Abstract
The interaction of Pseudomonas aeruginosa type IV pili and the glycosphingolipid asialo-GM1 (aGM1) can mediate bacterial adherence to epithelial cells, but the steps subsequent to this adherence have not been elucidated. To investigate the result of the interaction of pili and aGM1, we used polarized epithelial monolayers of Madin-Darby canine kidney (MDCK) cells in culture, which contained little detectable aGM1 on their apical surface but were able to incorporate exogenous aGM1. Compared to an untreated monolayer, P. aeruginosa PA103 displayed an eightfold increase in association with and fivefold more cytotoxicity toward MDCK cells pretreated with aGM1. Cytotoxicity of either carrier-treated or aGM1-treated monolayers required the type III secreted protein ExoU. Asialo-GM1 pretreatment of MDCK monolayers likewise augmented bacterial internalization of an isogenic invasive strain approximately fourfold. These increases were not seen in monolayers treated with GM1, the sialyated form of the glycolipid, and were inhibited by treatment with an antibody to aGM1. Also, the aGM1-mediated adhesion, cytotoxicity, and internalization required intact type IV pili since nonpiliated PA103 mutants were unaffected by aGM1 pretreatment of MDCK cells. These results demonstrate that epithelial cell injury and bacterial internalization can proceed from the same adhesin-receptor interaction, and they indicate that P. aeruginosa exoproducts solely determine the steps subsequent to adhesion.
Adherence to host cells is a crucial step in the initiation and establishment of infections caused by bacterial pathogens (14). Pseudomonas aeruginosa, an opportunistic pathogen of humans and the major cause of death in patients with cystic fibrosis (CF), utilizes type IV pili as an adhesin for binding to host cells. Early in the course of infection, P. aeruginosa encounters epithelial tissues where cells are organized in polarized monolayers with distinct apical and basolateral surfaces that differ in their protein and lipid compositions by nature of differential trafficking of membrane components (36). Pili have been demonstrated to mediate adherence to polarized and nonpolarized epithelial cell types in culture, including human buccal cells (11, 51), lung pneumocytes (7, 15), exfoliating trachael cells (52), immortalized human airway cells (16), and HeLa and Madin-Darby canine kidney (MDCK) cells (6). As a further indication of the role for pili in infection, nonpiliated mutants have been shown to have decreased virulence relative to their parental strains in animal models of pulmonary (16, 46), intraperitoneal (15), and burned skin (42) infections. Also, defects in pilus production have been shown to block entry of adherent P. aeruginosa into epithelial and endothelial cell lines (7, 39). Thus, type IV pili are considered to be factors significant in the early stages of P. aeruginosa chronic and acute infections (reviewed in reference 24).
P. aeruginosa pili are polar, flexible organelles that are composed of a single protein subunit, PilA. In vitro studies have shown that P. aeruginosa pili bind to glycolipids contained within epithelial cell membranes (3, 33) and show a specificity toward those with the Galβ1-4GlcNAc disaccharide available such as asialo-GM1(aGM1) and aGM2 (23, 40, 45). This disaccharide moiety is specifically recognized by the C-terminal domain of the PilA subunit (29, 34), the amino acid sequence of which can differ between P. aeruginosa strains (30). Several studies have indicated a direct correlation between the presence of aGM1 on host cells and P. aeruginosa adherence, thus demonstrating the role of this glycosphingolipid as a bacterial receptor. Specifically, increased bacterial binding to scarified corneal epithelium was shown to be coincident with the presence of greater quantities of aGM1 on these cells, and this adherence was attenuated by addition of an anti-aGM1 monoclonal antibody (27). It has also been determined that pilus-dependent P. aeruginosa association with immortalized nasal polyp epithelial monolayers was able to be competed specifically with aGM1 and that this glycolipid was found to be more prevalent on the surface of primary CF cells than on wild-type airway cells (41). Imundo et al. (28) further demonstrated that the aGM1-specific binding of P. aeruginosa was greater to a CF bronchial cell line than to an isogenic cell line expressing the wild-type CF transmembrane receptor. Finally, P. aeruginosa binding to regenerating respiratory epithelial cells isolated from either CF or non-CF patients was inhibited by treatment with anti-aGM1 antisera (9, 10). These previous results have led some investigators to propose that an increase in aGM1 on the epithelial surfaces of CF patients may contribute to chronic bacterial infection (28, 41). Also implied is a role for aGM1 in P. aeruginosa infection of epithelial surfaces that have been injured and are regenerating due to increased exposure of the glycosphingolipid.
With this in mind, we sought to determine whether the adherence of P. aeruginosa to epithelial cells through the interaction of pili and aGM1 could contribute to the disease process by causing cytotoxicity and/or bacterial internalization. MDCK cells, which differentiate into a well-polarized epithelial monolayer in culture, are susceptible to P. aeruginosa-induced cell damage when the bacteria are added to the apical surface (2). The bacteria appear to associate with a subset of apically exposed cells and then preferentially accumulate on the basolateral surface (2), which appears to be more susceptible to injury (19). The observed cytotoxicity correlated to virulence in a respiratory model of acute pneumonia for a variety of strains (2, 43) and for transposon-generated isogenic mutants (25). We have found that glycosphingolipid added to these cells will incorporate into their apical surface; using this system, we demonstrate that adherence of P. aeruginosa to MDCK cells mediated by the specific interaction between type IV pili and aGM1 can contribute to both the killing of epithelial cells by an ExoU-dependent mechanism as well as to internalization of invasion-proficient P. aeruginosa into epithelial cells.
MATERIALS AND METHODS
Bacterial growth conditions.
P. aeruginosa PA103 was propagated on Vogel-Bonner medium (VBM) agar (48) containing 100 μg of gentamicin or tetracycline ml−1 when appropriate. Escherichia coli XL1-Blue (for cloning) and S17-1 (for conjugation) were grown in LB broth or on LB agar containing ampicillin (50 μg ml−1), gentamicin (15 μg ml−1), or tetracycline (20 μg ml−1) when necessary. Strains used and constructed are listed in Table 1.
TABLE 1.
