Abstract
Despite the antimicrobial mechanisms of vertebrate phagocytes, mycobacteria can survive within the phagosomes of these cells. These organisms use various strategies to evade destruction, including inhibition of acidification of the phagosome and inhibition of phagosome-lysosome fusion. In contrast to mycobacteria, Coxiella burnetii, the etiologic agent of Q fever, inhabits a spacious acidified intracellular vacuole which is prone to fusion with other vacuoles of the host cell, including phagosomes containing mycobacteria. The Coxiella-infected cell thus provides a unique model for investigating the survival of mycobacteria in an acidified phagosome-like compartment. In the present study, murine bone marrow-derived macrophages were infected with either Mycobacterium avium or Mycobacterium tuberculosis and then coinfected with C. burnetii. We observed that the majority of phagocytosed mycobacteria colocalized to the C. burnetii-containing vacuole, which maintained its acidic properties. In coinfected macrophages, the growth of M. avium was not impaired following fusion with the acidified vacuole. In contrast, the growth rate of M. tuberculosis was reduced in acidified vacuoles. These results suggest that although both species of mycobacteria inhibit phagosome-lysosome fusion, they may be differentially susceptible to the toxic effects of the acidic environment in the mature phagolysosome.
When macrophages ingest particulate material, an intracellular vacuole (phagosome) which undergoes a stepwise maturation is formed. This maturation consists of a progressive acidification and several fusion and fission events, eventually leading to fusion with lysosomes (7, 40). Ultimately, the fully mature phagosome contains enzymes and the acidic environment necessary to denature and degrade phagocytosed material (17). This pathway is an important component of the macrophage’s defense against invading organisms. Thus, it is not surprising to find that successful intracellular pathogens have developed different strategies to escape the phagosomal maturation pathway. Two bacterial genera that have developed alternative strategies for their survival are Mycobacterium and Coxiella.
Virulent strains of Mycobacterium avium and Mycobacterium tuberculosis survive and grow exponentially inside nonactivated murine macrophages (3–5), despite the potent antimicrobial mechanisms of the host cell (22–24, 36). Mycobacteria arrest the maturation of the early endosome to a phagolysosome by inhibiting fusion of the mycobacterium-containing phagosome with lysosomes (4, 5, 12, 13, 22, 25). This is demonstrated by the observation that mycobacterium-containing phagosomes have low levels of the molecules characteristic of lysosomal membranes, including CD63, LAMP-1, LAMP-2, and the GTP-binding protein rab7, while retaining the markers of the early endocytic compartment, including the transferrin receptor and rab5 (12, 47, 49). Also, M. avium-containing phagosomes interact extensively with endosomes, as indicated by the ready acquisition of gangliosides from the cell surface (41). Although the lysosomal acid protease cathepsin D might be present in the mycobacterium-containing phagosomes in a small amount (12), the enzyme remains in an inactive form (44). Mycobacterium-containing phagosomes do not acidify (13, 37), which may be due at least in part to the exclusion of the vacuolar proton-ATPase from the membrane (43, 49). This lack of acidification is probably responsible for the failure of enzymes such as cathepsin D to be activated. The inhibition of phagosome maturation is assumed to be an active process controlled by mycobacteria, since only viable bacilli can accomplish it (5). When either human or murine macrophages are treated with cytokines leading to inhibition of mycobacterial growth, the arrest of maturation of the mycobacterium-containing phagosomes is overridden (3, 16, 23, 24, 30, 42, 48).
In contrast to the situation described above, Coxiella burnetii, the etiologic agent of Q fever, is an obligate intracellular pathogen that multiplies inside eukaryotic cells within a spacious vacuole with phagolysosome-like properties (29, 32). C. burnetii requires an acidic environment for its multiplication: pharmacologic agents that raise phagolysosome pH inhibit the growth of C. burnetii (27, 39). In addition, vacuoles containing C. burnetii are prone to fusion with other vesicles of the phagocytic-endocytic system (45, 46). This fusion has been observed in bone marrow-derived macrophages (BMM) infected with M. avium and coinfected with C. burnetii. In this system, progressive colocalization of the mycobacteria to the C. burnetii-containing phagosome-like vacuole was noted (15).
In the present study, we used the unique mycobacterium-C. burnetii coinfection model to investigate whether M. avium or M. tuberculosis could survive after transfer to an acidified phagolysosome-like vacuole. We report here that in murine BMM infected with M. avium 25291 and later coinfected with C. burnetii Nine Mile, the colocalization of mycobacteria in the acidified, C. burnetii-containing phagosome-like vacuole did not affect M. avium growth. In contrast, colocalization of M. tuberculosis H37Rv in the acidified vacuole of C. burnetii-infected cells impaired the growth of the tubercle bacillus.
MATERIALS AND METHODS
Macrophage culture.
BMM were prepared as follows. The femurs of C57BL/6 mice were flushed with Hanks’ balanced salt solution (HBSS) (GIBCO Laboratories, Grand Island, N.Y.) to obtain the bone marrow. Cells were washed once in HBSS, resuspended in RPMI 1640 (GIBCO) supplemented with 10% fetal calf serum (GIBCO) and 10% L929 cell-conditioned medium as a source of macrophage colony-stimulating factor (complete medium), plated in tissue culture dishes, and incubated at 37°C overnight. Adherent cells (mature macrophages and fibroblasts) were discarded; nonadherent cells were collected, washed once with HBSS, and resuspended in complete medium. Either 5 × 105 cells per well were seeded in a 24-well plate (Falcon, Becton Dickinson, Lincoln Park, N.J.) for CFU assay and fluorescence microscopy (see below) or 3.5 × 106 cells per well were seeded in 6-well plates for electron microscopy (EM). L929 cell-conditioned medium (10%) was added to the culture on days 3 and 6 of culture. Cells were used for infection on day 9 of culture.
