Abstract
Amniotic membrane is arguably one of the most popular biological wound dressings on the market today. Various growth factors and cytokines inherent to amniotic membrane tissue have been recognized as key mediators in wound healing and tissue regeneration, giving the tissue its clinical utility. Sterilization methodologies using irradiation are recognized as the gold standard in the field and routinely used to prepare tissue allografts, including amniotic membrane for transplantation. However, irradiation is not always compatible in preserving the physical structure or biochemical factors of biological materials and can potentially result in detrimental effects to the critical quality attributes of allograft tissues. Alternatively, a novel sterilization technique involving supercritical carbon dioxide (SCCO2) has been shown to have minimal effect on the inherent biophysical properties of sensitive biological tissues and tissue-derived products. At BioBridge Global, we have developed a process utilizing SCCO2 technology for the sterilization of an amniotic membrane tissue allograft product. This process, first and foremost, meets industry standards for sterilization while simultaneously maintaining the biochemical composition of the tissue. Our results show that upon SCCO2 sterilization, most of the growth factors tested were conserved, with many at quantities significantly greater than commercially available gamma and electron beam irradiated tissue. The SCCO2-sterilized amniotic membrane allograft is unique in that it is designed to overcome limitations associated with traditional tissue sterilization methodologies, namely, the conservation of key biological factors inherent to native amniotic membrane tissue. It is anticipated that by retaining these biological factors, clinical outcomes associated with the use of SCCO2-sterilized amniotic membrane will be improved.
Keywords: Amniotic membrane allograft tissue, Supercritical carbon dioxide, Sterilization, Spore inactivation, Validation, Irradiation
Introduction
The clinical application of amniotic membrane tissue allografts continues to increase in popularity as evidenced by its diversity of use. Traditionally used in the management of skin disorders, such as burns (Walker et al. 1977) and chronic, non-healing ulcers (Singh et al. 2004), amniotic membrane has recently found utility in a number of wound healing and regenerative medicine applications. The therapeutic benefit of amniotic membrane transplantation has been reported in the areas of orthopedics, ophthalmology, urology, dentistry, otolaryngology, and oncology, to name a few (Fairbairn et al. 2014, Nejad et al. 2021, Stirt et al. 2022, Nemati et al. 2023). Amniotic membrane has been shown to possess many beneficial properties that are advantageous to its clinical success. Reports show the amniotic tissue is anti-inflammatory, (Marsh et al. 2017) anti-scarring, (Gholipourmalekabadi et al. 2019) and anti-microbial (Ziaeian and Roudbari 2004; Zare-Bidaki et al. 2017) when used in wound healing and tissue regeneration applications. Unlike other commercially available bandages, amniotic membrane is a natural material composed primarily of the structural protein family of collagens, and a plethora of angiogenic, regenerative, and immune modulating cytokines and growth factors (McQuilling et al. 2017). Key growth factors and cytokines present in amniotic membrane favorable to wound healing include basic fibroblast growth factor, transforming growth factor alpha, platelet derived growth factor, tissue inhibitors of metalloproteinases, and members of the interleukin family, among others (Koob et al. 2015).
While the unique biochemical composition of amniotic membrane is credited for the clinical efficacy, it also presents a challenge to traditional tissue processing and sterilization methodologies where conservation of these factors is of paramount importance to functional performance. The terminal sterilization of tissue allografts is an unavoidable process that is required to ensure patient safety and prevent infection in the recipient due to microbial contamination. Thus, the impact of sterilization on sensitive biomaterials, like amniotic membrane, must be understood and any detrimental effects minimized during the development of such products. The FDA has recognized dry heat, steam, ethylene oxide, and irradiation as established sterilization methods, demonstrated by their “long history of safe and effective use” (Food and Drug Administration, CDRH et al. 2016). However, each of these sterilization methods is not without its limitations, especially with respect to their application to sensitive biomaterials which often have insufficient thermal, physical, and chemical stability to withstand these types of processing. Thermal sterilization processes are unsuitable for materials intolerant of high autoclave temperatures and susceptible to thermal degradation (Rogers 2012). Ethylene oxide is capable of leaving toxic chemical residuals in the sterilized material resulting in unwarranted downstream effects (Buben et al. 1999). Gamma irradiation has been shown to alter the biomechanical properties of different types of allograft tissues, including bone, tendon, amniotic membrane, and skin (Harrell et al. 2018). While gamma irradiation may be the most utilized method of tissue allograft sterilization, many studies have demonstrated that upon exposure, the structural proteins (i.e. collagen) of both hard and soft tissues breakdown, leading to reduced toughness, compressive strength, and tensile strength (Kaminski et al. 2012; Faragó et al. 2020). Changes in the extracellular matrix of amniotic membrane after exposure to irradiation have also been reported (Mrázová et al. 2014). With regard to the biochemical factors present in amniotic membrane allograft tissue, Suroto et al. reported reduction in basic fibroblast growth factor (bFGF) in with 15 kGy of gamma irradiation. Increasing the dose of irradiation, resulted in a 30% reduction in the amount of bFGF as compared to the non-irradiated controls (Suroto et al. 2021).
Alternatively, supercritical carbon dioxide (SCCO2) is a novel sterilization technique gaining traction for use with sensitive biomaterials, including tissue allografts. SCCO2 is highly pressurized carbon dioxide (CO2) that exhibits properties of both a gas and a liquid when held at conditions above its critical temperature and pressure (Tc and Tp). Compared to other supercritical fluids, CO2 has relatively mild critical coordinates (Tc = 31 °C, Tp = 73 atm) which make it appealing for use in temperature-sensitive applications. Furthermore, SCCO2 has highly tunable transport properties that cause it to simultaneously behave like a liquid and a gas. Small changes in pressure and temperature result in significant changes to the density of supercritical CO2 thus affecting its mass transfer properties (Nikolai et al. 2019). The ability of SCCO2 to permeate extremely small crevices and pores is advantageous when combining SCCO2 with antimicrobials or chemicals for the inactivation of contaminating microorganisms. While the use of SCCO2 alone is not practical for achieving industry standards of sterilization for biological materials, its combination with agents such as hydrogen peroxide, ethanol, and peracetic acid allow for these additives to permeate the material; increasing the effectiveness of the sterilization process (White et al. 2006).