Strains, phage, and plasmids used in this study
| Strain, phage, or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL1-Blue | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIq ZΔM15 Tn10(Tetr)]c | Stratagene |
| S17-1 | RP4 2-Tc::Mu-Km::Tn7 pro res mod+ (Tpr Smr) | 51 |
| P. aeruginosa | ||
| PA103 | Cytotoxic respiratory clinical isolate, Fla− | 38 |
| PA103ΔpilA | PA103 with StuI/Bsu36I region of pilA replaced with a Gmr cassette | This work |
| PA103exoU::Tn5 | Loss-of-cytotoxicity mutant 8; Tn5 Gmr inserted into exoU | 34 |
| PA103pscJ::Tn5 | Loss-of-cytotoxicity mutant N; Tn5 Gmr inserted into pscJ; type III secretion defective, internalization competent | 34 |
| PA103ΔpilApscJ::Tn5 | PA103pscJ::Tn5 with StuI/Bsu36I region of pilA replaced with a Gmr cassette | This work |
| Bacteriophage | ||
| PO4 | P. aeruginosa bacteriophage | 7 |
| Plasmids | ||
| pEX100T | Allelic replacement suicide plasmid; AprsacB oriT | 49 |
| pX1918GT | Plasmid containing axylE/aacC1 insertional cassette; Apr | 49 |
| pP103 | pUC18 containing PA103 pilA | 33 |
| pLW01 | pEX100T containing a 1.2-kb HindIII fragment of pilA with the StuI/Bsu36I region replaced with the xylE/aacC1 cassette of pX1918GT | This work |
| pRIC276 | Tetr cassette; luxAB-containing plasmid | 55 |
| pLW02 | pLW01 with the XhoI/ClaI fragment of the aacC1 gene replaced with the Tetr cassette of pRIC276 | This work |
Allelic replacement of the PA103 pilA gene.
The plasmids used are listed in Table 1. For allelic replacement of the pilA gene of PA103, a 1.24-kb HindIII fragment of the vector pP103 containing the PA103 pilA gene (30) was blunted with T4 polymerase and cloned into the SmaI site of pEX100T, a suicide vector containing the Bacillus subtilis sacB gene (44). A 458-bp StuI/Bsu36I fragment comprising the majority of the pilA open reading frame was then replaced with the 2.4-kb xylE/aacC1 cassette of pX1918GT (44). The resulting plasmid (pLW01) was transformed into E. coli S17-1 for conjugal transfer to PA103. Matings were performed on LB agar overnight at 37°C, and exoconjugants were selected on VBM agar plus 100 μg of gentamicin ml−1 and 5% sucrose (to eliminate single recombinants by nature of the sacB gene contained on pEX100T). Allelic replacement of the pilA gene (to generate PA103ΔpilA) was confirmed by assay of the Gmr strains for carbenicillin sensitivity, loss of twitching motility on VBM agar, resistance to the bacteriophage PO4, and absence of PilA by anti-PilA Western blotting and for cassette insertion by Southern blotting (data not shown). pilA is the terminal pilin-related gene in its operon, so the insertion of the xylE/aacC1 cassette is not expected to affect expression of downstream genes involved in pilin biogenesis or function.
Inactivation of the pilA gene in the invasion competent strain PA103pscJ::Tn5 (26, 31) was performed in a similar fashion. However, due to the Gmr cassette contained within the Tn5 transposon used to generate this mutant, an EcoRV fragment of pLW01 that contained a portion of the aacC1 gene was replaced with a XhoI/ClaI fragment (blunted with T4 polymerase) from pRIC276 (49) that contained a Tetr cassette, creating pLW02. In this case, exconjugants were selected on VBM agar plus 100 μg of tetracycline ml−1 and 5% sucrose and then screened as described. The resulting strain was designated PA103ΔpilApscJ::Tn5.
Mammalian cell culture and glycosphingolipid addition.
Approximately 5 × 106 MDCK type II cells cultured in minimal essential medium Eagle (MEM) plus Earle’s balanced salt solution (Sigma Chemical Co.) and 5% fetal calf serum (GIBCO) were seeded onto 12-mm-diameter, 0.4-μm-pore-size polycarbonate Transwell filters (Corning Costar Corporation). Cells were grown for 3 days at 37°C in 5% CO2 to allow for the formation of highly polarized monolayers. For their use in adherence, internalization, or cytotoxicity assays, the monolayers were placed in MEM supplemented with 20 mM HEPES buffer pH 7.4 (MEM-lite) and maintained in room air.
Monosialoganglioside (GM1) or gangliotetraosyl ceramide (aGM1) (Matreya Inc.) was suspended at 10 mg ml−1 in dimethyl sulfoxide (DMSO); 5 μl of this stock solution, the appropriate dilution in DMSO, or DMSO only was added to 195 μl of fresh MEM-lite on the apical surface of a 3-day-old MDCK monolayer, followed by incubation at 37°C for 1 h with gentle rocking. After this treatment and before addition of bacteria, the monolayers were washed twice with MEM-lite.
Immunofluorescence microscopy.
MDCK cells were cultured as indicated, and immunofluorescence was performed as previously described (47), with the following modifications. A 1:1,000 dilution of rabbit polyclonal anti-GM1 or anti-aGM1 antiserum (Wako Bioproducts) diluted in 200 μl of phosphate-buffered saline (PBS) containing 0.7% fish skin gelatin (PBS-FSG) was added to the apical surface of MDCK monolayers for 60 min at 37°C. The monolayers were then washed three times with PBS, fixed with 4% paraformaldehyde in PBS for 1 h at 37°C, quenched with 75 mM NH4Cl and 20 mM glycine in PBS for 15 min at room temperature, washed with PBS, then permeabilized for 30 min at room temperature with PBS-FSG containing 0.005% saponin and 100 μg of RNase ml−1. The filters were cut from their plastic supports, and monolayers were treated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody (Jackson Immunoresearch Laboratories Inc.) diluted 1:100 in PBS-FSG-saponin plus 2 μg of propidium iodide (a fluorescent nucleic acid stain) ml−1 for 1 h at 37°C, washed in PBS, fixed with 4% paraformaldehyde in 100 mM sodium cacodylate for 15 min at room temperature, rinsed again in PBS, and then mounted under coverslips for visualization by immunofluorescence microscopy using a fluorescein or rhodamine filter set.
To measure the integrity of MDCK monolayers, an inulin diffusion assay was used. FITC-inulin (Sigma) was added to the apical medium of a filter-grown MDCK monolayer (20 μg/ml, final concentration), and the percentage that diffused into the basal medium was measured with a fluorescence plate reader (excitation of 485 nm and emission of 530 nm) after incubation for 2 h at 37°C. At this time, 1% or less of the apically applied FITC inulin diffused across an intact monolayer into the basal medium.
Antibody treatment for blocking experiments.
