Abstract
Background
Bloodstream infections (BSIs) caused by KPC-producing Klebsiella pneumoniae (KPC-Kp) are still associated with high mortality, and the game-changing drug ceftazidime/avibactam has shown suboptimal pharmacokinetics in some clinical settings. Ceftazidime/avibactam renal dose adjustment has recently been identified as an independent risk factor for mortality.
Objectives
To investigate the effect of ceftazidime/avibactam renal dose adjustment on mortality.
Methods
Patients with KPC-Kp BSI treated with a ceftazidime/avibactam-based regimen were retrospectively collected and analysed. The primary outcome was mortality at 7, 14 and 30 days after the start of definitive ceftazidime/avibactam antibiotic therapy. Renal function was estimated using the CKD-EPI equation.
Results
One hundred and ten patients with KPC-Kp BSI treated with a ceftazidime/avibactam-based regimen were included. Full-dose ceftazidime/avibactam (7.5 g daily) was prescribed to 82 patients (74.5%), while 28 patients (25.5%) received a renal-adjusted dose (17 patients due to chronic renal disease or haemodialysis, 11 patients due to infection-related acute kidney injury), with a median of 1.9 g daily. At multivariable analysis, receiving a reduced dose of ceftazidime/avibactam was independently associated with mortality (HR 4.47, 95% CI 1.09–18.03, P = 0.037), along with intra-abdominal or lower respiratory tract infections as source of BSI (HR 5.42, 95% CI 1.77–16.55, P = 0.003), septic shock (HR 6.99, 95% CI 1.36–35.87, P = 0.020) and SARS-CoV-2 coinfection (HR 10.23, 95% CI 2.69–38.85, P = 0.001).
Conclusions
Dose reduction of ceftazidime/avibactam according to renal function in patients with KPC-Kp BSI seems to be independently associated with higher mortality. This may be possibly due to inadequate exposure provided by the recommended doses for renal impairment.
Introduction
The introduction of ceftazidime/avibactam has provided an effective weapon against KPC-producing pathogens compared with regimens based on older drugs,1 but bloodstream infections (BSIs) caused by KPC-producing Klebsiella pneumoniae (KPC-Kp) still carry a high mortality rate.2
β-Lactam agents such as ceftazidime/avibactam are hydrophilic molecules with low volume of distribution, predominantly urinary excretion, and time-dependent pharmacodynamics (PD), making them highly susceptible to the pharmacokinetic (PK) changes typical of patients with BSI, with or without sepsis.3
Since its introduction, the PK of ceftazidime/avibactam has been considered suboptimal in certain conditions, such as pneumonia and continuous renal replacement therapy (CRRT).4 More recently, renal adjustment of ceftazidime/avibactam dose was identified as a risk factor for mortality.5
We conducted an observational, retrospective, single-centre study in patients with KPC-Kp BSI treated with a ceftazidime/avibactam-based antibiotic regimen to assess whether renal adjustment has an impact on mortality. We further assessed whether mortality in the group receiving adjusted dose of ceftazidime/avibactam depended on sepsis severity.
Materials and methods
Ethics
The study was approved by the local Ethical Committees (no. 0069/2022) and conducted according to the guidelines of the Declaration of Helsinki.
Study population
We retrospectively analysed a cohort of 110 patients hospitalized at a tertiary University Hospital of Rome between 2018 and 2021. Inclusion criteria were: (i) age ≥18 years; (ii) patients affected by KPC-Kp BSI; and (iii) patients treated with a ceftazidime/avibactam-based regimen for definitive therapy.
Exclusion criteria were: (i) not receiving ceftazidime/avibactam for the treatment of KPC-Kp BSI; (ii) ceftazidime/avibactam duration of treatment of <48 h; (iii) BSI due to carbapenem-resistant K. pneumoniae other than KPC producers (i.e. OXA, MBL producers); (iv) polymicrobial BSI, except for CoNS isolation, which has been considered as contamination; or (v) non-availability of clinical and microbiological data.
After case identification in the hospital information system, demographic, anamnestic, microbiological, clinical and therapeutic data were extrapolated from clinical records. We further divided the study population into patients receiving full dosage of ceftazidime/avibactam and patients receiving adjusted doses of ceftazidime/avibactam according to the renal function.
The primary outcome was mortality at 7, 14 and 30 days after the start of ceftazidime/avibactam definitive antibiotic therapy.
Renal function assessment
Renal function was defined by estimated glomerular filtration rate (eGFR), considering serum creatinine (sCr) values. To estimate GFR, we used the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation expressed as a single equation, using the sCr value as follows: eGFRcr = 142 × min(sCr/κ, 1)α × max(sCr/κ, 1) − 1.200 × 0.9938age × 1.012 (if female), where sCr is standardized sCr in mg/dL, κ is 0.7 for female individuals or 0.9 for male individuals, α is −0.241 for female individuals or −0.302 for male individuals, min(sCr/κ, 1) is the minimum of sCr/κ or 1.0, and max(sCr/κ, 1) is the maximum of sCr/κ or 1.0.6
Acute kidney injury (AKI) was diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) definitions by an absolute increase in sCr, at least 0.3 mg/dL (26.5 μmol/L) within 48 h or by a 50% increase in sCr from baseline within 7 days, or a urine volume of less than 0.5 mL/kg/h for at least 6 h.7 AKI was evaluated at the time of hospital admission and prescription of ceftazidime/avibactam. The initiation of CRRT in patients with AKI-related infection was also collected.
The presence and stage of chronic kidney disease (CKD) were characterized according to the KDIGO guidelines as follows: stage 1 (>90 mL/min), stage 2 (60–89 mL/min), stage 3a (45–59 mL/min), stage 3b (30–44 mL/min), stage 4 (15–29 mL/min) and stage 5 (<15 mL/min). The guidelines define CKD as either kidney damage or a decreased GFR of less than 60 mL/min/1.73 m2 for at least 3 months.8
Ceftazidime/avibactam dosage scheme
The full dose of ceftazidime/avibactam was 2.5 g every 8 h administered for a 2 h infusion, for a total of 7.5 g daily. Dose adjustments for renal impairment were made according to the manufacturer’s recommendations, and described as follows: 1.25 g every 8 h for eGFR of 31–50 mL/min; 0.94 g every 12 h for eGFR of 16–30 mL/min; 0.94 g every 24 h for eGFR of 6–15 mL/min; and 0.94 g every 48 h for end-stage renal disease including being on haemodialysis.9
Definitions
Infections were defined according to standard ECDC definitions.10 KPC-Kp BSI was diagnosed when KPC-Kp was isolated from blood cultures (BCs) in the presence of clinical signs of infection, and BSI onset was defined as the date of collection of the index BC.
The probable or established source of infection was reported in the medical record by the attending physician or the infectious disease consultant and defined according to guidelines.10,11 Primary BSIs were defined as BSIs occurring in patients without a recognized source of infection. Central line-related BSIs (CLRBSIs) were considered if semiquantitative culture of the catheter tip was positive for the same KPC-Kp isolated from the BC.12
The burden of underlying comorbidities was assessed using the Charlson comorbidity index (CCI).13 Immunosuppression was defined as either steroid therapy with prednisone (or equivalent) at a dose of >0.5 mg/kg/day for at least 1 month or receipt of chemotherapy, TNF-α inhibitors, cyclophosphamide, azathioprine, methotrexate or mycophenolate mofetil in the previous 90 days.
Infection severity was determined using the increment-CPE score (ICS) calculated at the time of infection onset.14 The Pitt bacteraemia score (PBS) was also calculated for each patient.15 Sepsis and septic shock were defined according to the SEPSIS-3 criteria.16
Therapy was considered appropriate if exhibited in vitro activity according to the breakpoints established by EUCAST.17 Time (days) from index BC to the start of appropriate antibiotic treatment and to the start of ceftazidime/avibactam, as well as the appropriateness of empirical therapy, were also taken into account.