Several studies have looked extensively at the effect of SCCO2 exposure on the biochemical and mechanical properties of both hard (bone) and soft tissue (musculoskeletal and amnion) allografts. Russell et al. performed extensive compression testing on bone samples and found that there were no significant differences in the yield stress, ultimate stress, or stiffness of the tissue post SCCO2 treatment and with the addition of peracetic acid (Russell et al. 2015). Sun et al. showed that tendons exposed to SCCO2 (with sterilizing agent NovaKill) treatment maintained their collagen morphology and extracellular matrix (ECM) structure as compared to gamma irradiated tendons which exhibited separation of collagen fiber bundles and degradation of the ECM (Lovric et al. 2020). Other studies have shown amniotic membrane exposed to SCCO2 maintains key ECM proteins like type IV collagen, glycosaminoglycans, and elastin (Wehmeyer et al. 2014) as well as growth factors and pentraxin X3 (McDaniel et al. 2021), a key cytokine found in amniotic membrane shown to have an anti-inflammatory effect during wound healing (Zhu et al. 2020). However, previous studies have not evaluated the growth factors in amniotic membrane after exposure to a sterilization rigorous enough to meet industry standards.
Tissue banking industry standards dictate that a validated sterilization processes for allograft tissue must define the allowable risk of contamination for a given product. This risk is expressed in terms of a probability and is referred to as the Sterility Assurance Level (SAL). For most allograft tissues, this is recognized as a SAL of 10–6 (U.S. Department of Health and Human Services, Food and Drug Administration et al. 2011). Not to be confused with spore log reduction (SLR), a SAL of 10–6 is the probability of encountering one viable organism out of one million final products sterilized. The SLR, on the other hand, is a measure of the effectiveness of a sterilization process and can be used to determine the operational parameters required to achieve the desired SAL for a given sterilization process (Wagner et al. 2021). Therefore, the objectives of the present study were to first determine the SCCO2 operating parameters that could achieve industry standards for sterility, that is, a SAL of 10–6. Secondly, we evaluated the effect of different sterilization procedures through the analysis of inherent biochemical properties of the tissue. These findings were benchmarked with commercially available amniotic membrane tissue sterilized by gamma and electron beam irradiation.
Materials and methods
Microbial inactivation of Bacillus atrophaeus spore strips
To determine the operating parameters required to sterilize amniotic membrane allograft tissue, a series of experiments were carried out in a Nova 2200 sterilizer (NovaSterilis, Lansing, NY). This equipment consists of a 20-L stainless steel chamber to hold samples for sterilization using SCCO2. Inside the chamber are housed two wire baskets for product placement, and a third basket to hold the disinfectant additive NovaKill. This is a peracetic acid (PAA) based additive provided by NovaSterilis and often used in conjunction with SCCO2 to achieve microbial inactivation. Bacillus atrophaeus spore strips were selected for the microbial inactivation experiments.
First, Bacillus atrophaeus spore strips were used in experiments to determine the sterilization cycle parameters. Increasing concentrations of NovaKill were pipetted onto the additive pad at the start of each SCCO2 cycle to determine the minimum inhibitory concentration of NovaKill effective against the Bacillus atrophaeus spores. Along with the concentration of NovaKill, the duration of exposure to SCCO2 (i.e., cycle dwell time) was also evaluated pertinent to spore strip growth inhibition. At the completion of each sterilization cycle, a sampling of four of the packaged spore strips were selected for testing. The number of microorganisms remaining on the spore strips post-SCCO2 exposure were extracted and enumerated using a bioburden assay. Spore strips not exposed to SCCO2 treatment served as controls for all of the experiments.
Inactivation of Bacillus atrophaeus spores on amniotic membrane
The screening of starting material was in accordance with testing criteria established as per section D4.230 of American Association of Tissue Banks (AATB), 14th edition. The materials were negative for human immunodeficiency virus (HIV)-I and -II, hepatitis B surface antigen (HBsAg), hepatitis B virus (HBV), hepatitis C virus (HCV), Syphilis, human T-lymphotropic virus (HTLV)-I and -II, etc. The SCCO2 sterilization cycle parameters as determined from the spore strip studies were used for process validation studies to establish a SAL of 10–6 for amniotic membrane allograft tissue. Briefly, amniotic membrane was inoculated with a spore suspension of Bacillus atrophaeus, packaged into gas-permeable pouches, and exposed to the SCCO2 processing parameters. At the completion of three independent sterilization runs, a random sampling of three amniotic membranes per run were selected for testing. Any remaining microorganisms were extracted from the amnion and plated on agar plates. The agar plates were incubated at 30–35 °C and bacterial growth was monitored over a period of 24–72 h. At the end of the incubation period, the plates were observed for the presence or absence of bacterial colonies.
Histology
Fresh, SCCO2-treated, and irradiated samples of amniotic membrane tissue were prepared for histology. Briefly, tissue sections were fixed in 10% neutral buffered formalin overnight, dehydrated in a series of ethanol, and blocked in paraffin following standard embedding procedures. Cut Sects. 7 µm thick were stained with hematoxylin and eosin (H&E) following standard staining procedures. Images of the stained tissue sections were acquired with a Zeiss Axio Observer microscope and accompanying software (White Plains, NY).
