + 20 μL ViralBoost to maximize viral production.
Place the plate back in the tissue culture incubator.
Store the collected lentivirus-containing medium at 4°C.
Day 4
Collect lentivirus-containing medium into 50 mL conical tube from previous day.
Discard HEK293T cell plate.
Store the collected lentivirus-containing medium at 4°C.
Titration
Count Jurkat cells with Trypan Blue and seed at a density of 104 cells per well of a 96-well round-bottom plate in 200 μl RPMI10 complete medium.
Add 10, 3, 2, or 1 μL lentivirus-containing medium per well with 104 Jurkat cells. Make sure to leave wells with untransduced Jurkat cells as negative controls.
Place the plate with transduced Jurkat cells in tissue culture incubator for 72 hours.
Harvest cells and analyze via flow cytometry to measure transduction efficiency. In this specific case, determine % GFP+ cells in each condition (Figure 4C).
Use transduction efficiencies with different lentivirus-containing medium volumes to calculate the lentivirus titer, i.e. the number of lentivirus particles per volume of lentivirus-containing medium.
Perform a linear regression and extrapolate to determine volume required to transduce a desired number of T cells.
Centrifuge tubes with lentivirus-containing medium at 500 g for 5 min at 4°C.
Carefully transfer the lentivirus-containing supernatant to fresh conical tubes. This step eliminates cellular debris.
Aliquot lentivirus-containing medium and store at −80°C as needed. We transduce multiples of 2.5×105 T cells with CAR lentivirus for immune assays and hence make aliquots with the number of lentivirus particles needed to transduce 2.5×105 Jurkat cells.
3.3. T cell transduction
Count T cells with Trypan Blue.
Centrifuge cells at 500 g for 5 minutes at room temperature (RT).
Resuspend cells in RPMI10 complete medium at 1.25×106 cells/ml.
Thaw lentivirus aliquots on ice.
Add lentivirus aliquots to 2.5×105 T cells in 200 μl in a 1.5 ml microcentrifuge tube. CD4+ and CD8+ T cells are harder to transduce than Treg cells, so add 1 lentivirus aliquot per 2.5×105 Treg cells and 2 lentivirus aliquots per 2.5×105 CD4+ or CD8+ T cells.
Add rhIL-2 for a final concentration of 1000 IU/ml for Treg cells, 100 IU/ml for CD4+ T cells, and 300 IU/ml for CD8+ T cells.
Centrifuge at 1000 g for 1 hour at 32°C.
Transfer transduced T cells from 1.5 ml microcentrifuge tubes to a 24-well plate, each 1.5 microcentrifuge tube to one well.
Place the plate with transduced T cells in tissue culture incubator.
The following day, top up each well of the 24-well to 2 ml RPMI10 complete medium with rhIL-2 (1000 IU/ml for Treg cells, 100 IU/ml for CD4+ T cells, and 300 IU/ml for CD8+ T cells). Continue expanding transduced T cell cultures over time by splitting cells and adding fresh medium and rhIL-2 as needed.
3.4. CAR+ T cell sorting
Five days post transduction, count transduced T cells with Trypan Blue.
Centrifuge cells at 500 g for 5 minutes at room temperature (RT).
Resuspend cells at 15×106 cells/ml in DPBS.
Filter cell suspension through a 40 μm filter cap into a FACS tube and put on ice.
Prepare collection tubes for sorting by adding 3 mL RPMI10 complete medium per 15 mL conical tube and put on ice. Collection tube type and complete medium volume will depend on the specific cell sorting instrument used.
Sort CAR reporter-expressing cells, in this case GFP+, using FACS (Figure 5).
Count the cells post-sort in a 1:1 ratio with Trypan Blue.
Plate sorted CAR+ T cells at 5×105 cells per well of a 24-well plate in 1 ml RPMI10 complete medium and rhIL-2 and put in the tissue culture incubator. Continue expanding CAR+ T cell cultures over time by splitting cells and adding fresh RPMI10 complete medium and rhIL-2 as needed.
Figure 5. Purifying human CAR+ T cells.
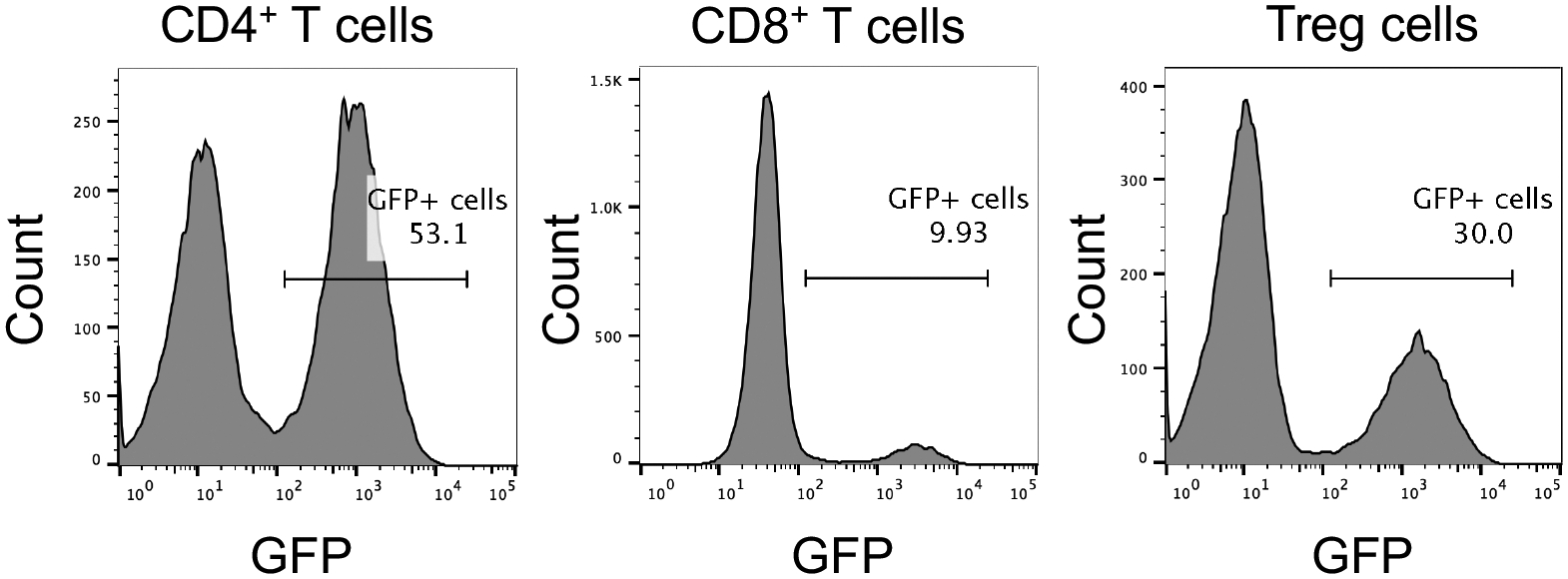
FACS sorting of CD19CAR-2A-GFP-expressing human CD4+ T cells, CD8+ T cells, and Treg cells one week post transduction.
3.5. Immune assays
Once CAR+ T cells have been generated, the time comes to submit them to a battery of in vitro immune assays to assess CAR functionality. Here, we describe protocols to measure CAR-mediated T cell activation, cytotoxicity, expansion, and exhaustion. In the specific case of CAR+ Treg cells, CAR Treg suppressive function and lineage stability are also assessed.
3.5.1. Activation
T cell activation via the TCR in response to its cognate antigen results in marked changes in T cell size, morphology, and proliferative patterns (Figure 3). Hence, the first step to demonstrate the functionality of a new CAR is to show that it leads to T cell activation in the presence of its target antigen. To do this, we generate stable K562 cell lines with the CAR target protein on their surface via lentiviral transduction (in this case, CD19-K562), irradiate them to inhibit their proliferation, and co-incubate them with CAR T cells (Figure 6A). K562 cells are an easy-to-maintain human myelogenous leukemia cell line devoid of MHC and co-stimulatory ligand (CD80 and CD86) expression, providing a blank canvas for CAR target expression. Anti-CD3/CD28 dynabeads are included as positive control for T cell activation. To quantify activation 48h post co-incubation, in addition to visual inspection of the CAR+ T cell and CAR target+ cell co-cultures, upregulation of the surface expression of the early activation markers CD25 and CD71 [11] is monitored by flow cytometry (Figure 6B), and IFN-γ (conventional CD4+ T cells) and IL-10 (Treg cells) levels are measured via enzyme-linked immunosorbent assay (ELISA) in the co-culture supernatants (Figure 7).
Figure 6. CAR-mediated activation of human CAR+ T cells.