Rabbit anti-aGM1 polyclonal antisera (Wako Bioproducts) was added to glycosphingolipid- or DMSO-treated monolayers prior to the addition of bacteria. To do so, the monolayers were washed with PBS and treated by apical addition of a 1:5 or 1:10 dilution of antibody in 100 μl of PBS for 2 h at 37°C. The cells were then washed twice with MEM-lite and placed in fresh medium prior to the addition of bacteria for association or cytotoxicity assays.
Association, internalization, and cytotoxicity assays.
P. aeruginosa strains were grown overnight at 37°C in LB broth without shaking, pelleted at room temperature, resuspended in MEM-lite, and then diluted to an A600 of 0.1; 150 μl of this suspension (containing approximately 1.5 × 107 CFU as measured by dilution plating) was added to the apical surface of MDCK monolayers in MEM-lite (multiplicity of infection [MOI] of 10).
Bacterial association with 3-day-old MDCK monolayers was measured after 2 h of cocultivation at 37°C by washing the monolayers three times with MEM-lite, cutting each filter from its support, rinsing the filter again in MEM-lite, and then placing the filter in 1 ml of MEM-lite containing 0.25% Triton X-100 to lyse the MDCK cells. After 15 min at 25°C, this mixture was vortexed with glass beads for 15 s, the lysis procedure was repeated, and the lysate was serially diluted onto LB agar for bacterial quantitation. Internalization was assessed by an aminoglycoside exclusion assay after incubation of the bacteria with 3-day-old MDCK monolayers for 3 h at 37°C (MOI of 10). At this time, the cells were washed three times with MEM-lite and incubated with amikacin (400 mg ml−1) in MEM-lite. After 2 h at 37°C, the MDCK cells were washed again in MEM-lite and lysed by the procedure described for the association assay, and internalized bacteria were quantitated by dilution plating. Each association or internalization assay was performed in triplicate, and the number of bacteria recovered in each sample was standardized to the number of bacteria in the inoculum (quantitated by dilution plating onto LB agar).
For cytotoxicity assays, approximately 1.5 × 107 bacteria were added to the apical medium of a treated MDCK monolayer (MOI of 10). After incubation for 3 to 3.5 h at 37°C in room air, cytotoxicity was quantitated by the release of lactate dehydrogenase (LDH) into the medium. Aliquots of the apical and basal medium were removed and assayed for LDH activity by measuring pyruvate reduction as instructed by the manufacturer (Sigma). LDH activity represents the total apical and basal pyruvate reduced per milliliter after subtraction of LDH released by exposure of cells to medium only (MEM-lite). Each assay was performed on three individual monolayers, and the error bars represent standard deviation from the mean.
RESULTS
Exogenous glycolipids can be incorporated into the apical membrane of MDCK cells.
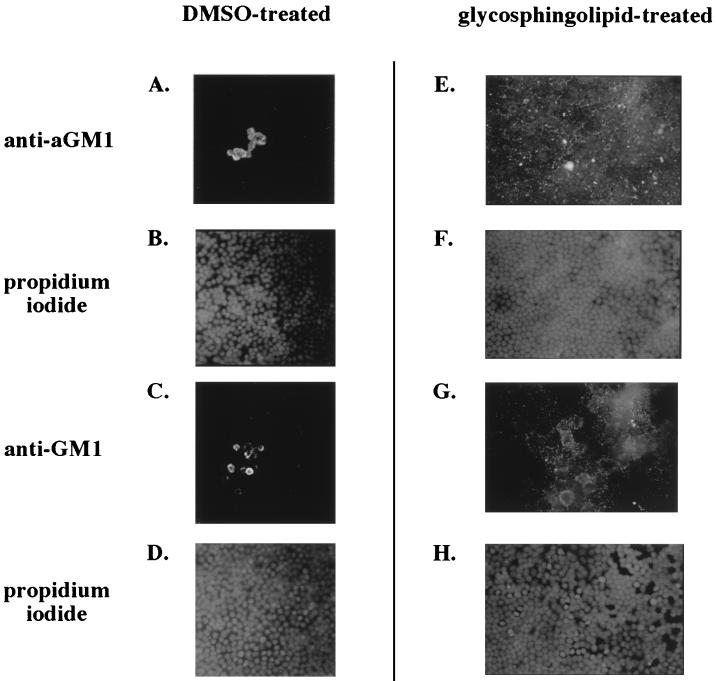
P. aeruginosa can adhere to and subsequently cause damage to, or become internalized in, filter-grown MDCK cells (2, 19, 26). To determine the contribution of aGM1 receptor to these processes, the apical surface of uninfected cells grown on permeable supports was stained with an anti-aGM1 antibody and visualized by immunofluorescence microscopy. The majority of the cells in a monolayer showed no significant staining, but isolated clumps of cells were heavily stained with anti-aGM1 (Fig. 1A). These regions consisted of approximately 5 to 20 adjacent cells and comprised less than 5% of the total cells in an MDCK monolayer. Examination of the anti-aGM1-stained cells at a higher magnification (×1,000) revealed a punctate distribution of the aGM1 signal on their apical surface. These stained cells did not appear different from other MDCK cells in the monolayer when stained with the nucleic acid dye propidium iodide (Fig. 1B). Although the opaque nature of the permeable filters prevented analysis by phase microscopy, the propidium iodide staining pattern was indicative of a uniform and intact monolayer. Furthermore, the cells that stained with anti-aGM1 were viable since they excluded a membrane-impermeable fluorescent dye (ethidium homodimer-1; Molecular Probes) when it was added to cells prior to permeabilization (data not shown). A similar staining pattern to that obtained with the anti-aGM1 antibody was seen when MDCK monolayers were treated with the fluorescein-labeled lectin peanut agglutinin, which recognizes the carbohydrate available in aGM1 (data not shown).
FIG. 1.
Immunofluorescent visualization of aGM1 or GM1 on the apical surface of MDCK monolayers with or without glycosphingolipid pretreatment. Antisera directed against aGM1 (A and E) or GM1 (C and G) was added apically prior to cell fixation and permeabilization, and an FITC-linked secondary antibody was used to visualize antibody staining by fluorescence microscopy at a magnification of ×400. MDCK monolayers were treated with DMSO carrier (A to D), aGM1 (E and F), and GM1 (G and H). Panels B, D, F, and H represent the propidium iodide staining of the fields seen in panels A, C, E, and G, respectively.
When a polyclonal antiserum directed against the sialylated ganglioside (anti-GM1) was used, the results resembled those obtained with the anti-aGM1 antibody. Only isolated groups of cells displayed anti-GM1 staining; the remainder showed little or no signal (Fig. 1C). The monolayer also appeared intact in this case (Fig. 1D). It should be noted that although this antibody is generated against the ganglioside GM1, it can also recognize the asialylated form of the glycolipid (aGM1).