Bacteria.
M. avium 25291 (American Type Culture Collection, Manassas, Va.) and M. tuberculosis H37Rv (Trudeau Institute, Saranac Lake, N.Y.) were cultivated in Middlebrook 7H9 medium (Difco Laboratories, Detroit, Mich.) supplemented with 0.04% Tween 80 (Sigma, St. Louis, Mich.). The bacteria were harvested in mid-log phase, washed, resuspended in saline, and kept frozen (−80°C) in aliquots until use. Before infection, the mycobacterial aliquots were probe sonicated as described previously (38) to disperse clumps. Phase II C. burnetii Nine Mile was provided by T. Hackstadt, Rocky Mountain Laboratories (Hamilton, Mont.). The phase II variant of C. burnetii (truncated lipopolysaccharide) is avirulent for humans and easily phagocytosed by mononuclear phagocytes (28). C. burnetii was propagated in Vero cells (African green monkey kidney fibroblasts; American Type Culture Collection). Heavily infected Vero cells were scraped off the culture plates and lysed by passing the cell suspension through a 26-gauge needle 100 times. This suspension was centrifuged at 630 × g to eliminate cell debris and then at 22,000 × g to pellet the bacteria. Highly enriched C. burnetii lysates were resuspended in RPMI 1640 medium in aliquots and kept frozen at −80°C until they were used.
Infections.
Nine-day-old BMM were infected with M. avium or M. tuberculosis at a multiplicity of infection of 8 mycobacteria per cell (M. avium) or 1 mycobacterium per cell (M. tuberculosis). After 4 h of incubation, cells were washed with warm HBSS to remove noninternalized organisms and afterward maintained in fresh culture medium. Four days after mycobacterial infection, cell monolayers were coinfected for 4 h with C. burnetii-enriched cell lysates and washed. Cultures were monitored for 3 days afterward.
CFU assay.
The BMM monolayer was disrupted with a solution of water containing 0.04% Tween 80 and 0.008% digitonin (Sigma) at different time points after infection. Bacterial suspensions were serially diluted and plated onto Middlebrook 7H10 agar plates supplemented with oleic acid-albumin-dextrose-catalase enrichment (Difco). Plates were incubated for 7 to 10 days at 37°C. Colonies were counted under a dissecting microscope and reported as CFU. Growth rates of the mycobacteria were calculated with the following formula: g = 0.69t/(lnM − lnMi), where M is the number of bacilli at the end of the study, Mi is the number of bacilli at the start of the study, and t is the study time.
Fluorescence microscopy.
BMM were cultured on 12-mm glass coverslips placed in 24-well plates. At 24-h intervals following infection, monolayers were fixed in periodate-lysine-paraformaldehyde and double stained with DAPI (4′,6 diamidino-2-phenylindole hydrochloride; Molecular Probes Inc., Eugene, Oreg.) for 3 min at room temperature, which stained the nuclei of the cells and the C. burnetii organisms, and with auramine-rhodamine acid-fast stain (Difco), which stained mycobacteria. The number of acid-fast bacilli (AFB) found inside and outside the C. burnetii-bearing vacuoles of 200 cells were then counted under a fluorescence microscope.
EM.
Cells were fixed in 2% glutaraldehyde–0.1 M sucrose–0.1 M cacodylate buffer (Polysciences, Warrington, Pa.) for 1 h at 4°C and processed for EM as previously described (38). For immunogold studies, cell samples were dehydrated in alcohol and embedded in LR White, hard-grade acrylic resin (London Resin Co., Reading, United Kingdom). Ultrathin sections were examined with a JEOL JEM 100CX transmission electron microscope.
Measurement of lysosomal acidification.
The acidification of intracellular compartments was determined by the method of Anderson et al. (1). Briefly, cells were incubated for 30 min with the weak base 3-(2,4-dinitroanilo)-3′-amino-N-methyl dipropylamine (DAMP) (Molecular Probes Inc.), washed three times with RPMI, and fixed with 1% glutaraldehyde in 0.1 M cacodylate buffer. After incubation with an ammonium chloride solution to remove nonspecifically bound DAMP, cells were scraped off the culture plates and processed for EM as described above. The ultrathin sections were placed on EM grids and incubated with a monoclonal anti-2,4-dinitrophenol mouse immunoglobulin G (Oxford Biomedical Research, Oxford, Mich.) for 30 min, followed by incubation with a secondary antibody conjugated with gold (Auroprobe goat anti-mouse immunoglobulin G; Amersham, Arlington Heights, Ill.). The grids were stained with uranyl and lead acetate and examined in a transmission electron microscope as described previously (38).
RESULTS
Fusion of M. avium vacuoles with the C. burnetii-containing vacuole.
