Abstract
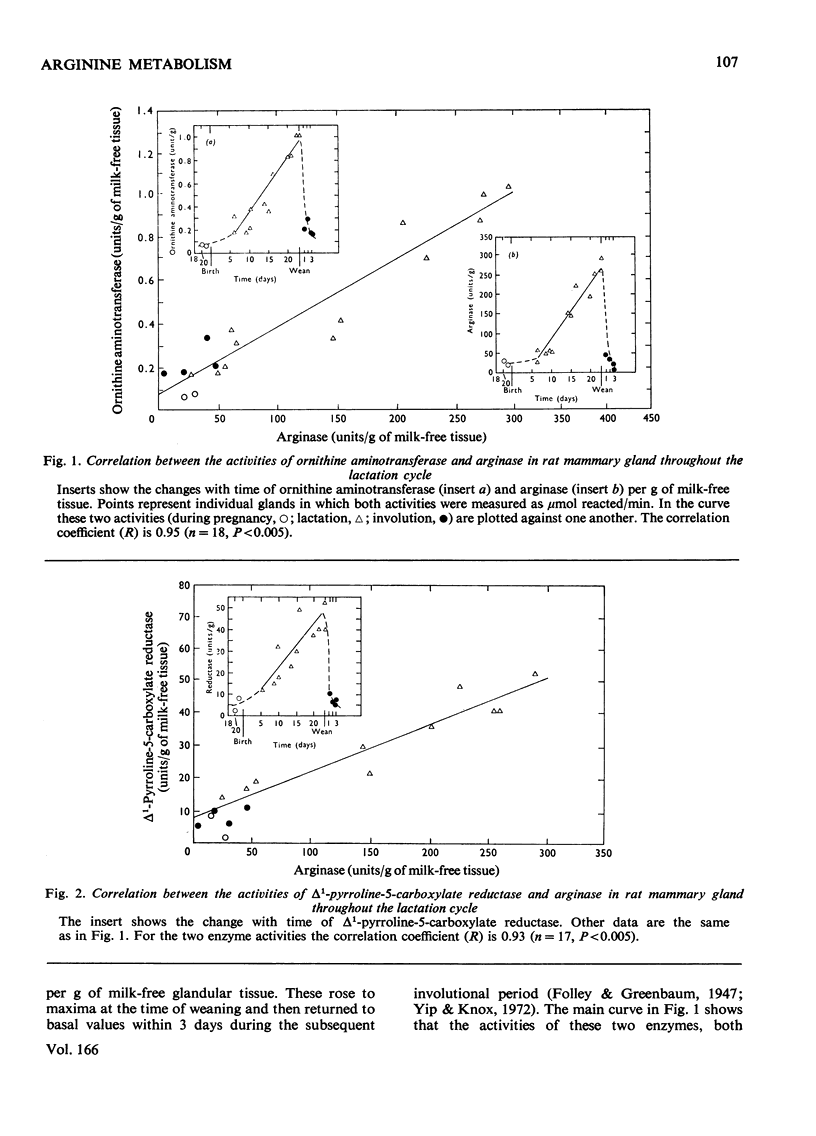
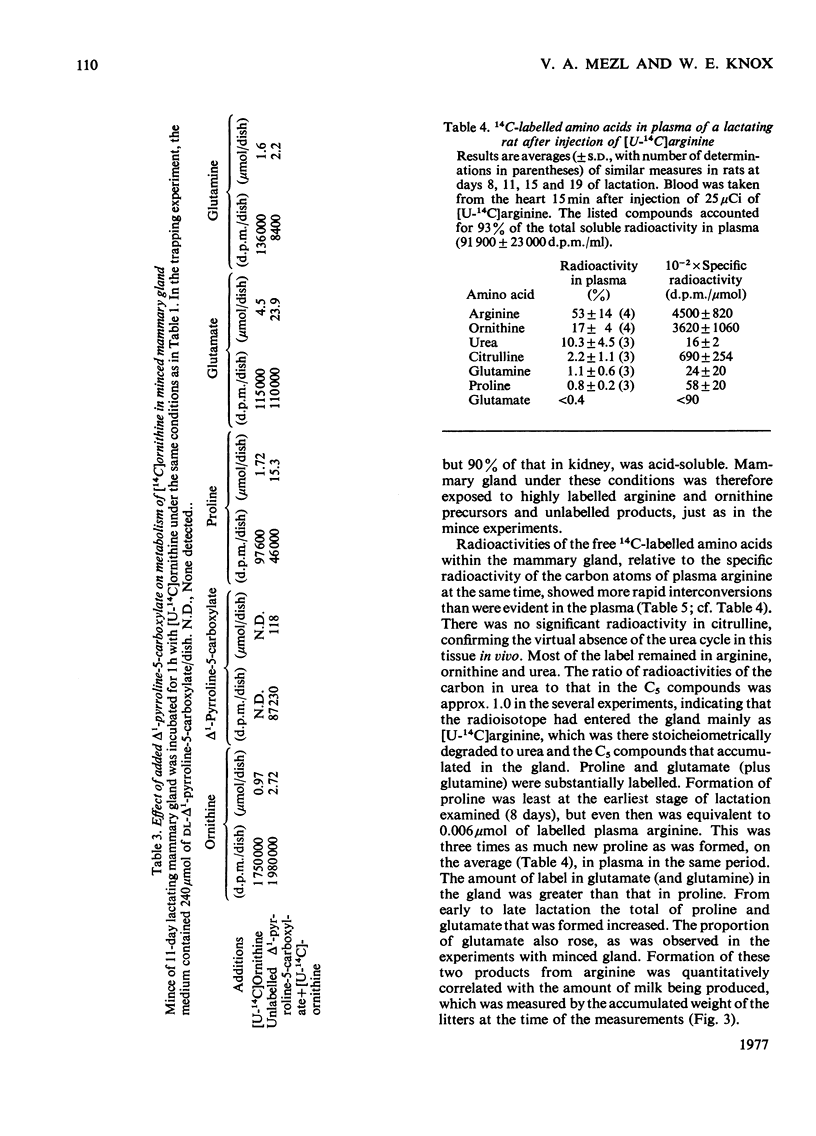
Significant activities of the four enzymes needed to convert arginine into proline and glutamate (arginase, ornithine aminotransferase, pyrroline-5-carboxylate reductase and pyrroline-5-carboxylate dehydrogenase) develop co-ordinately in lactating rat mammary glands in proportion to the increased production of milk. No enzymes were detected to carry out the reactions of proline oxidation or reduction of glutamate to pyrroline-5-carboxylate. Minces of the gland converted ornithine into proline and into glutamate plus glutamine. These conversions increased during the cycle of lactation in proportion to the increased milk production and to the content of the necessary enzymes. The minced gland did not convert labelled ornithine into citrulline, confirming the absence from the gland of a functioning urea cycle, and did not convert labelled proline or glutamate into ornithine. A metabolic flow of labelled arginine to proline and glutamate in mammary gland was confirmed in intact animals with experiments during which the specific radioactivity of proline in plasma remained below that of the proline being formed from labelled arginine within the gland. It was concluded that arginase in this tissue had a metabolic role in the biosynthesis of extra proline and glutamate needed for synthesis of milk proteins.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams E. Metabolism of proline and of hydroxyproline. Int Rev Connect Tissue Res. 1970;5:1–91. doi: 10.1016/b978-0-12-363705-5.50007-5. [DOI] [PubMed] [Google Scholar]
- Applegarth D. A., Ingram P., Hingston J., Hardwick D. F. Hyperprolinemia type II. Clin Biochem. 1974 Mar;7(1):14–28. doi: 10.1016/s0009-9120(74)90174-x. [DOI] [PubMed] [Google Scholar]
- BARRY J. M. The use of glutamine and glutamic acid by the mammary gland for casein synthesis. Biochem J. 1956 Aug;63(4):669–676. doi: 10.1042/bj0630669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson J. V., Jr, Patterson J. A. Accelerated chromatographic analysis of amino acids commonly found in physiological fluids on a spherical resin of specific design. Anal Biochem. 1965 Nov;13(2):265–280. doi: 10.1016/0003-2697(65)90196-x. [DOI] [PubMed] [Google Scholar]
- Eagle H., Washington C. L., Levy M. End product control of amino acid synthesis by cultured human cells. J Biol Chem. 1965 Oct;240(10):3944–3950. [PubMed] [Google Scholar]
- Folley S. J., Greenbaum A. L. Changes in the arginase and alkaline phosphatase contents of the mammary gland and liver of the rat during pregnancy, lactation and mammary involution. Biochem J. 1947;41(2):261–269. doi: 10.1042/bj0410261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitonde M. K., Nixey R. W. Sources of error in the determination of specific radioactivity of amino acids isolated by ion-exchange chromatography. Anal Biochem. 1972 Dec;50(2):416–429. doi: 10.1016/0003-2697(72)90050-4. [DOI] [PubMed] [Google Scholar]