The relative lack of aGM1 or GM1 on the apical surface of MDCK cells allowed us to determine if glycolipid could be added and incorporated into a monolayer. Glycosphingolipid (250 μg of GM1 or aGM1 ml−1) was added to the apical medium of MDCK cells, and its incorporation into the apical surface of the monolayer was monitored by immunofluorescence microscopy. As displayed in Fig. 1, anti-GM1 or anti-aGM1 staining on the apical surface of an MDCK monolayer treated with glycosphingolipid was much greater than that of a DMSO-treated monolayer (compare Fig. 1E and G to Fig. 1A and C), a clear indication that the added glycolipid was associated with the apical membrane of the epithelial cells. At a magnification of ×400, the antiglycosphingolipid staining with either antibody was punctate and dispersed inequally throughout the apical surface of the monolayer. At higher magnification (×1,000), the staining of exogenous aGM1 still appeared punctate and not concentrated on particular cells (data not shown). This staining was obviously distinct from that of the clusters of aGM1-stained cells that were evident in the monolayer before addition of glycosphingolipid. Again, propidium iodide staining was indicative of an undamaged MDCK monolayer (Fig. 1D and H). Also, the addition of aGM1 or GM1 did not increase the permeability of the monolayer to the small molecule FITC-inulin (data not shown), nor did it cause measurable cell damage as assayed by LDH release into the medium (see Fig. 3).
FIG. 3.
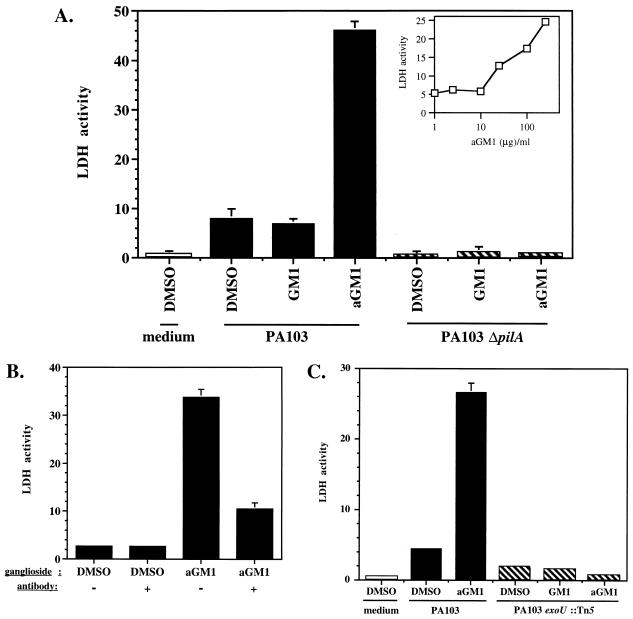
P. aeruginosa cytotoxicity was specifically increased by binding of pili to aGM1. (A) PA103, PA103ΔpilA, or medium only was added to MDCK monolayers that had been pretreated with exogenous GM1, aGM1, or DMSO carrier. Cytotoxicity was measured in triplicate by LDH release after 3.5 h of incubation with the bacteria; error bars indicate standard deviation. The inset shows that the amount of cytotoxicity measured was dependent on the amount of aGM1 added to the MDCK monolayers. Apically added PA103 was incubated for 3.5 h with the MDCK monolayers (MOI = 10) that had been pretreated with DMSO carrier or the indicated amounts of aGM1 for 1 h. (B) Cytotoxicity attributed to exogenous aGM1 was blocked by anti-aGM1 treatment. The apical surface of an MDCK cell monolayer was pretreated with DMSO carrier or aGM1 followed by anti-aGM1 antibody or PBS. Apically added PA103 was then incubated with the MDCK monolayer for 3 h (MOI = 10). Cell death was measured in triplicate by LDH release; error bars, present for all samples, indicate standard deviation. (C) ExoU is required for aGM1-dependent cytotoxicity. MDCK monolayers pretreated with exogenous GM1, aGM1, or DMSO were exposed to PA103, PA103exoU::Tn5, or medium only for 3 h. Cytotoxicity was quantitated by LDH release; error bars, shown for all samples, indicate standard deviation from the average of three replicates.
These experiments demonstrate that the amount of glycosphingolipid present on the apical surface of MDCK monolayer can be increased by experimental manipulation. Although the mechanism by which the exogenous glycolipid becomes incorporated into the MDCK apical surface is not clear, this system enables measurement of the biological consequences of P. aeruginosa binding to the receptor aGM1 in the context of host epithelial cells.
Treatment of MDCK monolayers with aGM1 can increase the adherence of P. aeruginosa in a pilus-dependent manner.
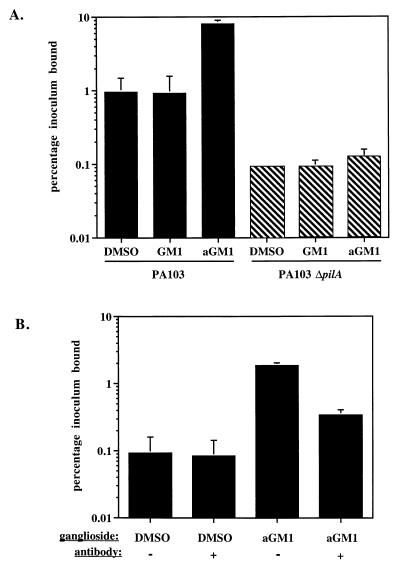
Isolated P. aeruginosa pili have been demonstrated to bind to aGM1 in vitro, and adherence of whole bacteria has been correlated to the presence of aGM1 on several mammalian cell types (10, 27, 28, 41). To determine if aGM1 in the context of an MDCK monolayer could increase P. aeruginosa binding, an MDCK monolayer treated with DMSO carrier or with 250 μg of GM1 or aGM1 ml−1 was apically infected with the P. aeruginosa PA103, and the number of adherent bacteria was quantitated. After 2 h of incubation, approximately 1% of the added inoculum adhered to the apical surface of MDCK cells treated with DMSO carrier, while eightfold more, or 8% of the inoculum, bound to monolayers pretreated with aGM1 (see Fig. 3A). This effect was specific to aGM1, since association with a monolayer receiving GM1 was approximately equal to that of a monolayer treated with the DMSO carrier (see Fig. 3A). The ganglioside GM1 is identical to aGM1 except that it contains a sialic acid residue protecting the Galβ1-4GlcNAc carbohydrate which is recognized by P. aeruginosa pili. To determine if the bacterial interaction with aGM1 was dependent on the presence of pili, we produced a PA103 mutant devoid of pili (PA103ΔpilA) by insertional inactivation of the pilA gene and assayed its adherence. Association of this nonpiliated mutant to DMSO-treated MDCK monolayers was reduced 10-fold relative to the wild-type strain, as only 0.09% of the inoculum bound (Fig. 2A). Also, the number of adherent nonpiliated bacteria was not significantly changed by prior treatment of the MDCK cells with aGM1 or GM1 (Fig. 2A). Thus, the increased bacterial association to MDCK cells treated with exogenous aGM1 required P. aeruginosa pili.