Clinical cure was defined as the resolution of symptoms after the end of antibiotic treatment.
Microbiology
According to the hospital microbiology laboratory’s routine protocol, a bacterial pellet obtained from a positive BC was used for bacterial identification by MALDI-TOF MS (Bruker Daltonik, Bremen, Germany). Antimicrobial susceptibility was assessed using the MicroScan WalkAway system (Beckman Coulter Inc., Brea, CA, USA). Subsequent molecular analysis to search for the blaKPC gene was performed by the GeneXpert® System (Cepheid, CA, USA).
Statistical analysis
Data are presented as medians with IQRs for continuous variables and as simple frequencies, proportions and percentages for categorical variables. The Mann–Whitney test was used for unpaired samples. Dichotomous variables were compared using Fisher’s exact test or chi-squared test, as appropriate. Survival was analysed using Kaplan–Meier curves and the statistical significance of the differences between the two groups was assessed using the log-rank test. Factors with a univariable value of P < 0.05 and those deemed clinically significant were included in the final multivariable Cox regression model to identify the independent predictors of 30 day mortality. P value analyses were two-sided and a P value of less than 0.05 was considered statistically significant. All statistical analyses were performed using the Statistical Program for the Social Sciences software package (IBM SPSS Statistics for Windows, version 27.0. Armonk, NY, USA).
Results
Study population
In total, we collected and analysed data from 110 adults with a median age of 69 (IQR 58.0–78.3) years and a median CCI score of 6 (IQR 5–9), affected by hospital-acquired KPC-Kp BSI (Table 1). Most of the population were previously colonized with KPC-Kp (79.1%), and a consistent proportion were in the ICU (30.9%) and/or in septic shock (25.5%) at the time of BSI diagnosis. CKD was present in 30% of hospitalized patients and five patients were on haemodialysis at admission. Nine patients had SARS-CoV-2 coinfection: all received a combination of remdesivir and corticosteroid, while none received IL-6 antagonists. In general, treatment of KPC-Kp BSI was started empirically at the onset of sepsis symptoms (determined to be appropriate in 38.2%). It was then adjusted once microbial identification and susceptibility testing results were available (appropriate treatment received after a median of 1 day after BC collection) and continued for a median of 14 days. Ceftazidime/avibactam was combined with another antimicrobial (most commonly meropenem or fosfomycin) in a substantial proportion (88.2%) of cases. The overall 30 day mortality from ceftazidime/avibactam treatment start was 18.2%.
Table 1.
Descriptive analysis of whole population and comparison according to full or reduced dose of ceftazidime/avibactam (CZA)
| Variable | Whole population (n = 110) | CZA full dose (n = 82) | CZA reduced dose (n = 28) | P value |
|---|---|---|---|---|
| Gender male, n (%) | 78 (70.9) | 58 (70.7) | 20 (71.4) | 0.863 |
| Age, years, median (IQR) | 69.0 (58.0–78.3) | 68.5 (57.0–78.0) | 69.0 (62.3–80.5) | 0.333 |
| Hospitalized in ICU, n (%) | 34 (30.9) | 25 (30.5) | 9 (32.1) | 0.901 |
| SARS-CoV-2 coinfection, n (%) | 9 (8.2) | 8 (9.8) | 1 (3.6) | 0.443 |
| Diabetes, n (%) | 28 (25.5) | 20 (24.4) | 8 (28.6) | 0.802 |
| Cardiovascular disease, n (%) | 77 (70.0) | 57 (69.5) | 20 (71.4) | 0.930 |
| COPD, n (%) | 38 (34.5) | 30 (36.6) | 8 (28.6) | 0.497 |
| CKD, n (%) | 33 (30.0) | 15 (18.3) | 18 (64.3) | <0.001 |
| Chronic haemodialysis, n (%) | 5 (4.5) | 0 | 5 (17.9) | <0.001 |
| Chronic liver disease, n (%) | 12 (10.9) | 8 (9.8) | 4 (14.3) | 0.497 |
| Solid neoplasia, n (%) | 28 (25.5) | 19 (23.2) | 9 (32.1) | 0.451 |
| Haematological neoplasia, n (%) | 7 (6.4) | 6 (7.3) | 1 (3.6) | 0.676 |
| Immunodeficiency, n (%) | 28 (25.5) | 20 (24.4) | 8 (28.6) | 0.802 |
| Solid organ transplant, n (%) | 7 (6.4) | 4 (4.9) | 3 (10.7) | 0.368 |
| CCI, median (IQR) | 6.0 (5.0–9.0) | 6.0 (5.0–7.3) | 8.0 (6.0–10.8) | 0.006 |
| Previous KPC-Kp rectal colonization, n (%) | 87 (79.1) | 65 (79.3) | 22 (78.6) | 0.952 |
| CKD-EPI eGFR at CZA start, median (IQR) | 78.6 (43.2–103.9) | 91.9 (70.2–113.1) | 28.5 (19.0–39.5) | <0.001 |
| KDIGO class at CZA prescription, n (%) | ||||
| ≥90 (G1) | 42 (38.2) | 42 (51.2) | 0 | <0.001 |
| 60–89 (G2) | 32 (29.1) | 32 (39.0) | 0 | |
| 45–59 (G3a) | 6 (5.5) | 4 (4.9) | 2 (7.1) | |
| 30–44 (G3b) | 12 (10.9) | 3 (3.7) | 9 (32.1) | |
| 15–29 (G4) | 13 (11.8) | 1 (1.2) | 12 (42.9) | |
| <15 (G5) | 5 (4.5) | 0 | 5 (17.9) | |
| Source of BSI, n (%) | ||||
| Primary bacteraemia | 32 (29.1) | 24 (29.3) | 8 (28.6) | 0.741 |
| CLRBSI | 5 (4.5) | 5 (6.1) | 0 | |
| Urinary tract | 31 (28.2) | 23 (28.0) | 8 (28.6) | |
| IAI | 20 (18.2) | 14 (17.1) | 6 (21.4) | |
| LRTI (VAP included) | 22 (20.0) | 16 (19.5) | 6 (21.4) | |
| CZA MIC of the isolated KPC-Kp (mg/L), median (IQR) | 2.0 (2.0–4.0) | 2.0 (2.0–4.0) | 2.5 (2.0–4.0) | 0.296 |
| Serum lactate (mmol/L), median (IQR) | 2.3 (1.7–3.5) | 2.7 (1.8–3.6) | 1.9 (1.4–3.5) | 0.509 |
| C-reactive protein (mg/dL), median (IQR) | 11.3 (5.9–18.1) | 10.8 (5.7–16.2) | 15.3 (6.2–24.1) | 0.101 |
| Procalcitonin (ng/dL), median (IQR) | 4.8 (0.9–31.1) | 2.6 (0.7–24.6) | 28.2 (4.1–33.0) | 0.010 |
| CZA dose (g), median (IQR) | 7.5 (3.75–7.5) | 7.5 (7.5–7.5) | 1.9 (1.9–3.8) | <0.001 |
| CZA full dose, n (%) | 82 (74.5) | — | — | — |
| CZA combination therapy, n (%) | 97 (88.2) | 73 (89.0) | 24 (85.7) | 0.736 |
| Treatment regimen, n (%) | 0.619 | |||
| CZA monotherapy | 13 (11.8) | 5 (6.10) | 4 (14.3) | |
| CZA + meropenem | 43 (39.1) | 31 (37.8) | 12 (42.9) | |
