Abstract
Objective
To investigate the impact of transition interval length when switching from natalizumab (NTZ) to anti‐CD20 monoclonal antibodies (antiCD20) on recurrent disease activity and safety in relapsing multiple sclerosis (RMS).
Methods
Aggregating data from 8 MS centres in Austria, Switzerland, and Germany, we included RMS patients who (i) continuously received NTZ for ≥3 months, (ii) were switched to antiCD20, and (iii) had ≥12 months follow‐up after switch. The primary endpoint was occurrence of relapse after switch, secondary endpoints included severe infections (CTCAE grade ≥3).
Results
Overall, 139 RMS patients were included (70.5% females, mean age at switch 38.8 years [SD 9.7], mean disease duration at switch 11.3 years [SD 6.2], median duration on NTZ 4.4 years [range: 0.3–16.4], median transition interval 58 days [0–180]). Relapse occurred in 18 patients (12.9%) after NTZ discontinuation. Of those, 11 (61.1%) patients relapsed during the transition interval. No patient with a transition interval below 30 days experienced a relapse, compared to 11.1% and 16.1% with transition intervals of 30–44 days and ≥ 45 days, respectively. In multivariable Cox regression, a transition interval ≥ 45 days predicted a 4.73‐fold increased risk of relapse. Over approximately 4 years of follow‐up, six severe infections were reported without any noticeable effect of transition interval length. No PML occurred.
Conclusions
Switching from NTZ to antiCD20 is generally both effective and safe. Keeping the transition interval below 30 days provides the optimal balance between preventing recurrent disease activity and ensuring safety.
Keywords: CD20, multiple sclerosis, natalizumab, prediction, risk, switch
INTRODUCTION
Natalizumab (NTZ), a monoclonal antibody blocking lymphocyte migration across the blood–brain barrier by binding to the α4 subunit of α4β1‐integrin, is one of the most effective disease‐modifying therapies (DMT) for relapsing multiple sclerosis (RMS) [1]. While generally providing a favourable safety profile, discontinuation of NTZ is common in clinical routine, typically in patients seropositive for John Cunningham virus (JCV) due to risk of developing progressive multifocal leukoencephalopathy (PML), less frequently also due to insufficient control of disease activity or family planning [1]. However, discontinuation of NTZ is complicated by the risk of reoccurrence or even rebound disease activity, which requires an exit strategy involving an alternative DMT [2]. While randomized controlled studies are lacking, real‐world observational data suggest B‐cell depleting anti‐CD20 monoclonal antibodies (antiCD20) as the preferred NTZ exit strategy due to their efficacy and favorable safety profile, with only rare PML occurrences [3].
However, no consensus exists on safely transitioning patients from NTZ to anti‐CD20, particularly regarding the optimal washout interval to balance preventing disease recurrence and ensuring safety. This study aimed to evaluate the impact of transition interval length on disease activity and infections to identify the optimal balance between efficacy and safety.
METHODS
This is a retrospective cohort study performed by eight specialized MS centres in Austria, Switzerland, and Germany [4]. Local MS databases of these centers were screened (database closure: MARCH 30, 2024) by the following criteria: RMS according to concurrent McDonald criteria aged ≥18 years, who (i) continuously received treatment with NTZ for ≥3 months, (ii) were switched from NTZ to any antiCD20 (ocrelizumab [OCR], ofatumumab [OFM], rituximab [RTX]), and (iii) had ≥12 months of follow‐up after switch [5].
The primary endpoint was time to relapse within 12 months after the last application of NTZ. Secondary endpoints were time to Expanded Disability Status Scale (EDSS) worsening and occurrence of a severe infection during follow‐up (for detailed definitions see supplemental methods). Transition interval was defined as the number of days between the last application of NTZ and the first application of antiCD20.
Statistical analysis was performed using R‐Statistical Software (Version 4.0.0). To evaluate the influence of transition interval length, we first used individual line diagrams plotting transition interval length and subsequent time on CD20 against relapses, EDSS worsening and severe infections before and after the last application of NTZ. Multivariable Cox regression models were performed with time to relapse and time to severe infection as the dependent variables and the transition interval as the independent variable adjusted for relevant covariables. A detailed description of statistical analyses is provided in the supplemental methods.