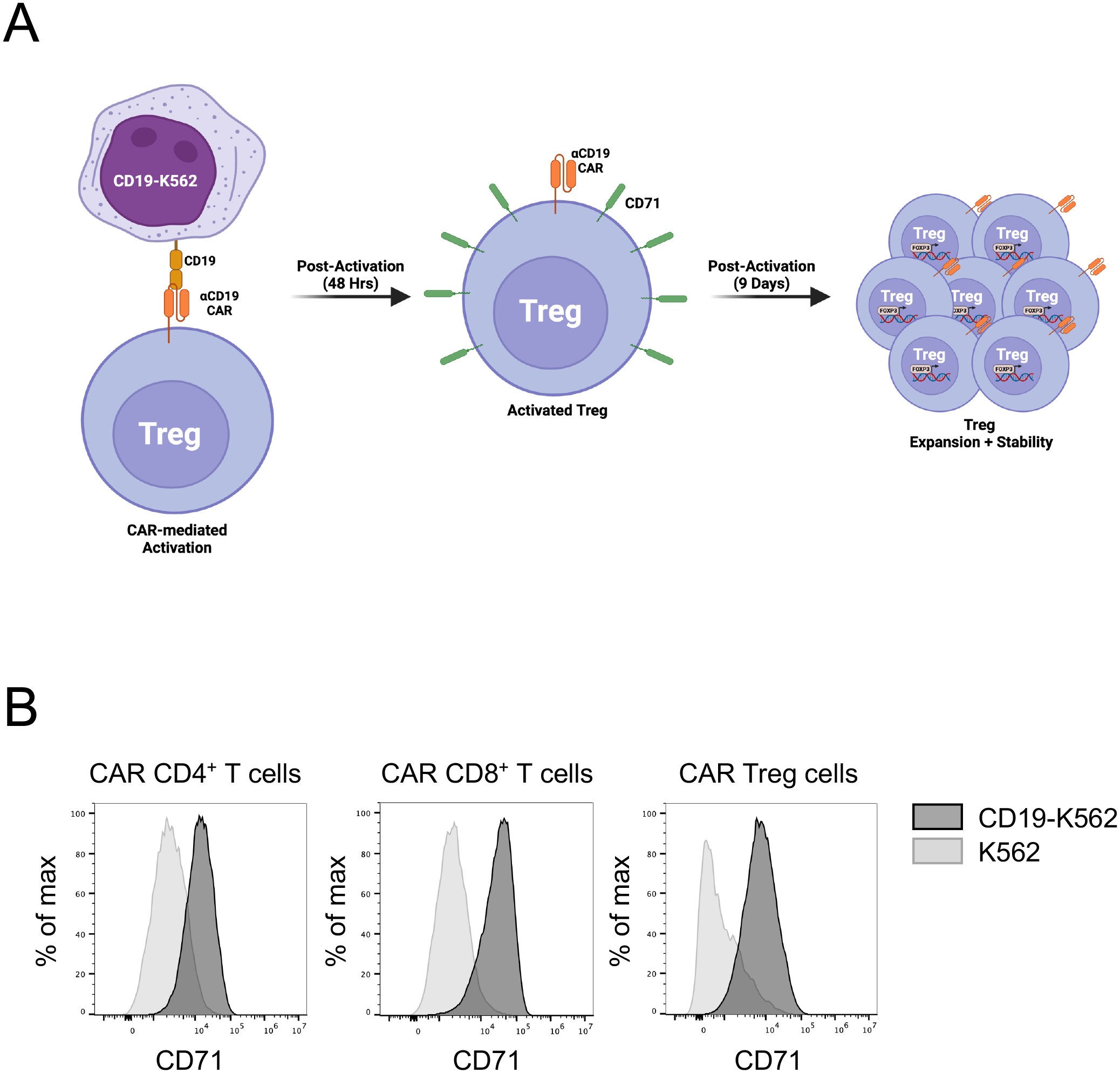
A. The workflow for evaluating CAR-mediated early activation of CAR T cells, e.g. CD19CAR Tregs, involves co-incubating CD19CAR Tregs with irradiated CD19-expressing K562 for 48h, followed by flow cytometry to detect upregulation of the activation marker CD71 (transferrin receptor). Continued expansion of the cells for 9 days allows for assessment of CAR-mediated expansion (CAR Treg cell number), as well as assess the phenotypic stability of the CAR Tregs (e.g. FOXP3 expression). B. CD19CAR-mediated activation of human CD4+ T cells, CD8+ T cells, and Treg cells after 48h co-incubation with either irradiated K562 or CD199-K562, as assessed by flow cytometry with anti-human CD71 FITC. Histograms represent CD71 surface expression of live CD4+GFP+ cells.
Figure 7. Cytokine secretion by activated human CAR+ T cells.
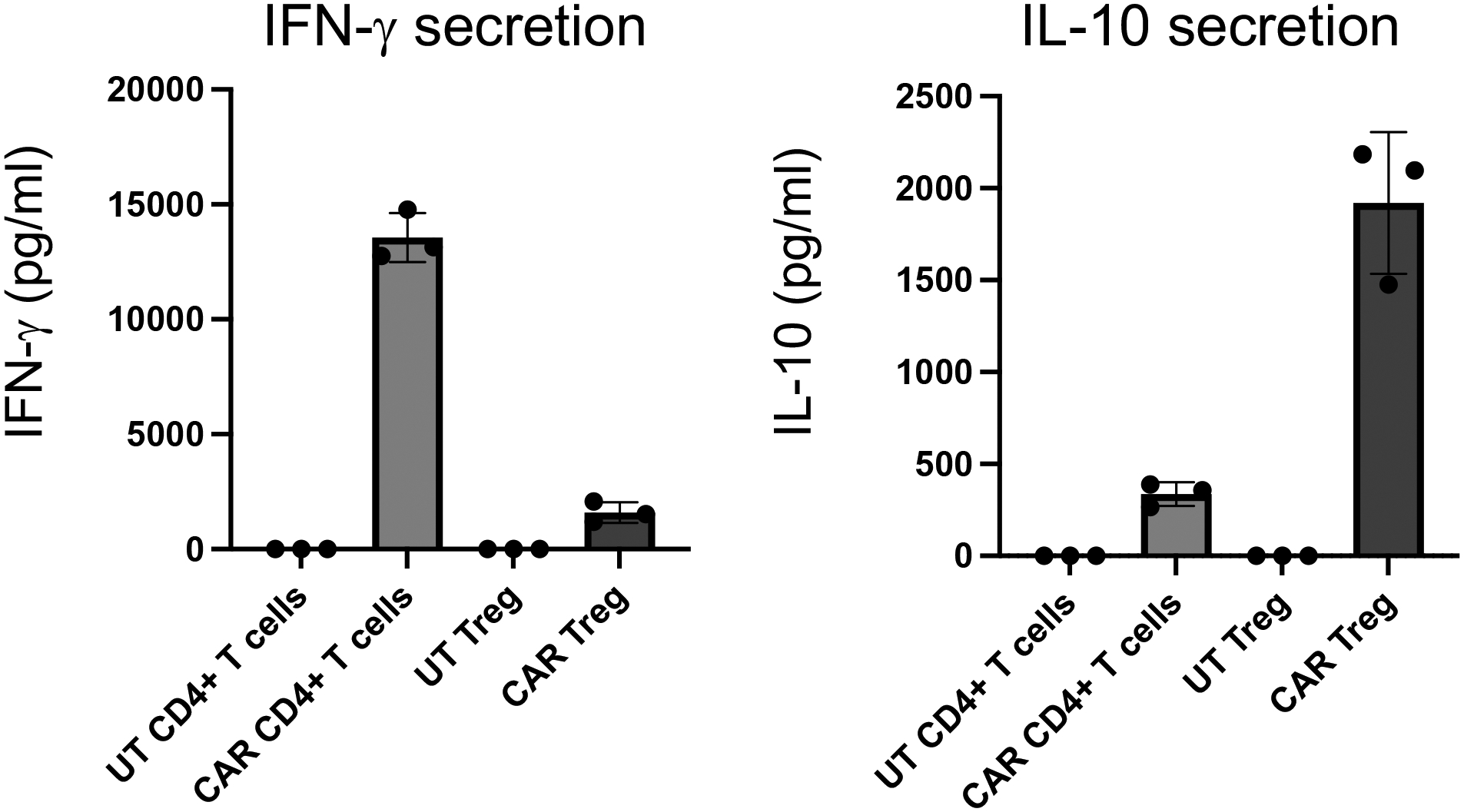
IFN-γ and IL-10 secretion by either untransduced (UT) or CD19CAR-expressing human CD4+ T cells and Treg cells activated by irradiated CD19-K562 cells for 48h. Cytokine levels were measured in the co-culture supernatants using enzyme-linked immunosorbent assay (ELISA).
Day 0 – Co-culture plate setup
Collect K562 cell lines (e.g. parental K562 and CD19-K562) into conical tubes.
Irradiate K562 cell lines with 4000 rad in Cesium-137 irradiator.
Collect CAR+ T cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded CAR+ T cell suspension into a new 15 ml conical tube.
Count irradiated K562 cells and debeaded CAR+ T cells with Trypan Blue.
Centrifuge K562 cells and CAR+ T cells at 500 g for 5 minutes at RT.
Resuspend cells in RPMI10 complete medium at 106 cells/mL.
If working with CAR Tregs, add rhIL-2 for a final concentration of 1000 IU/mL. Do not add rhIL-2 if working with either CAR CD4+ or CAR CD8+ T cells.
Co-incubate 1×105 CAR+ T cells with 1×105 parental K562 cells, CAR-antigen expressing K562 cells, or anti-CD3/CD28 dynabeads (2.5 μL) in triplicates in a 96-well round bottom plate (1:1 ratio).
Incubate plate for 48 hours in the tissue culture incubator.
Day 2 – Antibody staining and flow cytometry
Resuspend and transfer the contents of each 96-well round bottom plate well into a labeled 1.5 ml microcentrifuge tube.
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare antibody master mix with DPBS, anti-human CD4 PE/Cy7 1:200, anti-human CD8 PerCP 1:100, anti-human CD25 APC 1:100, anti-human CD71 PE 1:100, and Ghost Viability Dye BV510 1:500. Each sample will require 50 μl of antibody master mix. Hence, if staining 10 samples as an example, add 2.5 μl anti-human CD4 PE/Cy7, 5 μl anti-human CD8 PerCP, 5 μl anti-human CD25 APC, 5 μl anti-human CD71 PE, and 1 μl Ghost Viability Dye BV510 to 0.5 ml DPBS in a 1.5 ml microcentrifuge tube and put on ice in the dark.
Carefully collect supernatant from centrifuged microcentrifuge tubes into new labeled microcentrifuge with a micropipette without disturbing the cell pellet and store at 4°C if analyzing on the same day or at −20°C for long term storage.