FIG. 2.
aGM1 specifically increased the pilus-dependent association of PA103 with MDCK cells. (A) Association was assayed 2 h after addition of PA103 or PA103ΔpilA to MDCK monolayers (MOI = 10) that had been pretreated with exogenous GM1, aGM1, or DMSO carrier. Association is expressed as the average percentage of the bacteria initially added, and error bars represent the standard deviation of three replicate assays. (B) Anti-aGM1 antiserum blocked the increased association due to exogenous aGM1. The apical surface of an MDCK cell monolayer was pretreated with DMSO or aGM1 and then incubated with anti-aGM1 antibody or PBS carrier. Apically added PA103 was incubated with the monolayer for 2 h (MOI = 10), and measured cell association is expressed as percentage of the bacteria initially added. Error bars, shown for all samples, represent the standard deviation of three replicate assays.
To further assess the specificity of this interaction, DMSO- or aGM1-treated MDCK monolayers were incubated with anti-aGM1 antibody. This antibody reduced the binding of PA103 to cells treated with aGM1 approximately twofold (Fig. 2B). Interestingly, there was no significant effect of anti-aGM1 treatment on the association of PA103 to DMSO-treated MDCK cells. This result is consistent with our observation that the apical surface of these monolayers does not significantly stain with anti-aGM1 antibody (Fig. 1A) and suggests that aGM1 is not a significant receptor on the apical surface of an MDCK monolayer. These observations are in agreement with published results demonstrating that relative to membranes of other cell types, little aGM1 is contained in MDCK cell membranes (35).
The interaction of pili and aGM1 can mediate cytotoxicity through an ExoU-dependent process.
To determine if P. aeruginosa binding to host cells through the interaction of pili and aGM1 could contribute to bacterium-mediated cytotoxicity, we added PA103 or PA103ΔpilA to MDCK monolayers pretreated with aGM1, GM1, or DMSO carrier and quantitated cell damage by LDH release. Consistent with previously published data (31), the addition of PA103 to control MDCK cells caused significant damage compared to monolayers that were not exposed to bacteria (7.9 versus 0.7 U of LDH ml−1 released [Fig. 3A]). A further fivefold increase in LDH release, to 46 U ml−1, was observed upon addition of PA103 to monolayers pretreated with 250 μg of aGM1 ml−1 (Fig. 3A). In contrast, incubation of PA103 with MDCK cells pretreated with 250 μg of GM1 ml−1 caused no more cytotoxicity than with DMSO treatment, as 6.9 U of LDH ml−1 was released. The increase in cytotoxicity due to aGM1 addition was concentration dependent, and the amount of LDH released from MDCK cells correlated to the quantity of aGM1 (0 to 250 μg ml−1) that was present in the pretreatment (Fig. 3A, inset).
As with adherence, cytotoxicity due to aGM1 treatment was dependent on the presence of pili. PA103ΔpilA caused no significant damage to MDCK monolayers (0.5 U of LDH ml−1 released), and no increase in cytotoxicity was observed when the monolayers were pretreated with aGM1 or GM1 (Fig. 3A). This result also indicates that aGM1 treatment or incorporation itself caused no damage to MDCK cells.
As an additional measure of the specificity of the PA103 pili-aGM1 interaction, anti-aGM1 antibody was used in an attempt to block MDCK cell damage. Antibody treatment of MDCK monolayers that had received aGM1 caused a threefold reduction in the ability of PA103 to cause cytotoxicity (Fig. 3B). However, without glycosphingolipid addition, the cytotoxicity of PA103 was unaltered by an identical anti-aGM1 treatment. While this finding is indicative of the specificity for aGM1, it also suggests that this receptor may not significantly contribute to cell damage in untreated MDCK monolayers. Similar results were obtained in the bacterial adherence assay, where there was also minimal effect of anti-aGM1 treatment on DMSO-treated MDCK monolayers (Fig. 2B).
Damage to MDCK cells was previously shown to require the putative cytotoxin ExoU, which is exported by type III secretion (17, 25). This was also the case with aGM1-treated MDCK cells. A PA103 mutant unable to produce ExoU due to a transposon insertion into the structural gene (PA103exoU::Tn5; mutant 8 in reference 25) had minimal cytotoxicity toward MDCK cells treated with DMSO carrier. Cytotoxicity was not increased when the MDCK monolayer was pretreated with aGM1 or GM1 (Fig. 3C). As in the previous experiment, the cytotoxicity of the wild-type strain was increased approximately fivefold in aGM1-treated MDCK cells, but the total amount of LDH released in this experiment was reduced due to the shorter incubation time of the bacteria with the MDCK cells (3 h versus 3.5 h). Since ExoU is required for MDCK cell damage with or without aGM1 supplementation, the same mechanism of cytotoxicity appears to be utilized in each case.
The interaction of pili and aGM1 can mediate the internalization of PA103 into epithelial cells.
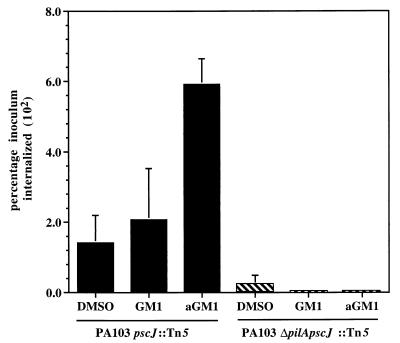
P. aeruginosa has been demonstrated to be internalized into epithelial cells (7, 20, 21, 37–39), though this process is not a prerequisite for cytotoxicity (13). We sought to determine if the adherence of P. aeruginosa to MDCK cells via pili binding to aGM1 could also act as an initial step in the internalization process. Since strain PA103 is not internalized into epithelial cells as efficiently as many other P. aeruginosa strains, an invasion-competent isogenic mutant defective in type III secretion, PA103pscJ::Tn5 (mutant N in reference 31), was used to quantify internalization into MDCK cells by an aminoglycoside exclusion assay. The inactivation of the type III secretion system in this mutant has been shown to augment PA103 internalization presumably by preventing translocation of an anti-internalization factor (22, 26). While 1.4% of the added PA103pscJ::Tn5 was detected inside MDCK cells treated with DMSO carrier, this was increased approximately fourfold to 5.9% by pretreatment of the monolayer with aGM1 (Fig. 4). Pretreatment of a monolayer with GM1 did not have a substantial effect on internalization, and 2.1% of the inoculum was taken into the epithelial cells (Fig. 4).