The study was approved by the ethics committees of the Medical University Vienna (ethical approval number: 1286/2022; on behalf of all Austrian centers), Bern (2017‐01369) and Munich (24‐0310), which waived the need for written informed consent from study participants. This study adheres to “Strengthening the Reporting of Observational Studies in Epidemiology” (STROBE) guidelines.
RESULTS
A total of 139 patients were included (Figure S1 and Table S1). Median transition interval between last NTZ infusion and first antiCD20 therapy application was 58 days (IQR 42–92, range 0–180), with the majority of patients switching to OCR (67.6%), followed by OFM (24.5%) and RTX (7.9%).
Disease activity
Relapse occurred in 18 patients (12.9%) within 12 months after NTZ discontinuation, of whom 11 (61.1%) happened during the respective transition interval (Figure 1a).
FIGURE 1.
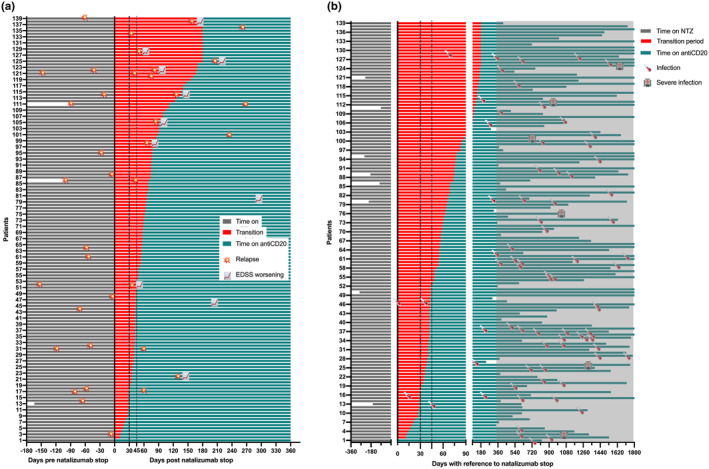
(a) Timing of clinical disease activity following switch from natalizumab to antiCD20 monoclonal antibodies. The vertical dashed line marks the split between transition intervals ≤/>30 days between natalizumab and antiCD20, the vertical dotted line marks the split between transition intervals ≤/>45 days; red and teal coloring indicate the treatment status: between treatments (red) or after the first antiCD20 application (teal). Data truncated at 360 days after natalizumab discontinuation. (b) Timing of infections following switch from natalizumab to antiCD20 monoclonal antibodies. The vertical dashed line marks the split between transition intervals ≤/>30 days between natalizumab and antiCD20, the vertical dotted line marks the split between transition intervals ≤/>45 days; red and teal coloring indicate the treatment status: between treatments (red) or after the first antiCD20 application (teal). Grey area marks observation period longer than 360 days after switch, that is, unlikely to be influenced by transition interval length. Data truncated at 1800 days after natalizumab discontinuation.
None of the patients with a transition interval below 30 days had a relapse (0/16), compared to 11.1% (4/36) with a transition interval of 30–44 days and 16.1% (14/87) with a transition interval ≥ 45 days. In the multivariable Cox regression, longer transition intervals were linked to a higher relapse risk after NTZ discontinuation (HR 1.14 per 7 days, p < 0.001), with patients having intervals ≥45 days showing a 4.73‐fold increased relapse risk (Table 1).
TABLE 1.
Multivariable Cox regression predicting relapse in patients switching from natalizumab to antiCD20.
| Relapse | Hazard ratio | 95% confidence interval | p‐value |
|---|---|---|---|
| Female (referenced to male) | 1.44 | 0.50–4.15 | 0.505 |
| Age at switch (per year) | 0.93 | 0.88–0.99 | 0.019 |
| Annualized relapse rate under NTZ (per 1 relapse/year) | 3.45 | 1.27–9.38 | 0.015 |
| MRI activity under NTZ (referenced to no MRI activity) | 1.67 | 0.50–5.72 | 0.401 |
|
Transition interval (last NTZ to first CD20) | |||
| Per 7 days | 1.14 | 1.07–1.21 | <0.001 |
|
≥30 days (referenced to <30 days) |
Not calculable (no relapse observed in reference group) | ||
| ≥45 days (referenced to <45 days) a | 4.73 | 1.28–17.4 | 0.020 |
Note: Calculated by multivariable Cox regression (Nagelkerke's Pseudo R squared: 0.872, p < 0.001) with time to relapse after discontinuing natalizumab to switch to antiCD20 as the dependent variable.