Briefly vortex cell pellet.
Add 50 μL of antibody master mix into each microcentrifuge tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
Wash by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Resuspend the cell pellet in 300 μL of PBS.
Transfer to FACS tubes and put on ice in the dark.
Analyze by flow cytometry (Note 4.4). The expected outcome is upregulation of early activation marker surface expression in activated CAR+ T cells (Figure 6B).
Day 2 – Measure cytokine secretion
If needed, thaw CAR T cell and K562 cell co-culture supernatants on ice.
Follow Human IFN-γ Quantikine ELISA Kit protocol.
Measure absorbance in microplate reader. The expected outcome is detection of IFN-γ production by conventional CAR CD4+ T cells but not by CAR Treg cells (Figure 7).
Follow Human IL-10 Quantikine ELISA Kit protocol.
Measure absorbance in microplate reader. The expected outcome is detection of IL-10 production by CAR Treg cells but not by conventional CAR CD4+ T cells (Figure 7).
3.5.2. Cytotoxicity
The ability to kill antigen-expressing target cells is the most important functional readout for conventional CAR T cells (Note 4.5). In this protocol, we co-incubated CAR+ T cells with CAR target-expressing K562 in progressively increasing T cell:target cell ratios. CAR T cell cytotoxicity was then determined through the indirect measurement of extracellular lactate dehydrogenase (LDH) activity in the supernatant of co-cultures [12]. LDH is an intracellular enzyme and thus is only present in the extracellular milieu in the event of cell lysis. Addition of a substrate to supernatant samples that is converted into a chromogenic product by LDH allows for accurate quantification of supernatant LDH activity and, by proxy, target cell lysis (Figure 8).
Figure 8. CAR-mediated cytotoxicity of human CAR+ T cells.
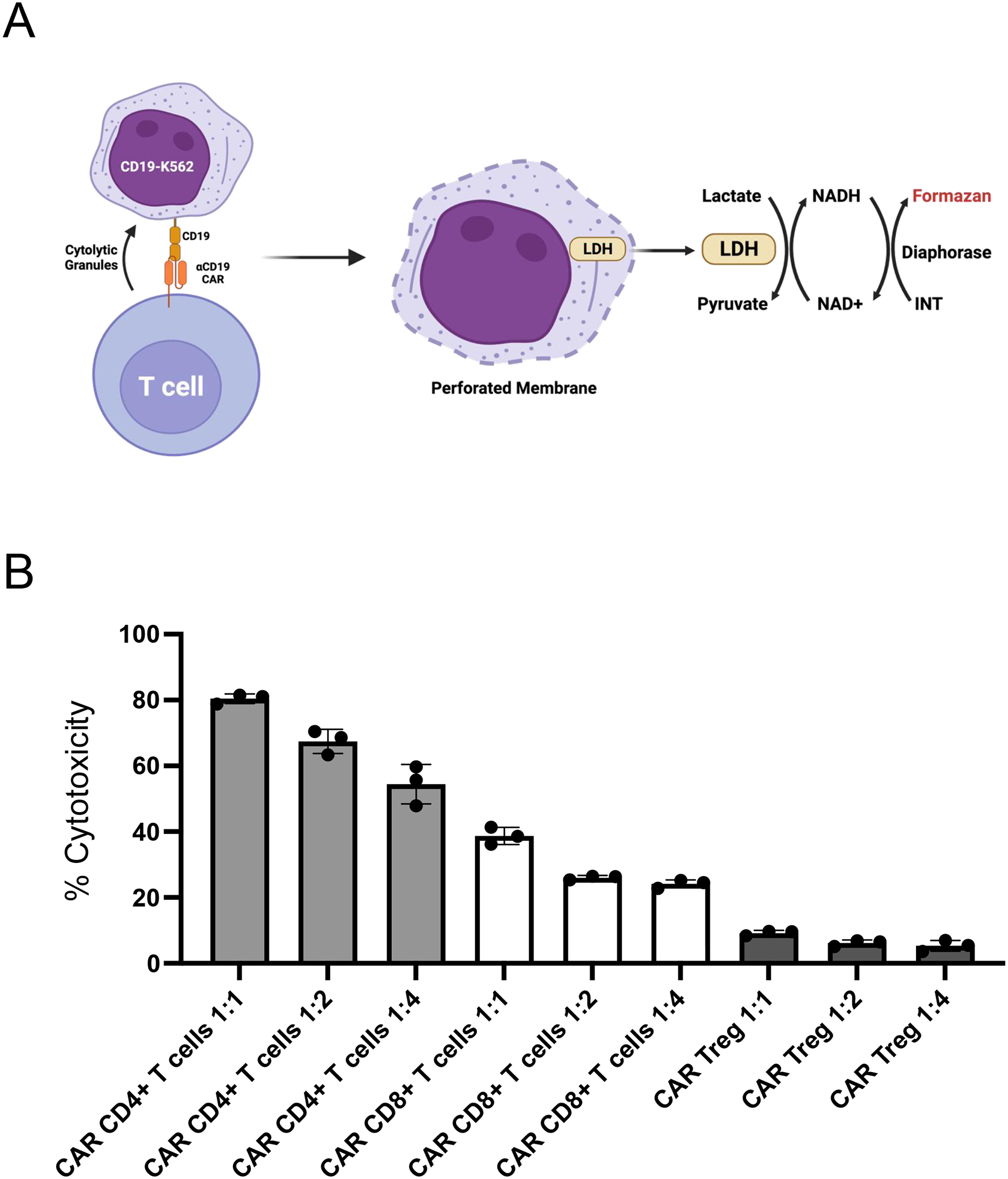
A. The workflow for evaluating CAR-mediated cytotoxicity of CAR T cells, e.g. CD19CAR T cells, involves co-incubating CD19CAR T cells with CD19-K562 cells at different ratios. Following co-incubation for a given period of time (e.g. 48h), the co-culture supernatants are collected and their content in lactate dehydrogenase (LDH, an intracellular enzyme that is only released into the supernatant upon cell lysis) is measured based on a reaction that quantitatively uses LDH to generate a colored product, formazan. B. Relative cytotoxicity of CD19CAR human CD4+ T cells, CD8+ T cells, and Treg cells towards CD19-K562 cells for 48h co-incubation. The ratios (1:1, 1:2, 1:4) indicate the ratio of CAR T cell number to CD19-K562 cell number per condition.
Collect CAR+ T cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded CAR+ T cell suspension into a new 15 ml conical tube.
Count CAR+ T cells and CAR target-expressing K562 cells (e.g. CD19-K562) with Trypan Blue.
Obtain 3×105 CAR+ T cells. This allows to cover triplicates of 3 T cell:target cell ratios, 1:1, 1:2, and 1:4, at 5×104 target cells per 96-well round-bottom plate well, as 5×104×3 + 2.5×104×3 + 1.25×104×3 = 2.63×104 ~3×105 CAR+ T cells.
Centrifuge at 500 g for 5 min at RT.
Resuspend 3×105 CAR+ T cells in 600 μL RPMI10 complete medium. This yields 200 μL for each of the 1:1 triplicates. If working with CAR Tregs, add rhIL-2 to a final concentration of 1000 IU/ml. If working with CAR CD4+ or CD8+ T cells, do not add rhIL-2.
- Perform serial dilution on the 96-well round-bottom plate as follows:
- Transfer 200 μL of CAR T cell suspension into each of the 3 1:1 ratio wells.
- Add 100 μL of RPMI10 complete medium into each of the 1:2 and 1:4 ratio wells.
- For each of the triplicates, transfer 100 μL of cell suspension from the 1:1 wells into the associated 1:2 ratio wells.
- For each of the triplicates, transfer 100 μL of cell suspension, from the 1:2 wells, into the associated 1:4 ratio wells.
- Discard 100 μL of cell suspension from the 1:4 ratio wells into a waste container. Each well now has 100 μL CAR T cell suspension with either 5×104 cells (1:1), 2.5×104 (1:2), or 1.25×104 cells (1:4).
Resuspend CAR target-expressing K562 cells at 5×105 cells/ml in RPMI10 complete medium. If working with CAR Tregs, add rhIL-2 to a final concentration of 1000 IU/ml. If working with CAR CD4+ or CD8+ T cells, do not add rhIL-2.
Add 5×105 CAR target-expressing K562 cells in 100 μL on top of every sample well, bringing the volume of each sample well to 200 μL.
Add 5×105 CAR target-expressing K562 cells in 100 μL to 6 empty wells, and then add 100 μL of RPMI10 complete medium on top. These will be used later as minimum and maximum LDH release controls in triplicate.
Incubate for 48h in the tissue culture incubator.
Two days later, add 20 μL of 10X concentrated Lysis Buffer from the CyQUANT LDH Cytotoxicity Assay kit to 3 wells with CAR target-expressing K562 cells alone.
Incubate for 45 min in the tissue culture incubator.
Carefully remove 50 μL of supernatant from each sample using a multichannel pipette into a new 96-well plate.
Follow the CyQUANT LDH Cytotoxicity Assay kit protocol.
Measure absorbance in microplate reader. The expected outcome is an increase in supernatant LDH activity, hence target cell lysis, with increased CAR T cell number per well (Figure 8B). Of note, as this assay utilizes the supernatant and not the co-incubated cells, it allows for sampling multiple time points.