FIG. 4.
Bacterial internalization into MDCK cells was specifically augmented by exogenous aGM1. PA103pscJ::Tn5 and PA103ΔpilApscJ::Tn5 were added to MDCK monolayers (MOI = 10) after treatment with GM1, aGM1, or DMSO. Internalization was assayed by aminoglycoside exclusion after 3 h and is expressed as percentage of the bacteria initially added. Error bars, shown for all samples, represent standard deviation from the average of three replicate samples.
To assess if the increase in internalization due to aGM1 treatment of the MDCK monolayer was dependent on the presence of pili, the pilA gene of the invasion-competent mutant of PA103 was inactivated. The resulting strain, PA103ΔpilApscJ::Tn5, was internalized into DMSO-treated MDCK cells approximately sixfold less effectively than PA103pscJ::Tn5 (0.23% of the inoculum), and treatment of MDCK monolayers with GM1 or aGM1 did not alter the internalization of this nonpiliated strain (Fig. 4). Thus, as with cytotoxicity, internalization of P. aeruginosa into epithelial cells was mediated by the specific interaction of type IV pili and aGM1.
DISCUSSION
The type IV pili of P. aeruginosa are thought to assist in establishing an interaction between the pathogen and epithelial cells during the early stages of infection. This work indicates that the association of P. aeruginosa pili and the aGM1 present on epithelial cells can contribute to the pathogenic process by leading to host cell damage or to bacterial internalization. To demonstrate this, we used filter-grown MDCK cell monolayers which incorporate exogenous aGM1 or GM1 into their apical surfaces, as indicated by an increase in immunostaining with an antiglycosphingolipid antibody in comparison to a monolayer not receiving glycosphingolipid (Fig. 1). Monolayers pretreated with aGM1 were susceptible to eightfold more P. aeruginosa adherence, fivefold more cytotoxicity, and fourfold greater bacterial internalization than monolayers pretreated with DMSO carrier (Fig. 2A, 3A, and 4). Several criteria indicate that these effects were due to the specific interaction between type IV pili and aGM1. First, pretreatment of MDCK monolayers with the ganglioside GM1, which differs from aGM1 in that it has a sialylic acid protecting the Galβ1-4GlcNAc disaccharide to which P. aeruginosa pili bind (42, 48), did not increase bacterial adherence, cytotoxicity, or internalization (Fig. 2A, 3A, and 4). Second, the elimination of pili by disruption of the PA103 pilA structural gene abolished the increases in adherence, cytotoxicity, and internalization due to aGM1 pretreatment (Fig. 2A, 3A, and 4). Finally, the effect of aGM1 pretreatment on adherence and cytotoxicity was attenuated by a polyclonal antibody directed against anti-aGM1 (Fig. 2B and 3B). Thus, we have demonstrated that P. aeruginosa adherence to aGM1 on epithelial cells via their type IV pili can act as an initial step leading to cell damage or to internalization.
This observation that cytotoxicity or internalization can occur subsequent to the same adherence step (pili binding to aGM1) is significant since it indicates that the attributes of the bacterial strain determine the pathway taken. It has been postulated that these events utilize entirely separate pathways, based on the demonstration that cytotoxicity does not require internalization (13, 18). Our data suggest, however, that each of these can proceed from an identical adhesin-receptor interaction and the outcome depends on factors produced by the bacterium. Given that P. aeruginosa isolates differ in the types and amounts of exoproteins produced and that some of these factors act as cytotoxins (17, 25) whereas others influence internalization (13, 26), it is reasonable that the properties of a particular strain, and not the type of host cell interaction, define the progression of an infection.
Though we demonstrate the relevance of P. aeruginosa binding to aGM1, the participation of other host cell receptors in cytotoxicity or internalization is not excluded. In fact, our finding that antibodies to aGM1 do not abrogate adherence or cytotoxicity in DMSO-treated MDCK cells (Fig. 2B and 3B) suggests that receptors other than aGM1 are largely responsible for P. aeruginosa-mediated cytotoxicity or internalization in this particular cell type. These may be other glycolipids or glycoproteins since mutant MDCK cell monolayers that had altered apical surface glycosylation (concanavalin A-resistant or ricin-resistant cells) were previously shown to be less susceptible to PA103-induced cell damage than wild-type MDCK monolayers (2). Whether these other receptors colocalize with the aGM1-staining cells is under investigation. Regardless, our results indicate that type IV pili appear to be the dominant adhesin toward MDCK monolayers since nonpiliated strains were noncytotoxic and approximately 10-fold less adherent and invasive than wild-type bacteria. In other cell types this may not be the case, as it has been shown that the lipopolysaccharide-CF transmembrane regulator interaction may also play an important role in bacterial uptake (41, 42).
In addition to their roles as adhesins, P. aeruginosa pili may also contribute to the pathogenic process in other ways. Type IV pili are responsible for twitching motility, a type of surface locomotion that is thought to depend on the dynamic extension and retraction of pili (35). Interestingly, mutant strains with pili that appear nonretractile not only lost twitching motility (5, 50) but also had reduced adherence to and cytotoxicity toward epithelial cells as well as decreased virulence in a mouse pneumonia model (8). This finding suggests that additional functions of pili, presumably dependent on their extension and retraction, are necessary in host cell interactions. This is further supported by our observation that adhesion of non-piliated PA103 mutants due to centrifugation of the bacteria onto MDCK monolayers was not sufficient to rescue the noncytotoxic phenotype (48a). One possibility is that the dynamic nature of pili assists in type III effector translocation. Our work demonstrates that secretion of the putative cytotoxin ExoU by a type III mechanism (17, 25) was required for the PA103-mediated damage to MDCK cells due to the binding of pili to added aGM1 (and without added aGM1) (Fig. 3). Precedence for a requirement for type IV pili function in type III effector translocation has been noted in the interaction of enteropathogenic E. coli and epithelial cells. In this case, bundle-forming pili have been postulated to initiate host cell contact prior to the establishment of more intimate association via the bacterial adhesin intimin and the bacterium-produced receptor Tir (1, 4, 32). This intimate adherence ultimately results in the formation of the attaching and effacing lesions, which require type III-secreted effector molecules (12). For P. aeruginosa adherence, pili binding to aGM1 could lead to closer contact and promote secretion of factors such as ExoU.