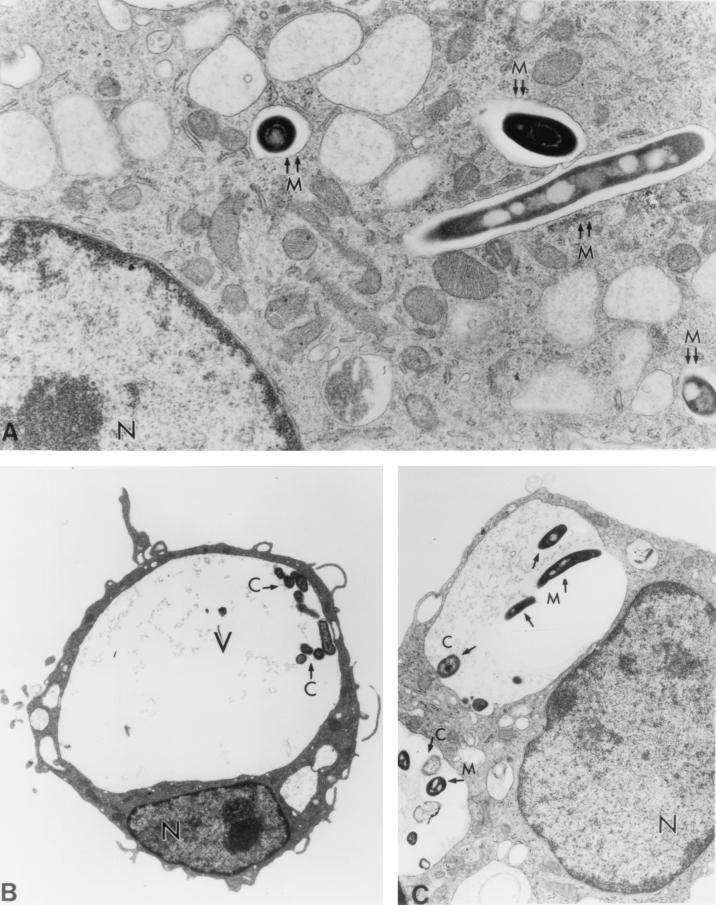
When murine BMM were infected with M. avium 25291, the mycobacteria localized in individual, tight vacuoles in the cytoplasm of the cell (Fig. 1A). Infection of murine BMM with C. burnetii Nine Mile, on the other hand, resulted in the formation of large, spacious vacuoles, which were easily observed with light microscopy and/or EM approximately 24 h after infection. The C. burnetii-containing vacuoles increased in volume over time and soon occupied up to the entire cytoplasm of the cell (Fig. 1B). When BMM were infected first with M. avium for 4 days, followed by infection with C. burnetii, the mycobacterial vacuoles fused with the large vacuoles containing C. burnetii. This was apparent as early as 24 h after coinfection. Colocalization of the mycobacteria and C. burnetii in the same vacuole was observed by EM (Fig. 1C) and by fluorescence microscopy (not shown). The degree of fusion of M. avium to the C. burnetii-containing vacuole, as evaluated by light microscopy, was 38% ± 11% (mean ± standard deviation) at 24 h, and it increased to 49% ± 19% at 48 h after the double infection. Examination of the electron micrographs of the infected cells showed that the majority of mycobacteria were within the C. burnetii-containing vacuoles. Many mycobacteria remained adjacent to the inner side of the phagosome membrane (Fig. 1 and 2). By day 4 after coinfection, macrophages began to spontaneously detach from the culture plates. Further coinfection experiments were therefore carried out for 3 days.
FIG. 1.
(A) Electron micrograph of murine BMM infected with M. avium (M). Note the tight vacuole containing the bacterium (double arrows). Magnification, ×19,800. (B) Electron micrograph of murine BMM infected with C. burnetii (C). Note the large vacuole (V) occupying almost the entire cytoplasm of the cell and eccentric nucleus (N). Magnification, ×3,300. (C) Electron micrograph at 24 h after coinfection of murine BMM with M. avium and C. burnetii. Note colocalization of the two organisms in the same large vacuole (arrows point to the organisms). Magnification, ×8,300.
FIG. 2.
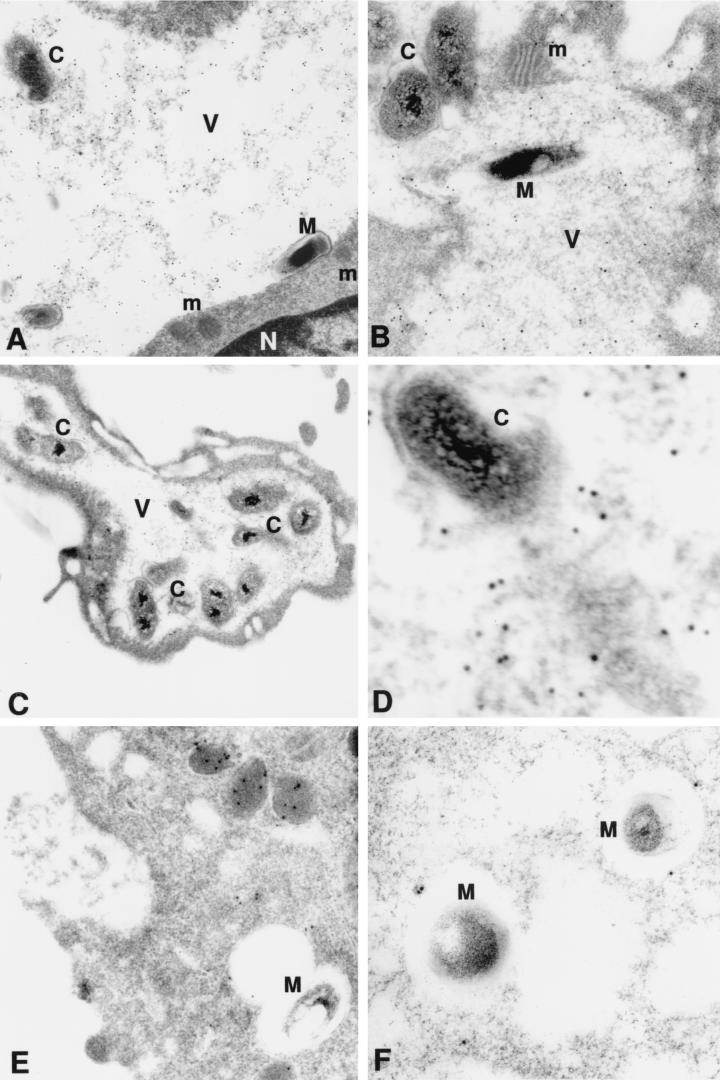
Electron micrograph of murine BMM infected with M. avium (M) and/or C. burnetii (C) and stained with DAMP and gold-conjugated antibody. (A and B) Coinfected cells with vacuoles containing M. avium and C. burnetii; (C and D) cells with vacuole containing C. burnetii only; (E and F) cells with vacuoles containing M. avium only. Note presence of gold particles, indicating the low-pH environment within the vacuoles (V) occupied by the two organisms and by C. burnetii only. Only a few gold particles are visible within the vacuoles containing M. avium only and in the nucleus (N) or mitochondria (m) of the cells. Magnifications, ×30,000 (A), ×30,000 (B), ×16,000 (C), ×86,000 (D), ×46,000 (E), and ×32,000 (F). Panel D is a higher magnification of panel C.