3.5.3. Suppression
Treg cells are a subset of CD4+ T cells dedicated to suppressing immune responses. Treg cells constitute a form of dominant tolerance, directly inhibiting the activation, expansion, and function of effector immune cells. Assessing the capacity of Treg cells to suppress the proliferation of CD4+ and CD8+ T cells in vitro has long been the method of choice for initial assessment of Treg cell function [13]. In its simplest form, CD4+ and CD8+ T responder (Tresp) cells are labeled with a cell-permeable fluorescent dye, typically carboxyfluorescein succinimidyl ester (CFSE) or, more recently, CellTrace Violet (CTV), and co-incubated with Treg cells at different Treg:Tresp ratios and anti-CD3/28 activating beads (Figure 9A). Here, we first activate the Tregs via the CAR (with CAR target molecule-expressing irradiated K562 cells) and the Tresp cells via TCR/CD28 (by stimulation with anti-CD3/CD28 dynabeads) separately overnight (Figure 9B), as previously reported [14]. The following day, anti-CD3/CD28 dynabead-activated CTV-labeled Tresp cells are magnetically debeaded, re-counted, and added to the CAR-activated Tregs. CTV dilution by Tresp is evaluated 3–5 days later using flow cytometry (Figure 10).
Figure 9. CAR Treg-mediated suppression assay setup.
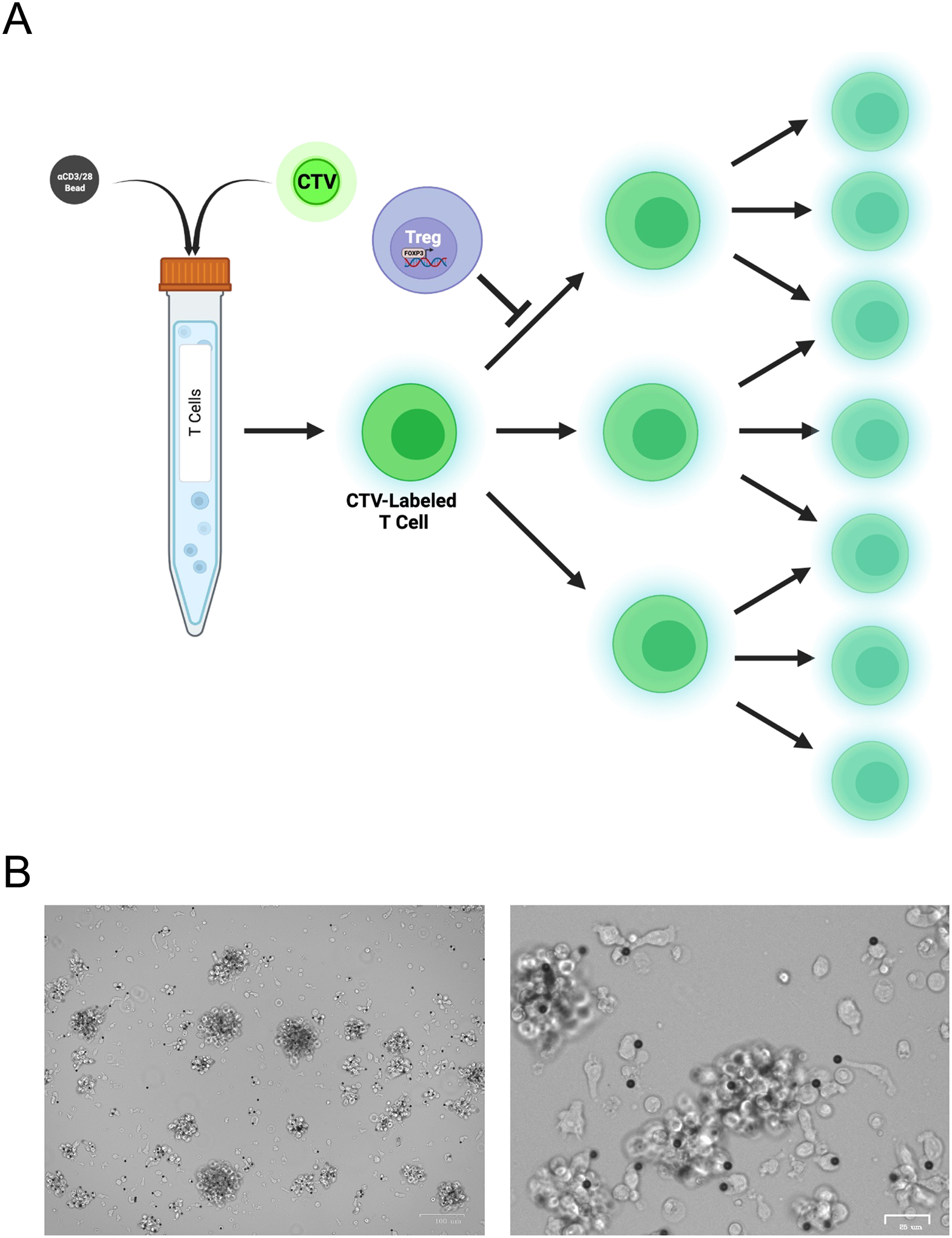
A. Bulk T cells (human CD4+ T cells and CD8+ T cells mixed at a 1:1 ratio) are labeled with a fluorescent dye, e.g. CellTrace Violet (CTV) and incubated with anti-CD3/CD28 dynabeads at a 1:10 bead to cell ratio overnight. The following day, these CTV-labeled anti-CD3/CD28-activated T cells are debeaded and co-incubated with CAR-activated CAR Tregs at different Treg:T cell ratio. Suppression of T cell proliferation by CAR Tregs is measured by inhibition of CTV dilution, detected by flow cytometry. B. Anti-CD3/CD28 bead-mediated activation (1:10 bead to cell ratio) of human bulk T cells (CD4+ T cells and CD8+ T cells mixed at a 1:1 ratio) overnight. Images at 5x (left) and 20x (right) magnification.
Figure 10. CAR-mediated suppression of human CAR+ Treg cells.
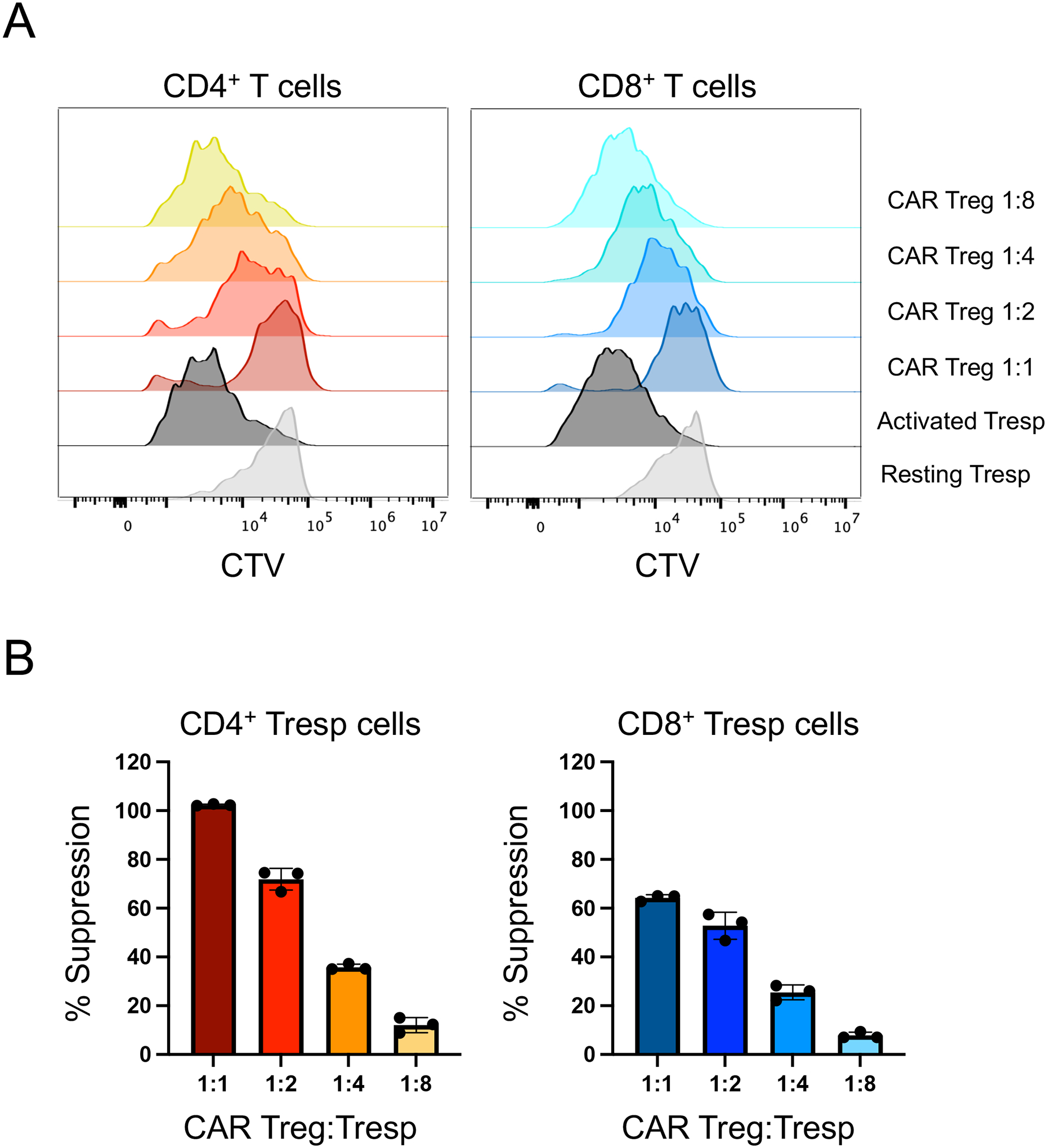
A. CTV dilution by anti-CD3/CD28 bead-activated human T responder (Tresp) cells (human CD4+ T cells and CD8+ T cells mixed at a 1:1 ratio) alone (“activated Tresp”) or in the presence of different numbers of CD19CAR Tregs (1:1, 1:2, 1:4), detected using flow cytometry. Unactivated Tresp cells are included as a negative control for proliferation. Data for CD4+ Tresp cells are on the left and data for CD8+ Tresp cells are on the right. CTV dilution for CD4+ Tresp cells is obtained from CD4+GFP-CTV+ cells (left), whereas CTV dilution for CD4+ Tresp cells is obtained from CD8+CTV+ cells (right). B. Summary data for histogram plots displayed in A.