| CZA + fosfomycin | 46 (41.8) | 37 (45.1) | 9 (32.1) | |
| CZA + other (co-trimoxazole, tigecycline, gentamicin or colistin) | 8 (7.3) | 5 (6.1.0) | 3 (10.7) | |
| sCr at admission (mg/dL), median (IQR) | 1.0 (0.7–1.4) | 0.9 (0.7–1.3) | 1.6 (0.9–4.8) | <0.001 |
| sCr at CZA prescription (mg/dL), median (IQR) | 0.9 (0.6–1.4) | 0.7 (0.5–1.0) | 2.2 (1.6–3.2) | <0.001 |
| sCr after 48–72 h of CZA administration (mg/dL), median (IQR) | 0.8 (0.5–1.2) | 0.6 (0.5–0.9) | 1.6 (1.2–2.9) | <0.001 |
| sCr after 7 days of CZA administration (mg/dL), median (IQR) | 0.7 (0.4–1.1) | 0.6 (0.4–0.8) | 1.4 (0.8–2.8) | <0.001 |
| sCr at the end of CZA treatment (mg/dL), median (IQR) | 0.7 (0.4–1.1) | 0.6 (0.4–0.9) | 1.5 (0.8–2.3) | <0.001 |
| CRRT required due to infection, n (%) | 10 (9.1) | 6 (7.3) | 4 (14.3) | 0.227 |
| Vasoactive drugs required, n (%) | 37 (33.6) | 24 (29.3) | 13 (46.4) | 0.110 |
| Septic shock, n (%) | 28 (25.5) | 17 (20.7) | 11 (39.3) | 0.077 |
| PBS, median (IQR) | 3.0 (2.0–4.0) | 3.0 (2.0–4.0) | 4.0 (2.0–4.0) | 0.223 |
| ICS, median (IQR) | 6.0 (3.0–8.0) | 6.0 (3.0–8.0) | 8.0 (6.0–11.0) | 0.042 |
| Time (days) from index BC to appropriate (in vitro active) therapy, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.685 |
| Time (days) from index BC to CZA prescription, median (IQR) | 1 (0–2) | 1 (0–2) | 1 (0–2) | 0.482 |
| Appropriate empirical therapy, n (%) | 42 (38.2) | 31 (37.8) | 11 (39.3) | 0.885 |
| Performed source control, n (%) | 91 (82.7) | 68 (83.0) | 23 (82.2) | 0.359 |
| Duration of definitive therapy (days), median (IQR) | 14 (12–20) | 15 (12.0–20.0) | 13 (10.3–14.8) | 0.026 |
| Clinical cure, n (%) | 68 (61.8) | 53 (64.6) | 15 (53.6) | 0.369 |
| KPC-Kp BSI recurrence within 30 days, n (%) | 11 (10.0) | 11 (13.4) | 0 | 0.063 |
| Secondary infections within 30 days, n (%) | 38 (34.5) | 30 (36.6) | 8 (28.6) | 0.497 |
| Subsequent finding of CZA MIC increase (≥8 mg/L) | 7 (6.4) | 6 (7.3) | 1 (3.6) | 0.590 |
| Negative BCs 72 h after treatment start, n (%) [performed in n (%)] | 63 (57.3) [73 (66.4)] | 49 (59.8) [58 (70.7)] | 14 (50.0) [15 (53.6)] | 0.180 |
| Negative BCs 7 days after treatment start, n (%) [performed in n (%)] | 52 (47.3) [56 (50.9)] | 40 (48.8) [44 (54.3)] | 12 (42.9) [12 (42.9)] | 0.397 |
| Negative BCs 14 days after treatment start, n (%) [performed in n (%)] | 39 (35.5) [42 (38.2)] | 33 (40.2) [36 (45.6)] | 6 (21.4) [6 (21.4)] | 0.085 |
| Adverse reactions to antibiotic treatment, n (%) | 11 (10.0) | 9 (11.0) | 2 (7.1) | 0.726 |
| Cumulative mortality 7 days after CZA start, n (%) | 6 (5.5) | 3 (3.7) | 3 (10.7) | 0.171 |
| Cumulative mortality 14 days after CZA start, n (%) | 12 (10.9) | 5 (6.1) | 7 (25.0) | 0.011 |
| Cumulative mortality 30 days after CZA start, n (%) | 20 (18.2) | 11 (13.4) | 9 (32.1) | 0.044 |
| Overall in-hospital mortality, n (%) | 37 (33.6) | 25 (30.5) | 12 (42.9) | 0.253 |
| Length of stay from CZA start (days), median (IQR) | 28.0 (14.0–53.3) | 31.5 (17.0–54.8) | 15.0 (12.3–46.0) | 0.049 |
Values in bold indicate P<0.05.
CKD, chronic kidney disease; CLRBSI, Central line-related BSIs; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CZA, ceftazidime/avibactam; IAI, intra-abdominal infection; ICS, increment CPE score; KDIGO, kidney disease improving global outcomes; LRTI, lower respiratory tract infection; PBS, Pitt bacteremia score; VAP, ventilator-associated pneumonia.
Comparison between patients receiving full and renal-adjusted dosage of ceftazidime/avibactam
Full-dose ceftazidime/avibactam (7.5 g daily) was prescribed to 82 patients (74.5%), while 28 received a renal-adjusted dose (25.5%) with a median of 1.9 g daily (IQR 1.9–3.8) (Table 1). Ceftazidime/avibactam was dosed according to the renal function estimated on the day of ceftazidime/avibactam start.
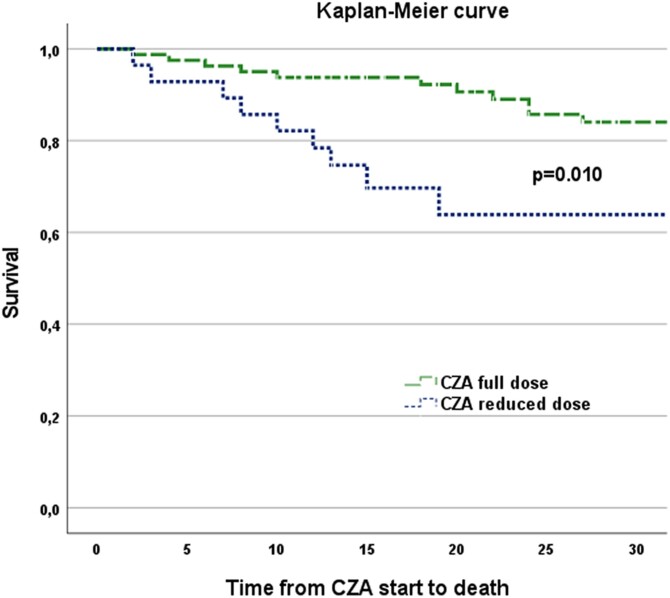
Those who received a reduced dose were predominantly in group 3 of the KDIGO classification, had significantly higher values of CCI score, ICS and serum procalcitonin levels and had significantly higher 14 day and 30 day mortality (25.0% versus 6.1%, P = 0.011%; and 32.1% versus 13.4%, P = 0.044, respectively), while clinical cure was similar between the two groups (64.6% and 53.6% in full and renal-adjusted ceftazidime/avibactam dosage, respectively). The Kaplan–Meier survival curve showed a significant (P = 0.006) difference in survival between patients receiving full and reduced doses of ceftazidime/avibactam (Figure 1).
Figure 1.
Kaplan–Meier survival curve comparing those treated with a full dose of ceftazidime/avibactam with those who received a dose reduced according to renal function. CZA, ceftazidime/avibactam.
Comparison between patients with acute or chronic renal impairment
We then analysed the population treated with a reduced dose of ceftazidime/avibactam (median daily dose of 1.9 g), comparing patients with CKD and/or chronic haemodialysis (n=17) with those who had normal renal function on admission but developed AKI, or even the need for CRRT, concomitantly with the KPC-Kp BSI (n=11) (Table 2). Patients with CKD had less severe sepsis and received more effective empirical treatment. Patients with AKI developed more secondary infections within 30 days after BSI and had a higher, although not significant, median value of ceftazidime/avibactam MIC. However, there was no significant difference in ceftazidime/avibactam median dose, ceftazidime/avibactam combination or monotherapy (82.4% versus 90.9%, P = 0.527), ceftazidime/avibactam combination regimen, survival (30 day mortality of 29.4% versus 36.4%, P = 0.700) or persistence of positive BCs (47.1% versus 54.5% at 72 h, P = 0.696) between these two subgroups.