Characterization of the mycobacterium-C. burnetii-containing vacuole.
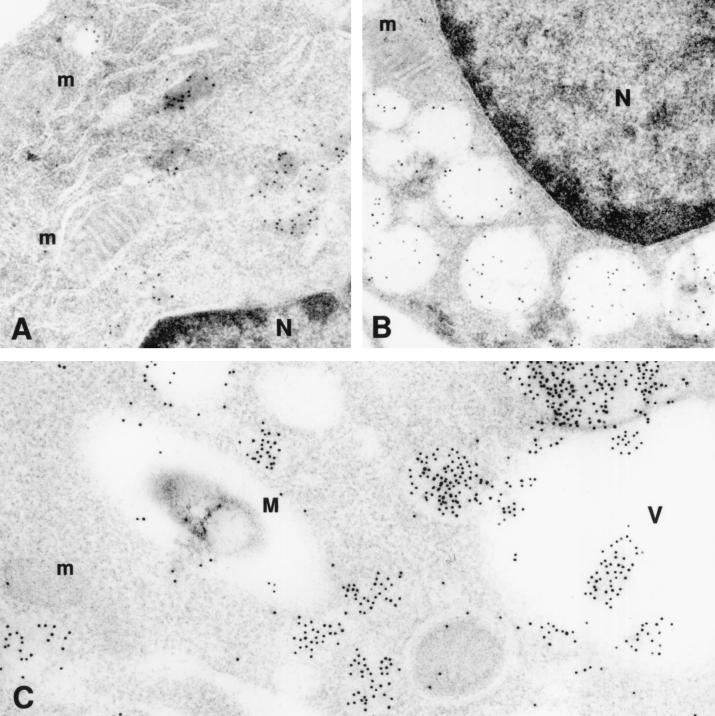
We next determined whether the phagosomal vacuole containing C. burnetii would maintain its acidic characteristics after fusing with the vacuole containing mycobacteria. We used the DAMP stain technique to facilitate visualization of an acidic environment. DAMP is a weak base which accumulates exclusively in the acidic compartments of cells. After fixation and processing for EM, the presence of DAMP can be revealed by immunostaining of the sections with gold-conjugated antibodies. The density of gold particles in a given cell compartment is inversely proportional to its pH. Virtually no gold particles were seen in mycobacterium-containing vacuoles by this method (Fig. 2). On the other hand, gold particles were easily detected in large numbers inside the vacuoles containing both C. burnetii and M. avium as well as in those vacuoles containing C. burnetii alone (Fig. 2). Gold particles were also seen in vacuoles which appeared to be in the process of fusing with the large Coxiella-containing vacuoles but not with the mycobacterium-containing vacuoles, suggesting that lysosomes fused with the former but not the latter vacuoles (Fig. 3). These results indicate that mycobacteria were colocalized with C. burnetii in vacuoles with low pH.
FIG. 3.
Electron micrograph of murine BMM stained with DAMP and gold-conjugated antibody. (A) Uninfected cell (24 h in culture) with small vacuoles (lysosomes) containing gold particles; (B) uninfected cell (5 days in culture) with larger vacuoles (secondary lysosomes) containing gold particles; (C) cell coinfected with C. burnetii (not seen in the figures) and M. avium (M). Vacuoles containing gold particles are seen fusing with the large vacuole (V) containing C. burnetii but are not seen fusing with the vacuole containing M. avium. Gold particles are not seen in the nucleus (N) or mitochondria (m) of the cells. Magnifications, ×43,000 (A), ×40,000 (B), ×48,000 (C).
Growth of M. avium after fusion with the acidified vacuole.