Day 0 – Responder T cell (Tresp) fluorescent dye staining and overnight activation
Collect CAR+ Treg cells, untransduced CD4+ T cells, and untransduced CD8+ T cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded cell suspensions into new 15 ml conical tubes.
Collect K562 cell lines (e.g. parental K562 and CD19-K562) into conical tubes.
Irradiate K562 cell lines with 4000 rad in Cesium-137 irradiator.
Count debeaded T cells and irradiated K562 cells with Trypan Blue.
Mix untransduced CD4+ T cells and untransduced CD8+ T cells 1:1
Centrifuge mixed T cells at 500 g for 5 min at RT.
Resuspend T cells in 1 ml DPBS.
Add 1 μl CellTrace Violet (CTV) dye reconstituted in DMSO to T cells.
Incubate in water bath at 37°C for 20 min.
Add 9 ml of RPMI10 complete medium and centrifuge at 500 g for 5 min at RT.
Activate CTV-labeled T cells with anti-CD3/CD28 dynabeads at a 1:10 bead:T cell ratio without rhIL-2. Strong anti-CD3/CD28 activation and presence of IL-2 greatly decrease the likelihood of the suppression assay working. Assume a 30–50% cell loss with overnight anti-CD3/CD28 T cell overnight activation. Hence, as 2.5×104 CD4+ T cells and 2.5×104 CD8+ T cells will be needed per well of a full 96-well round-bottom plate, mix 5×106 CD4+ T cells with 5×106 CD8+ T cells and 106 anti-CD3/CD28 dynabeads (25 μl) in 10 ml RPMI10 complete medium.
Incubate in a T25 cell culture flask in the tissue culture incubator overnight.
In parallel, add 5×104 CTV-labeled non-activated T cells in 200 μl RPMI10 complete medium in three wells of a 96-well round-bottom plate as minimum proliferation controls.
Day 1 – Co-culture plate setup
Obtain 9×105 CAR+ Treg cells. This covers triplicates of 4 CAR Treg:Tresp ratios, 1:1, 1:2, 1:4, 1:8, at 5×104 target cells per 96-well round-bottom plate well, as 5×104×3 + 2.5×104×3 + 1.25×104×3 = 2.63×104 ~3×105 CAR+ Treg cells. The three CAR Treg condition requiring 3×105 CAR Tregs each are: anti-CD3/CD28 activation (positive control, polyclonal activation), irradiated K562 (negative control, no activation), and irradiated CAR target-expressing K562 (experiment, CAR activation).
Obtain 3×105 irradiated K562 cells and 3×105 irradiated CAR target-expressing K562 cells
Combine 3×105 CAR Tregs with 3×105 irradiated K562 cells (1:1 ratio), 3×105 CAR Tregs with 3×105 irradiated CAR target-expressing K562 cells (1:1 ratio), and 3×105 CAR Tregs with 6×104 anti-CD3/CD28 dynabeads (1.5 μl, for a 1:10 ratio of beads to Tresp cells, as there will be 12 wells total with 5×104 Tresp cells per well, hence 6×105 Tresp cells total) in three separate 15 ml conical tubes and centrifuge at 500 g for 5 min at RT.
Resuspend each one of the cell mixtures above in 600 μL RPMI10 complete medium. This yields 200 μL for each of the 1:1 Treg:Tresp triplicates.
- Perform serial dilution on the 96-well round-bottom plate from earlier as follows:
- Transfer 200 μL of cell suspension into each of the 3 1:1 ratio wells.
- Add 100 μL of RPMI10 complete medium into each of the 1:2, 1:4, and 1:8 ratio wells.
- For each of the triplicates, transfer 100 μL of cell suspension from the 1:1 wells into the associated 1:2 ratio wells.
- For each of the triplicates, transfer 100 μL of cell suspension, from the 1:2 wells, into the associated 1:4 ratio wells.
- For each of the triplicates, transfer 100 μL of cell suspension, from the 1:4 wells, into the associated 1:8 ratio wells.
- Discard 100 μL of cell suspension from the 1:8 ratio wells into a waste container. Each well now has 100 μL of cell suspension with either 5×104 (1:1), 2.5×104 (1:2), 1.25×104 (1:4), or 0.625 ×104 (1:8) CAR Tregs and an equal number of either irradiated K562, irradiated CAR target-expressing K562 or 5×103 anti-CD3/CD28 dynabeads.
Collect activated CD4+ and CD8+ T cells into a 15 ml conical tube and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded T cell suspension into a new 15 ml conical tube.
Count debeaded T cells with Trypan Blue.
Centrifuge 2×106 T cells at 500 g for 5 min at RT.
Resuspend 2×106 T cells in 4 ml RPMI10 complete medium.
Add 100 μL of T cell suspension (5×104 T cells) to each well with CAR Tregs, as well as to three wells with RPMI10 complete medium alone for maximum proliferation controls. The plate now has 3 wells with non-activated T cells alone (minimum proliferation control), 3 wells with activated T cells alone (maximum proliferation control) and activated T cells in the presence of decreasing numbers of CAR Tregs stimulated either via the CAR or via anti-CD3/CD28 dynabeads.
Incubate plate in the tissue culture incubator for 4 days.
Day 5 – Antibody staining and flow cytometry
Resuspend and transfer the contents of each 96-well round bottom plate well into a labeled 5 ml FACS tube.
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare antibody master mix with DPBS, anti-human CD4 PE/Cy7 1:200 and anti-human CD8 PerCP. Each sample will require 50 μl of antibody master mix. Hence, if staining 40 samples as an example, add 10 μl anti-human CD4 PE/Cy7 and 20 μl anti-human CD8 PerCP to 2 ml DPBS in a 15 ml conical tube and put on ice in the dark.
Briefly vortex cell pellet.
Add 50 μL of antibody master mix into each microcentrifuge tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
Wash by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Resuspend the cell pellet in 300 μL of PBS.
Put tubes on ice in the dark.
- Analyze by flow cytometry (Note 4.6). The expected outcome is non-activated CD4+ and CD8+ T cells alone to display a uniformly high CTV fluorescence peak, activated CD4+ and CD8+ T cells alone displaying multiple peaks of CTV intensity, one corresponding to each cell division, and activated CD4+ and CD8+ T cells in the presence of activated CAR Tregs displaying a reduction in the number of CTV peaks, hence in proliferation (Figure 10A). Treg cell-mediated suppression (Figure 10B) is calculated as follows:
3.5.4. Expansion
A notable indicator of a successful CAR construct is the expansion of the CAR+ T cell population upon stimulation with the appropriate antigen. Ideally, this expansion will either be comparable to or higher than that induced upon TCR/CD28 stimulation. With an expansive population, more cells will be available for grafting into hosts, which in return may have a more effective response. In this protocol, T cell subsets (CD4+ T cells, CD8+ T cells, and CD4+CD25+CD127− Treg cells) and conditions (untransduced and CAR+ cells) are activated via either the CAR (with target molecule-expressing irradiated K562 cells) or via TCR/CD28 (with anti-CD3/CD28 dynabeads). At 9 days post-activation (or Day 18 since T cell purification day), each sample receives flow cytometry counting beads and is stained with anti-CD4, anti-CD8, and a viability dye to evaluate expansion and viability via flow cytometry.
Day 0 – Co-culture plate setup
Collect K562 cell lines (e.g. parental K562 and CD19-K562) into conical tubes.
Irradiate K562 cell lines with 4000 rad in Cesium-137 irradiator.
Collect CAR+ T cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded CAR+ T cell suspension into a new 15 ml conical tube.
Count irradiated K562 cells and debeaded CAR+ T cells with Trypan Blue.
Centrifuge K562 cells and CAR+ T cells at 500 g for 5 minutes at RT.
Resuspend cells in RPMI10 complete medium at 106 cells/mL.
If working with CAR Tregs, add rhIL-2 for a final concentration of 1000 IU/mL. Do not add rhIL-2 if working with either CAR CD4+ or CAR CD8+ T cells.
Co-incubate 1×105 CAR+ T cells with 1×105 parental K562 cells, CAR-antigen expressing K562 cells, or anti-CD3/CD28 dynabeads (2.5 μL) in triplicates in a 96-well round bottom plate (1:1 ratio).
Incubate plate for 48 hours in the tissue culture incubator.
Days 2–9 – Co-culture expansion
Two days later, transfer cells from each 96-well round-bottom plate well into a 24-well plate well with 1 ml RPMI10 complete medium. If working with Tregs, include rhIL-2 at 1,000 IU/ml. If working with either CD4+ T cells or CD8+ T cells, do not add rhIL-2.
Continue to expand co-cultures by adding fresh RPMI10 (with rhIL-2, if needed) and splitting into additional 24-well plates as needed.
Day 9 – Antibody staining and flow cytometry
Resuspend and transfer the contents of each group of 24-well plate wells well into a conical tube.
Count cells with Trypan Blue.
Transfer up to 106 cells to a 5 ml FACS tube
Add 5,000 Precision Count Beads (5 μl) per tube.
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare antibody master mix with DPBS, anti-human CD4 PE/Cy7 1:200, anti-human CD8 PerCP, and Ghost viability dye BV510. Each sample will require 50 μl of antibody master mix. Hence, if staining 10 samples as an example, add 2.5 μl anti-human CD4 PE/Cy7, 5 μl anti-human CD8 PerCP, and 1 μl Ghost viability dye BV510 to 0.5 ml DPBS in a 1.5 ml microcentrifuge tube and put on ice in the dark.