Microarray growth factor analysis
Proteins were extracted from SCCO2-sterilized and commercially available amniotic membrane using methods previously established in our laboratory. At the completion of a SCCO2 sterilization run, a random sampling of amniotic membranes from three donors were selected for testing. Samples of amniotic membranes derived from three donors sterilized by gamma-irradiation, and two donors treated by e-beam sterilization were purchased and used for comparison. Amniotic membrane tissue was placed in RIPA buffer containing 1X protease inhibitors, minced, and sonicated in an ice bath. The protein extracts were analyzed for 40 different growth factors and receptors using the Quantibody® Human Growth Factor Array from RayBiotech (Norcross, GA). Fluorescent images were acquired using an Innoscan 710 laser scanner equipped with a Cy3 (green) channel (Innopsys, France). The images were analyzed, and the fluorescent intensity quantified using the microarray analysis software, Magpix. The concentrations of proteins in each sample tested were determined using a standard curve of fluorescence versus known concentrations of proteins and cytokines run in parallel with the experimental samples.
The growth factors analyzed in this study include: amphiregulin (AR), brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (bFGF), bone morphogenetic protein-4 (BMP-4), bone morphogenetic protein-5 (BMP-5), bone morphogenetic protein-7 (BMP-7), beta-nerve growth factor (β-NGF), epidermal growth factor (EGF), epidermal growth factor receptor (EGFR), epidermal growth factor vascular endothelial growth factor (EG-VEGF), fibroblast growth factor-4 (FGF-4, fibroblast growth factor-7 (FGF-7), growth differentiation factor-15 (GDF-15), glial cell derived neurotrophic factor (GDNF), growth hormone (GH), heparin-binding epidermal growth factor-like growth factor (HB-EGF), hepatocyte growth factor (HGF), insulin-like growth factor binding protein-1 (IGFBP-1), insulin-like growth factor binding protein-2 (IGFBP-2), insulin-like growth factor binding protein-3 (IGFBP-3), insulin-like growth factor binding protein-4 (IGFBP-4), insulin-like growth factor binding protein-6 (IGFBP-6), insulin-like growth factor-1 (IGF-1), insulin, macrophage colony stimulating factor receptor (MCSF R), nerve growth factor receptor (NGF R), neurotrophic factor-3 (NT-3), neurotrophic factor-4 (NT-4), osteoprotegerin (OPG), platelet derived growth factor-AA (PDGF-AA), placental growth factor (PlGF), stem cell factor (SCF), stem cell factor receptor (SCF R), transforming growth factor alpha (TGFα), transforming growth factor beta 1, (TGFβ1), transforming growth factor beta 3 (TGFβ3), vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor 2 (VEGF R2), vascular endothelial growth factor receptor 3 (VEGF R3), and vascular endothelial growth factor D (VEGF-D).
Statistical analysis
The numerical results are reported as mean ± standard error of the mean (mean ± SEM). For the microarray analysis, statistical differences between the groups were determined using GraphPad statistical analyses software. Comparisons between groups were made using unpaired Student’s t-tests with values of p < 0.05 considered statistically significant. Welch’s correction was used when the variances of the experimental groups were not equivalent.
Results
Microbial inactivation of Bacillus atrophaeus spores
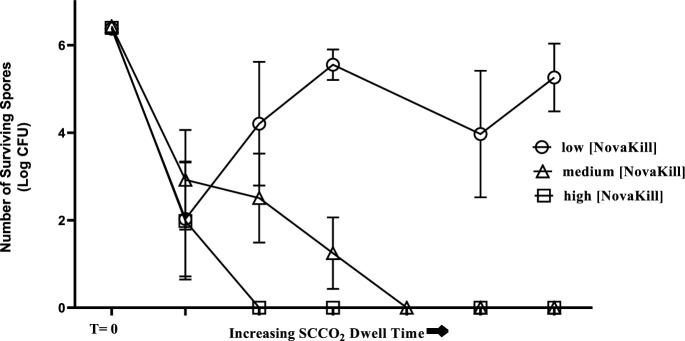
The susceptibility of Bacillus atrophaeus to the supercritical sterilization process was evaluated and a sterilization process was determined. The relationship between Bacillus atrophaeus inactivation, SCCO2 cycle dwell time, and NovaKill concentration are shown in the survivor curves in Fig. 1. These graphs demonstrate bacterial inactivation was dependent primarily on the NovaKill concentration in the system. At a low concentration of NovaKill, greater than 90% of the spores survived treatment, regardless of increasing SCCO2 cycle dwell time. As expected, increasing the concentration of NovaKill in the system resulted in greater microbial inactivation.
Fig. 1.
Survivor curves of Bacillus atrophaeus exposed to SCCO2 sterilization process. The number of surviving Bacillus atrophaeus spores was plotted against the SCCO2 cycle dwell time. Three different concentrations of the NovaKill additive were evaluated
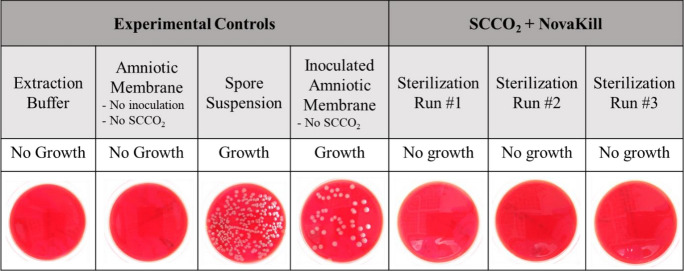
Amniotic membrane allografts inoculated with Bacillus atrophaeus spores were subjected to SCCO2 exposure under the operating parameters that were established with the Bacillus atrophaeus spore strips and conducive to a SAL of 10–6. Also known as process validation, the results of this experiment establish a sterilization process whereby SCCO2 and NovaKill can be delivered effectively and reproducibly to the amniotic membrane (Fig. 2).
Fig. 2.