Table 2.
Comparison of patients with chronic and acute kidney impairment
| Variable | Reduced CZA dose for CKD or haemodialysis (n = 17) | Reduced CZA dose for sepsis-related AKI (n = 11) | P value |
|---|---|---|---|
| Gender male, n (%) | 12 (70.6) | 8 (72.7) | 0.903 |
| Age, years, median (IQR) | 67.0 (62.0–80.0) | 70.0 (68.0–82.0) | 0.547 |
| Hospitalized in ICU, n (%) | 4 (23.5) | 5 (45.5) | 0.409 |
| SARS-CoV-2 coinfection, n (%) | 1 (5.9) | 0 | 0.607 |
| Diabetes, n (%) | 4 (23.5) | 4 (36.4) | 0.671 |
| Cardiovascular disease, n (%) | 11 (64.7) | 9 (81.8) | 0.419 |
| COPD, n (%) | 6 (35.3) | 2 (18.2) | 0.419 |
| CKD, n (%) | 17 (100.0) | 3 (27.3) | <0.001 |
| Chronic haemodialysis, n (%) | 5 (29.4) | 0 (0) | 0.125 |
| Chronic liver disease, n (%) | 3 (17.6) | 1 (9.1) | 0.527 |
| Solid neoplasia, n (%) | 5 (29.4) | 4 (36.4) | 0.700 |
| Haematological neoplasia, n (%) | 1 (5.9) | 0 | 0.413 |
| Immunodeficiency, n (%) | 4 (23.5) | 4 (36.4) | 0.671 |
| Solid organ transplant, n (%) | 2 (11.8) | 1 (9.1) | 0.823 |
| CCI, median (IQR) | 8.0 (6.0–11.0) | 7.0 (6.0–12.0) | 0.963 |
| Previous KPC-Kp rectal colonization, n (%) | 13 (76.5) | 9 (81.8) | 0.736 |
| CKD-EPI eGFR at CZA start, median (IQR) | 28.2 (19.1–38.6) | 29.3 (13.7–41.1) | 0.926 |
| KDIGO class at CZA prescription, n (%) | |||
| ≥90 (G1) | 0 | 0 | |
| 60–89 (G2) | 0 | 0 | |
| 45–59 (G3a) | 1 (5.9) | 1 (9.1) | |
| 30–44 (G3b) | 5 (29.4) | 4 (36.4) | |
| 15–29 (G4) | 9 (52.9) | 3 (27.3) | |
| <15 (G5) | 2 (11.8) | 3 (27.3) | 0.547 |
| Source of BSI, n (%) | 0.236 | ||
| Primary bacteraemia | 4 (23.5) | 4 (36.4) | |
| CLRBSI | 0 | 0 | |
| Urinary tract | 6 (35.3) | 2 (18.2) | |
| IAI | 5 (29.4) | 1 (9.1) | |
| LRTI (VAP included) | 2 (11.8) | 4 (36.4) | |
| CZA MIC of the isolated KPC-Kp (mg/L), median (IQR) | 2.0 (2.0–4.0) | 4.0 (2.5–4.0) | 0.073 |
| Serum lactate (mmol/L), median (IQR) | 2.2 (1.3–3.8) | 1.8 (1.8–1.8) | 0.711 |
| C-reactive protein (mg/dL), median (IQR) | 14.1 (5.0–24.0) | 19.7 (6.4–27.4) | 0.329 |
| Procalcitonin (ng/dL), median (IQR) | 28.2 (5.0–43.5) | 16.5 (2.5–32.8) | 0.556 |
| CZA dose (g), median (IQR) | 1.9 (1.9–3.8) | 1.9 (0.9–3.8) | 0.677 |
| CZA full dose, n (%) | — | — | / |
| CZA combination therapy, n (%) | 14 (82.4) | 10 (90.9) | 0.527 |
| Treatment regimen, n (%) | |||
| CZA monotherapy | 3 (17.6) | 1 (9.1) | 0.778 |
| CZA + meropenem | 6 (35.3) | 6 (54.5) | |
| CZA + fosfomycin | 6 (35.3) | 3 (27.3) | |
| CZA + other (co-trimoxazole, tigecycline, gentamicin or colistin) | 2 (11.8) | 1 (9.1) | |
| sCr at admission (mg/dL), median (IQR) | 3.1 (1.5–6.8) | 0.9 (0.8–1.3) | <0.001 |
| sCr at CZA prescription (mg/dL), median (IQR) | 2.3 (1.6–3.3) | 2.1 (1.6–3.2) | 0.853 |
| sCr after 48–72 h of CZA administration (mg/dL), median (IQR) | 1.6 (1.2–3.0) | 1.6 (1.0–2.5) | 0.640 |
| sCr after 7 days of CZA administration (mg/dL), median (IQR) | 1.4 (1.1–3.2) | 0.7 (0.6–2.7) | 0.318 |
| sCr at the end of CZA treatment (mg/dL), median (IQR) | 1.6 (1.1–3.4) | 1.0 (0.5–2.1) | 0.290 |
| CRRT required due to infection, n (%) | 0 | 4 (36.4) | 0.016 |
| Vasoactive drugs required, n (%) | 6 (35.3) | 7 (63.6) | 0.246 |
| Time from index BC to empirical therapy, median (IQR) | 0 | 0 | 0.749 |
| Septic shock, n (%) | 4 (23.5) | 7 (63.6) | 0.053 |
| PBS, median (IQR) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | 0.019 |
| ICS, median (IQR) | 6.0 (3.0–10.0) | 10.0 (8.0–11.0) | 0.053 |
| Time (days) from index BC to appropriate (in vitro active) therapy, median (IQR) | 1 (0–2) | 1 (1–2) | 0.111 |
| Time (days) from index BC to CZA prescription, median (IQR) | 1 (0–2) | 1 (1–2) | 0.329 |
| Appropriate empirical therapy, n (%) | 10 (58.8) | 1 (9.1) | 0.016 |
| Performed source control, n (%) | 14 (82.4) | 9 (81.9) | 0.966 |
| Duration of definitive therapy (days), median (IQR) | 14 (10.0–15.0) | 12 (11.0–14.0) | 0.817 |
| Clinical cure, n (%) | 11 (64.7) | 4 (36.7) | 0.246 |
| KPC-Kp BSI recurrence within 30 days, n (%) | 0 | 0 | / |
| Secondary infections within 30 days, n (%) | 2 (11.8) | 6 (54.5) | 0.030 |
| Subsequent finding of CZA MIC increase (≥8mg/L) | 1 (5.9) | 0 | 0.333 |
| Negative BCs 72 h after treatment start, n (%) [performed in n (%)] | 8 (47.1) [9 (52.9)] | 6 (54.5) [6 (54.5)] | 0.696 |
| Negative BCs 7 days after treatment start, n (%) [performed in n (%)] | 5 (29.4) [5 (29.4)] | 7 (63.6) [7 (63.6)] | 0.130 |
| Negative BCs 14 days after treatment start, n (%) [performed in n (%)] | 3 (17.6) [3 (17.6)] | 3 (27.3) [3 (27.3)] | 0.601 |
| Adverse reactions to antibiotic treatment, n (%) | 1 (5.9) | 1 (9.1) | 0.752 |
| Cumulative mortality 7 days from CZA start, n (%) | 3 (17.6) | 0 | 0.258 |
| Cumulative mortality 14 days from CZA start, n (%) | 3 (17.6) | 4 (36.4) | 0.381 |
| Cumulative mortality 30 days from CZA start, n (%) | 5 (29.4) | 4 (36.4) | 0.700 |
| Overall in-hospital mortality, n (%) | 7 (41.2) | 5 (45.5) | 0.826 |
| Length of stay from CZA start (days), median (IQR) | 16.0 (12.0–51.0) | 16.0 (14.0–47.0) | 0.846 |
Values in bold indicate P<0.05.