Briefly vortex cell pellet.
Add 50 μL of antibody master mix to each FACS tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
Wash by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Resuspend the cell pellet in 300 μL of PBS.
Put tubes on ice in the dark.
Analyze by flow cytometry (Note 4.7.). Precision Count Beads fluoresce in multiple channels and can thus be identified and gated on by their high signal intensity on the diagonal of FITC and PE channels, for example. Since the absolute number of beads per tube is known, 5,000, these can be used to use the number of events of interest, for instance live CD4+GFP+ events, to obtain the absolute number of CAR+ cells. Please ensure to not only normalize the number of cells using the number of Precision Count Beads events per tube, but also to consider the total number of cells per condition vs. the number of cells used for the staining. Different CAR constructs, different blood donors, and different T cell subsets (Figure 11) are expected to grow at different rates.
Figure 11. CAR-mediated expansion of human CAR+ T cells.
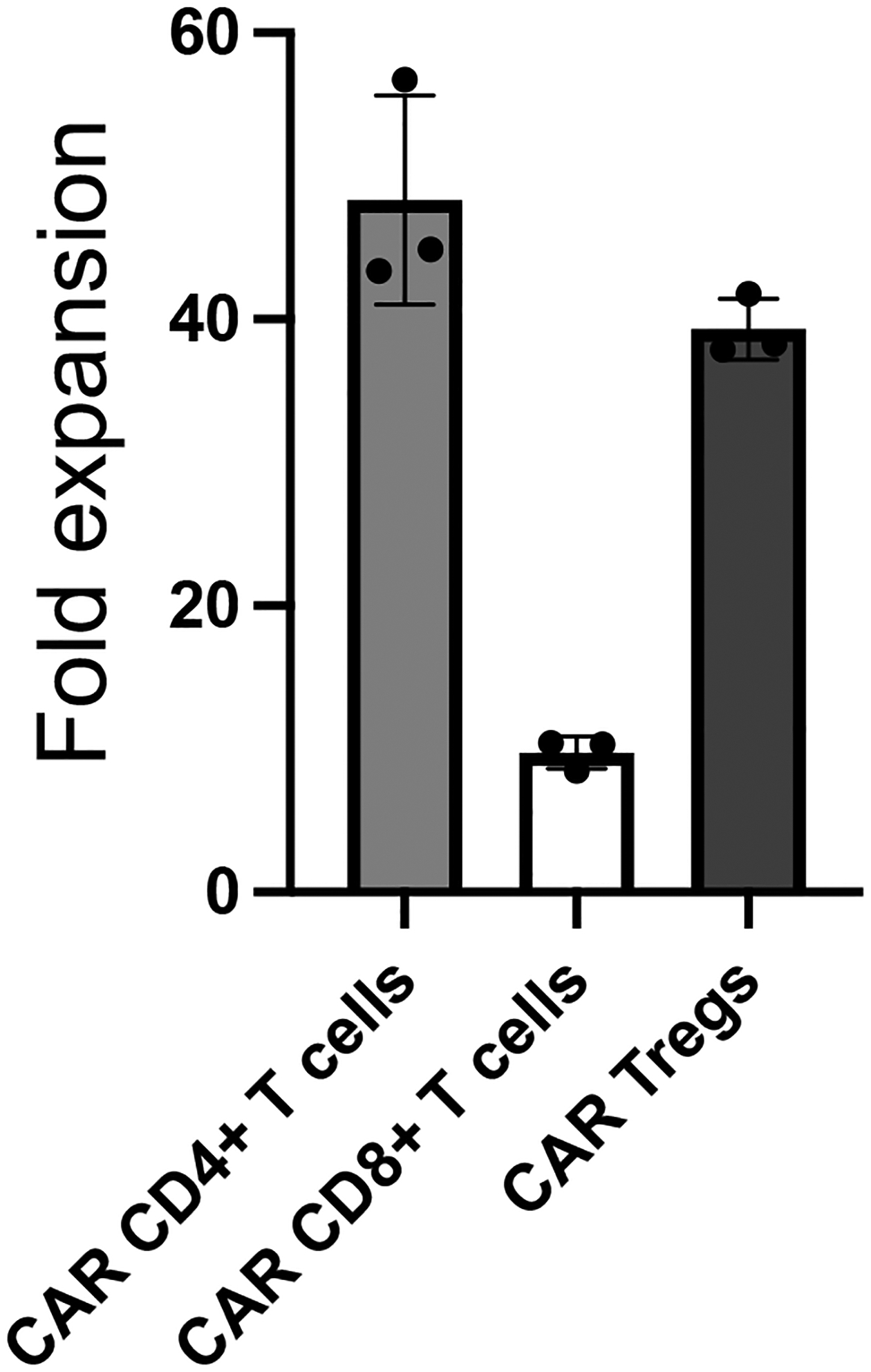
CAR+ T cell fold-expansion for CD19CAR CD4+ T cells, CD19CAR CD8+ T cells, and CD19CAR Tregs after 9-day co-incubation with irradiated CD19-K562 cells. Absolute cell numbers were calculated using flow cytometry by recording event number for either live CD4+GFP+ or live CD8+GFP+ cells, followed by normalization using precision count beads added number ratio to event number ratio and any dilution factor from using only a fraction of the expanded cells for flow cytometry.
3.5.5. Exhaustion
Upon constant stimulus from inflammatory conditions or lasting antigen exposure, T cells exhibit exhaustion. T cell exhaustion results in less cytokine production, upregulation of inhibitory signals, growth arrest, metabolic deficiencies, and, eventually, apoptosis. Consequently, exhausted T cells lack the ability to eliminate antigen-expressing cells. When assessing a candidate CAR construct, it is important to evaluate the potential for CAR activation to induce T cell exhaustion. Well known exhaustion markers include PD-1, CTLA-4, LAG3, TIM-3, and TIGIT [15].
Day 0 – Co-culture plate setup
Collect K562 cell lines (e.g. parental K562 and CD19-K562) into conical tubes.
Irradiate K562 cell lines with 4000 rad in Cesium-137 irradiator.
Collect CAR+ T cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded CAR+ T cell suspension into a new 15 ml conical tube.
Count irradiated K562 cells and debeaded CAR+ T cells with Trypan Blue.
Centrifuge K562 cells and CAR+ T cells at 500 g for 5 minutes at RT.
Resuspend cells in RPMI10 complete medium at 106 cells/mL.
If working with CAR Tregs, add rhIL-2 for a final concentration of 1000 IU/mL. Do not add rhIL-2 if working with either CAR CD4+ or CAR CD8+ T cells.
Co-incubate 1×105 CAR+ T cells with 1×105 parental K562 cells, CAR-antigen expressing K562 cells, or anti-CD3/CD28 dynabeads (2.5 μL) in triplicates in a 96-well round bottom plate (1:1 ratio).
Incubate plate for 48 hours in the tissue culture incubator.
Days 2–9 – Co-culture expansion
Two days later, transfer cells from each 96-well round-bottom plate well into a 24-well plate well with 1 ml RPMI10 complete medium. If working with Tregs, include rhIL-2 at 1,000 IU/ml. If working with either CD4+ T cells or CD8+ T cells, do not add rhIL-2.
Continue to expand co-cultures by adding fresh RPMI10 (with rhIL-2, if needed) and splitting into additional 24-well plates as needed.
Day 9 – Antibody staining and flow cytometry
Resuspend and transfer the contents of each group of 24-well plate wells well into a conical tube.
Count cells with Trypan Blue.
Transfer up to 106 cells to a 5 ml FACS tube
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare antibody master mix with DPBS, anti-human CD4 Alexa 700 1:200, anti-human CD8 APC/Cy7, anti-human PD-1 PerCP, and Ghost viability dye BV510. Each sample will require 50 μl of antibody master mix. Hence, if staining 10 samples as an example, add 2.5 μl anti-human CD4 Alexa 700, 5 μl anti-human CD8 APC/Cy7, 5 μl anti-human PD-1 PerCP, and 1 μl Ghost viability dye BV510 to 0.5 ml DPBS in a 1.5 ml microcentrifuge tube and put on ice in the dark.
Briefly vortex cell pellet.
Add 50 μL of antibody master mix to each FACS tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
Wash by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Resuspend the cell pellet in 300 μL of PBS.
Put tubes on ice in the dark.
Analyze by flow cytometry (Note 4.8). The expected outcome is the observation of two populations: PD-1 negative and PD-1 positive cells (Figure 12).
Figure 12. CAR-mediated exhaustion of human CAR+ T cells.