Amniotic membrane was inoculated with Bacillus atrophaeus spores (106 CFU) and exposed to SCCO2 sterilization. The experimental controls exhibited either growth or no growth of the spores. The inoculated amniotic membrane pieces do not exhibit growth of the Bacillus atrophaeus spores after exposure to SCCO2/NovaKill
Histology
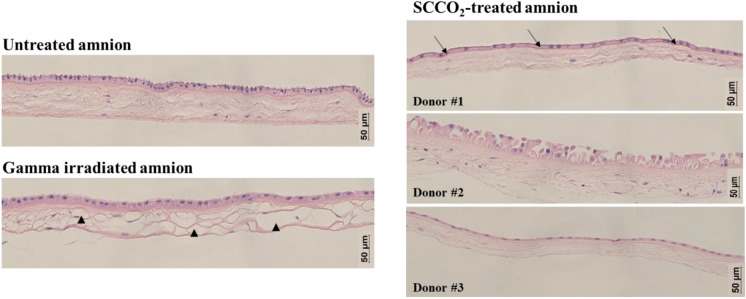
General histology staining using H&E was used to qualitatively evaluate the effect of supercritical sterilization on the gross structure of amniotic membrane and compared with untreated and gamma irradiated amnion. In the untreated amnion section, H&E staining showed a well-defined epithelial layer atop a basement membrane and followed by the collagenous stroma. The epithelial layer remained intact for both gamma irradiated amnion as well as the SCCO2-sterilized amnion. The basement membrane, however, was far less distinguishable in the gamma-sterilized tissues with only the SCCO2-treated tissues showing evidence of a basement membrane. The staining showed that the collagenous stroma was mostly preserved in SCCO2-treated amniotic membrane tissue (Fig. 3). This is in contrast to that of gamma irradiated tissue whereby some areas of the collagen stroma showed significant areas of degradation and fiber compaction. Although not present in every tissue section, there were places where degradation of the collagen stroma could be observed in the gamma irradiated amnion. This trend was not observed for the SCCO2-treated amnion, with the structure resembling that of the fresh amniotic membrane across multiple donors.
Fig. 3.
Sections of fresh, gamma irradiated, and SCCO2-treated amniotic membrane were processed for hematoxylin and eosin staining. In addition to the untreated amnion, the basement membrane was visible in one of the SCCO2 treated tissues as indicated by the arrows. Unlike the stroma of the untreated and SCCO2-treated amnion, numerous gaps were present in the stroma of the gamma irradiated tissue, indicated by the arrowheads
Human cytokine antibody array
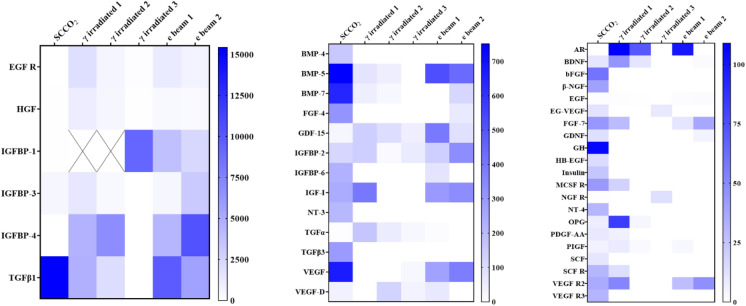
An antibody microarray analysis of 40 different growth factors and receptors was performed on amniotic membrane stored at − 80 °C prior to SCCO2 sterilization, SCCO2-sterilized amnion, commercially available gamma-irradiated amnion, and electron beam irradiated amnion. In comparison to commercially available gamma and electron beam irradiated amniotic membrane, SCCO2-sterilized amniotic membrane demonstrated significantly higher levels of many of the analytes analyzed (Fig. 4). Table 1 highlights the analytes that were statistically significant between SCCO2-sterilized amniotic membrane and gamma and electron beam irradiated amniotic membrane, respectively.
Fig. 4.
Heat maps showing the amount of growth factors (reported in pg/mL) present in tissue extracts from amniotic membrane that was sterilized by SCCO2 as compared to commercially available amniotic membrane tissue. More growth factors were conserved in the SCCO2-sterilized amnion compared to commercially available gamma irradiated and electron beam irradiated tissues. Values occurring above the upper limit of detection for the microarray assay are denoted by X
Table 1.
Concentration of growth factors in SCCO2 sterilized amniotic membrane
| Growth factor/receptor | Concentration (pg/mL ± SD) | Average fold increase above gamma irradiated | Average fold increase above e-beam irradiated |
|---|---|---|---|
| BDNF | 11.0 ± 1.0 | - | 12X |
| bFGF | 59.6 ± 5.0 | 60X | 60X |
| BMP-4 | 161.6 ± 20.0 | 162X | 162X |
| BMP-5 | 751.1 ± 120.0 | 17X | 2X |
| BMP-7 | 640.9 ± 143.0 | 26X | 11X |
| β-NGF | 40.5 ± 2.0 | 41X | 41X |
| EG-VEGF | 11.8 ± 2.0 | 4X | 12X |
| FGF-4 | 312.7 ± 163.0 | 313X | 10X |
| FGF-7 | 44.3 ± 8.0 | 5X | 2X |
| GDNF | 13.5 ± 3.0 | 14X | 6X |
| GH | 106.3 ± 36.0 | 106X | 106X |
| HB-EGF | 15.0 ± 3.0 | 15X | 15X |
| IGFBP-2 | 118.4 ± 9.0 | 2X | - |
| IGFBP-6 | 228.7 ± 105.0 | 229X | 6X |
| IGF-1 | 242.1 ± 87.0 | 2X | - |
| Insulin | 24.3 ± 2.0 | 24X | 24X |
| MCSF R | 43.9 ± 2.0 | 7X | 44X |
| NT-3 | 207.4 ± 11.0 | 207X | 207X |
| NT-4 | 31.1 ± 3.0 | 31X | 31X |
| PlGF | 5.9 ± 1.0 | 2X | 4X |
| SCF | 10.4 ± 2.0 | 10X | 10X |
| SCF-R | 31.0 ± 8.0 | 7X | 31X |
| TGF β1 | 15,422.5 ± 4245.0 | 7X | 2X |
| TGF β3 | 294.3 ± 12.0 | 294X | 294X |
| VEGF | 671.1 ± 47.0 | 88X | 2X |
| VEGF R2 | 36.2 ± 8.0 | 2X | - |
| VEGF R3 | 31.5 ± 3.0 | 32X | 32X |
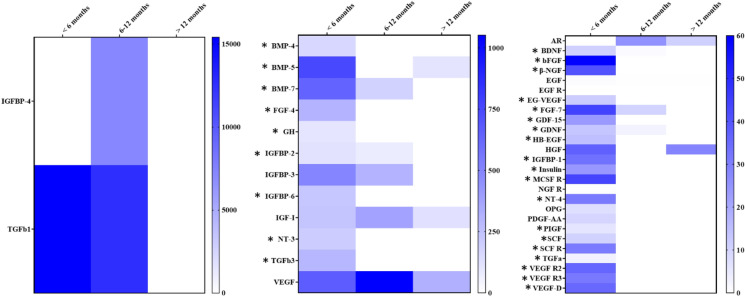
The Quantibody® Growth Factor microarray analyses revealed the storage conditions significantly impacted the amount of growth factors present in the SCCO2-sterilized amnion tissue (Fig. 5). Delaminated amniotic membrane tissue stored at − 80 °C without sterilization for six months or longer had significantly lower levels of many growth factors associated with wound healing. This included bFGF, GH, BMPs -4, -5, and -7, FGF-7, TGFα, and TGF β3. The neurotrophic factors BDNF, GDNF, NT-3, and NT-4 were also higher in amnion that was stored for less than six months. Tissue storage greater than one year further exacerbated the loss of growth factor, and the majority of the analytes were undetectable by the assay. An example of this can be seen with TGFβ1.