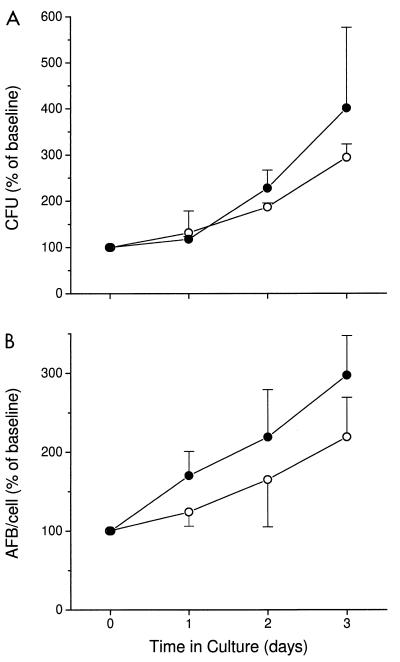
When BMM were infected with M. avium only, the mycobacteria grew exponentially inside the murine phagocytes, multiplying about sixfold in 7 days. Following coinfection of BMM with M. avium and C. burnetii and the fusion of the mycobacterial vacuoles with the C. burnetii-containing vacuole, no significant change in the growth rate of M. avium was observed (Fig. 4). Both the CFU assay (Fig. 4A) and direct counting of auramine-stained AFB in the light microscope (Fig. 4B), showed that M. avium grew as well or even slightly faster in the doubly infected cells. The doubling time for M. avium during 7 days of culture was 1.9 days in cells infected with M. avium alone, compared to 1.5 days in cells coinfected with C. burnetii. These results suggest that fusion of M. avium with the acidified phagosome-like vacuoles containing C. burnetii does not impair the ability of this organism to multiply.
FIG. 4.
Growth of M. avium in murine BMM (open circles) and in cells coinfected with C. burnetii (closed circles). BMM were infected with M. avium and then coinfected with C. burnetii on day 4 (designated in the graph as time zero). Growth of M. avium was evaluated by number of CFU (A) and by direct counting of AFB by light microscopy (B). Results are expressed as mean percentages of baseline values for three independent experiments. Error bars indicate standard deviations. Baseline values (measured at time zero) were (55 ± 11) × 104 and (56 ± 6) × 104 CFU per cell and 8.2 and 7.4 AFB per cell for M. avium alone and M. avium with C. burnetii, respectively. By an independent t test, P was >0.05 at all time points.
Growth of M. tuberculosis after fusion with the acidified vacuole.
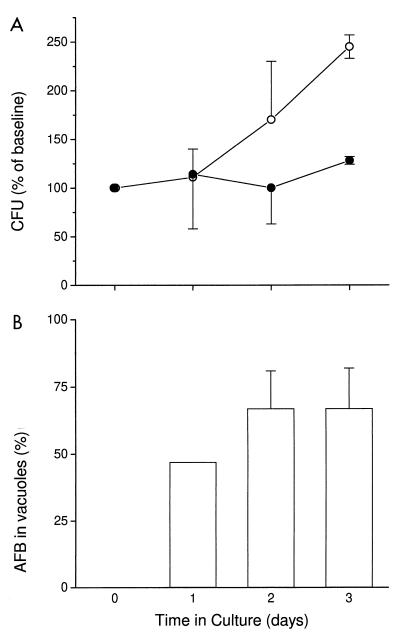
We next determined the effects of colocalization in an acidified C. burnetii vacuole on M. tuberculosis. Using fluorescent microscopy, the percentage of colocalization of M. tuberculosis H37Rv in the C. burnetii-containing vacuole was assessed. Similar to the experiments with M. avium, there was a progressive colocalization of M. tuberculosis in the C. burnetii-containing vacuole, which was observed as early as 24 h after coinfection. Up to 67% of the M. tuberculosis organisms were colocalized to the C. burnetii vacuole by 48 h (Fig. 5B). When BMM were infected with M. tuberculosis only, the mycobacteria multiplied with a doubling time of 2.2 days. Following coinfection of BMM with C. burnetii and fusion of the majority of M. tuberculosis vacuoles with the acidified phagosome-like vacuole of C. burnetii, the growth of M. tuberculosis was inhibited (Fig. 5A). The doubling time for M. tuberculosis in cultures coinfected with C. burnetii was 7.1 days. Thus, colocalization of M. tuberculosis to an acidified phagosome-like vacuole appeared to impair the growth of this organism.
FIG. 5.
(A) Growth of M. tuberculosis in murine BMM (open circles) and in cells coinfected with C. burnetii (closed circles). BMM were infected with M. tuberculosis and then coinfected with C. burnetii on day 4 (designated in the graph as time zero). (B) Percent of M. tuberculosis colocalized within the C. burnetii-containing vacuole. Results are expressed as mean percentages of baseline values for three independent experiments. Error bars indicate standard deviations. Baseline values (measured at time zero) were (3.3 ± 0.8) × 104 and (4.6 ± 1.6) × 104 CFU for M. tuberculosis alone and for M. tuberculosis with C. burnetii, respectively. By an independent t test, P was 0.02 on day 3.
DISCUSSION
Mycobacteria can affect the characteristics of the phagosome in which they reside by preventing phagosome maturation and acidification and phagosome-lysosome fusion. The ability of the mycobacteria to block fusion has been interpreted as a protective mechanism, since it prevents their exposure to potentially toxic low pH and activated lysosomal enzymes. Here, we report that although both M. avium 25291 and M. tuberculosis H37Rv can inhibit the maturation of the macrophage phagosome, they may be differentially susceptible to the antimicrobial effects of the phagosome-like compartment. In contrast to M. avium, which grows in the C. burnetii-containing vacuoles, M. tuberculosis growth appears to be inhibited. However, we do not know how long the growth inhibition of M. tuberculosis would have been maintained, since the experiments could not be continued beyond day 3 after coinfection. Fusion of either M. avium- or M. tuberculosis-containing phagosomes with an acidified phagosome-like compartment did not result in death or degradation of the bacteria by the low pH and hydrolytic activity of the vacuole, as indicated by both EM and light microscopy combined with the CFU data.