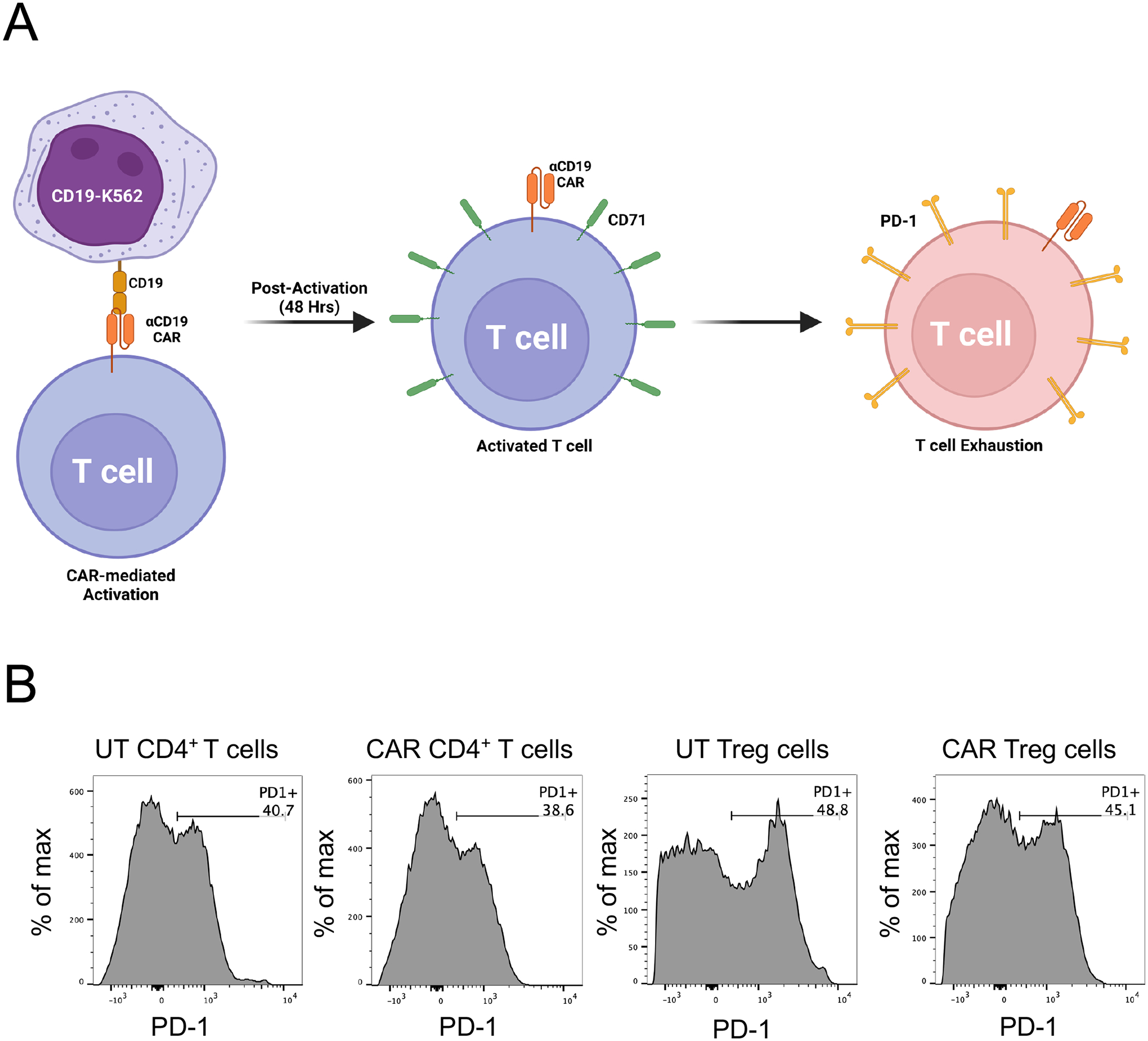
A. The workflow for evaluating exhaustion of CAR T cells, e.g. CD19CAR T cells, involves co-incubating CD19CAR T cells with irradiated CD19-expressing K562 for 9 days, followed by surface staining for PD-1 and detection using flow cytometry. B. Surface PD-1 expression by human CD19CAR CD4+ T cells or CD19CAR Treg cells activated either via anti-CD3/CD28 beads or CD19-K562, as detected by flow cytometry with anti-human PD-1 PerCP. Histograms represent PD-1 expression in live CD4+GFP+ cells.
3.5.6. Stability
Treg cells display plasticity, making them susceptible to transdifferentiation into pro-inflammatory effector T cells upon certain environmental cues, including prolonged ex vitro expansion and exposure to inflammatory cytokines [16]. Thus, it is important to ensure that CAR-mediated activation does not compromise Treg stability over time. Established Treg cell functional markers, such as CD25 and CTLA-4, as well as the Treg cell lineage-specific transcription factors FOXP3 and HELIOS, allow for adequate assessment of the phenotypic stability of Tregs over time [16].
Day 0 – Co-culture plate setup
-
10.
Collect K562 cell lines (e.g. parental K562 and CD19-K562) into conical tubes.
-
11.
Irradiate K562 cell lines with 4000 rad in Cesium-137 irradiator.
-
12.
Collect CAR+ Treg cells into 15 ml conical tubes and magnetically remove anti-CD3/CD28 dynabeads by incubating in a DynaMag-15 magnet for 3 min and transferring the debeaded CAR+ Treg suspension into a new 15 ml conical tube.
-
13.
Count irradiated K562 cells and debeaded CAR+ Treg cells with Trypan Blue.
-
14.
Centrifuge K562 cells and CAR+ Treg cells at 500 g for 5 minutes at RT.
-
15.
Resuspend cells in RPMI10 complete medium at 106 cells/mL.
-
16.
Add rhIL-2 for a final concentration of 1000 IU/mL.
-
17.
Co-incubate 1×105 CAR+ Treg cells with 1×105 parental K562 cells, CAR-antigen expressing K562 cells, or anti-CD3/CD28 dynabeads (2.5 μL) in triplicates in a 96-well round bottom plate (1:1 ratio).
-
18.
Incubate plate for 48 hours in the tissue culture incubator.
Days 2–9 – Co-culture expansion
-
3.
Two days later, transfer cells from each 96-well round-bottom plate well into a 24-well plate well with 1 ml RPMI10 complete medium with rhIL-2 at 1,000 IU/ml.
-
4.
Continue to expand co-cultures by adding fresh RPMI10 with rhIL-2 at 1,000 IU/ml and splitting into additional 24-well plates as needed.
Day 9 – Antibody staining and flow cytometry
Resuspend and transfer the contents of each group of 24-well plate wells well into a conical tube.
Count cells with Trypan Blue.
Transfer up to 106 cells to a 5 ml FACS tube
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare surface staining antibody master mix with DPBS, anti-human CD4 PE/Cy7 1:200, anti-human CD25 APC, and Ghost viability dye BV510. Each sample will require 50 μl of antibody master mix. Hence, if staining 10 samples as an example, add 2.5 μl anti-human CD4 PE/Cy7, 5 μl anti-human CD25 APC, and 1 μl Ghost viability dye BV510 to 0.5 ml DPBS in a 1.5 ml microcentrifuge tube and put on ice in the dark.
Briefly vortex cell pellet.
Add 50 μL of surface staining antibody master mix to each FACS tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
In the meantime, prepare Fixation/Permeabilization buffer by adding 3 volumes of Fixation/Permeabilization diluent to 1 volume of Fixation/Permeabilization concentrate from the eBioscience Foxp3 transcription factor staining buffer set. Each sample requires 50 μL of Fixation/Permeabilization buffer. Hence, if staining 10 samples, as an example, mix 750 μL of Fixation/Permeabilization diluent with 750 μL of Fixation/Permeabilization concentrate in a 1.5 ml microcentrifuge tube to obtain 1 ml Fixation/Permeabilization buffer and keep on ice.
Wash stained cells by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Briefly vortex cell pellet.
Add 50 μL of Fixation/Permeabilization buffer to each FACS tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
In the meantime, prepare Permeabilization buffer by adding 9 volumes of DPBS to 1 volume to Permeabilization Buffer 10x concentrate from the eBioscience Foxp3 transcription factor staining buffer set. Each sample requires 500 μL of Permeabilization buffer to wash the Fixation/Permeabilization buffer and 50 μL of Permeabilization buffer for staining with antibodies targeting intracellular proteins. Hence, if staining 10 samples, as an example, mix 4.95 ml DPBS with 550 μL of Permeabilization Buffer 10x concentrate in a 15 ml conical tube to obtain 5.5 ml of Permeabilization buffer and keep on ice.
Wash fixed and permeabilized cells by adding 500 μL Permeabilization buffer.
Centrifuge at 500 g for 5 minutes at RT.
In the meantime, prepare intracellular staining antibody master mix with Permeabilization buffer, anti-human FOXP3 eFluor 450 1:40, anti-human HELIOS PE 1:40, and CTLA-4 PerCP-e710 1:40. Each sample will require 50 μl of antibody master mix. Hence, if staining 10 samples as an example, add 12.5 μl anti-human FOXP3 eFluor 450, 12.5 μl anti-human HELIOS PE, and 12.5 μl anti-human CTLA-4 PerCP-e710 to 0.5 ml Permeabilization buffer in a 1.5 ml microcentrifuge tube and put on ice in the dark.
Decant supernatant of centrifuged washed cells.
Briefly vortex cell pellet.
Add 50 μL of intracellular staining antibody master mix to each FACS tube.
Briefly vortex and incubate at 4°C for 30 minutes in the dark.
Wash stained cells by adding 500 μL PBS.
Centrifuge at 500 g for 5 minutes at RT.
Decant supernatant.
Resuspend the cell pellet in 300 μL of PBS.
Put tubes on ice in the dark.
Analyze by flow cytometry (Note 4.9). The expected outcome is that the majority of CAR Tregs are FOXP3+HELIOS+ cells. Use bulk CD4+ T cells as a negative control for FOXP3 and HELIOS staining (Figure 13).
Figure 13. CAR-mediated impact of human CAR+ Treg cells.