Our observations suggest a model for the establishment of a P. aeruginosa infection. The polarized sorting of molecules in highly differentiated epithelial layers results in the paucity of aGM1 or other receptor on their apical surfaces, and thus these cells are relatively resistant to infection by P. aeruginosa. Consistent with this, it has previously been shown that epithelial cell polarity affects susceptibility to P. aeruginosa injury and invasion; in polarized MDCK cells, the basolateral surface is more sensitive to damage (19). The present studies, which demonstrate only a small amount of anti-aGM1 immunofluorescence staining of untreated MDCK monolayers and a lack of an effect of anti-aGM1 treatment on adherence to and cytotoxicity toward DMSO-treated monolayers, support this hypothesis. However, apical aGM1 could become available by epithelial monolayer injury and regeneration (10) or in the context of CF (41). The increased amount of aGM1 receptor could be recognized by P. aeruginosa expressing type IV pili, and that adherence could result in either cell injury or bacterial internalization, depending on the factors produced by the particular strain. Cytotoxicity would subsequently allow the bacteria to gain access to the basolateral surface of epithelial cells, where they would be able to more rapidly spread and eventually disseminate to distant sites of infection. Alternatively, as suggested by others (37, 38), increased P. aeruginosa internalization may assist in the establishment of other forms of infection. Experiments to test this model are in progress.
ACKNOWLEDGMENTS
We thank members of the Engel laboratory for critical reading of the manuscript and for scientific advice.
J.C.C. was supported by the Bank of America-Gianinni Foundation. J.N.E. was supported by grants from the University Wide AIDS Research Program, the NIH (R01 AI42806), and the American Lung Association. K.E.M. was supported by NIH grant R01 HL55980. J.N.E. is a Career Investigator of the American Lung Association.
REFERENCES
- 1.Anantha R P, Stone K D, Donnenberg M S. Role of BfpF, a member of the PilT family of putative nucleotide-binding proteins, in type IV pilus biogenesis and in interactions between enteropathogenic Escherichia coli and host cells. Infect Immun. 1998;66:122–131. doi: 10.1128/iai.66.1.122-131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apodaca G, Bomsel M, Lindstedt R, Engel J, Frank D, Mostov K, Wiener-Kronish J. Characterization of Pseudomonas aeruginosa-induced MDCK cell injury: glycosylation defective host cells are resistant to bacterial killing. Infect Immun. 1995;63:1541–1551. doi: 10.1128/iai.63.4.1541-1551.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker J, Hansson G, Leffler H, Riise G, Svanborg-Eden C. Glycosphingolipid receptors for Pseudomonas aeruginosa. Infect Immun. 1990;58:2361–2366. doi: 10.1128/iai.58.7.2361-2366.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bieber D, Ramer S W, Wu C Y, Murray W J, Tobe T, Fernandez R, Schoolnik G K. Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science. 1998;280:2114–2118. doi: 10.1126/science.280.5372.2114. [DOI] [PubMed] [Google Scholar]
- 5.Bradley D E. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974;51:489–492. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- 6.Cervin M A, Simpson D A, Smith A L, Lory S. Differences in eucaryotic cell binding of Pseudomonas. Microb Pathog. 1994;17:291–299. doi: 10.1006/mpat.1994.1075. [DOI] [PubMed] [Google Scholar]
- 7.Chi E, Mehl T, Nunn D, Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991;59:822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Comolli J C, Hauser A R, Waite L, Whitchurch C B, Mattick J S, Engel J N. Pseudomonas aeruginosa gene products PilU and PilT are required for cytotoxicity in vitro and virulence in a mouse model of acute pneumonia. Infect Immun. 1999;67:3625–3630. doi: 10.1128/iai.67.7.3625-3630.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Bentzmann S, Plotkowski C, Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am J Respir Crit Care Med. 1996;154:S155–S162. doi: 10.1164/ajrccm/154.4_Pt_2.S155. [DOI] [PubMed] [Google Scholar]
- 10.de Bentzmann S, Roger P, Dupuit F, Bajolet-Laudinat O, Fuchey C, Plotkowski M C, Puchelle E. Asialo GM1 is a receptor for Pseudomonas aeruginosa adherence to regenerating respiratory epithelial cells. Infect Immun. 1996;64:1582–1588. doi: 10.1128/iai.64.5.1582-1588.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doig P, Todd T, Sastry P A, Lee K K, Hodges R S, Paranchych W, Irvin R T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988;56:1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnenberg M S. Entry of enteropathogenic Escherichia coli into host cells. Curr Top Microbiol Immunol. 1996;209:79–98. doi: 10.1007/978-3-642-85216-9_5. [DOI] [PubMed] [Google Scholar]
- 13.Evans D J, Frank D W, Finck-Barbançon V, Wu C, Fleiszig S M. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Falkow S, Isberg R R, Portnoy D A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- 15.Farinha M A, Conway B D, Glasier L M G, Ellert N W, Irvin R T, Sherburne R, Paranchych W. Alteration of the pilin adhesin of Pseudomonas aeruginosa PAO results in normal pilus biogenesis but a loss of adherence to human pneumocyte cells and decreased virulence in mice. Infect Immun. 1994;62:4118–4123. doi: 10.1128/iai.62.10.4118-4123.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Finck-Barbancon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M J, Wu C, Mende-Mueller L, Frank D. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 18.Fleiszig S, Vallas V, Jun C, Mok L, Balkovetz D, Roth M, Mostov K. Susceptibility of epithelial cells to Pseudomonas aeruginosa invasion and cytotoxicity is upregulated by hepatocyte growth factor. Infect Immun. 1998;66:3443–3446. doi: 10.1128/iai.66.7.3443-3446.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fleiszig S M, Evans D J, Do N, Vallas V, Shin S, Mostov K. Epithelial cell polarity affects susceptibility to Pseudomonas aeruginosa invasion and cytotoxicity. Infect Immun. 1997;65:2861–2867. doi: 10.1128/iai.65.7.2861-2867.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fleiszig S M, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleiszig S M, Zaidi T S, Pier G B. Pseudomonas aeruginosa invasion of and multiplication within corneal epithelial cells in vitro. Infect Immun. 1995;63:4072–4077. doi: 10.1128/iai.63.10.4072-4077.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K, Kanada D, Sawa T, Yen T S B, Frank D. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S K, Berk R S, Masinick S, Hazlett L D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hahn H P. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 25.Hauser A R, Kang P J, Engel J. PepA, a novel secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]