Abbreviation: NTZ, natalizumab.
Calculated with a separate multivariable Cox regression model substituting transition interval as a continuous variable to a dichotomous categorical variable (≥45 vs. <45 days) as an independent variable with the model otherwise set up identically (Nagelkerke's Pseudo R squared: 0.813, p < 0.001).
Infections
Over a median follow‐up of 3.6 years, 80 infections were documented in 62 patients. Of those, six were reported as severe infections (two SARS‐CoV2 pneumonia, bacterial pneumonia, pyelonephritis, severe herpes zoster, clostridia enteritis). No PML occurred and no deaths were reported. There was no difference in the number of severe infections in relation to transition interval length with one (SARS‐CoV2 pneumonia) occurring in patients with a transition interval below 30 days (1/16, 6.3%), compared to one (bacterial pneumonia) with transition interval 30–44 days (1/36, 2.8%) and four with ≥45 days (4/87, 4.6%, p = 0.832). No severe infection occurred in the first year after switch with no significant correlation between interval length and time to severe infection (Spearman rho 0.182, p = 0.152) (Figure 1b).
Sensitivity analyses did not indicate a relevant effect for different antiCD20 agents or study center on disease activity or infections.
DISCUSSION
While switching to antiCD20 is emerging as a preferred exit strategy from NTZ, there is uncertainty concerning the optimal balance between efficacy and safety.
Here, relapses occurred in 13% of patients after NTZ discontinuation, with 61% during the transition to anti‐CD20 and 55% leading to relapse‐associated EDSS worsening. Shorter transition intervals improved disease activity control, with no relapses or EDSS worsening observed below 30 days, while intervals ≥45 days predicted a nearly five‐fold increased relapse risk. Over 4 years, six severe infections were reported, with no impact from transition interval length and no PML cases.
Current guidelines recommend a transition interval of 1 to 3 months after NTZ when switching to another second‐line therapy like antiCD20 [6]. Real‐world studies report NTZ‐to‐anti‐CD20 intervals averaging 6–8 weeks, consistent with our cohort [6, 7, 8].
The upper limit of 12 weeks is based on previous observations, where disease activity post‐NTZ discontinuation peaked at 4–7 months [2]. However, risk of relapse already starts to increase weeks after discontinuation of NTZ and desaturation of α4β1‐integrin receptors occurs already 6–8 weeks following NTZ withdrawal with considerable inter‐individual variation [9, 10]. Further, antiCD20 treatment requires around 4–12 weeks to achieve clinical and radiological efficacy [11]. Thus, a subgroup of patients remains at risk of disease recurrence when NTZ‐to‐anti‐CD20 transition intervals exceed 4 weeks. Our findings highlight the clinical relevance, with a considerable proportion of patients experiencing relapses and disability worsening during 4‐ to 12‐week intervals. Thus, keeping the transition interval below 4 weeks appears crucial to optimizing disease control.
The recommendation of a 4‐week minimum transition interval after NTZ discontinuation aims to prevent infections from cumulative immunosuppression [3]. However, NTZ does not cause systemic immunosuppression and is rarely linked to infections other than PML in JCV‐positive patients [1]. Since no severe infections in our cohort occurred within the first year post‐switch, transition length likely has little impact on infection risk, which appears primarily driven by antiCD20‐mediated B‐cell depletion.
In our cohort, no PML occurred during the transition interval or nearly 4 years of follow‐up. Similarly, in five studies involving 331 patients switching from NTZ to antiCD20, no PML was reported during the transition interval. However, the cumulative incidence of carry‐over PML, attributed to NTZ but diagnosed after anti‐CD20 initiation, was 0.6% (2/331) [3].
While the risk of carry‐over PML when switching from NTZ to antiCD20 is not negligible, it appears independent of transition interval length. A well‐established monitoring regimen, including clinical assessment, frequent MRI, and lumbar puncture for high anti‐JCV antibody index or suspicion, helps mitigate this risk [1, 12, 13].
A thorough monitoring regimen when switching from NTZ to antiCD20 can rule out PML within 1–2 weeks of the last NTZ dose, eliminating any necessity for transition intervals longer than 4 weeks.
In this light, we advocate that the transition interval from last application of NTZ to first application of antiCD20 should be kept as short as possible to a maximum of 4 weeks, depending on time required to rule out PML, to balance preventing disease recurrence and ensuring safety.