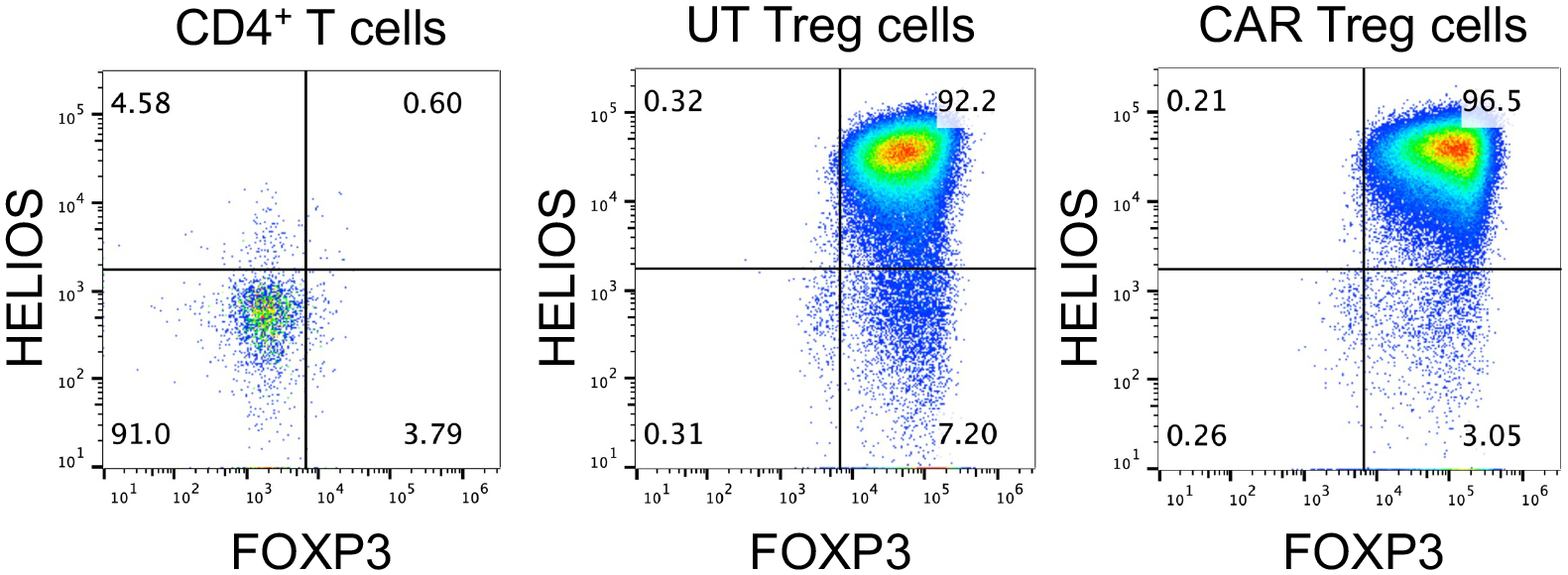
Expression of the Treg cell lineage transcription factors FOXP3 and HELIOS in CD4+ T cells activated via anti-CD3/CD28 dynabeads, untransduced (UT) Treg cells activated via anti-CD3/CD28 dynabeads, or CD19CAR Treg cells activated with irradiated CD19-K562 cells for 9 days. Dot plots represent FOXP3 and HELIOS expression in viable CD4+ cells, as detected by flow cytometry with anti-human FOXP3 eFluor 450 and anti-human HELIOS PE.
4. Notes
4.1. RPMI10 complete medium is prepared by adding 50 ml FBS, 5 ml penicillin-streptomycin solution, 5 ml GlutaMAX, 5 ml sodium pyruvate, 5 ml NEAA, and 5 ml 1M HEPES to 500 ml RPMI 1640 medium. After adding all the components to the basal RPMI 1640 medium, RPMI10 can be filtered using a vacuum filtration system with a 20 μm pore size membrane to ensure sterility.
4.2. While freshly isolated and plated T cells (Day 0) have a round morphology, over the course of 48h following activation (Day 2) they acquire an elongated shape, grow in size, and form dense clusters around the anti-CD3/CD28 dynabeads (Figure 3).
4.3. DMEM10 complete medium is prepared by adding 50 ml FBS, 5 ml GlutaMAX, 5 ml sodium pyruvate, and 5 ml NEAA to 500 ml DMEM medium. After adding all the components to the basal DMEM medium, RPMI10 can be filtered using a vaccum filtration system with a 20 μm pore size membrane to ensure sterility. Penicilin-streptomycin and HEPES have both been reported to negatively impact DNA transfection and are thus not included in DMEM10 complete medium.
4.4. Multi-color flow cytometry requires preparation of single-color controls for the cytometer to perform compensation, i.e. correct for spectral overlap between different channels. Using cells is preferable to using beads for single-color antibody stains, to better match the intensity of the fluorophores used. In this specific case, 6 single-color controls are needed: unstained, GFP, PE/Cy7, PerCP, BV510, PE, and APC.
4.5. There are a multitude of in vitro cytotoxicity assays with readouts based on radioactivity, microscopy, flow cytometry, luminescence, and absorbance. The most cost-effective and scalable readout is absorbance, which we used in this protocol.
4.6. In this specific case, 5 single-color controls are needed for compensation: unstained, GFP, PE/Cy7, PerCP, and CTV.
4.7. In this specific case, 5 single-color controls are needed for compensation: unstained, GFP, PE/Cy7, PerCP, and BV510.
4.8. In this specific case, 6 single-color controls are needed for compensation: unstained, GFP, Alexa 700, APC/Cy7, PerCP, and BV510.
4.9. In this specific case, 8 single-color controls are needed for compensation: unstained, GFP, PE/Cy7, APC, BV510, eFluor 450, PE, and PerCP-e710.
Table 1:
Timeline of HEK293T transfection for lentivirus production.
| Monday (D0) | Tuesday (D1) | Wednesday (D2) | Thursday (D3) | Friday (D4) |
|---|---|---|---|---|
| Seed cells at 3.8 × 106 cells per 10 cm dish | Add chloroquine Incubate 5 hours Transfect with DNA/PEI complexes | Change medium Add ViralBoost | Collect viral medium Add fresh medium + ViralBoost | Collect viral medium Transduce Jurkat cells for virus titration |
Table 2:
Plasmid DNA concentrations used in second generation CAR lentivirus production.
| Plasmid | DNA amount (μg) | Concentration (ng/μL) | Volume to add (μL) |
|---|---|---|---|
| Packaging plasmid (psPAX2) | 6.66 μg | ||
| Envelope plasmid (pCMV-VSV-G) | 3.33 μg | ||
| Transfer plasmid (pSLCAR-CD19-28z) | 10 μg |
Acknowledgements
This work was funded by Human Islet Research Network (HIRN) Emerging Leader in Type 1 Diabetes grant U24DK104162-07 (LMRF), American Cancer Society (ACS) Institutional Research Grant IRG-19-137-20 (LMRF), South Carolina Clinical and Translational Research (SCTR) Pilot Project Discovery Grant 1TL1TR001451-01 (LMRF), Diabetes Research Connection (DRC) Grant IPF 22-1224 (LMRF), and Cellular, Biochemical and Molecular Sciences training grant T32GM132055 (RWC). Supported in part by the Flow Cytometry and Cell Sorting Shared Resource, Hollings Cancer Center, Medical University of South Carolina (P30 CA138313).
References
- 1.Neefjes J, Jongsma ML, Paul P, Bakke O. Towards a systems understanding of MHC class I and MHC class II antigen presentation. Nat Rev Immunol. 2011;11(12):823–36. doi: 10.1038/nri3084. [DOI] [PubMed] [Google Scholar]
- 2.Montemurro A, Schuster V, Povlsen HR, Bentzen AK, Jurtz V, Chronister WD, et al. NetTCR-2.0 enables accurate prediction of TCR-peptide binding by using paired TCRalpha and beta sequence data. Commun Biol. 2021;4(1):1060. doi: 10.1038/s42003-021-02610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.June CH, Sadelain M. Chimeric Antigen Receptor Therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hou AJ, Chen LC, Chen YY. Navigating CAR-T cells through the solid-tumour microenvironment. Nat Rev Drug Discov. 2021;20(7):531–50. doi: 10.1038/s41573-021-00189-2. [DOI] [PubMed] [Google Scholar]
- 5.Amor C, Feucht J, Leibold J, Ho YJ, Zhu C, Alonso-Curbelo D, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature. 2020;583(7814):127–32. doi: 10.1038/s41586-020-2403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rurik JG, Tombacz I, Yadegari A, Mendez Fernandez PO, Shewale SV, Li L, et al. CAR T cells produced in vivo to treat cardiac injury. Science. 2022;375(6576):91–6. doi: 10.1126/science.abm0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32(2):520–31. doi: 10.1038/leu.2017.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38(8):947–53. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muller YD, Ferreira LMR, Ronin E, Ho P, Nguyen V, Faleo G, et al. Precision Engineering of an Anti-HLA-A2 Chimeric Antigen Receptor in Regulatory T Cells for Transplant Immune Tolerance. Front Immunol. 2021;12:686439. doi: 10.3389/fimmu.2021.686439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discov. 2019;18(10):749–69. doi: 10.1038/s41573-019-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller YD, Nguyen DP, Ferreira LMR, Ho P, Raffin C, Valencia RVB, et al. The CD28-Transmembrane Domain Mediates Chimeric Antigen Receptor Heterodimerization With CD28. Front Immunol. 2021;12:639818. doi: 10.3389/fimmu.2021.639818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64(3):313–20. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 13.Collison LW, Vignali DA. In vitro Treg suppression assays. Methods Mol Biol. 2011;707:21–37. doi: 10.1007/978-1-61737-979-6_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fung VCW, Rosado-Sanchez I, Levings MK. Transduction of Human T Cell Subsets with Lentivirus. Methods Mol Biol. 2021;2285:227–54. doi: 10.1007/978-1-0716-1311-5_19. [DOI] [PubMed] [Google Scholar]
- 15.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12(6):492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 16.Skartsis N, Peng Y, Ferreira LMR, Nguyen V, Ronin E, Muller YD, et al. IL-6 and TNFalpha Drive Extensive Proliferation of Human Tregs Without Compromising Their Lineage Stability or Function. Front Immunol. 2021;12:783282. doi: 10.3389/fimmu.2021.783282. [DOI] [PMC free article] [PubMed] [Google Scholar]