The robust characteristics of M. avium, which continues growing well in the acidified phagosome-like compartment, are of interest. The strain used in these experiments was originally selected for its ability to grow in mice and may not be representative of the behavior of other laboratory strains or clinical isolates of M. avium. In contrast to our findings, other studies have shown a correlation between increased phagolysosome fusion and M. avium growth inhibition. Slower growth of M. avium was observed in macrophages from a resistant mouse strain (bearing the R allele of the Nramp-1 gene), compared to that observed in macrophages from a susceptible strain (Nramp-1 S). The slower growth was correlated with more extensive phagosome-lysosome fusion (14). The growth of clinical isolates of M. avium has been shown to be restricted in human or murine macrophages activated with gamma interferon and tumor necrosis factor alpha, but not in macrophages activated with macrophage colony-stimulating factor (16, 30). In addition, when mouse macrophages activated by gamma interferon and lipopolysaccharide were infected with M. avium (42) or M. bovis (48), reduced intracellular growth concomitant with an increase in the degrees of phagosome-lysosome fusion and of acidification was observed.
In cell-free culture, M. tuberculosis has been reported to be more sensitive to an acidic pH than M. avium (10). We observed a similar differential susceptibility of these mycobacteria to pH within the host cell vacuole. Furthermore, our results suggest that when mycobacteria enter a compartment already rendered acidic (by C. burnetii), the mycobacteria do not appear to change the pH of the vacuole. Whether mycobacteria can continuously modify the vacuoles in which they reside in vivo is not known. It has been observed that when mycobacteria divide, the vacuole appears to divide with them, sequestering each bacillus in a separate tight vacuole (34, 35). Also, when clumps of mycobacteria (Mycobacterium smegmatis) are phagocytosed, as the bacilli divide, they appear to segregate to individual tight vacuoles (6). This ability of mycobacteria to remain within a tight vacuole may facilitate their control of fusion of their phagosome with other vacuoles within the cells.
In their interaction with macrophages, M. avium and M. tuberculosis have been found to differ widely. M. tuberculosis has been reported to be more sensitive than M. avium to other potential bactericidal pathways, including nitric oxide (8, 9, 19, 20, 31). On the other hand, M. avium is more sensitive to the effects of expression of Nramp-1 than M. tuberculosis (2, 33). Despite these differences in sensitivity to macrophage protective mechanisms, many investigators assume that these mycobacteria are analogous because they appear to inhabit the same intracellular compartment. Taken together, the present studies and those of others suggest that observations made for one mycobacterium may not necessarily apply to other mycobacteria.
A limitation of our model is the apparent incomplete fusion of mycobacterial vacuoles with the C. burnetii-containing phagosome-like vacuoles. Using light microscopy, we have shown that 49% of intracellular M. avium and 67% of M. tuberculosis organisms colocalize to the C. burnetii-containing vacuole by 48 h. However, our EM studies show that many mycobacteria remain adjacent to the vacuole membrane (Fig. 2A). Thus, it is difficult to determine solely by light microscopy exactly where the mycobacteria are located, suggesting that the light microscopy count may be an underestimation of colocalization. Examination of the electron micrographs from the present study showed that there was indeed a higher degree (80 to 90%) of colocalization of the two organisms. In addition, de Chastellier and Rabinovitch examined hundreds of coinfected cells using quantitative EM and observed a progressive colocalization of M. avium and C. burnetii which reached up to 90% by 48 h (15). Many of the vacuoles seen in those preparations were too small to be detected by light microscopy. We therefore assume that the percentage of colocalization reported here is an underestimation of the actual colocalization of mycobacteria and C. burnetii in the coinfected cells.
The ability of mycobacteria to block phagosome-lysosome fusion may not only save the organisms from a hostile low-pH environment but also confer a survival advantage to mycobacteria by facilitating the access to nutrients. Russell et al. have shown that mycobacterial phagosomes interact with plasmalemma-derived vesicles and acquire molecules from outside the cell (41, 44). Also, Clemens and Horwitz have shown that M. tuberculosis phagosomes contain transferrin receptors from the cell surface which allow transfer of iron into the vacuole (11, 12). The traffic of transferrin-bound iron occurs at the early endosome stage, the stage at which mycobacteria arrest the maturation of the phagosome. Iron is an essential nutrient for mycobacteria, so access to this element may facilitate growth (18, 21, 26).
The sensitivity of M. tuberculosis to low pH may contribute to the control of this infection in humans. This control is dependent on macrophage activation by an emerging immune response. Therefore, if the infecting mycobacteria are recognized and there is an early immune response that triggers the activation of phagocytes, facilitating phagosome-lysosome fusion, the infection may be controlled. However, if immune activation is delayed and the number of organisms increases before acidification of the vacuole occurs, extensive growth of the mycobacteria may take place and clinical disease will eventually become manifest.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants AI 22616 and AI42056. Maria Salome Gomés was supported by Fundação para a Ciência e Tecnologia, Portugal.
We thank Victoria H. Freedman for help in preparation of the manuscript, Wilhelmine Hellmann for expert assistance in all aspects of EM, Marguerite Nulty for secretarial help, and Judy Adams for preparation of the figures.