This study's strengths include high‐quality data from quality‐controlled databases across specialized MS centers in Austria, Switzerland, and Germany, ensuring rigorous follow‐up and a well‐characterized cohort [4, 14]. However, the sample size, while the largest for NTZ‐to‐anti‐CD20 transitions, remains moderate. Low event rates limited multivariable analyses, such as the association between transition intervals and EDSS worsening, severe infections, or MRI activity. Sensitivity analyses showed no significant differences between antiCD20 agents, but subgroup analyses were underpowered. Future larger studies should address these gaps. Additionally, the retrospective analysis introduces a range of well‐known potential confounders [15].
Contrary to a randomized controlled study, selection and indication bias likely influenced transition interval length, driven by patient and physician characteristics. This is mitigated by broad inclusion criteria designed to represent real‐world clinical scenarios, aiding counseling efforts. Detection/reporting bias for infections is also possible due to data collection methods but is reduced by rigorous follow‐up procedures likely to capture most adverse events, including severe infections. However, generalizability is limited as over 95% of the population was Caucasian, limitations future research should address.
In conclusion, switching from NTZ to antiCD20 is generally both effective and safe. Keeping the transition interval as short as possible with a maximum of 4 weeks, depending on time required to rule out PML, is likely to provide the optimal balance between preventing recurrent disease activity and ensuring safety.
AUTHOR CONTRIBUTIONS
Gabriel Bsteh: Formal analysis; conceptualization; methodology; writing – original draft; funding acquisition. Robert Hoepner: Conceptualization; data curation; writing – review and editing; methodology; supervision. Jonathan A. Gernert: Data curation; writing – review and editing; methodology. Klaus Berek: Data curation; methodology; writing – review and editing. Christiane Gradl: Data curation; methodology; writing – review and editing. Dariia Kliushnikova: Data curation; methodology; writing – review and editing. Anna Damulina: Data curation; methodology; writing – review and editing. Gerhard Traxler: Data curation; methodology; writing – review and editing. Fabian Föttinger: Data curation; methodology; writing – review and editing. Sebastian Habernig: Data curation; methodology; writing – review and editing. Nik Krajnc: Data curation; methodology; writing – review and editing. Alejandro Xavier León Betancourt: Data curation; methodology; writing – review and editing. Markus Ponleitner: Data curation; methodology; writing – review and editing. Tobias Zrzavy: Data curation; methodology; writing – review and editing. Florian Deisenhammer: Data curation; supervision; resources; writing – review and editing. Franziska Di Pauli: Data curation; supervision; writing – review and editing; resources; methodology. Joachim Havla: Resources; writing – review and editing. Michael Khalil: Writing – review and editing; resources; supervision. Tania Kümpfel: Resources; writing – review and editing; supervision. Peter Wipfler: Supervision; writing – review and editing; resources. Andrew Chan: Resources; supervision; writing – review and editing. Thomas Berger: Supervision; writing – review and editing; resources. Helly Hammer: Conceptualization; data curation; supervision; writing – review and editing. Harald Hegen: Conceptualization; data curation; supervision; writing – review and editing.
FUNDING INFORMATION
This study was funded by the Austrian MS Research Society and a strategic research fund from the medical faculty, University of Bern, Bern, Switzerland.