- Glass R. D., Knox W. E. Arginase isozymes of rat mammary gland, liver, and other tissues. J Biol Chem. 1973 Aug 25;248(16):5785–5789. [PubMed] [Google Scholar]
- Goodman S. I., Mace J. W., Miles B. S., Teng C. C., Brown S. B. Defective hydroxyproline metabolism in type II hyperprolinemia. Biochem Med. 1974 Aug;10(4):329–336. doi: 10.1016/0006-2944(74)90036-2. [DOI] [PubMed] [Google Scholar]
- Herzfeld A., Knox W. E. The properties, developmental formation, and estrogen induction of ornithine aminotransferase in rat tissues. J Biol Chem. 1968 Jun 25;243(12):3327–3332. [PubMed] [Google Scholar]
- Herzfeld A., Mezl V. A., Knox W. E. Enzymes metabolizing delta1-pyrroline-5-carboxylate in rat tissues. Biochem J. 1977 Jul 15;166(1):95–103. doi: 10.1042/bj1660095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfeld A., Raper S. M. The heterogeneity of arginases in rat tissues. Biochem J. 1976 Feb 1;153(2):469–478. doi: 10.1042/bj1530469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Glutathione, metabolism and function via the gamma-glutamyl cycle. Life Sci. 1974 Jul 15;15(2):177–190. doi: 10.1016/0024-3205(74)90206-9. [DOI] [PubMed] [Google Scholar]
- Mepham T. B., Linzell J. L. A quantitative assessment of the contribution of individual plasma amino acids to the synthesis of milk proteins by the goat mammary gland. Biochem J. 1966 Oct;101(1):76–83. doi: 10.1042/bj1010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mepham T. B., Linzell J. L. Urea formation by the lactating goat mammary gland. Nature. 1967 Apr 29;214(5087):507–508. doi: 10.1038/214507b0. [DOI] [PubMed] [Google Scholar]
- Mezl V. A., Knox W. E. Properties and analysis of a stable derivative of pyrroline-5-carboxylic acid for use in metabolic studies. Anal Biochem. 1976 Aug;74(2):430–440. doi: 10.1016/0003-2697(76)90223-2. [DOI] [PubMed] [Google Scholar]
- Oka T., Perry J. W. Arginase affects lactogenesis through its influence on the biosynthesis of spermidine. Nature. 1974 Aug 23;250(5468):660–661. doi: 10.1038/250660a0. [DOI] [PubMed] [Google Scholar]
- Olson A. C., White L. M., Noma A. T. Scintillation counting of the ninhydrin-amino acid-C14 reaction products from an automatic amino acid analyzer. Anal Biochem. 1968 Jul;24(1):120–127. doi: 10.1016/0003-2697(68)90066-3. [DOI] [PubMed] [Google Scholar]
- Peterson D. E., Charon N. W., Johnson R. C. Quantitative decarboxylation of (14C) amino acids using automatic amino acid analyzer. Anal Biochem. 1976 May 7;72:305–309. doi: 10.1016/0003-2697(76)90534-0. [DOI] [PubMed] [Google Scholar]