Fig. 5.
Heat maps showing the amount of growth factors (reported in pg/mL) present in tissue extracts from amniotic membrane subjected to different storage times. Although the samples are from different donors, the trend is a drastic decrease in the number of growth factors present (as indicated by the white rectangles) upon prolonged storage of the amnion at − 80 °C prior to SCCO2 sterilization. Statistically significant (p < 0.01) higher quantities of growth factors found in SCCO2 amnion stored for less than six months are indicated by an “*”
Discussion
The goal of this study was to develop a process utilizing SCCO2 to sterilize amniotic membrane while simultaneously conserving the biological properties of the tissue. The endospore Bacillus atrophaeus was used to derive the SCCO2 operating conditions necessary to achieve a SAL 10–6 for amniotic membrane tissue (Spilimbergo et al. 2002; White et al. 2006; Setlow et al. 2016). Amongst the spore-forming bacteria, Bacillus atrophaeus was chosen as the model organism in this study because it is harder to kill than the other expected contaminating microorganisms, and B. atrophaeus endospore could switch back to a vegetative state at 37 °C. When the process is optimized to achieve a sterility assurance level (SAL) of 10–6, the probability of amnion membrane being contaminated with the hard-to-kill B. atrophaeus after sterilization is one in a million. In extension, other microbial contaminants will also be killed by this process. Hence, Bacillus atrophaeus is a good biological indicator for this process.
Furthermore, our results demonstrate that the structure of the amniotic membrane tissue and inherent growth factors and receptors were maintained after processing. These findings corroborate the work of others and support the feasibility of using SCCO2 to achieve industry standards of sterilization for sensitive, biological matrices as an alternative to traditional sterilization methods, such as irradiation. Amniotic membrane is now part of an expanding list of biological tissues that can successfully be sterilized using SCCO2, others which include porcine acellular dermal matrix, (Qiu et al. 2009) decellularized lung tissue, (Balestrini et al. 2016) and bone and tendon allograft tissue (Burns et al. 2009).
There are many advantages to using supercritical CO2 as a sterilization method for allograft tissues. First, the biocidal effects of SCCO2 against a plethora of microorganisms are well documented thus making this sterilization methodology applicable to a variety of sensitive biomaterials. Soares et al. Riberio et al. and Zhang et al. have provided comprehensive reviews of the effects of SCCO2 on the inactivation of different types of microorganisms including gram positive, gram negative, endospores, and fungi. (Zhang et al. 2006; Soares et al. 2019; Ribeiro et al. 2020). The effectiveness of supercritical sterilization has also been evaluated against viruses, including coronaviruses (Fages et al. 1998; Perrut 2012; Bernhardt et al. 2015; Bennet et al. 2021). Endospores, however, typically demonstrate the greatest resistance to SCCO2 sterilization due to the presence of a spore coat which allows them to survive under severe environmental conditions. For this reason, they are often chosen to challenge sterilization equipment and processes (Dillow et al. 1999; Watanabe et al. 2003; Haas et al. 2007; AATB 2016). In the current study, the inactivation of the spore forming bacteria Bacillus atrophaeus was used to determine the SCCO2 processing parameters needed to achieve the industry standard SAL 10–6. This organism was chosen to measure the effectiveness of the sterilization process because (1) it poses a greater microbial challenge than the native flora found on the amniotic membrane tissue, and (2) being that it is an endospore, it has demonstrated increased resistance to SCCO2 sterilization as compared to other types of bacteria (Zhang et al. 2006; Qiu et al. 2009; Wehmeyer et al. 2014).
Another benefit of using SCCO2 for sterilization is the relatively mild and tunable critical coordinates of CO2, specifically with regards to temperature (Setlow et al. 2016). The critical temperature of CO2 is 31.1 °C, which is below the degradation limit of most thermally liable biological materials i.e. proteins (White et al. 2006). The amount of NovaKill used in our process is several folds lower than the concentrations that could potentially cause skin irritation as per Sect. 4.4.5 in the exposure guideline levels (NRC 2010). Also, the concentration of NovaKill in our process is several folds lower than the studies reporting lack of cytotoxicity or detrimental effects to the structural integrity of the skin allografts (Huang et al. 2004).