CONFLICT OF INTEREST STATEMENT
Gabriel Bsteh has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, BMS/Celgene, Lilly, Merck, Novartis, Roche, Sanofi, and Teva, and received honoraria for consulting Biogen, BMS/Celgene, Novartis, Roche, Sanofi, and Teva. He has received unrestricted research grants from BMS/Celgene and Novartis. Robert Hoepner has received speaker/advisor honorary from Alexion, Almirall, Biogen, BMS/Celgene, Janssen, Merck, Novartis, Roche, Sanofi, and Teva/Mepha. He received research support within the last 5 years from Biogen, BMS/Celgene, Chiesi, Merck, Sanofi, and Roche. He also received research grants from the Swiss MS Society, the SITEM Insel Support Fund and is a member of the Advisory Board of the Swiss and International MS Society. He also serves as deputy editor in chief for Journal of Central Nervous System disease. All conflicts are not related to this work. Jonathan A. Gernert has participated in meetings sponsored by, received travel funding and non‐financial support from Merck. He received a research grant from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; SFB/TRR 274, ID 408885537). Klaus Berek has participated in meetings sponsored by and received travel funding or speaker honoraria from Biogen, Merck, Novartis, Roche, Sanofi, and Teva. He is associate editor of Frontiers in Immunology /Neurology, Section Multiple Sclerosis and Neuroimmunology. Christiane Gradl has participated in meetings sponsored by or received honoraria (lectures, consultations) and/or travel funding from Alexion, Almirall, Amgen/Horizon, Biogen, BMS/Celgene, D‐Pharma, Merck, Novartis, Roche, Sanofi, and Teva. Dariia Kliushnikova has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, Sanofi, and Teva. All conflicts are not related to this work. Anna Damulina has participated in meetings sponsored by, received speaker honoraria or travel funding from Sanofi‐Aventis, Novartis, and Janssen. Gerhard Traxler has participated in meetings sponsored by, received honoraria (lectures, advisory boards, and consultations) or travel funding from Alexion, Amgen/Horizon, Biogen, BMS/Celgene, Janssen, Lilly, Merck, Novartis, Roche, and Sanofi. Fabian Föttinger has nothing to disclose. Sebastian Habernig has nothing to disclose. Nik Krajnc has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen, Merck, Novartis, Roche, and Sanofi. He held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Alejandro Xavier León Betancourt has nothing to disclose. Markus Ponleitner has participated in meetings sponsored by, received speaker or consulting honoraria from Amicus and travel funding from Amicus, Merck, Novartis, and Sanofi. Tobias Zrzavy has participated in meetings sponsored by or received travel funding from Biogen, Merck, Novartis, Roche, and Teva. Florian Deisenhammer has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, BMS/Celgene, Merck, Novartis, Roche, and Sanofi. His institution received scientific grants from Biogen and Sanofi. Franziska Di Pauli has participated in meetings sponsored by, received honoraria (lectures, advisory boards, and consultations) or travel funding from Bayer, Biogen, BMS/Celgene, Merck, Novartis, Sanofi, Roche, and Teva. Her institution has received research grants from Roche. Joachim Havla has received travel funding, speaker honoraria or nonfinancial support from Alexion, Amgen/Horizon, Bayer, Biogen, BMS/Celgene, Merck, Novartis and Roche, and nonfinancial support of the Sumaira‐Foundation and Guthy‐Jackson Charitable Foundation, all outside the submitted work. He holds research grants from the Friedrich‐Baur‐Stiftung, Amgen/Horizon, Merck and Sanofi. Michael Khalil has received travel funding and speaker honoraria from Bayer, Biogen, Novartis, Merck, Sanofi and Teva, and serves on scientific advisory boards for Biogen, BMS/Celgene, Gilead, Merck, Novartis, and Roche. He received research grants from Biogen, Novartis and Teva. Tania Kümpfel has received speaker honoraria and/or personal fees for advisory boards from Alexion/Astra Zeneca, Amgen/Horizon, Biogen, Chugai, Merck, Novartis, and Roche. The Institution she works for has received compensation for serving as a member of a steering committee from Roche. TK is a site principal investigator in several randomized clinical trials (BMS/Celgene, Novartis, Roche, and Sanofi) and in a randomized clinical trial supported by the BMBf (funding code: 01GM1908E). Her institution has received compensation for clinical trials all outside the present work. Peter Wipfler has participated in meetings sponsored by, received honoraria (lectures, advisory boards, and consultations) or travel funding from Alexion, Amgen/Horizon, Biogen, BMS/Celgene, Janssen, Merck, Novartis, Roche, Sanofi, SQUIBB, and Teva. Andrew Chan has received speakers'/board honoraria from Actelion (Janssen/J&J), Alexion, Almirall, Bayer, Biogen, BMS/Celgene, Merck, Novartis, Roche, Sanofi, and Teva, all for hospital research funds. He received research support from Biogen, CSL Behring, Sanofi, and UCB, the European Union, and the Swiss National Foundation. Thomas Berger has participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Amgen/Horizon, Bayer, Biogen, Bionorica, BMS, Genesis, GSK, Jazz Pharma, Janssen, MedDay, Merck, Neuraxpharma, Novartis, Octapharma, Roche, Sandoz, Sanofi, Teva and UCB. His institution has received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS, Merck, Novartis, Roche, Sanofi, Teva) and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi, Teva. Helly Hammer has received speaker/advisor honorary from Biogen, Janssen, Merck, and Teva. She received research support within the last 5 years from Biogen. She received travel grants from Biogen, Janssen, Merck, and Roche. Harald Hegen has participated in meetings sponsored by, received speaker honoraria or travel funding from Amgen/Horizon, Bayer, Biogen, BMS/Celgene, Janssen, Merck, Novartis, Sanofi, Siemens, Teva, and received honoraria for acting as consultant for Biogen, BMS/Celgene, Novartis, Roche, Sanofi, and Teva.