- Raghupathi Reddy S. R., Campbell J. W. Arginine metabolism in insects. Role of arginase in proline formation during silkmoth development. Biochem J. 1969 Nov;115(3):495–503. doi: 10.1042/bj1150495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roets E., Verbeke R., Massart-Leën A. M., Peeters G. Metabolism of (14C)citrulline in the perfused sheep and goat udder. Biochem J. 1974 Dec;144(3):435–446. doi: 10.1042/bj1440435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers Q. R., Freedland R. A., Symmons R. A. In vivo synthesis and utilization of arginine in the rat. Am J Physiol. 1972 Jul;223(1):236–240. doi: 10.1152/ajplegacy.1972.223.1.236. [DOI] [PubMed] [Google Scholar]
- Russell D. H., McVicker T. A. Polyamine biogenesis in the rat mammary gland during pregnancy and lactation. Biochem J. 1972 Nov;130(1):71–76. doi: 10.1042/bj1300071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T. F., Strecker H. J. Synthesis of proline and hydroxyproline in human lung (WI-38) fibroblasts. Biochem J. 1975 Sep;150(3):453–461. doi: 10.1042/bj1500453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki J., Taguchi I., Yamanaka H., Mayuzumi T., Shida K. Effect of testosterone administration on the free amino acids and conversion of arginine to proline and glutamate in the rat ventral prostate. Endocrinol Jpn. 1973 Oct;20(5):455–462. doi: 10.1507/endocrj1954.20.455. [DOI] [PubMed] [Google Scholar]
- Smith A. D., Benziman M., Strecker H. J. The formation of ornithine from proline in animal tissues. Biochem J. 1967 Aug;104(2):557–563. doi: 10.1042/bj1040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeke R., Peeters G., Massart-Leën A. M., Cocquyt G. Incorporation of DL-[2-14C]ornithine and DL-[5-14C]arginine in milk constituents by the isolated lactating sheep udder. Biochem J. 1968 Feb;106(3):719–724. doi: 10.1042/bj1060719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E., McLean P. Effect of thyroidectomy on pathways of glucose metabolism in lactating rat mammary gland. Biochem J. 1967 Nov;105(2):615–623. doi: 10.1042/bj1050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Intestinal metabolism of glutamine and glutamate from the lumen as compared to glutamine from blood. Arch Biochem Biophys. 1975 Dec;171(2):662–672. doi: 10.1016/0003-9861(75)90078-8. [DOI] [PubMed] [Google Scholar]
- Windmueller H. G., Spaeth A. E. Uptake and metabolism of plasma glutamine by the small intestine. J Biol Chem. 1974 Aug 25;249(16):5070–5079. [PubMed] [Google Scholar]
- Yip M. C., Knox W. E. Function of arginase in lactating mammary gland. Biochem J. 1972 May;127(5):893–899. doi: 10.1042/bj1270893. [DOI] [PMC free article] [PubMed] [Google Scholar]