Studies have investigated the effects of SCCO2 processing parameters on hard and soft tissue material properties and have found the degradation of proteins to be minimal. Balestrini, et al. (2016) found the structural proteins collagen, elastin, glycosaminoglycans, and laminin were preserved in decellularized lung matrices subjected to SCCO2 comparable to decellularized controls. In addition to the aforementioned structural proteins, a study of SCCO2-treated adipose tissue showed the growth factors bFGF and VEGF were also preserved (Wang et al. 2017). Specific to amniotic membrane, Wehmeyer et al. showed the structural proteins of the tissue were also preserved with SCCO2 treatment, however, there was no investigation into the effect of processing on the presence of growth factors (Wehmeyer et al. 2014). The current study is one of the first to quantitatively assess the effect of SCCO2 sterilization on the presence of growth factors in amniotic membrane tissue. The preservation of select proteins in SCCO2-sterilized amniotic membrane was evident by the results of microarray analysis which demonstrated SCCO2 sterilization maintains a number of growth factors and receptors inherent to amniotic membrane tissue at levels comparable to, if not greater than, amniotic membrane sterilized by means of gamma or electron beam irradiation. Key growth factors that have been identified in the literature as essential biochemical mediators of physiological and pathological wound healing, including the epidermal growth factor (EGF), transforming growth factor beta (TGF-b), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF) families (Barrientos et al. 2008). Our data shows that examples of growth factors from each of these families are present in SCCO2 sterilized amniotic membrane. Furthermore, many of these factors are present in SCCO2 sterilized amniotic membrane at levels over 100 times higher than that of commercially available amniotic membrane sterilized by irradiation. The increased availability of these growth factors in SCCO2 sterilized amniotic membrane make the tissue uniquely suited for wound healing applications as these factors collectively promote re-epithelialization, granulation tissue formation/ECM deposition, and neovascularization (Greenhalgh 1996).
Factors such as bFGF, BMP-4, GH, and SCF were completely absent from the gamma and electron beam irradiated samples tested. Many of the growth factors analyzed in this study were not consistently detected in commercially available amniotic membrane sterilized either by gamma or electron beam irradiation. The findings of our study support other published reports that also show gamma irradiation is detrimental to select growth factors typically present in amniotic membrane. Suroto et al. (2021), observed significant losses of TGF-β and bFGF upon sterilizing lyophilized amniotic membrane using gamma irradiation at levels as low as 15 kilogray (kGy) . Paolin et al. (2016) also reported significant decreases in bFGF, and EGF upon gamma irradiation of lyophilized amniotic membrane . It is not just growth factors of the tissue that are affected by irradiation. Others have shown the collagenous connective tissue layer, often referred to as the stroma, is damaged by gamma irradiation. In a light microscopy study, von Versen-Höynck et al. (2004) showed the stroma had been destroyed with the collagen fibers resolving into thick bundles and losing all continuity . We also observed damage to the collagen stroma in our studies of gamma irradiated amniotic membrane. The SCCO2 treated amnion, however maintained the architecture of its collagen stroma.
One of the unique and unexpected finding of this study was the impact freezing amniotic membrane tissue prior to sterilization had on tissue quality with regards to the presence of growth factors.. The majority of the growth factors present in amniotic membrane frozen for less than six months disappeared after storage at − 80 °C for 1 year. This is in stark contrast to other studies that have reported that growth factor concentrations are not affected by freezing. Thomasen et al. (2011) for example, reported no statistically significant differences in protein analyzed by Western blotting. While sterilization is of paramount importance in the manufacturing process, the observed results of this study highlight the criticality of other processing parameters and their role in maintaining the biophysical properties of amniotic membrane tissue. An understanding of tissue specific storage timeframes is critical to ensuring transplant quality especially when the use of cryoprotective agents is not feasible.
In summary, BioBridge Global has developed a process utilizing SCCO2 technology for the sterilization of an amniotic membrane tissue allograft product. The amniotic membrane allograft is designed to overcome limitations associated with traditional tissue sterilization, that is the maintenance of key biological properties that make the tissue advantageous for use clinical applications.
Author contribution
J.O. designed the study, conducted the experiments, reviewed the results, and drafted the manuscript. K.P., J.F., X.L., A.T., D.V. assisted with the experiments. A.S. contributed to the design of the study, and drafting the manuscript. All authors reviewed the manuscript.
Funding
This work was internally funded through BioBridge Global. The authors did not receive external support from any organization for the submitted work. The authors have no financial or non-financial interests to disclose.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- AATB (2016). Standards for tissue banking, 14th edition.