- 26.Hauser A R, Kang P J, Fleiszig S J M, Mostov K, Engel J. Defects in type III secretion correlate with internalization of Pseudomonas aeruginosa by epithelial cells. Infect Immun. 1998;66:1413–1420. doi: 10.1128/iai.66.4.1413-1420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hazlett L D, Barrett M S, R, Rosol K. Evidence for asialo GM1 as a corneal glycolipid receptor for Pseudomonas aeruginosa adhesion. Infect Immun. 1993;61:5164–5173. doi: 10.1128/iai.61.12.5164-5173.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imundo L, Barasch J, Prince A, Al-Awqati Q. Cystic fibrosis epithelial cells have a receptor for pathogenic bacteria on their apical surface. Proc Natl Acad Sci USA. 1995;92:3019–3023. doi: 10.1073/pnas.92.7.3019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irvin R T, Doig P, Lee K K, Sastry P A, Paranchych W, Todd T, Hodges R S. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural subunit contains a human epithelial cell-binding domain. Infect Immun. 1989;57:3720–3726. doi: 10.1128/iai.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson K, Parker M L, Lory S. Nucleotide sequence and transcriptional initiation site of two Pseudomonas aeruginosa pilin genes. J Biol Chem. 1986;261:15703–15708. [PubMed] [Google Scholar]
- 31.Kang P J, Hauser A R, Apodaca G, Fleiszig S, Wiener-Kronish J, Mostov K, Engel J N. Identification of Pseudomonas aeruginosa genes required for epithelial cell injury. Mol Microbiol. 1997;24:1249–1262. doi: 10.1046/j.1365-2958.1997.4311793.x. [DOI] [PubMed] [Google Scholar]
- 32.Kenny B, DeVinney R, Stein M, Reinsheid J, Frey E A, Finaly B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 33.Krivan H C, Roberts D D, Ginsburg V. Many pulmonary pathogenic bacteria bind specifically to the carbohydrate sequence GalNAcβ1-4Gal found in some glycolipids. Proc Natl Acad Sci USA. 1988;85:6157–6161. doi: 10.1073/pnas.85.16.6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee K K, Sheth H B, Wong W Y, Sherburne R, Paranchych W, Hodges R S, Lingwood C A, Krivan H, Irvin R T. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 35.Mattick J S, Alm R A. Common architecture of type 4 fimbriae and complexes involved in macromolecular traffic. Trends Microbiol. 1995;3:411–413. [Google Scholar]
- 36.Mostov K E. Regulation of protein traffic in polarized epithelial cells. Histol Histopathol. 1995;10:423–431. [PubMed] [Google Scholar]
- 37.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pier G B, Grout M, Zaidi T S, Olsen J C, Johnson L G, Yankaskas J R, Goldberg J B. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science. 1996;271:64–67. doi: 10.1126/science.271.5245.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plotkowski M-C, Saliba A M, Pereira S H M, Cervante M P, Bajolet-Laudinat O. Pseudomonas aeruginosa selective adherence to and entry into human endothelial cells. Infect Immun. 1994;62:5456–5463. doi: 10.1128/iai.62.12.5456-5463.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramphal R, Carnoy C, Fievre S, Michalski J C, Houdret N. Pseudomonas aeruginosa recognizes carbohydrate chains containing type 1 (Galβ1-3GlcNAc) or type 2 (Galβ1-4GlcNAc) dissaccharide units. Infect Immun. 1991;59:700–704. doi: 10.1128/iai.59.2.700-704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saiman L, Prince A. Pseudomonas aeruginosa pili bind to asialoGM1 which is increased on the surface of cystic fibrosis epithelial cells. J Clin Investig. 1993;92:1875–1880. doi: 10.1172/JCI116779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sata H, Okinda K, Saiton H. Role of pilin in the pathogenesis of Pseudomonas aeruginosa burn infection. Microbiol Immunol. 1988;32:131–139. doi: 10.1111/j.1348-0421.1988.tb01372.x. [DOI] [PubMed] [Google Scholar]
- 43.Sawa T, Ohara M, Kurahashi K, Twining S, Frank D, Doroques D, Long T, Gropper M, Wiener-Kronish J. In vitro cellular cytotoxicity predicts Pseudomonas aeruginosa virulence in lung infections. Infect Immun. 1998;66:3242–3249. doi: 10.1128/iai.66.7.3242-3249.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer H P, Hoang T T. An improved system for gene replacement and xylE fusion analysis in Pseudomonas aeruginosa. Gene. 1995;158:15–22. doi: 10.1016/0378-1119(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 45.Sheth H B, Lee K K, Wong W Y, Srivastava G, Hindsgaul O, Hodges R S, Paranchych W, Irvin R T. The pili of Pseudomonas aeruginosa strains PAK and PAO bind specifically to the carbohydrate sequence βGalNac(1-4)βGal found in glycolipids asialo-GM1 and asialo-GM2. Mol Microbiol. 1994;11:715–723. doi: 10.1111/j.1365-2958.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 46.Tang H, Kays M, Prince A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect Immun. 1995;63:1278–1285. doi: 10.1128/iai.63.4.1278-1285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Ooij C, Apodaca J, Mostov K, Engel J. Characterization of the Chlamydia trachomatis vacuole and its interaction with the host endocytic pathway in HeLa cells. Infect Immun. 1997;65:758–766. doi: 10.1128/iai.65.2.758-766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel H J, Bonner D M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956;218:97–106. [PubMed] [Google Scholar]
- 48a.Waite, L., and J. Engel. Unpublished data.
- 49.Watson A A, Mattick J S, Alm R A. Functional expression of heterologous type 4 fimbriae in Pseudomonas aeruginosa. Gene. 1996;175:143–150. doi: 10.1016/0378-1119(96)00140-0. [DOI] [PubMed] [Google Scholar]
- 50.Whitchurch C B, Mattick J S. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol Microbiol. 1994;13:1079–1081. doi: 10.1111/j.1365-2958.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 51.Woods D E, Straus D C, Johanson W G, Berry V K, Bass J A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980;29:1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zoutman D E, Hulbert W, Pasloske B, Joffe A, Volpel K, Trebilcock M, Paranchych W. The role of polar pili in the adherence of Pseudomonas aeruginosa to injured canine tracheal cells: a semiquantitative morphological study. Scanning Microsc. 1991;5:109–126. [PubMed] [Google Scholar]