Supporting information
Figure S1. CONSORT flow chart.
Appendix S1: Supporting Information.
Appendix S2: Supporting Information.
Appendix S3: Supporting Information.
Bsteh G, Hoepner R, Gernert JA, et al. Switching from natalizumab to antiCD20 monoclonal antibodies: Short transition interval is associated with improved outcome. Eur J Neurol. 2025;32:e16587. doi: 10.1111/ene.16587
Helly Hammer and Harald Hegen contributed equally.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Krajnc N, Bsteh G, Berger T, Mares J, Hartung HP. Monoclonal antibodies in the treatment of relapsing multiple sclerosis: an overview with emphasis on pregnancy, vaccination, and risk management. Neurotherapeutics. 2022;19(3):753‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Prosperini L, Kinkel RP, Miravalle AA, Iaffaldano P, Fantaccini S. Post‐natalizumab disease reactivation in multiple sclerosis: systematic review and meta‐analysis. Ther Adv Neurol Disord. 2019;12:1756286419837809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brown JD, Muston BT, Massey J. Switching from natalizumab to an anti‐CD20 monoclonal antibody in relapsing remitting multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2024;86:105605. [DOI] [PubMed] [Google Scholar]
- 4. Bsteh G, Aicher ML, Walde J, et al. Association of disease‐modifying treatment with outcome in patients with relapsing multiple sclerosis and isolated MRI activity. Neurology. 2024;103:e209752. [DOI] [PubMed] [Google Scholar]
- 5. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162‐173. [DOI] [PubMed] [Google Scholar]
- 6. Alping P, Frisell T, Novakova L, et al. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950‐958. [DOI] [PubMed] [Google Scholar]
- 7. van Lierop Z, Toorop A, Coerver E, et al. Ocrelizumab after natalizumab in JC‐virus positive relapsing remitting multiple sclerosis patients. Mult Scler J Exp Transl Clin. 2021;7(2):20552173211013830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bigaut K, Kremer L, Fabacher T, et al. Ocrelizumab versus fingolimod after natalizumab cessation in multiple sclerosis: an observational study. J Neurol. 2022;269(6):3295‐3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Harrer A, Pilz G, Oppermann K, et al. From natalizumab to fingolimod in eight weeks—immunological, clinical, and radiological data in quest of the optimal switch. Clin Immunol. 2017;176:87‐93. [DOI] [PubMed] [Google Scholar]
- 10. Roos I, Malpas C, Leray E, et al. Disease reactivation after cessation of disease‐modifying therapy in patients with relapsing‐remitting multiple sclerosis. Neurology. 2022;99(17):e1926‐e1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barkhof F, Kappos L, Wolinsky JS, et al. Onset of clinical and MRI efficacy of ocrelizumab in relapsing multiple sclerosis. Neurology. 2019;93(19):e1778‐e1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa GD, Comi G. A safety review of current monoclonal antibodies used to treat multiple sclerosis. Expert Opin Drug Saf. 2023;22(11):1011‐1024. [DOI] [PubMed] [Google Scholar]
- 13. Meinl I, Adams O, Warnke C, et al. Very early detection of progressive multifocal leukoencephalopathy: should we perform cerebrospinal fluid analysis before switching from natalizumab to ocrelizumab? ECTRIMS. 2019;P665. Available from: https://www.ectrims‐congress.eu/2019/abstracts/publication.html [Google Scholar]
- 14. Bsteh G, Hegen H, Riedl K, et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: the VIAADISC score. Eur J Neurol. 2021;28:1609‐1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kalincik T, Butzkueven H. Observational data: understanding the real MS world. Mult Scler J. 2016;22(13):1642‐1648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. CONSORT flow chart.
Appendix S1: Supporting Information.
Appendix S2: Supporting Information.
Appendix S3: Supporting Information.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