REFERENCES
- 1.Anderson R G W, Falck J R, Goldstein J L, Brown M S. Visualization of acidic organelles in intact cells by electron microscopy. Proc Natl Acad Sci USA. 1984;81:4838–4842. doi: 10.1073/pnas.81.15.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg R, Sarmento A M. The role of macrophage activation and Bcg-encoded function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990;88:324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg R, Orme I M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993;80:352–359. [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong J A, Hart P D. Response of cultured macrophages to mycobacterium tuberculosis with observation on fusion of lysosomes with phagosomes. J Exp Med. 1971;134:713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armstrong J A, Hart P D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975;142:1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barker K, Fan H, Carroll C, Kaplan G, Barker J, Hellmann W, Cohn Z A. Nonadherent cultures of human monocytes kill Mycobacterium smegmatis, but adherent cultures do not. Infect Immun. 1996;64:428–433. doi: 10.1128/iai.64.2.428-433.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berón W, Alvarez-Dominguez C, Mayorga L, Stahl P D. Membrane trafficking along the phagocytic pathway. Trends Cell Biol. 1995;5:100–104. doi: 10.1016/s0962-8924(00)88958-8. [DOI] [PubMed] [Google Scholar]
- 8.Chan J, Xing Y, Magiozzo R S, Bloom B R. Killing of virulent Mycobacterium tuberculosis by reactive nitrogen intermediates produced by activated murine macrophages. J Exp Med. 1992;175:1111–1122. doi: 10.1084/jem.175.4.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan J, Tanaka K, Carroll D, Flynn J, Bloom B R. Effects of nitric oxide synthase inhibitors on murine infection with Mycobacterium tuberculosis. Infect Immun. 1995;63:736–740. doi: 10.1128/iai.63.2.736-740.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman J S, Bernard J S. The pH tolerance of unclassified mycobacteria. Am Rev Respir Dis. 1962;86:582–583. doi: 10.1164/arrd.1962.86.4.582. [DOI] [PubMed] [Google Scholar]
- 11.Clemens D L, Horwitz M A. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crowle A J, Dahl R, Ross E, May M H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991;59:1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Chastellier C, Fréhel C, Offredo C, Skamene E. Implication of phagosome-lysosome fusion in restriction of Mycobacterium avium growth in bone marrow macrophages from genetically resistant mice. Infect Immun. 1993;61:3775–3784. doi: 10.1128/iai.61.9.3775-3784.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Chastellier, C., M. Thibon, and M. Rabinovitch. Construction of chimeric phagosomes that shelter Mycobacterium avium and Coxiella burnetii (phase II) in doubly infected mouse macrophages: an ultrastructural study. Eur. J. Cell Biol., in press. [DOI] [PubMed]
- 16.Denis M. Growth of Mycobacterium avium in human monocytes: identification of cytokines which reduce and enhance intracellular microbial growth. Eur J Immunol. 1991;21:391–395. doi: 10.1002/eji.1830210221. [DOI] [PubMed] [Google Scholar]
- 17.Desjardins M. Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends Cell Biol. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 18.Dhople A I, Poirier T C. Role of iron in the pathogenesis of Mycobacterium avium infection in mice. Microbios. 1996;87:77–87. [PubMed] [Google Scholar]
- 19.Doherty T M, Sher A. Defects in cell-mediated immunity affect chronic, but not innate, resistance of mice to Mycobacterium avium infection. J Immunol. 1997;158:4822–4831. [PubMed] [Google Scholar]
- 20.Doi T, Ando M, Akaike T, Suga M, Sato K, Maeda H. Resistance to nitric oxide in Mycobacterium avium complex and its implication in pathogenesis. Infect Immun. 1993;61:1980–1989. doi: 10.1128/iai.61.5.1980-1989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Douvas G S, May M H, Crowle A J. Transferrin, iron and serum lipids enhance or inhibit Mycobacterium avium replication in human macrophages. J Infect Dis. 1993;167:857–864. doi: 10.1093/infdis/167.4.857. [DOI] [PubMed] [Google Scholar]
- 22.Fenton M J, Vermeulen M W. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64:683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flesch I E A, Kaufmann S H E. Attempts to characterize the mechanisms involved in mycobacterial growth inhibition by gamma-interferon-activated bone marrow macrophages. Infect Immun. 1988;56:1464–1469. doi: 10.1128/iai.56.6.1464-1469.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flesch I E, Kaufmann S H E. Activation of tuberculostatic macrophage functions by gamma interferon, interleukin-4, and tumor necrosis factor. Infect Immun. 1990;58:2675–2677. doi: 10.1128/iai.58.8.2675-2677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frehel C, de Chastellier C, Lang T, Rastogi N. Evidence for inhibition of fusion of lysosomal and prelysosomal compartments with phagosomes in macrophages infected with pathogenic Mycobacterium avium. Infect Immun. 1986;52:252–262. doi: 10.1128/iai.52.1.252-262.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomes M S, Appelberg R. Evidence for a link between iron metabolism and Nramp1 gene function in innate resistance against Mycobacterium avium. Immunology. 1998;95:165–168. doi: 10.1046/j.1365-2567.1998.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hackstadt T, Williams J C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci USA. 1981;78:3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hackstadt T. Biosafety concerns and Coxiella burnetii. Trends Microbiol. 1996;4:341–342. doi: 10.1016/0966-842x(96)81555-1. [DOI] [PubMed] [Google Scholar]