CKD, chronic kidney disease; CLRBSI, Central line-related BSIs; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CZA, ceftazidime/avibactam; IAI, intra-abdominal infection; ICS, increment CPE score; KDIGO, kidney disease improving global outcomes; LRTI, lower respiratory tract infection; PBS, Pitt bacteremia score; VAP, ventilator-associated pneumonia.
Comparison between survivors and non-survivors at 30 days from ceftazidime/avibactam start
Patients who died within 30 days from the start of ceftazidime/avibactam (18.2%) were more likely to have SARS-CoV-2 coinfection (25% versus 4.4%), a higher CCI (median value of 8.0 versus 6.0) and in particular a higher prevalence of diabetes (45.0% versus 21.1%) and lower eGFR values (median value of 45.6 versus 83.3 mL/min) and were less likely to receive full dose of ceftazidime/avibactam (55.0% versus 78.9%) (Table 3). They also had more severe sepsis (ICS value of 8.0 versus 6.0), with a higher incidence of septic shock (55.0% versus 18.9%), need for vasoactive drugs (60.0% versus 27.8%) and need for CRRT (30.0% versus 4.4%) (Table 3). Intra-abdominal infections (IAIs) and lower respiratory tract infections (LRTIs) were significantly more common as a source of BSI in patients with a negative outcome (14.4% and 15.6% of surviving patients versus 35.0% and 40.0% of deceased patients, P = 0.004). The treatment regimen (monotherapy or ceftazidime/avibactam-based combination therapy) did not differ significantly (Table 3).
Table 3.
Survival analysis
| Variable | Survived at 30 days from CZA start (n = 90) | Died at 30 days from CZA start (n = 20) | P value |
|---|---|---|---|
| Gender male, n (%) | 64 (71.1) | 14 (70.0) | 0.921 |
| Age, years, median (IQR) | 68.0 (57.0–78.3) | 72.5 (62.3–78.8) | 0.210 |
| Hospitalized in ICU, n (%) | 25 (27.8) | 9 (45.0) | 0.180 |
| SARS-CoV-2 coinfection, n (%) | 4 (4.4) | 5 (25.0) | 0.010 |
| Diabetes, n (%) | 19 (21.1) | 9 (45.0) | 0.044 |
| Cardiovascular disease, n (%) | 62 (68.9) | 15 (75.0) | 0.788 |
| COPD, n (%) | 28 (31.1) | 10 (50.0) | 0.124 |
| CKD, n (%) | 27 (30.0) | 8 (40.0) | 0.431 |
| Chronic haemodialysis, n (%) | 3 (3.3) | 2 (10.0) | 0.223 |
| Chronic liver disease, n (%) | 8 (8.9) | 4 (20.0) | 0.226 |
| Solid neoplasia, n (%) | 22 (24.4) | 6 (30.0) | 0.583 |
| Haematological neoplasia, n (%) | 4 (4.4) | 3 (15.0) | 0.111 |
| Immunodeficiency, n (%) | 21 (23.3) | 7 (35.0) | 0.273 |
| Solid organ transplant, n (%) | 6 (6.7) | 1 (5.0) | 0.782 |
| CCI, median (IQR) | 6.0 (5.0–8.0) | 8.0 (5.3–10.0) | 0.024 |
| Previous KPC-Kp rectal colonization, n (%) | 74 (82.2) | 13 (65.0) | 0.125 |
| CKD-EPI eGFR, median (IQR) | 83.3 (52.8–109.7) | 45.6 (28.9–85.5) | 0.009 |
| KDIGO class at CZA prescription, n (%) | |||
| ≥90 (G1) | 38 (42.2) | 4 (20.0) | 0.075 |
| 60–89 (G2) | 28 (31.1) | 4 (20.0) | |
| 45–59 (G3a) | 4 (4.4) | 2 (10.0) | |
| 30–44 (G3b) | 7 (7.8) | 5 (25.0) | |
| 15–29 (G4) | 10 (11.1) | 3 (15.0) | |
| <15 (G5) | 3 (3.3) | 2 (10.0) | |
| Source of BSI, n (%) | |||
| Primary bacteraemia | 28 (31.1) | 4 (20.0) | 0.004 |
| CLRBSI | 5 (5.6) | 0 | |
| Urinary tract | 30 (33.3) | 1 (5.0) | |
| IAI | 13 (14.4) | 7 (35.0) | |
| LRTI (VAP included) | 14 (15.6) | 8 (40.0) | |
| CZA MIC of the isolated KPC-Kp (mg/L), median (IQR) | 2.0 (2.0–4.0) | 2.0 (2.0–3.8) | 0.529 |
| Serum lactate (mmol/L), median (IQR) | 2.3 (1.6–3.3) | 3.2 (1.8–4.2) | 0.346 |
| C-reactive protein (mg/dL), median (IQR) | 10.2 (5.7–16.6) | 14.3 (11.9–24.2) | 0.051 |
| Procalcitonin (ng/dL), median (IQR) | 4.6 (0.7–30.7) | 6.9 (2.2–33.0) | 0.171 |
| CZA dose (g), median (IQR) | 7.5 (7.5–7.5) | 7.5 (2.4–7.5) | 0.026 |
| CZA full dose, n (%) | 71 (78.9) | 11 (55.0) | 0.044 |
| CZA combination therapy, n (%) | 80 (88.9) | 17 (85.0) | 0.702 |
| Treatment regimen, n (%) | |||
| CZA monotherapy | 10 (11.1) | 3 (15.0) | 0.865 |
| CZA + meropenem | 35 (38.9) | 8 (40.0) | |
| CZA + fosfomycin | 39 (43.3) | 7 (35.0) | |
| CZA + other (co-trimoxazole, tigecycline, gentamicin or colistin) | 6 (6.7) | 2 (10.0) | |
| sCr at admission (mg/dL), median (IQR) | 1.0 (0.7–1.4) | 1.1 (0.8–2.8) | 0.162 |
| sCr at CZA prescription (mg/dL), median (IQR) | 0.8 (0.5–1.3) | 1.4 (0.8–2.3) | 0.010 |
| sCr after 48–72 h of CZA administration (mg/dL), median (IQR) | 0.7 (0.5–1.1) | 1.2 (0.7–1.9) | 0.016 |
| sCr after 7 days of CZA administration (mg/dL), median (IQR) | 0.6 (0.4–1.0) | 1.4 (0.5–2.2) | 0.027 |
| sCr at the end of CZA treatment (mg/dL), median (IQR) | 0.6 (0.4–1.0) | 1.2 (0.5–1.9) | 0.032 |
| CRRT required due to infection, n (%) | 4 (4.4) | 6 (30.0) | 0.002 |
| Vasoactive drugs required, n (%) | 25 (27.8) | 12 (60.0) | 0.009 |
| Septic shock, n (%) | 17 (18.9) | 11 (55.0) | 0.003 |
| PBS, median (IQR) | 3.0 (2.0–4.0) | 4.0 (2.0–5.0) | 0.203 |
| ICS, median (IQR) | 6.0 (3.0–8.0) | 8.0 (6.0–11.0) | 0.004 |
| Time (days) from index BC to appropriate (in vitro active) therapy, median (IQR) | 1 (0–2) | 1 (0–2) | 0.714 |
| Time (days) from index BC to CZA prescription, median (IQR) | 1 (0.75–2) | 1 (0–2) | 0.309 |
| Appropriate empiric therapy, n (%) | 36 (40.0) | 6 (30.0) | 0.456 |
| Performed source control, n (%) | 76 (84.4) | 15 (75.0) | 0.310 |
| Duration of definitive therapy (days), median (IQR) | 15.0 (12.0–20.0) | 10.5 (6.3–18.8) | 0.005 |
| Clinical cure, n (%) | 68 (75.6) | 0 | <0.001 |
| KPC-Kp BSI recurrence within 30 days, n (%) | 11 (12.2) | 0 | 0.210 |
| Secondary infections within 30 days, n (%) | 30 (33.3) | 8 (40.0) | 0.609 |
| Subsequent finding of CZA MIC increase (≥8 mg/L) | 7 (7.8) | 0 | 0.168 |
| Negative BCs 72 h after treatment start, n (%) [performed in n (%)] | 53 (58.9) [63 (68.9)] | 10 (50.0) [11 (55.5)] | 0.446 |
| Negative BCs 7 days after treatment start, n (%) [performed in n (%)] | 44 (48.9) [48 (53.3)] | 8 (40.0) [8 (40.0)] | 0.575 |
| Negative BCs 14 days after treatment start, n (%) [performed in n (%)] | 36 (40.0) [38 (42.2)] | 3 (15.0) [4 (20.0)] | 0.211 |
| Adverse reactions to antibiotic treatment, n (%) | 10 (11.1) | 1 (5.0) | 0.685 |
| Cumulative mortality 7 days from CZA start, n (%) | 0 | 6 (30.0) | <0.001 |
| Cumulative mortality 14 days from CZA start, n (%) | 0 | 12 (60.0) | <0.001 |
| Overall in-hospital mortality, n (%) | 17 (18.9) | 20 (100.0) | <0.001 |
| Length of stay from CZA start (days), median (IQR) | 38.0 (16.8–60.3) | 11.0 (6.3–19.8) | <0.001 |
Values in bold indicate P<0.05.