- Balestrini JL, Liu A, Gard AL, Huie J, Blatt KM, Schwan J, Zhao L, Broekelmann TJ, Mecham RP, Wilcox EC, Niklason LE (2016) Sterilization of lung matrices by supercritical carbon dioxide. Tissue Eng Part C Methods 22(3):260–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M (2008) Growth factors and cytokines in wound healing. Wound Repair Regen 16(5):585–601 [DOI] [PubMed] [Google Scholar]
- Bennet D, Harris AF, Lacombe J, Brooks C, Bionda N, Strickland AD, Eisenhut T, Zenhausern F (2021) Evaluation of supercritical CO(2) sterilization efficacy for sanitizing personal protective equipment from the coronavirus SARS-CoV-2. Sci Total Environ 780:146519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt A, Wehrl M, Paul B, Hochmuth T, Schumacher M, Schütz K, Gelinsky M (2015) Improved sterilization of sensitive biomaterials with supercritical carbon dioxide at low temperature. PLoS ONE 10(6):e0129205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buben I, Melichercíková V, Novotná N, Svitáková R (1999) Problems associated with sterilization using ethylene oxide. Residues in treated materials. Cent Eur J Public Health 7(4):197–202 [PubMed] [Google Scholar]
- Dillow AK, Dehghani F, Hrkach JS, Foster NR, Langer R (1999) Bacterial inactivation by using near-and supercritical carbon dioxide. Proc Natl Acad Sci 96(18):10344–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fages J, Poirier B, Barbier Y, Frayssinet P, Joffret ML, Majewski W, Bonel G, Larzul D (1998) Viral inactivation of human bone tissue using supercritical fluid extraction. Asaio J 44(4):289–293 [DOI] [PubMed] [Google Scholar]
- Fairbairn NG, Randolph MA, Redmond RW (2014) The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg 67(5):662–675 [DOI] [PubMed] [Google Scholar]
- Faragó D, Szebényi G, Temesi T, Mária Kiss R, Pap K (2020) Evaluation of the effect of freezing and gamma irradiation on different types of tendon allografts by DIC assisted tensile testing. Appl Sci 10(15):5369 [Google Scholar]
- Food and Drug Administration, CDRH and CBER (2016). "Submission and review of sterility information in premarket notification (510(k)) submissions for devices labeled as sterile: Guidance for industry and food and drug administration staff."
- Gholipourmalekabadi M, Khosravimelal S, Nokhbedehghan Z, Sameni M, Jajarmi V, Urbanska AM, Mirzaei H, Salimi M, Chauhan NPS, Mobaraki M, Reis RL, Samadikuchaksaraei A, Kundu SC (2019) Modulation of hypertrophic scar formation using amniotic membrane/electrospun silk fibroin bilayer membrane in a rabbit ear model. ACS Biomater Sci Eng 5(3):1487–1496 [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG (1996) The role of growth factors in wound healing. J Trauma Acute Care Surg 41(1):159–167 [DOI] [PubMed] [Google Scholar]
- Haas G, Prescott H, Dudley E, Dik R, Hintlian C, Keane L (2007) Inactivation of microorganisms by CO2 under pressure. J Food Saf 9:253–265 [Google Scholar]
- Harrell CR, Djonov V, Fellabaum C, Volarevic V (2018) Risks of using sterilization by gamma radiation: the other side of the coin. Int J Med Sci 15(3):274–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Dawson RA, Pegg DE, Kearney JN, Macneil S (2004) Use of peracetic acid to sterilize human donor skin for production of acellular dermal matrices for clinical use. Wound Repair Regen 12(3):276–287 [DOI] [PubMed] [Google Scholar]
- Jildeh ZB, Wagner PH, Schöning MJ (2021) Sterilization of objects, products, and packaging surfaces and their characterization in different fields of industry The status in 2020. Phys Status Solidi (a) 218(13):2000732 [Google Scholar]
- Kaminski A, Jastrzebska A, Grazka E, Marowska J, Gut G, Wojciechowski A, Uhrynowska-Tyszkiewicz I (2012) Effect of gamma irradiation on mechanical properties of human cortical bone: influence of different processing methods. Cell Tissue Bank 13(3):363–374 [DOI] [PubMed] [Google Scholar]
- Koob TJ, Lim JJ, Zabek N, Massee M (2015) Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing. J Biomed Mater Res B Appl Biomater 103(5):1133–1140 [DOI] [PubMed] [Google Scholar]
- Marsh KM, Ferng AS, Pilikian T, Desai AA, Avery R, Friedman M, Oliva I, Jokerst C, Schipper D, Khalpey Z (2017) Anti-inflammatory properties of amniotic membrane patch following pericardiectomy for constrictive pericarditis. J Cardiothorac Surg 12(1):6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel JS, Wehmeyer JL, Cornell LE, Johnson AJ, Zamora DO (2021) Amniotic membrane allografts maintain key biological properties post SCCO2 and lyophilization processing. J Biomater Appl 35(6):592–601 [DOI] [PubMed] [Google Scholar]
- McQuilling JP, Vines JB, Kimmerling KA, Mowry KC (2017) Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds 29(6):E36-e40 [PMC free article] [PubMed] [Google Scholar]
- Mrázová H, Koller J, Fujeríková G, Babál P (2014) Structural changes of skin and amnion grafts for transplantation purposes following different doses of irradiation. Cell Tissue Banking 15:429–433 [DOI] [PubMed] [Google Scholar]
- Nejad AR, Hamidieh AA, Amirkhani MA, Sisakht MM (2021) Update review on five top clinical applications of human amniotic membrane in regenerative medicine. Placenta 103:104–119 [DOI] [PubMed] [Google Scholar]
- Nemati M, Nowrouzi M, Nemati F, Alizadeh A (2023) The improving effects of the amnion and chorion membranes on tissue regeneration in periodontal disorders: a systematic review. Tissue Cell 83:102147 [DOI] [PubMed] [Google Scholar]
- Nichols A, Burns D, Christopher R (2009) Studies on the sterilization of human bone and tendon musculoskeletal allograft tissue using supercritical carbon dioxide. J Orthop 6:e9 [Google Scholar]
- Nikolai P, Rabiyat B, Aslan A, Ilmutdin A (2019) Supercritical CO2: properties and technological applications - a review. J Therm Sci 28(3):394–430 [Google Scholar]