- 29.Heinzen R A, Scidmore M A, Rockey D D, Hackstadt T. Differential interaction with endocytic and exocytic pathways distinguish parasitophorous vacuoles of Coxiella burnetii and Chlamydia trachomatis. Infect Immun. 1996;64:796–809. doi: 10.1128/iai.64.3.796-809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu N, Young L S, Bermudez L E. Response to stimulation with recombinant cytokines and synthesis of cytokines by murine intestinal macrophages infected with the Mycobacterium avium complex. Infect Immun. 1995;63:528–533. doi: 10.1128/iai.63.2.528-533.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.MacMicking J D, LaCourse R, Mudgett J S, Shah S K, Nathan C F. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc Natl Acad Sci USA. 1997;94:5243–5248. doi: 10.1073/pnas.94.10.5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maurin M, Benoliel A M, Bongrand P, Raoult D. Phagolysosomes of Coxiella burnetii-infected cell lines maintain an acidic pH during persistent infection. Infect Immun. 1992;60:5013–5016. doi: 10.1128/iai.60.12.5013-5016.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Medina E, Rogerson B J, North R J. The Nramp1 antimicrobial resistance gene segregates independently of resistance to virulent Mycobacterium tuberculosis. Immunology. 1996;88:479–481. doi: 10.1046/j.1365-2567.1996.d01-700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molloy A, Laochumroonvorapong P, Kaplan G. Apoptosis, but not necrosis, of infected monocytes is coupled with killing of intracellular bacillus Calmette-Guerin. J Exp Med. 1994;180:1499–1509. doi: 10.1084/jem.180.4.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreira A L, Wang J, Tsenova-Berkova L, Hellmann W, Freedman V H, Kaplan G. Sequestration of Mycobacterium tuberculosis in tight vacuoles in vivo in lung macrophages of mice infected by the respiratory route. Infect Immun. 1997;65:305–308. doi: 10.1128/iai.65.1.305-308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nathan C, Hibbs J., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991;3:65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 37.Oh Y-K, Straubinger R M. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization on phagosomal pH and phagosome-lysosome interaction. Infect Immun. 1996;64:319–325. doi: 10.1128/iai.64.1.319-325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul S, Laochumroonvorapong P, Kaplan G. Comparable growth of virulent and avirulent Mycobacterium tuberculosis in human macrophages in vitro. J Infect Dis. 1996;174:105–112. doi: 10.1093/infdis/174.1.105. [DOI] [PubMed] [Google Scholar]
- 39.Raoult D, Drancourt M, Vestris G. Bactericidal effect of doxycycline associated with lysosomotropic agents on Coxiella burnetii in P388D1 cells. Antimicrob Agents Chemother. 1990;34:1512–1514. doi: 10.1128/aac.34.8.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robinson M S, Watts C, Zerial M. Membrane dynamics in endocytosis. Cell. 1996;84:13–21. doi: 10.1016/s0092-8674(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 41.Russell D G, Dant J, Sturgill-Koszycki S. Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J Immunol. 1996;156:4764–4773. [PubMed] [Google Scholar]
- 42.Schaibe U E, Sturgill-Koszycki S, Schlesinger P H, Russell D G. Cytokine activation leads to acidification and increases maturation of Mycobacterium avium-containing phagosomes in murine macrophages. J Immunol. 1998;160:1290–1296. [PubMed] [Google Scholar]
- 43.Sturgill-Koszycki S, Schlesinger P H, Chakraborty P, Haddix P L, Collins H L, Fok A K, Allen R D, Gluck S L, Heuser J, Russell D G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994;263:678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- 44.Sturgill-Koszycki S, Schaible U E, Russell D G. Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 1996;15:6960–6968. [PMC free article] [PubMed] [Google Scholar]
- 45.Veras P S, de Chastellier C, Moreau M F, Villiers V, Thibon M, Mattei D, Rabinovitch M. Fusion between large phagocytic vesicles: targeting of yeast and other particulates to phagososomes that shelter the bacterium Coxiella burnetii or the protozoan Leishmania amazonensis in Chinese hamster ovary cells. J Cell Sci. 1994;107:3065–3076. doi: 10.1242/jcs.107.11.3065. [DOI] [PubMed] [Google Scholar]
- 46.Veras P S T, Moulia C, Dauguet C, Tunis C T, Thibon M, Rabinovitch M. Entry and survival of Leishmania amazonensis amastigotes within phagolysosome-like vacuoles that shelter Coxiella burnetii in Chinese hamster ovary cells. Infect Immun. 1995;63:3502–3506. doi: 10.1128/iai.63.9.3502-3506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Via L E, Deretic D, Ulmer R J, Hibler N S, Huber L A, Deretic V. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem. 1997;272:13326–13331. doi: 10.1074/jbc.272.20.13326. [DOI] [PubMed] [Google Scholar]
- 48.Via L E, Fratti R A, McFalone M, Pagán-Ramos E, Deretic D, Deretic V. Effects of cytokines in mycobacterial phagosome maturation. J Cell Sci. 1998;111:897–905. doi: 10.1242/jcs.111.7.897. [DOI] [PubMed] [Google Scholar]
- 49.Xu S, Cooper A, Sturgill-Koszycki S, van Heyningen T, Chatterjee D, Orme I M, Allen P, Russell D G. Intracellular trafficking in Mycobacterium tuberculosis and Mycobacterium avium-infected macrophages. J Immunol. 1994;153:2568–2578. [PubMed] [Google Scholar]