CKD, chronic kidney disease; CLRBSI, Central line-related BSIs; COPD, chronic obstructive pulmonary disease; CRRT, continuous renal replacement therapy; CZA, ceftazidime/avibactam; IAI, intra-abdominal infection; ICS, increment CPE score; KDIGO, kidney disease improving global outcomes; LRTI, lower respiratory tract infection; PBS, Pitt bacteremia score; VAP, ventilator-associated pneumonia.
Multivariable analysis evaluating the risk factors for 30 day mortality from ceftazidime/avibactam start was performed on the whole population: a reduced dose of ceftazidime/avibactam (HR 4.47, 95% CI 1.09–18.03, P = 0.037), IAI or LRTI as source of BSI (HR 5.42, 95% CI 1.77–16.55, P = 0.003), septic shock (HR 6.99, 95% CI 1.36–35.87, P = 0.020) and SARS-CoV-2 coinfection (HR 10.23, 95% CI 2.69–38.85, P = 0.001) were identified as factors independently associated with 30 day mortality (Table 4). Considering the potential difference between CKD and AKI patients, we conducted two additional multivariable models by excluding patients with CKD [n = 17, Table S1(a) (available as Supplementary data at JAC-AMR Online)] and those with AKI [n = 11, Table S1 (b)]. Specifically, after excluding patients with CKD, ceftazidime/avibactam-adjusted dosage was not associated with the main outcome (HR 4.07, 95% CI 0.59–27.74, P = 0.152), while, after excluding patients with AKI (and therefore including those receiving a reduced dosage of ceftazidime/avibactam due to CKD), there was a trend towards significance (HR 5.16, 95% CI 0.87–30.56, P = 0.071).
Table 4.
Multivariable analysis of independent predictors of 30 day mortality in patients with BSI from KPC-Kp
| Variables | HR (95% CI) | P value |
|---|---|---|
| Adjusted dose of CZA | 4.47 (1.09–18.03) | 0.037 |
| Source of BSI: IAI or LRTI | 5.42 (1.77–16.55) | 0.003 |
| SARS-CoV-2 coinfection | 10.23 (2.69–38.85) | 0.001 |
| Septic shock | 6.99 (1.36–35.87) | 0.020 |
| CRRT required due to infection | 2.27 (0.71–7.28) | 0.165 |
| CCI, one point increment | 1.02 (0.84–1.23) | 0.809 |
| ICS, one point increment | 0.90 (0.71–1.15) | 0.419 |
| Hospitalization in ICU | 0.84 (0.28–2.51) | 0.762 |
| sCr at CZA prescription (0.1 mg/mL increment) | 0.94 (0.62–1.45) | 0.812 |
Values in bold indicate P<0.05.
BSI, bloodstream infection; CCI, Charlson comorbidity index; CRRT, continuous renal replacement therapy; CZA, ceftazidime/avibactam; IAI, intra-abdominal infection; ICS, increment CPE score; ICU, intensive care unit; LRTI, lower respiratory tract infection; sCr, serum creatinine.
Discussion
In patients with sepsis, early and adequate antibiotic exposure is considered essential to maximize clinical success and prevent resistance.18,19 Unfortunately, the septic state induces many unpredictable PK changes, ranging from increased renal clearance to transient AKI, often resulting in prescriptions that miss PD targets.3,20 However, even standard doses of β-lactams such as meropenem result in inadequate exposure in a significant proportion of critically ill patients, with large inter-individual variability and a heavy and dynamic influence of renal function. The discrepancy is greater in patients with moderate renal impairment.21 In theory, the aim of dose adjustment should be to minimize toxicity (i.e. overexposure) without jeopardizing efficacy (PD target achievement), but current recommendations for renal adjustment are often based on small studies in healthy volunteers or people with stable chronic renal impairment.22 Previous studies have shown that for most antibiotics excreted by kidneys, there is no good-quality evidence to support the recommended dose reduction in patients with renal impairment to ensure adequate drug exposure.23 In addition, estimates of renal function in patients with AKI may appear worse than actual renal function, leading to underexposure.23 In fact, in the kidney the dynamic changes are difficult to characterize with sCr because this biomarker is not sensitive to rapid fluctuations over time.24 As a result, renal adjustment of antibiotic dose has been associated with increased failure and mortality in ICU patients.25
We presented a population with KPC-Kp BSI and compared those treated with a full dose of ceftazidime/avibactam with those treated with a reduced dose for renal adaptation. Among those treated with a reduced dose, we then compared patients with acute renal impairment with those with chronic renal impairment.
KPC-Kp BSIs affected a population with a high number of comorbidities (expressed by the elevated CCI), occurring in the ICU in approximately one-third of cases and manifested as severe sepsis syndrome with elevated severity indices such as serum lactates, need for CRRT, PBS and ICS. However, the frequency of prescription of ceftazidime/avibactam-based combination therapy for the treatment of KPC-Kp infection (88.2%) and the overall 30 day mortality (18.2%) were comparable to those previously shown in similar studies.5
The survival analysis identified reduced ceftazidime/avibactam dose as one of the factors associated with death, along with comorbidities, sepsis severity indices, and IAI and LRTI as BSI source. The multivariable analysis supports the independent effect of ceftazidime/avibactam dose on mortality.
The next objective of the study was to assess whether the higher mortality in the group treated with an adjusted dose of ceftazidime/avibactam was due to a higher sepsis severity or, rather, depended on renal failure itself. Comparison of the two subgroups of patients treated with reduced ceftazidime/avibactam (CKD and AKI patients, Table 2) showed that they did not differ significantly in terms of demographics, comorbidities, source of infection, source control effectiveness, mono- or combination therapy, type of combination regimen and recurrence. As expected, the AKI group probably suffered from a more severe sepsis syndrome, as evidenced by a higher PBS and the higher need for CRRT. AKI patients also were more commonly already admitted to the ICU at the time of BSI diagnosis and their isolates tended to have a higher MIC of ceftazidime/avibactam. Likewise, their higher number of secondary infections could be attributed to the greater need for device and invasive treatment, such as the use of CRRT. Interestingly, patients with AKI less frequently received adequate empirical treatment (9.1% versus 58.8%). This was probably because the consulting infectious disease physician is more likely to assume the involvement of MDR pathogens when faced with a chronic dialysis patient with sepsis. Despite these differences between the population of patients with known CKD and those with sepsis-associated AKI, there were no significant differences in length of stay, BC clearance or mortality. Therefore, it can be hypothesized that the role of the reduced dose of ceftazidime/avibactam in increasing mortality does not depend only on the acute, infection-related status of renal impairment. Interestingly, after excluding patients with AKI (and thus retaining only those receiving a reduced dosage of ceftazidime/avibactam due to CKD), a trend towards a significant association with the outcome was observed, suggesting that the risk of failure in attaining optimal ceftazidime/avibactam joint PK/PD target may be present also in patients with CKD. We believe that the loss of statistical significance is likely due to the reduced number of patients remaining in the analysis. Therefore, further studies are needed to investigate the role of ceftazidime/avibactam dosage adjustment due to CKD and its association with poorer outcomes.