- Noël J, Stirt D, Moschovas MC, Reddy S, Jaber AR, Sandri M, Bhat S, Rogers T, Ahmed S, Mascarenhas A, Patel E, Patel V (2022) Oncologic outcomes with and without amniotic membranes in robotic-assisted radical prostatectomy: a propensity score matched analysis. Asian J Urol 11:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (2010) Peracetic Acid Acute Exposure Guideline Levels Acute Exposure Guideline Levels for Selected Airborne Chemicals. National Academies Press, Washington [PubMed] [Google Scholar]
- Paolin A, Trojan D, Leonardi A, Mellone S, Volpe A, Orlandi A, Cogliati E (2016) Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes. Cell Tissue Banking 17(3):399–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrut M (2012) Sterilization and virus inactivation by supercritical fluids (a review). J Supercrit Fluids 66:359–371 [Google Scholar]
- Qiu Q-Q, Leamy P, Brittingham J, Pomerleau J, Kabaria N, Connor J (2009) Inactivation of bacterial spores and viruses in biological material using supercritical carbon dioxide with sterilant. J Biomed Mater Res B Appl Biomater 91B(2):572–578 [DOI] [PubMed] [Google Scholar]
- Ribeiro N, Soares GC, Santos-Rosales V, Concheiro A, Alvarez-Lorenzo C, García-González CA, Oliveira AL (2020) A new era for sterilization based on supercritical CO(2) technology. J Biomed Mater Res B Appl Biomater 108(2):399–428 [DOI] [PubMed] [Google Scholar]
- Rogers WJ (2012) 2 - Steam and dry heat sterilization of biomaterials and medical devices. In: Lerouge S, Simmons A (eds) Sterilisation of Biomaterials and Medical Devices. Woodhead Publishing, pp 20–55 [Google Scholar]
- Russell N, Rives A, Pelletier MH, Wang T, Walsh WR (2015) The effect of supercritical carbon dioxide sterilization on the anisotropy of bovine cortical bone. Cell Tissue Banking 16(1):109–121 [DOI] [PubMed] [Google Scholar]
- Setlow B, Korza G, Blatt KMS, Fey JP, Setlow P (2016) Mechanism of Bacillus subtilis spore inactivation by and resistance to supercritical CO2 plus peracetic acid. J Appl Microbiol 120(1):57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Chouhan US, Purohit S, Gupta P, Kumar P, Kumar A, Chacharkar MP, Kachhawa D, Ghiya BC (2004) Radiation processed amniotic membranes in the treatment of non-healing ulcers of different etiologies. Cell Tissue Banking 5(2):129–134 [DOI] [PubMed] [Google Scholar]
- Soares GC, Learmonth DA, Vallejo MC, Davila SP, González P, Sousa RA, Oliveira AL (2019) Supercritical CO2 technology: The next standard sterilization technique? Mater Sci Eng, C 99:520–540 [DOI] [PubMed] [Google Scholar]
- Spilimbergo S, Elvassore N, Bertucco A (2002) Microbial inactivation by high-pressure. J Supercrit Fluids 22(1):55–63 [Google Scholar]
- Sun Y, Lovric V, Wang T, Oliver RA, Walsh WR (2020) Effects of SCCO(2), Gamma irradiation, and sodium dodecyl sulfate treatments on the initial properties of tendon allografts. Int J Mol Sci 21(5):1565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suroto H, Aryawan DM, Prakoeswa CA (2021) The influence of the preservation method and gamma irradiation sterilization on TGF-β and bFGF levels in freeze-dried amnion membrane (FD-AM) and amnion sponge. Int J Biomater 2021:6685225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomasen H, Pauklin M, Noelle B, Geerling G, Vetter J, Steven P, Steuhl KP, Meller D (2011) The effect of long-term storage on the biological and histological properties of cryopreserved amniotic membrane. Curr Eye Res 36(3):247–255 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services, Food and Drug Administration and Center for Biologics Evaluation and Research (2011). Guidance for Industry: Current Good Tissue Practice (CGTP) and Additional Requirements for Manufacturers of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps).
- von Versen-Höynck F, Syring C, Bachmann S, Möller DE (2004) The influence of different preservation and sterilisation steps on the histological properties of amnion allografts — light and scanning electron microscopic studies. Cell Tissue Banking 5(1):45–56 [DOI] [PubMed] [Google Scholar]
- Walker AB, Cooney DR, Allen JE (1977) Use of fresh amnion as a burn dressing. J Pediatr Surg 12(3):391–395 [DOI] [PubMed] [Google Scholar]
- Wang JK, Luo B, Guneta V, Li L, Foo SEM, Dai Y, Tan TTY, Tan NS, Choong C, Wong MTC (2017) Supercritical carbon dioxide extracted extracellular matrix material from adipose tissue. Mater Sci Eng, C 75:349–358 [DOI] [PubMed] [Google Scholar]
- Watanabe T, Furukawa S, Hirata J, Koyama T, Ogihara H, Yamasaki M (2003) Inactivation of Geobacillus stearothermophilus spores by high-pressure carbon dioxide treatment. Appl Environ Microbiol 69(12):7124–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehmeyer JL, Natesan S, Christy RJ (2014) Development of a sterile amniotic membrane tissue graft using supercritical carbon dioxide. Tissue Eng Part C Methods 21(7):649–659 [DOI] [PubMed] [Google Scholar]
- White A, Burns D, Christensen TW (2006) Effective terminal sterilization using supercritical carbon dioxide. J Biotechnol 123(4):504–515 [DOI] [PubMed] [Google Scholar]
- Zare-Bidaki M, Sadrinia S, Erfani S, Afkar E, Ghanbarzade N (2017) Antimicrobial properties of amniotic and chorionic membranes: a comparative study of two human fetal sacs. J Reprod Infertil 18(2):218–224 [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davis TA, Matthews MA, Drews MJ, LaBerge M, An YH (2006) Sterilization using high-pressure carbon dioxide. J Supercrit Fluids 38(3):354–372 [Google Scholar]
- Zhu YT, Li F, Zhang Y, Chen SY, Tighe S, Lin SY, Tseng SCG (2020) HC-HA/PTX3 Purified from human amniotic membrane reverts human corneal fibroblasts and myofibroblasts to keratocytes by activating BMP signaling. Invest Ophthalmol vis Sci 61(5):62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaeian B, Roudbari M (2004) The comparison of biologic dressing with amniotic membrane and anti-microbial dressing in wounds of burned patients. Intern Med Today 10(3):15–19 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.