As shown in Tables 1–3, the median creatinine values tended to decrease in the days following prescription, especially in patients with AKI. The prescribed dose of ceftazidime/avibactam was certainly adjusted accordingly. However, this reduction in creatinine seems to occur at 48–72 h after prescription. In our opinion, this subsequent increase in ceftazidime/avibactam dose did not really change the outcome of the study. In fact, it has been shown previously that it is the treatment the patient receives in the first 24 h of treatment that mainly influences the outcome of KPC-Kp BSI.26
Our interpretation seems to be in line with previous findings. Indeed, several antibiotics approved in recent decades have shown a reduced efficacy in patients with moderate renal impairment, which is the most represented KDIGO category at the time of ceftazidime/avibactam prescription in the present study.27,28
At multivariable analysis, the reduced dose of ceftazidime/avibactam, septic shock, SARS-CoV-2 coinfection and IAI or LRTI as source of BSI were independently associated with 30 day mortality. This may not represent a coincidence. Indeed, the lung is a site where the PK of ceftazidime/avibactam are thought to be less efficient.4 In the RECLAIM 1 and 2 trials, ceftazidime/avibactam dose reduction in patients with IAI and moderate AKI was associated with a significantly lower response rate,29 which was attributed to the high rate (67.9%) of patients who recovered from moderate AKI within 72 h of starting treatment for sepsis.27,29 In addition, CRRT has been identified as one of the risk factors for clinical failure and resistance development in people treated with ceftazidime/avibactam.4 In a retrospective, multicentre cohort of carbapenem-resistant Enterobacterales infections (only 8.3% BSI) treated with ceftazidime/avibactam, patients who died were more likely to have had their ceftazidime/avibactam dose adjusted for renal impairment.30 In agreement with our findings, the large cohort of adults with KPC-Kp treated with a ceftazidime/avibactam-based regimen presented by Tumbarello et al. showed that patients with LRTI had a higher 30 day mortality and the ceftazidime/avibactam dose adjustment for renal function significantly impacted on 30 day survival rate of patients with LRTI or IAI. Also, ceftazidime/avibactam dose adjustment resulted one of the factors independently associated with 30 day mortality at multivariable analysis.5 However, data on renal function distribution and calculation, and on renal adjusted dosing are poorly described in the paper by Tumbarello et al.
A systematic review and meta-analysis examined the efficacy of ceftazidime/avibactam in patients with renal impairment who received the recommended dose adjustment compared with patients with normal renal function who received the full dose. Overall, ceftazidime/avibactam renal adjustment was associated with a higher risk of mortality. Of note, only 42.6% of patients enrolled had a BSI, but the ICU admission rate was high.31 In addition, the authors questioned whether the manufacturer’s recommendations for ceftazidime/avibactam dose adjustment were sufficiently focused on achieving optimal PK/PD targets with the currently most supported strategy, i.e. reducing the amount of each single dose while maintaining the dosing interval unmodified.31
Finally, in a recent retrospective study conducted on a severely ill population with difficult-to-treat Pseudomonas aeruginosa hospital-acquired pneumonia treated with ceftazidime/avibactam, multivariable analysis showed that clinical cure was positively associated with ceftazidime/avibactam loading dose and prolonged infusion, and negatively associated with an APACHE-II score of >15, septic shock and ceftazidime/avibactam dose adjustment for renal impairment.32
All these considered, our findings point in the same direction, suggesting that the currently recommended renal dose adjustment of ceftazidime/avibactam may not produce adequate drug exposure in patients with ongoing sepsis and that, accordingly, optimization of ceftazidime/avibactam administration [i.e. by loading dosage and/or extended/continuous infusion or drug dosage adjustment following real-time therapeutic drug monitoring (TDM) results, or reducing the amount of each single dose while maintaining the dosing interval unmodified] is needed in patients with both acute and chronic renal failure. It has already been shown that the use of ceftazidime/avibactam as a continuous infusion ensures a high rate of achievement of the PK/PD target in MDR pathogens.33 The possible underexposure to ceftazidime/avibactam could theoretically facilitate the development of resistance. In our study, a KPC-Kp strain with ceftazidime/avibactam MICs greater than or equal to the EUCAST breakpoint of 8 mg/L was subsequently isolated in 6.4% of the population. However, this did not differ significantly according to the dose of ceftazidime/avibactam received, the type of renal failure or the outcome.
This study has many limitations. The retrospective nature of the study limits our ability to collect certain types of data. The sample size is small, so subgroup analysis has an elevated risk of bias. CLCR was estimated using the CKD-EPI equation because weight was frequently not available in the medical records. However, the lack of weight in the medical records suggests that it was also unavailable at the time of the ceftazidime/avibactam prescription and therefore our estimate of clearance is likely to be consistent with that used by the prescribers. We recognize that the renal function with CKD-EPI cannot be accurate in ICU patients. Usually, formulae used to estimate GFR assume both that sCr is in steady state and that the creatinine generation rate is the same as that in stable outpatients, assumptions which are most likely not maintained during the acute phase of critical illness. The subgroup receiving a reduced dose of ceftazidime/avibactam had higher CCI (possibly partially driven by history of CKD) and ICS (only partially driven by a CCI value of ≥2), raising some concerns about a possible imbalance in baseline conditions between the two groups, which was limited by their inclusion in the final multivariable model. Finally, in the absence of TDM, we could not verify whether renal-adjusted dosages of ceftazidime/avibactam actually corresponded to drug underexposure.
Conclusions
The introduction of ceftazidime/avibactam has improved the outcome of patients with carbapenem-resistant infections, but unfortunately some subgroups of patients do not appear to benefit from adequate exposure to the drug at the recommended doses. In the present study, patients with KPC-Kp BSIs treated with renal-adjusted doses of ceftazidime/avibactam, regardless of acute or chronic kidney disease, had a higher mortality rate. Further research is needed to determine the correct ceftazidime/avibactam dosage required to treat BSIs in patients with renal impairment.
Supplementary Material
Contributor Information
A Oliva, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
L Volpicelli, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
A Gigante, Department of Translational and Precision Medicine, Sapienza University of Rome, Viale dell’Università 37, Rome 00185, Italy.
M Di Nillo, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
S Trapani, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
A Viscido, Microbiology and Virology Unit, University Hospital Policlinico Umberto I, Viale del Policlinico, 155, Rome 00161, Italy.
F Sacco, Microbiology and Virology Unit, University Hospital Policlinico Umberto I, Viale del Policlinico, 155, Rome 00161, Italy.
C M Mastroianni, Department of Public Health and Infectious Diseases, Sapienza University of Rome, Piazzale Aldo Moro 5, Rome 00185, Italy.
Funding
This study was conducted as part of our routine work.
Transparency declarations
All authors have no conflicts of interest to declare with regard to this publication.
Supplementary data
Table S1 is available as Supplementary data at JAC-AMR Online.
References
- 1. Karampatakis T, Tsergouli K, Lowrie K. Efficacy and safety of ceftazidime-avibactam compared to other antimicrobials for the treatment of infections caused by carbapenem-resistant Klebsiella pneumoniae strains, a systematic review and meta-analysis. Microb Pathog 2023; 179: 106090. 10.1016/j.micpath.2023.106090 [DOI] [PubMed] [Google Scholar]
- 2. Boattini M, Bianco G, Charrier L et al. Rapid diagnostics and ceftazidime/avibactam for KPC-producing Klebsiella pneumoniae bloodstream infections: impact on mortality and role of combination therapy. Eur J Clin Microbiol Infect Dis 2023; 42: 431–9. 10.1007/s10096-023-04577-x [DOI] [PubMed] [Google Scholar]
- 3. Gatti M, Pea F. Pharmacokinetic/pharmacodynamic target attainment in critically ill renal patients on antimicrobial usage: focus on novel beta-lactams and beta lactams/beta-lactamase inhibitors. Expert Rev Clin Pharmacol 2021; 14: 583–99. 10.1080/17512433.2021.1901574 [DOI] [PubMed] [Google Scholar]
- 4. Shields RK, Nguyen MH, Chen L et al. Pneumonia and renal replacement therapy are risk factors for ceftazidime-avibactam treatment failures and resistance among patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2018; 62: e02497-17. 10.1128/AAC.02497-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tumbarello M, Raffaelli F, Giannella M et al. Ceftazidime-avibactam use for Klebsiella pneumoniae carbapenemase-producing K. pneumoniae infections: a retrospective observational multicenter study. Clin Infect Dis 2021; 73: 1664–76. 10.1093/cid/ciab176 [DOI] [PubMed] [Google Scholar]
- 6. Inker LA, Eneanya ND, Coresh J et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med 2021; 385: 1737–49. 10.1056/NEJMoa2102953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012; 2: 1–138. 10.1038/kisup.2012.2 [DOI] [Google Scholar]
- 8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2024 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2024; 105: S117–314. 10.1016/j.kint.2023.10.018 [DOI] [PubMed] [Google Scholar]
- 9. EMA . European Public Assessment Report (EPAR) for Zavicefta, product information. https://www.ema.europa.eu/en/documents/product-information/zavicefta-epar-product-information_en.pdf.
- 10. ECDC . EU case definitions. https://www.ecdc.europa.eu/en/all-topics/eu-case-definitions.
- 11. CDC, National Healthcare Safety Network (NHSN) . Bloodstream infection (BSI) events. https://www.cdc.gov/nhsn/psc/bsi/index.html.
- 12. Cleri DJ, Corrado ML, Seligman SJ. Quantitative culture of intravenous catheters and other intravascular inserts. J Infect Dis 1980; 141: 781–6. 10.1093/infdis/141.6.781 [DOI] [PubMed] [Google Scholar]
- 13. Charlson ME, Pompei P, Ales KL et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–83. 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 14. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17: 726–34. 10.1016/S1473-3099(17)30228-1 [DOI] [PubMed] [Google Scholar]
- 15. Korvick JA, Bryan CS, Farber B et al. Prospective observational study of Klebsiella bacteremia in 230 patients: outcome for antibiotic combinations versus monotherapy. Antimicrob Agents Chemother 1992; 36: 2639–44. 10.1128/AAC.36.12.2639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Singer M, Deutschman CS, Seymour CW et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016; 315: 801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. EUCAST . Clinical breakpoints—breakpoints and guidance. https://www.eucast.org/clinical_breakpoints.
- 18. Evans L, Rhodes A, Alhazzani W et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021; 47: 1181–247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gatti M, Cojutti PG, Pea F. Impact of attaining aggressive vs. conservative PK/PD target on the clinical efficacy of beta-lactams for the treatment of Gram-negative infections in the critically ill patients: a systematic review and meta-analysis. Crit Care 2024; 28: 123. 10.1186/s13054-024-04911-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fujii M, Karumai T, Yamamoto R et al. Pharmacokinetic and pharmacodynamic considerations in antimicrobial therapy for sepsis. Expert Opin Drug Metab Toxicol 2020; 16: 415–30. 10.1080/17425255.2020.1750597 [DOI] [PubMed] [Google Scholar]
- 21. Ehmann L, Zoller M, Minichmayr IK et al. Role of renal function in risk assessment of target non-attainment after standard dosing of meropenem in critically ill patients: a prospective observational study. Crit Care 2017; 21: 263. 10.1186/s13054-017-1829-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crass RL, Rodvold KA, Mueller BA et al. Renal dosing of antibiotics: are we jumping the gun? Clin Infect Dis 2019; 68: 1596–602. 10.1093/cid/ciy790 [DOI] [PubMed] [Google Scholar]
- 23. de Vroom SL, van Daalen FV, Zieck SE et al. Does dose reduction of renally cleared antibiotics in patients with impaired renal function lead to adequate drug exposure? A systematic review. Clin Microbiol Infect 2021; 27: 352–63. 10.1016/j.cmi.2020.11.032 [DOI] [PubMed] [Google Scholar]
- 24. Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009; 20: 672–9. 10.1681/ASN.2008070669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camargo MS, Mistro S, Oliveira MG et al. Association between increased mortality rate and antibiotic dose adjustment in intensive care unit patients with renal impairment. Eur J Clin Pharmacol 2019; 75: 119–26. 10.1007/s00228-018-2565-7 [DOI] [PubMed] [Google Scholar]
- 26. Falcone M, Bassetti M, Tiseo G et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit Care 2020; 24: 29. 10.1186/s13054-020-2742-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bidell MR, Lodise TP. Suboptimal clinical response rates with newer antibiotics among patients with moderate renal impairment: review of the literature and potential pharmacokinetic and pharmacodynamic considerations for observed findings. Pharmacotherapy 2018; 38: 1205–15. 10.1002/phar.2184 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Lovern M, Riccobene T et al. Considerations in the selection of renal dosage adjustments for patients with serious infections and lessons learned from the development of ceftazidime-avibactam. Antimicrob Agents Chemother 2020; 64: e02105-19. 10.1128/AAC.02105-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mazuski JE, Gasink LB, Armstrong J et al. Efficacy and safety of ceftazidime-avibactam plus metronidazole versus meropenem in the treatment of complicated intra-abdominal infection: results from a randomized, controlled, double-blind, phase 3 program. Clin Infect Dis 2016; 62: 1380–9. 10.1093/cid/ciw133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Jorgensen SCJ, Trinh TD, Zasowski EJ et al. Evaluation of the INCREMENT-CPE, Pitt bacteremia and qPitt scores in patients with carbapenem-resistant Enterobacteriaceae infections treated with ceftazidime-avibactam. Infect Dis Ther 2020; 9: 291–304. 10.1007/s40121-020-00288-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gatti M, Fornaro G, Viale P et al. Clinical efficacy of renal dosing adjustments of ceftazidime-avibactam in patients affected by carbapenem-resistant Gram-negative infections: a systematic review and meta-analysis of observational studies. Br J Clin Pharmacol 2023; 89: 617–29. 10.1111/bcp.15586 [DOI] [PubMed] [Google Scholar]
- 32. Xu C, Zeng F, Huang Y et al. Clinical efficacy of ceftazidime/avibactam combination therapy for severe hospital-acquired pulmonary infections caused by carbapenem-resistant and difficult-to-treat Pseudomonas aeruginosa. Int J Antimicrob Agents 2024; 63: 107021. 10.1016/j.ijantimicag.2023.107021 [DOI] [PubMed] [Google Scholar]
- 33. Fresan D, Luque S, Benítez-Cano A, et al. Pharmacokinetics/pharmacodynamics and therapeutic drug monitoring of ceftazidime/avibactam administered by continuous infusion in patients with MDR Gram-negative bacterial infections. J Antimicrob Chemother 2023; 78: 678–83. 10.1093/jac/dkac439 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.